Abstract
Background
This study aimed to investigate the effects of table tennis training (TTT) versus standard occupational therapy (SOT) on visual perception and executive functions in school-age children with mild intellectual disabilities and borderline intellectual functioning.
Subjects and methods
Children (n=91) were randomly assigned to intervention with either SOT (n=46, 20 females, mean age =10.9±3.9 years) or TTT (n=45, 21 females, mean age =10.6±3.6 years), while another 41 (18 females, mean age =10.7±4.0 years) served as controls. Both the SOT and TTT programs were administered 60 minutes per session, three times a week, for 16 weeks. The Test of Visual Perceptual Skill–third edition (TVPS-3) was used to evaluate visual perception, and executive functions were assessed by the Wisconsin Card Sorting Test 64-card version (WCST-64) and the Stroop test.
Results
At postintervention, the two intervention groups significantly outperformed the control group on all measures of visual perception and executive functions. Participants in the TTT group had significantly greater before–after changes on all measures of the TVPS-3, WCST-64, and the Stroop test compared to the SOT and controls.
Conclusion
Table tennis could be considered a therapy option while treating cognitive/perceptual problems in children with mild intellectual disabilities and borderline intellectual functioning. Implications for clinical professionals and recommendations for further research are discussed.
Keywords: visual perception, executive function, table tennis, intellectual disabilities
Introduction
In addition to significant limitations in intellectual functioning and adaptive behaviors, children with intellectual disabilities (IDs) are also characterized by delay of motor-milestone attainments, impairments of sensory modulation, and deficits in cognitive–perceptual–motor (CPM) functions.1,2 Around 60% of school-age children with ID are mild ID or borderline intellectual functioning (MID/BIF). CPM deficits are common in these children, and have a profound impact on their ability to perform age-appropriate self-care and academic tasks.3,4 CPM functions encompass a mixture of cognition (eg, executive functions), perception (eg, visual perception), and motor functions, as well as implicit knowledge of the reciprocal interaction between somatosensation and kinesthetically perceived information.1,5 There is no consensus concerning the definition of executive functions. Kozial and Lutz6 defined executive function in a simple way:
… as the functions an organism employs to act independently in its own best interest as a whole, at any point in time, for the purpose for survival.
It is featured as a process that is evident at both automatic and higher-order control behaviors. Among the executive functions, deficits in action planning, shifting between cognitive strategies, and purposeful behavior regulation have been commonly observed in children with ID.7–10 Impairments in executive functions have been reported in MID/BIF as well.11
Children with MID/BIF experience difficulties in various visual–perceptual functions, which have implications for a variety of tasks requiring gross or fine motor control, and visual–motor integration.2,12,13 Previous study results have shown that individuals with ID have deficits in the following visual–perceptual functions: mental rotation ability,14 visual organization ability,13 figure–ground and visual imagery abilities,15 and visuospatial memory.16
The deficits on the cognitive and motor domains are interrelated, and the impaired cognitive functions in children appear to be related to their physical performance and activity.2,17,18 Motor development in childhood generates procedural and declarative knowledge for the development of executive function.6 As a result, early interventions to boost the cognitive and perceptual functions through motor-oriented interventions are recommended.19 Previous studies have demonstrated that moderate physical activity (exercises) and complex motor learning increase the expressions of neurotrophins (eg, BDNF) in the cerebellum, motor cortex, and task-related areas.20 Different dimensions of cognitive performance, such as processing speed, planning and control strategies, and working memory, could be improved by physical exercise and regular physical activity.21,22 As well, exercise-centered interventions have been proven to be advantageous in enhancing visual–perceptual functions in children with various developmental disabilities.23–25
Table tennis is a racket sport that requires functional pairing between perceptual and action modalities under different spatial and temporal demands.26 Table tennis training (TTT) programs include complex motor-skill learning (eg, juggling or ball skills), numerous repetition (practice), and multisensory feedbacks, and all these features can foster the emergence of neural plasticity and executive functioning.24,27 TTT has been also applied in individuals with disabilities, and studies have lent support to its effectiveness in enhancing motor proficiencies,17 physiological outcome,28 praxis skills,24 and psychosocial status.29
Health and functioning interact with and influence activities and participation based on the International Classification Framework for Children and Youth.30,31 Enhancing functional abilities (eg, visual perception, executive functions) that could increase activity participation and independence has always been the main goal of occupational therapists working with ID.13 However, impairments in executive functions and visual perception in ID are seldom treated compared to their sensorimotor problems.32 Table tennis is a compulsory part of the school physical education curriculum in Taiwan; therefore, TTT could be beneficial in facilitating students with MID/BIF integrate into the mainstream school setting through participation in racket/bat sports with typically developing peers.33 Nevertheless, TTT has seldom been considered a therapeutic activity for individuals with ID, due to the limitations of their cognitive and motor competences, such as delayed response and slower movements.34,35
We tested the following hypotheses derived from clinical experiences with CPM treatment techniques in children with MID/BIF aged 6–12 years. Both the TTT and standard occupational therapy (SOT) were delivered as training programs. First, the TTT has therapeutic effects on improving visual–perceptual and executive functions. Second, the largest treatment effect of both visual perception and executive functions can be seen in TTT groups comparing to the SOT or control groups.
Subjects and methods
Participants
The inclusion criteria were age 6–12 years, IQ of 55–85 classified by the Wechsler Intelligence Scale for Children: Chinese Version (third edition),36 or MID or BIF classified by the social affairs bureau of the local city government. Participants were categorized into two clinical groups: MID (IQ of 55–70) and BIF (IQ of 71–85). Participants with associated cardiovascular conditions, blindness, deafness, or previous neurological impairments were excluded. Individuals who had received any physical or OT in the year preceding the study were also excluded. The sample did not include children with known genetic disorders (eg, Down syndrome, fragile X syndrome, Williams syndrome) or pediatric psychiatric-related illness (eg, ADHD, depression, anxiety, etc).
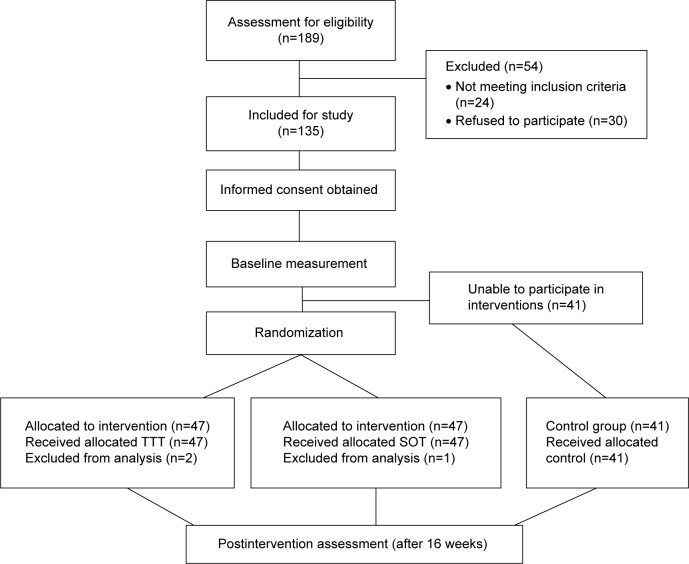
A total of 189 children with MID/BIF were recruited from three special schools and eleven regular schools in the Kaohsiung and Pingtung metropolitan areas. The parents/caregivers were informed that their children would be randomly assigned to different intervention groups: TTT or SOT. The participants would not receive the intervention program if they were chosen for the control group during the study period. Of the 189 participants recruited, 24 (12.7%) were ineligible, and 30 (15.9%) refused to participate. Of the remaining 135 participants with ID, 65 (48%) were classified as having MID, and 70 (52%) as having BIF. Forty-one of the 135 children (20 MID and 21 BIF) who had initially agreed to participate in the intervention but could not attend due to practical reasons (eg, time of the sessions) were assigned to the control group. All participants were right-handed, and they were not on any psychoactive medication.
Instruments
Study questionnaire
This study-specific questionnaire included child’s anthropometric variables (weight and height), demographic data (age, sex), received medications, treatments, and paramedical therapies.
Test of Visual Perceptual Skill – third edition
In the present study, the Test of Visual Perceptual Skill – third edition (TVPS-3)37 was used to evaluate the effectiveness of the TTT on children with MID/BIF. The TVPS-3 assesses visual perception for individuals aged 4–18 years in seven comprehensive domains: visual discrimination, visual memory, spatial relationship, visual form constancy, visual sequential memory, visual figure–ground, and visual closure. All seven subtests include two trial items and 16 formal items, which are presented in multiple-choice formats. The test–retest reliability was 0.97 in healthy individual aged 4–18 years participating in a standardization study.37 The TVPS-3 shows a moderately strong correlation (0.67) with a subtest of another similar test – the visual supplement of the Developmental Test of Visual-Motor Integration – in typically developing children.38,39
Instruments used to measure executive functioning Short form of the Wisconsin Card Sorting Test
The short form of the Wisconsin Card Sorting Test 64-card version (WCST-64)40 was used in the present study to shorten the administration time for individuals with intellectual limitations. The WCST-64 was developed to assess abstract reasoning and the ability to shift cognitive strategies in response to changing environmental contingencies. Convincing evidence has supported the comparability of the standard WCST41 and WCST-64 in various populations, including neurologic and psychiatric samples.42,43 The following indices were examined: total number correct (TNC), perseverative responses (PR), perseverative errors (PE), nonperseverative errors (NPE), conceptual-level responses (CLR), and number of categories completed (NCC). Generalizability coefficients for the WCST-64 scores on 33 healthy adults averaged 0.74, and that should be regarded as demonstrating very good scale reliability.44 Empirical validation in children and adolescents has been established as well.40
Stroop Color–Word Test, children’s version
The Stroop Color–Word Test, children’s version45 is designed to measure the cognitive abilities associated with cognitive flexibility, resistance to interference from outside stimuli, creativity, and psychopathology that influence the individual’s ability to cope with cognitive stress and process complex input. It is evident that the Stroop test is able to measure executive functions and is sensitive to the effects of exercise.46–48 Test–retest reliability coefficients with different stimuli are rather high (0.84–0.91) for the Stroop in 45 healthy female adults.49 Significant correlates of Stroop with other measures of response inhibition in children with learning disabilities demonstrated good concurrent validity of the Stroop.50 Discriminant validity of the Stroop has also been verified in screening between subgroups of disruptive children.51
Procedures
Informed consent was obtained from the participants’ parents or guardians using consent forms approved by the Institutional Review Board of Kaohsiung Medical University Hospital. All participants had the tests of visual perception and executive functions measured by the TVPS-3, WCST-64, and Stroop twice (pretest and posttest). Three research assistants majoring in OT administered all the assessments to the children according to standardized procedures provided by the appropriate test manuals. The examiners undertook an intensive 1-day training session led by the principal investigator. During training, particular attention was drawn to the tests’ explicit nature, administration, and scoring. After completing training, each of the three therapists viewed the recording of the assessment of one child and scored it individually. High levels of interrater reliability with all three instruments were reached: 0.98, 0.90, and 0.97 for the TVPS-3, WCST-64, and Stroop, respectively. All evaluators were blind to the group assignment. Testing was conducted on an individual basis in quiet locations identified at the child’s respective school or OT unit. All tests were administered on weekday mornings and completed within 3 days. Trial items of each instrument were provided to help the participants familiarize themselves with the tests. Test sessions lasted approximately 90–120 minutes, with a suitable number of breaks to minimize the effects of fatigue. The caregivers were required to keep a diary of the participants’ activities to avoid the learning effects of other related training programs or sports.
Training programs
Table tennis intervention
Intervention group 1 was enrolled in a 16-week TTT program consisting of three 60-minute sessions per week. The intervention program was administered on an individual basis in a table tennis center located at the university. All the training courses were conducted by two graduate students (coaches) with occupational therapist’s licenses; they were also members of school table tennis teams. The personnel were supervised by a senior occupational therapist with expertise in working with children with ID and who was familiar with TTT. The procedure of each single training session had the following structure: a warm-up, the main part of TTT, playing table tennis with a coach, and cooling down at the end. Adopted from the previous TTT protocol developed for developmental coordination disorder,24 the main part of TTT was simplified and had four major components: 1) serving (including how to apply topspin, sidespin, backspin, and no spin), 2) forehand bouncing, 3) backhand bouncing, and 4) continuously hitting back a ball 50 times, for which the interval, direction, and speed of the balls served by the coach were varied to provide different levels of complexity, delivered from the same end by the coach. The training intervention was implemented in a sequence of increasing difficulty where each component progressed from simple movement or practice to more complex variations. This approach was structured to achieve a particular type of training that was expected to relate to executive functions in general. The coach threw balls in three different colors (orange, white, black), and asked the participants to hit balls with the requested color in a random order. Since motor-imagery training is helpful in enhancing motor and cognitive skills,52,53 participants were taught to observe the movements of the coaches and mentally rehearse the movements. Verbal and kinetic feedback (process-oriented feedback) was consistently provided to the participants to promote their learning.
Standard occupational therapy
Intervention group 2 was enrolled in a 16-week SOT intervention program consisting of three 60-minute sessions per week. Occupational therapists use different therapeutic activities to improve the visual–perceptual and executive functions in children with disabilities. The theoretical approaches that guide treatment of visual–perceptual skills are developmental and neurophysiologic models54 that included the neurodevelopmental treatment (NDT) and perceptual motor (PM) approaches. Sensory integration (SI) approaches have often been used to improve the executive functions in children with MID/BIF.55 SOT comprises various activity combinations incorporating the principles of the SI, NDT, and PM approaches. The SI program includes activities such as linear and circular swing, tactile perception, bilateral integration and sequencing, and equilibrium reactions for the purpose of presenting the child with opportunities for various sensory experiences.56 The NDT program involves activities such as developmental movement patterns and fine motor skills.57 The PM program consists of fine and gross motor training. Unlike the NDT and SI approaches, no effort was made to control the degree or variety of sensory inputs in performing the PM training activities, nor were the inhibitory or facilitatory handling techniques directly incorporated into the PM program. The NDT, ST, and PM activities were evenly arranged during the intervention sessions.
Data analysis
SPSS 15.0 was used to analyze the data. To determine preintervention differences in test performance across three groups, multivariate analysis of variance (MANOVA) was applied, with preintervention test scores as dependent measures and group as a between-participants factor. A second MANOVA was conducted to investigate postintervention differences in test performance among groups. If the multivariate test indicated a significant group effect, follow-up univariate F-tests were performed with Scheffé post hoc comparisons.58 Follow-up univariate F-tests were performed accordingly. In light of the number of univariate analyses conducted, the α-level was set at 0.003 (0.05/17 variables) for all follow-up analyses to maintain a family-wise error rate of <0.05. Paired t-tests were used to investigate the before-and-after intervention difference of each group. To quantify the magnitude of the postintervention difference between intervention and control groups, effect sizes were calculated as d= (treatment mean – control mean)/standard deviation. As a guide to interpreting these values, Cohen59 labeled effect sizes of 0.2≤d<0.5, 0.5≤d<0.8, and d≥0.8 small, medium, and large, respectively. Effect sizes were again computed by dividing the mean change in a test score by the standard deviation of the test score at baseline to quantify the magnitude of change between pre- and postintervention test scores for each group.
Results
Group comparability
Using a computer-generated random table, the other 94 children were randomly assigned to two equal-size intervention groups (Figure 1). Due to the low attendance rate (absence rate >50%), three children dropped out and 91 completed all treatment sessions and assessments (with 45 and 46 for the TTT and SOT groups, respectively). Sample demographics and anthropometric details are presented in Table 1, and all the attributes were evenly distributed among the three groups. The three groups did not differ significantly in age (F=0.13, P=0.88), height (F=6.63, P=0.62), weight (F=14.79, P=0.75), sex (χ2=2.84, P=0.09), or numbers of ID classification (χ2=3.33, P=0.08). There was no significant difference between two of the three groups either. Before performing the MANOVA, Box’s M-test of equality of covariance matrices was conducted to test the assumptions of homogeneity of variance. Box’s M-test yielded a nonsignificant result (M=365.57, P=0.82); therefore, the assumption of homogeneity of variance–covariance matrices was supported. The MANOVA results demonstrated no significant overall group effect (Wilks’ λ=0.04, F32,254=122.33, P=0.07, partial η2=0.03). None of the univariate between-group comparisons for the TVPS-3, WCST-64, or Stroop was significant (all P>0.01). In other words, there was no significant preintervention difference in test scores between the control group and either of the intervention groups.
Figure 1.
Participant flowchart.
Abbreviations: TTT, table tennis training; SOT, standard occupational therapy.
Table 1.
Sample demographics
| Demographic | SOT (n=46) | TTT (n=45) | Control (n=41) | |||
|---|---|---|---|---|---|---|
| Age, years (mean, SD) | 10.9 | 3.9 | 10.6 | 3.6 | 10.7 | 4.0 |
| IQ classification, n (%) | ||||||
| Mild ID | 22 | 47 | 22 | 48 | 20 | 49 |
| Borderline ID | 24 | 53 | 23 | 52 | 21 | 51 |
| Height, m (mean, SD) | 1.41 | 0.06 | 1.43 | 0.08 | 1.45 | 0.06 |
| Body weight, kg (mean, SD) | 46.8 | 9.9 | 47.2 | 10.2 | 48.2 | 9.3 |
| Sex, n (%) | ||||||
| Female | 20 | 43 | 21 | 46 | 18 | 45 |
| Male | 26 | 57 | 24 | 54 | 23 | 55 |
Abbreviations: SOT, standard occupational therapy; TTT, table tennis training; SD, standard deviation; ID, intellectual disability.
Postintervention differences between groups
In regard to the group differences in postintervention test performance, the results of MANOVA revealed a significant overall group effect (Wilks’ λ=0.04, F32,254=66.12, P<0.01, partial η2=0.94). Three groups performed significantly different across test measures (Table 2). As shown in Table 3, the Scheffé multiple-comparison test showed that both the TTT and SOT groups had better performance on all measures than the controls, the TTT group outperformed the SOT group in spatial relationships (F=9.661, P=0.002), form constancy (F=6.100, P=0.002), sequential memory (F=22.848, P=0.002), and figure–ground (F=10.464, P<0.001) subtests and total scores (Table 3). There was no difference in visual discrimination (P=0.48), visual memory (P=0.51), or visual closure (P=0.66) between the TTT and SOT groups. On the WCST-64, the TTT group had better performance on all subtests than the SOT group (TNC, F=12.39, P<0.001; PR, F=15.47, P<0.001; PE, F=8.90, P<0.001; NPE, F=4.10, P=0.002; CLR, F=10.19, P<0.001; NCC, F=4.45, P=0.002). There were significant differences on the Stroop Color (F=10.63, P<0.001) and Color–Word (F=77.37, P<0.001) subtests between the two groups, and the TTT group performed better than the SOT group.
Table 2.
Summary of the postintervention standard scores for each group
| Tests | Group mean (SE) test scores
|
F* | Partial η2 | ||
|---|---|---|---|---|---|
| SOT | TTT | Controls | |||
| TVPS-3 | |||||
| DIS | 8.79 (0.97) | 9.09 (0.90) | 8.01 (1.10) | 2.95 | 0.43 |
| MEM | 6.86 (0.72) | 7.04 (0.79) | 6.13 (0.68) | 1.56 | 0.41 |
| SPA | 7.52 (0.83) | 8.07 (1.04) | 7.91 (1.08) | 9.66 | 0.08 |
| CON | 3.71 (1.12) | 4.23 (1.10) | 3.02 (1.21) | 6.10 | 0.35 |
| SEQ | 5.25 (0.88) | 5.95 (0.90) | 4.52 (0.95) | 17.10 | 1.04 |
| FGR | 5.68 (0.86) | 6.23 (0.95) | 4.61 (0.90) | 10.46 | 0.29 |
| CLO | 3.57 (0.57) | 3.55 (0.97) | 2.88 (0.92) | 8.28 | 0.10 |
| TRC | 41.38 (2.64) | 44.29 (2.47) | 37.08 (2.44) | 36.31 | 0.25 |
| WCST-64 | |||||
| TNC (+) | 37.38 (2.70) | 38.88 (1.70) | 34.12 (2.30) | 12.39 | 0.40 |
| PR (−) | 18.02 (1.82) | 16.71 (1.68) | 19.38 (4.18) | 15.47 | 0.32 |
| PE (−) | 17.75 (2.07) | 16.54 (2.24) | 18.12 (3.76) | 8.90 | 0.38 |
| NPE (−) | 11.91 (1.93) | 11.02 (2.68) | 12.89 (2.22) | 4.10 | 0.74 |
| CLR (+) | 35.39 (0.82) | 35.35 (2.59) | 33.12 (2.77) | 10.19 | 0.39 |
| NCC (+) | 3.86 (0.92) | 4.20 (0.77) | 3.13 (1.98) | 4.45 | 0.44 |
| Stroop | |||||
| Word (−) | 28.96 (1.32) | 28.52 (2.16) | 33.23 (3.33) | 1.74 | 0.22 |
| Color (−) | 42.16 (3.88) | 40.02 (3.02) | 45.98 (5.38) | 10.63 | 0.29 |
| Color–Word (−) | 77.93 (1.95) | 73.79 (2.93) | 80.71 (4.69) | 77.37 | 0.41 |
Note:
Univariate F-test significant at the 0.003 level.
Abbreviations: SE, standard error; SOT, standard occupational therapy; TTT, table tennis training; TVPS, Test of Visual Perceptual Skill; DIS, visual discrimination; MEM, visual memory; SPA, spatial relationships; CON, form constancy; SEQ, sequential memory; FGR, figure–ground; CLO, visual closure; TRC, total raw score; WCST, Wisconsin Card Sorting Test; TNC, total number correct; PR, perseverative responses; PE, perseverative errors; NPE, nonperseverative errors; CLR, conceptual-level responses; NCC, number of categories completed.
Table 3.
Post hoc Scheffé multiple comparisons at postintervention
| Test | SOT-TTT | SOT-control | TTT-control |
|---|---|---|---|
| TVPS-3 | |||
| DIS | −0.18 | 2.03* | 2.67* |
| MEM | −0.22‡ | 0.78† | 1.34* |
| SPA | −1.29* | 0.75† | 1.99* |
| CON | −0.19 | 0.61† | 1.09* |
| SEQ | −0.76† | 0.56† | 1.88* |
| FGR | −0.73† | 0.92* | 1.46* |
| CLO | −1.11* | 0.58† | 0.98* |
| TRC | −1.01* | 1.65* | 2.35* |
| WCST-64 | |||
| TNC | −2.81* | 1.34* | 1.17* |
| PR | 1.31* | 0.84* | 0.95* |
| PE | 0.99* | 0.55† | 0.99* |
| NPE | 0.87* | 0.67† | 1.23* |
| CLR | −1.26* | 0.73† | 1.01* |
| NCC | −1.33* | 0.66† | 1.26* |
| Stroop | |||
| Word | 2.11* | 0.66† | 0.92* |
| Color | 0.26‡ | 0.68† | 1.24* |
| Color–Word | 1.87* | 1.92* | 1.99* |
Notes: To quantify the magnitude of the difference between intervention and control groups at postintervention, effect sizes were calculated as d= (treatment mean – control mean)/SD. SD was calculated as the square root of the pooled estimate of population variance (SD2 = [N1 × SD12 + N2 × SD22]/[N1 + N2 – 2]).
Cohen’s d-value ≥0.8 indicates a large effect size;
Cohen’s d-value ≥0.5–0.8 indicates a medium effect size;
Cohen’s d-value ≥0.2–0.5 indicates a small effect size; P<0.003.
Abbreviations: SOT, standard occupational therapy; TTT, table tennis training; TVPS, Test of Visual Perceptual Skill; DIS, visual discrimination; MEM, visual memory; SPA, spatial relationships; CON, form constancy; SEQ, sequential memory; FGR, figure–ground; CLO, visual closure; TRC, total raw score; WCST-64, Wisconsin Card Sorting Test 64-card version; TNC, total number correct; PR, perseverative responses; PE, perseverative errors; NPE, nonperseverative errors; CLR, conceptual-level responses; NCC, number of categories completed; SD, standard deviation.
Effect sizes were provided to describe the magnitude of these between-group comparisons: SOT and TTT, SOT and control, and TTT and control (Table 3). Relative to the control group, large effect sizes were seen across all the measures for the TTT group. With regard to the SOT group, moderate-to-large effect sizes were achieved for all measures. Taken together, the SOT and TTT groups substantially outperformed the control group on most visual–perceptual and executive function measures at postintervention, and the TTT groups had larger effect sizes than the SOT groups.
Pre- and postintervention differences within groups
Results of paired t-tests indicated that both intervention groups had significant pre- and postintervention differences on all measures (all P<0.01). There was no pre- and postintervention difference in the control group, except for the CLR index of the WCST-64. Estimates of effect size for each group are summarized in Table 4. Cohen’s d-values for most before-and-after comparisons in the TTT groups noticeably exceeded 0.8 (seven of eight items of the TVPS-3, four of six in WCST-64, and two of three in Stroop), thereby reflecting robust effect sizes. On the TVPS-3, Cohen’s d-values for two before-and-after comparisons (visual discrimination, total raw score) exceeded 0.8 (large effect size) in the SOT group, four (visual discrimination, visual memory, figure–ground, and total score) exceeded 0.5 (ie, medium effect size), and three exhibited small effect sizes (form constancy, sequential memory, and visual closure). SOT had small effect sizes in most WCST-64 (four of seven) and Stroop (two of three) subtests, with the exception of large effect sizes in TNC and CLR of WCST-64 and Color–Word of Stroop. Relative to either the TTT or the SOT group, the control group showed little or decreased gains on all the measures of visual perception and executive functions.
Table 4.
Summary of intervention gains and effect sizes for each group
| Tests | SOT
|
TTT
|
Control
|
|||
|---|---|---|---|---|---|---|
| Change | Cohen’s d | Change | Cohen’s d | Change | Cohen’s d | |
| TVPS-3 | ||||||
| DIS | 2.83 | 1.03* | 2.95 | 1.67* | −0.08 | −0.13 |
| MEM | 0.65 | 0.75† | 0.98 | 1.04* | 0.02 | 0.02 |
| SPA | 0.75 | 0.65† | 1.57 | 1.71* | −0.08 | −0.09 |
| CON | 0.41 | 0.31‡ | 1.03 | 0.90* | −0.18 | −0.14 |
| SEQ | 0.34 | 0.31‡ | 0.97 | 0.88* | 0.14 | 0.11 |
| FGR | 0.79 | 0.74† | 1.28 | 1.16* | 0.22 | 0.19 |
| CLO | 0.25 | 0.46‡ | 0.93 | 0.53† | 0.08 | 0.09 |
| TRC | 6.0 | 1.33* | 10.11 | 2.23* | 0.02 | 0.13 |
| WCST-64 | ||||||
| TNC | 2.39 | 1.04* | 4.99 | 1.17* | 0.11 | 0.14 |
| PR | 1.00 | 0.44‡ | 3.04 | 0.80* | 0.05 | 0.15 |
| PE | 0.18 | 0.08 | 1.91 | 0.53† | −0.35 | −0.19 |
| NPE | 0.64 | 0.37‡ | 2.09 | 0.69† | 0.18 | 0.17 |
| CLR | 1.60 | 0.63† | 1.85 | 0.80* | 0.25 | 0.18‡ |
| NCC | 0.57 | 0.46‡ | 0.93 | 1.16* | 0.04 | 0.08 |
| Stroop | ||||||
| Word | 3.42 | 0.46‡ | 4.60 | 0.52† | 1.68 | 0.19 |
| Color | 0.98 | 0.28‡ | 3.02 | 0.84* | 1.48 | 0.18 |
| Color–Word | 1.93 | 0.92* | 5.42 | 1.99* | 0.99 | 0.08 |
Notes: To quantify the magnitude of the difference between intervention and control groups at postintervention, effect sizes were calculated as d= (treatment mean – control mean)/SD. SD was calculated as the square root of the pooled estimate of population variance (SD2 = [N1 × SD12 + N2 × SD22]/[N1 + N2 − 2]).
Cohen’s d-value ≥0.8 indicates a large effect size;
Cohen’s d-value ≥0.5–0.8 indicates a medium effect size;
Cohen’s d-value ≥0.2–0.5 indicates a small effect size; P<0.001.
Abbreviations: SOT, standard occupational therapy; TTT, table tennis training; TVPS, Test of Visual Perceptual Skill; DIS, visual discrimination; MEM, visual memory; SPA, spatial relationships; CON, form constancy; SEQ, sequential memory; FGR, figure–ground; CLO, visual closure; TRC, total raw score; WCST-64, Wisconsin Card Sorting Test 64-card version; TNC, total number correct; PR, perseverative responses; PE, perseverative errors; NPE, nonperseverative errors; CLR, conceptual-level responses; NCC, number of categories completed; SD, standard deviation.
Discussion
Although scientific evidence has supported the benefits of TTT on motor performance and psychosocial functions,24 studies regarding the effects of TTT in improving visual–perceptual and executive functions in individuals with ID is still scant. A control group and a reference group for another intervention (SOT) participated in the current study to exclude whether the improvements seen over time are simply a maturation effect. Our main finding of this study was that both visual–perceptual and executive functions in school-age children with MID/BIF improved significantly after a 16-week TTT compared to the SOT and controls. The observed effect sizes were in the moderate-to-large range. The high attendance rate in TTT (45 of 47 [96%] completed all the treatment sessions) implies that TTT was able to tap into the child’s inner drive; therefore, the therapeutic effects and psychosocial benefits could be maximized through actively participating in the TTT activities. The TTT program implemented in this study was feasible for school-age children with borderline-to-mild intellectual limitations.
Both the TTT and SOT groups had significant gains in visual–perceptual functions compared to the no-treatment control group. Of the two intervention groups, the TTT group demonstrated the largest increase in postintervention score on all TVPS-3 subtests. Significant improvement in visual–perceptual functions may be accounted for by the features characterizing TTT. First, the four components (serving, forehand bouncing, backhand bouncing, and hitting a ball) of TTT are filled with spatial–temporal challenges where individuals need to discriminate the visual object, memorize the object and location, predict the upcoming visual cues, and adjust the spatial relationships.26 Second, in terms of motor control, TTT requires precise hand–eye coordination and perception–action coupling.26,60 The individuals also have to deal with the time latency necessary to adjust motor commands based on visual information. Therefore, visual–perceptual functions contributing to visuomotor integration were improved as well.
The study revealed that both the WCST-64 and Stroop subindices were significantly improved after interventions, whereas greater influence was found in those performances in the TTT group. Success with skilled interceptive tasks relies upon sophisticated higher-level motor planning and online motor control.61,62 The context-specific visuomotor transformations of the TTT influence motor planning and movement execution for complex motor skills. Repetitive intensive training, random practice, and the observation of others were often used in the TTT intervention. These motor-learning strategies have been reported to be effective in facilitating brain plasticity of children that engage the mirror-neuron system, including areas of the frontal, parietal, and temporal lobes in the human brain.63,64 The hippocampal system and basal ganglia are also active during conditional and complex motor learning.65 Children may benefit more from exercise (eg, TTT) because their brain structures are developing.
With regard to WCST-64 performance, we found that performances on the postintervention test in TNC, PR, PE, NPE, CLR, and NCC were improved following TTT relative to the preintervention test. Greve et al indicated two distinguishing factors that could be measured in the WCST.41 The first factor, including TNC, PR, PE, CLR, and NCC, is concept formation/perseveration, and reflects the ability to shift cognitive strategies. The second factor, including only NPE, reflects inefficient and unsuccessful problem solving. As mentioned previously, children with different types of ID have revealed dysfunctions in the prefrontal cortex and subcortical areas,66–68 and these areas have been linked to executive function tapped by the WCST-64.69 The WCST-64-related executive functions include strategic planning, organized searching, utilizing environmental feedback to shift cognitive sets, directing behavior toward achieving a goal, and modulating impulsive responding.21,22,70
Compared with the SOT and control groups, the TTT group presented better performance on the Stroop test, particular in the Stroop Color–Word condition following exercise. The Stroop was used to assess processing speed, selective attention, and the ability to inhibit or shift a habitual response. Several studies have supported the view that moderate-intensity exercise (eg, TTT) benefits both lower-level cognitive processes (ie, speed of processing) and higher-level cognitive processes (ie, executive functions) in shifting the habitual response.46,48,71 The exercise-induced physiological arousal might be a potential mechanism to explain the cognitive benefits of exercise.46 While the participants were asked to hit the ball with a specific color during the TTT session, they also needed to use the anticipatory system in the brain (feed-forward mechanism) to anticipate the future spatial and temporal coordination of the flying ball; therefore, the complexity of this task and the temporal constraints have a training cognitive effect on executive functions measured by the Stroop.
Recent research supports the hypothesis that the motor and cognitive domains are highly interrelated in individuals with atypical development.72 The literature to date demonstrates that motor-activity programs can enhance both the improvement in cognitive performance (ie, speed of processing, executive function, and working memory) and the production of neurotrophins responsible for neural activities in ID.22 There is a causal link between physical activity and brain development in individuals with ID, particularly in the prefrontal cortical, rectal gyrus, medial frontal gyrus, and orbital gyrus.73 In addition, intervention aimed at improving cognitive abilities may further decrease motor impairments as well in individuals with ID.74
Although the present study has yielded findings that establish positive links among TTT, executive functions, and visual perception, there remain some limitations. First, the results of the present study reflect the TTT effects during a 16-week training intervention. Continued improvement or maintenance of visual–perceptual and executive functions would strengthen support for TTT. Therefore, replication of this study with a long-term follow-up (eg, 6 months or 1 year after intervention) is warranted to discern the long-term impact of the TTT intervention. Second, follow-up studies are also needed to verify the functional outcomes of racket sports (ie, the correlation between improved visual–perceptual and executive functions and school functions). More effort should be made to help these children generalize the training effects to functional tasks that demand similar cognitive and perceptual functions. Third, individuals with BIF and MID are different in terms of their intellectual functioning or plasticity to any type of intervention. Future study should specify if all participants are individuals with BIF or MID. Last, the subjective outcomes (eg, perceived fatigue or satisfaction level) are important, and should have been reported for clinical aspects. Future study should adopt subject-outcome measurement and provide an adverse-effect report.
Conclusion
This study is the first systematically to assess the effects of TTT on visual perception and executive functions in school-age children with MID/BIF. The present study findings provide empirical credence to the perceptions of parents, therapists, and teachers that therapeutic intervention using either TTT or SOT is effective in improving cognitive and visual–perceptual function to varying degrees in children with ID compared with no treatment. The TTT program had greater effects on visual–perceptual and executive function than the SOT or the control group; therefore, TTT could be used as adjuvant therapy to other proven successful interventions (eg, sensorimotor and PM approaches).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Batshaw ML, Shapiro B. Mental retardation. In: Batshaw ML, editor. Children with Disabilities. 5th ed. Baltimore: Paul H Brookes; 2002. pp. 287–305. [Google Scholar]
- 2.Wuang YP, Wang CC, Huang MH, Su CY. Profiles and cognitive predictors of motor functions among early school-age children with mild intellectual disabilities. J Intellect Disabil Res. 2008;52:1048–1060. doi: 10.1111/j.1365-2788.2008.01096.x. [DOI] [PubMed] [Google Scholar]
- 3.Avchen RN, Mervis CA, Yeargin-Allsopp M. Prevalence of mental retardation in children 6–10 years of age: Metropolitan Atlanta Developmental Disabilities Surveillance Program, 1991–1994; Poster presented at: 129th Annual Meeting of the American Public Health Association; October 21–25, 2001; Atlanta, GA. [Google Scholar]
- 4.Feder KP, Majnemer A. Handwriting development, competency, and intervention. Dev Med Child Neurol. 2007;49:312–317. doi: 10.1111/j.1469-8749.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilson P. Visuospatial, kinesthetic, visuomotor integration, and visuoconstructional disorders. In: Dewey D, Tupper DE, editors. Developmental Motor Disorders: A Neuropsychological Perspective. New York: Guilford Press; 2004. [Google Scholar]
- 6.Kozial LF, Lutz JT. From movement to thought: the development of executive function. Appl Neuropsychol Child. 2013;2:104–115. doi: 10.1080/21622965.2013.748386. [DOI] [PubMed] [Google Scholar]
- 7.Chen YW, Tseng MH, Hu FC, Cermak SA. Psychosocial adjustment and attention in children with developmental coordination disorder using different motor tests. Res Dev Disabil. 2009;30:1367–1377. doi: 10.1016/j.ridd.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Dewey D, Kaplan BJ, Crawford SG, Wilson BN. Developmental coordination disorder: associated problems in attention, learning, and psychosocial adjustment. Hum Mov Sci. 2002;21:905–918. doi: 10.1016/s0167-9457(02)00163-x. [DOI] [PubMed] [Google Scholar]
- 9.Loesch DZ, Bui QM, Grigsby J, et al. Effect of the fragile X status categories and the fragile X mental retardation protein levels on executive functioning in males and females with fragile X. Neuropsychology. 2003;17:646–657. doi: 10.1037/0894-4105.17.4.646. [DOI] [PubMed] [Google Scholar]
- 10.Oka K, Miura T. Allocation of attention and effect of practice on persons with and without mental retardation. Res Dev Disabil. 2005;29:165–175. doi: 10.1016/j.ridd.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Alloway TP. Working memory and executive function profiles of individuals with borderline intellectual functioning. J Intellect Disabil Res. 2010;54:448–456. doi: 10.1111/j.1365-2788.2010.01281.x. [DOI] [PubMed] [Google Scholar]
- 12.Rigoldi C, Galli M, Mainardi L, Crivellini M, Albertini G. Postural control in children, teenagers and adults with Down syndrome. Res Dev Disabil. 2011;32:170–175. doi: 10.1016/j.ridd.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Wuang YP, Su CY. Correlations of sensory processing and visual organization ability with participation in school-aged children with Down syndrome. Res Dev Disabil. 2011;32:2398–2407. doi: 10.1016/j.ridd.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Hinnell C, Virji-Babul N. Mental rotation abilities in individuals with Down syndrome – a pilot study. Downs Syndr Res Pract. 2004;9:12–16. [PubMed] [Google Scholar]
- 15.Vicari S, Bellucci S, Carlesimo GA. Evidence from two genetic syndromes for the independence of spatial and visual working memory. Dev Med Child Neurol. 2006;48:126–131. doi: 10.1017/S0012162206000272. [DOI] [PubMed] [Google Scholar]
- 16.Carretti B, Lanfranchi S, Mammarella IC. Spatial-simultaneous and spatial-sequential working memory in individuals with Down syndrome: the effect of configuration. Res Dev Disabil. 2013;34:669–675. doi: 10.1016/j.ridd.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Lin HC, Wuang YP. Strength and agility training in adolescents with Down syndrome: a randomized controlled trial. Res Dev Disabil. 2012;33:2236–2244. doi: 10.1016/j.ridd.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: a meta-analysis. Pediatr Exerc Sci. 2003;15:243–256. [Google Scholar]
- 19.Hartman E, Houwen S, Scherder E, Visscher C. On the relationship between motor performance and executive functioning in children with intellectual disabilities. J Intellect Disabil Res. 2010;54:468–477. doi: 10.1111/j.1365-2788.2010.01284.x. [DOI] [PubMed] [Google Scholar]
- 20.Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028:92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Best RJ. Effects of physical activity on children’s executive function: contributions of experimental research on aerobic exercise. Dev Rev. 2010;30:331–551. doi: 10.1016/j.dr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golubović Š, Maksimović J, Golubović B, Glumbić N. Effects of exercise on physical fitness in children with intellectual disability. Res Dev Disabil. 2012;33:608–614. doi: 10.1016/j.ridd.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Deutsch JE, Borbely M, Filler J, Huhn K, Guarrera-Bowlby P. Use of a low-cost, commercially available gaming console (Wii) for rehabilitation of an adolescent with cerebral palsy. Phys Ther. 2008;88:1196–1207. doi: 10.2522/ptj.20080062. [DOI] [PubMed] [Google Scholar]
- 24.Tsai CL. The effectiveness of exercise intervention on inhibitory control in children with developmental coordination disorder: using a visuospatial attention paradigm as a model. Res Dev Disabil. 2009;30:1269–1280. doi: 10.1016/j.ridd.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Wuang YP, Chiang CS, Su CY, Wang CC. Effectiveness of virtual reality using Wii gaming technology in children with Down syndrome. Res Dev Disabil. 2011;32:312–321. doi: 10.1016/j.ridd.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues ST, Vickers JN, Williams AM. Head, eye and arm coordination in table tennis. J Sport Sci. 2002;20:187–200. doi: 10.1080/026404102317284754. [DOI] [PubMed] [Google Scholar]
- 27.Tomporowski PD, Lambourne K, Okumura MS. Physical activity interventions and children’s mental function: an introduction and overview. Prev Med. 2011;52(Suppl 1):S3–S9. doi: 10.1016/j.ypmed.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers A, Furler BL, Brinks S, Darrah J. A systematic review of the effectiveness of aerobic exercise interventions for children with cerebral palsy: an AACPDM evidence report. Dev Med Child Neurol. 2008;50:808–814. doi: 10.1111/j.1469-8749.2008.03134.x. [DOI] [PubMed] [Google Scholar]
- 29.Taylor LM, Maddison R, Pfaeffli LA, Rawstorn JC, Gant N, Kerse NM. Activity and energy expenditure in older people playing active video games. Arch Phys Med Rehabil. 2012;93:2281–2286. doi: 10.1016/j.apmr.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 30.Simeonsson RJ, Carlson D, Hungtington GS, McMillen JS, Brent JL. Students with disabilities: a national survey of participation in school activities. Disabil Rehabil. 2001;23:49–63. doi: 10.1080/096382801750058134. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization . International Classification of Functioning, Disability and Health: Children and Youth Version. Geneva: WHO; 2007. [Google Scholar]
- 32.Wuang YP, Wang CC, Huang MH, Su CY. Efficacy of sensory integration, neuron-developmental treatment, and perceptual motor therapy on sensorimotor performance in children with mild mental retardation. Am J Occup Ther. 2009;63:439–450. doi: 10.5014/ajot.63.4.441. [DOI] [PubMed] [Google Scholar]
- 33.Fegan PL, Intellectual disabilities . In: Adapted Physical Education and Sport. 5th ed. Winnick JP, editor. Champaign (IL): Human Kinetics; 2011. pp. 151–167. [Google Scholar]
- 34.Lahtinen U, Rintala P, Malin A. Physical performance of individuals with intellectual disabilitiy: a 30-year follow-up. Adapt Phys Activ Q. 2007;24:125–143. doi: 10.1123/apaq.24.2.125. [DOI] [PubMed] [Google Scholar]
- 35.Lin YJ. The effect of adapted physical education teaching on table-tennis learning for students with mild intellectual disabilities [unpublished master’s thesis] Taipei: National Taipei University of Education; 2010. [Google Scholar]
- 36.Chen RH. Wechsler Intelligence Scale for Children: Chinese Version. 3rd ed. Taipei: Chinese Behavioral Science Corporation; 1997. [Google Scholar]
- 37.Martin N, Gardner MF. Test of Visual Perceptual Skills. 3rd ed. Novato (CA): Academic Therapy Publications; 2006. [Google Scholar]
- 38.Beery K, Beery NA. Development Test of Visual Motor Integration: Administration, Scoring, and Teaching Manual. 5th ed. Minneapolis: NCS Pearson; 2004. [Google Scholar]
- 39.Brown T, Mullins E, Stagnitti K. The reliability of performance of healthy adults on three visual perception tests. Br J Occup Ther. 2008;71:438–447. [Google Scholar]
- 40.Kongs SK, Thompson LL, Iverson GL, Heaton RK. WCST-64: Wisconsin Card Sorting Test – 64 Card Version: Professional Manual. Odessa (FL): Psychological Assessment Resources; 2000. [Google Scholar]
- 41.Greve KW, Stickle TR, Love J, Bianchini KJ, Stanford MS. Latent structure of the Wisconsin Card Sorting Test: a confirmatory factor analytic study. Arch Clin Neuropsychol. 2005;20:355–364. doi: 10.1016/j.acn.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Love JM, Greve KW, Sherwin E, Mathias P. Comparability of the standard WCST-64 in traumatic brain injury. Appl Neuropsychol. 2003;25:515–520. doi: 10.1207/s15324826an1004_7. [DOI] [PubMed] [Google Scholar]
- 43.Sherer M, Nick TG, Millis SR, Novack TA. Use of the WCST and the WCST-64 in the assessment of traumatic brain injury. J Clin Exp Neuropsychol. 2003;25:512–520. doi: 10.1076/jcen.25.4.512.13877. [DOI] [PubMed] [Google Scholar]
- 44.Cicchetti DV, Sparrow SS. Developing criteria for establishing inter-rater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic. 1981;86:127–137. [PubMed] [Google Scholar]
- 45.Golden CJ, Freshwater SM, Golden Z. Stroop Color and Word Test: Children’s Version for Ages 5–14 – A Manual for Clinical and Experimental Use. Wood Dale (IL): Stoelting Co; 2003. [Google Scholar]
- 46.Chang YK, Etnier JL. Effects of an acute bout of localized resistance exercise on cognitive performance in middle-aged adults: a randomized controlled trial study. Psychol Sport Exerc. 2009;10:19–24. [Google Scholar]
- 47.Chang YK, Etnier JL. Exploring the dose-response relationship between resistance exercise intensity and cognitive function. J Sport Exerc Psychol. 2009;31:640–656. doi: 10.1123/jsep.31.5.640. [DOI] [PubMed] [Google Scholar]
- 48.Chang YK, Liu S, Yu HH, Lee YH. Effects of acute exercise on executive function in children with attention deficit hyperactive disorder. Arch Clin Neuropsychol. 2012;27:225–237. doi: 10.1093/arclin/acr094. [DOI] [PubMed] [Google Scholar]
- 49.Siegrist M. Test-retest reliability of different versions of the Stroop test. J Psychol. 1997;131:299–306. [Google Scholar]
- 50.Cox CS, Chee E, Chase GA, et al. Reading proficiency affects the construct validity of the Stroop test interference score. Clin Neuropsychol. 1996;11:105–110. [Google Scholar]
- 51.Lavoie ME, Charlebois P. The discriminant validity of the Stroop color and word test: toward a cost-effective strategy to distinguish subgroups of disruptive preadolescents. Psychol Sch. 1994;31:98–107. [Google Scholar]
- 52.Munzert J, Lorey B, Zentgraf K. Cognitive motor processes: the role of motor imagery in the study of motor representations. Brain Res Rev. 2009;60:306–326. doi: 10.1016/j.brainresrev.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 53.Wilson PH, Thomas PR, Maruff P. Motor imagery training ameliorates motor clumsiness in children. J Child Neurol. 2002;17:491–498. doi: 10.1177/088307380201700704. [DOI] [PubMed] [Google Scholar]
- 54.Schneck CM. Visual perception. In: Case-Smith J, O’Brien JC, editors. Occupational Therapy for Children. 6th ed. Maryland Heights (MO): Mosby; 2011. pp. 389–340. [Google Scholar]
- 55.Schaaf RC, Miller LJ. Occupational therapy using a sensory integrative approach for children with developmental disabilities. Ment Retard Dev Disabil Res Rev. 2005;11:143–138. doi: 10.1002/mrdd.20067. [DOI] [PubMed] [Google Scholar]
- 56.Parham LD, Cohn ES, Spitzer S, et al. Fidelity in sensory integration intervention research. Am J Occup Ther. 2007;61:216–217. doi: 10.5014/ajot.61.2.216. [DOI] [PubMed] [Google Scholar]
- 57.Howle JM. Neurodevelopmental Treatment Approach: Theoretical Foundations and Principles of Clinical Practice. Seattle: Osseum Entertainment; 2002. [Google Scholar]
- 58.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3rd ed. Upper Saddle River (NJ): Pearson Prentice Hall; 2009. [Google Scholar]
- 59.Cohen J. Statistical Power for the Behavioral Sciences. New York: Academic Press; 1977. [Google Scholar]
- 60.Lees A. Science and the major racket sports: a review. J Sports Sci. 2003;21:707–732. doi: 10.1080/0264041031000140275. [DOI] [PubMed] [Google Scholar]
- 61.Mann DL, Abernethy B, Farrow D. Action specificity increases anticipatory performance and the expert advantage in natural interceptive tasks. Acta Psychol (Amst) 2010;135:17–23. doi: 10.1016/j.actpsy.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Mazyn LI, Savelsbergh GJ, Montagne G, Lenoir M. Planning and on-line control of catching as a function of perceptual-motor constraints. Acta Psychol (Amst) 2007;126:59–78. doi: 10.1016/j.actpsy.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 63.Buccino G, Solodkin A, Small SL. Functions of the mirror neuron system: implications for neurorehabilitation. Cogn Behav Neurol. 2006;19:55–63. doi: 10.1097/00146965-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Iacoboni M. Neural mechanisms of imitation. Curr Opin Neurobiol. 2005;15:632–637. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 65.Wise SP, Murray EA. Arbitrary associations between antecedents and actions. Trends Neurosci. 2000;23:271–276. doi: 10.1016/s0166-2236(00)01570-8. [DOI] [PubMed] [Google Scholar]
- 66.Campbell LE, Dalya E, Toal F, et al. Brain structural differences associated with the behavioural phenotype in children with Williams syndrome. Brain Res. 2009;1258:96–107. doi: 10.1016/j.brainres.2008.11.101. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto R, Backer KC, Tassone F, Hagerman RJ, Rivera SM. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS) J Psychiatr Res. 2011;45:36–43. doi: 10.1016/j.jpsychires.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teipel SJ, Hampel H. Neuroanatomy of Down syndrome in vivo: a model of preclinical Alzheimer’s disease. Behav Genet. 2006;36:405–415. doi: 10.1007/s10519-006-9047-x. [DOI] [PubMed] [Google Scholar]
- 69.Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage. 2006;30:1038–1049. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 70.Mouelhi Guizani S, Tenenbaum G, Bouzaouach I, Ben Kheder A, Feki Y, Bouaziz M. Information-processing under incremental levels of physical loads: comparing racquet to combat sports. J Sports Med Phys Fitness. 2006;46:335–343. [PubMed] [Google Scholar]
- 71.Sibley BA, Etnier JL, Le Masurier GC. Effects of an acute bout of exercise on cognitive aspects of Stroop performance. J Sport Exerc Psychol. 2006;28:285–299. [Google Scholar]
- 72.Alesi M, Battaglia G, Roccella M, Testa D, Palma A, Pepi A. Improvement of gross and cognitive abilities by an exercise training program: three case reports. Neuropsychiatry Dis Treat. 2014;10:479–485. doi: 10.2147/NDT.S58455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin I, Park EY. Meta-analysis of the effect of exercise programs for individuals with intellectual disabilities. Res Dev Disabil. 2012;33:1937–1947. doi: 10.1016/j.ridd.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Yildirim N, Erbahçeci F, Ergun N, Kenneth HP, Beets M. The effect of physical fitness training on reaction time in youth with intellectual disabilities. Percept Mot Skills. 2010;111:178–186. doi: 10.2466/06.10.11.13.15.25.PMS.111.4.178-186. [DOI] [PubMed] [Google Scholar]