Abstract
Background/Aims
Currently, the videofluoroscopic swallowing study (VFSS) is the standard tool for evaluating dysphagia. We evaluated whether the addition of endoscopist-directed flexible endoscopic evaluation of swallowing (FEES) to VFSS could improve the detection rates of penetration, aspiration, and pharyngeal residue, compared the diagnostic efficacy between VFSS and endoscopist-directed FEES and assessed the adverse events of the FEES.
Methods
In single tertiary referral center, a retrospective analysis of prospectively collected data was conducted. Fifty consecutive patients suspected of oropharyngeal dysphagia were enrolled in this study between January 2012 and July 2012.
Results
The agreement in the detection of penetration and aspiration between VFSS and FEES of viscous food (κ=0.34; 95% confidence interval [CI], 0.15 to 0.53) and liquid food (κ=0.22; 95% CI, 0.02 to 0.42) was “fair.” The agreement in the detection of pharyngeal residue between the two tests was “substantial” with viscous food (κ=0.63; 95% CI, 0.41 to 0.94) and “fair” with liquid food (κ=0.37; 95% CI, 0.10 to 0.63). Adding FEES to VFSS significantly increased the detection rates of penetration, aspiration, and pharyngeal residue. No severe adverse events were noted during FEES, except for two cases of epistaxis, which stopped spontaneously without requiring any packing.
Conclusions
This study demonstrated that the addition of endoscopist-directed FEES to VFSS increased the detection rates of penetration, aspiration, and pharyngeal residue.
Keywords: Deglutition disorders, Endoscopy, Videofluoroscopic swallowing study
INTRODUCTION
Oropharyngeal dysphagia (OPD) is significantly associated with nutritional deficiency and aspiration pneumonia, which can result in death.1–4 Therefore, the accurate assessment of penetration or aspiration is important in patients with suspected OPD. Unfortunately, clinical assessment alone underestimates the risk of aspiration by 50%.5 Currently, a videofluoroscopic swallowing study (VFSS) is the standard tool for evaluation of dysphagia.6 However, its accuracy depends on the examiner’s experience.7 VFSS performance is severely limited in patients who cannot sit. Furthermore, this limitation relates to prolonged exposure to radiation and the requirement for fluoroscopy equipment.
Endoscopy, which is used to identify benign and malignant lesions of the laryngopharynx and upper esophagus, may be limited in the field of OPD. In 1988, Langmore et al.8 published the first report describing the use of flexible endoscopy for the purpose of assessing dysphagia, termed flexible endoscopic evaluation of swallowing (FEES). FEES can directly observe the laryngopharyngeal structures and movement as well as delivery of various foods and liquids.9 In addition, the procedure can be performed at the bedside and does not require fluoroscopic equipment. To date, this procedure is administered using a nasal endoscope by an otolaryngologist or a speech language pathologist (SLP). A recent study reported that even when clinicians with no experience in nasal endoscopy performed FEES after only listening to a 30-minute lecture, they obtained reliable evaluation results.10 OPD is frequently encountered in gastroenterology practice, and the pharynx is a typical area within the practice field of an endoscopist. To our knowledge, there is no study regarding FEES performed in OPD patients by an endoscopist. Several studies have compared VFSS and otolaryngologist/SLP-directed FEES.11,12 However, there is no comparative study of the detection of penetration, aspiration, and pharyngeal residue by VFSS and endoscopist-directed FEES.
In this study, we evaluated whether the addition of endoscopist-directed FEES to VFSS could improve the detection rates of penetration, aspiration, and pharyngeal residue. This study also compared the diagnostic efficacy between VFSS and endoscopist-directed FEES and assessed any adverse FEES events.
MATERIALS AND METHODS
1. Subjects
Seventy-three consecutive participants were patients suspected of having OPD. The diagnosis of OPD was made by review of the clinical history, particularly difficulty in swallowing food or pills, changes in swallowing ability, coughing or choking when eating, shortness of breath during swallowing, food backing up into the mouth or nasal passage, fever or voice changes after swallowing, pain when swallowing, and unexplained weight loss. They recruited for the assessment of VFSS and endoscopist-directed FEES based on the taking of food or fluids by mouth and determined not to be at high risk of aspiration on the basis of clinical evaluations of swallowing. The two examinations were performed on the same day in the Department of Physical Medicine and Rehabilitation and Institute for Digestive Research, Soonchunhyang University Hospital, respectively. Patients were excluded if (1) they had absolute contraindications such as shock, peritonitis, perforated viscous, severe cardiac decompensation, (2) had relative contraindications including obtunded or uncooperative subjects, coma and cardiac arrhythmias, (3) were allergic to lidocaine, and (4) had active nasal bleeding which was uncontrolled at the time of evaluation. Twenty-three of 73 consecutive OPD patients were excluded. A total of 50 consecutive patients were enrolled in this study between January 2012 and July 2012. The study protocol was approved by the Institutional Review Board of Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea. All subjects gave written informed consent before commencement of the study.
2. Methods
1) VFSS protocol
Subjects initially sat laterally and were observed for 4 to 5 seconds to stabilize their positions. For the VFSS test diets, we first used a 5-mL liquid barium (barium sulfate; Solotop suspension 140a)-blended yogurt followed by 5-mL liquid barium diluted with water (when appropriate). While patients sat in a wheelchair, we used a fluoroscope to view lateral images of the head, neck, and upper chest. The lateral fluoroscopic screening field was set when vocal fold was located at the center and the mandibular angles of both sides were matched completely. The food bolus was kept on the screening field throughout the swallowing process. The entire clinical procedure was recorded on video, and the videotape of the procedure was analyzed by an experienced physiatrist (J.W.P.). The VFSS measures included penetration, aspiration, and pharyngeal residue.
Original eight-point Penetration-Aspiration Scale (PAS)13 considers not only invasive depth but also clearance and response. However, there are considerable difficulties in accurately determining elimination of the ingesta by the cough reflex. It is also unclear whether the different levels have clinically different meanings. It was necessary to facilitate statistical analysis and encourage observers to provide a definite statement. Therefore, we reconstructed the scale and redefined level 1 as normal, levels 2–5 as penetration (when material remained above the vocal cord or reached the vocal cord), and levels 6–8 as aspiration (when material passed the glottis).
We also used the binary parameters of presence versus absence in the assessment of the pharyngeal residue instead of its grading system.14 Pharyngeal residue was defined as retention of the material that exceeded a thin mucosal coating in the valleculae or pyriform sinuses after swallowing.
2) Endoscopist-directed FEES protocol
The FEES was performed within 24 hours of the VFSS by an experienced endoscopist (T.H.L.), who was blinded to the results of the VFSS. The endoscopist had 7 years of experience in endoscopy and attended a 2-hour FEES lecture at the Korean Dysphagia Society before this study. A thin video gastroscope (Olympus GIF-XP 260; Olympus, Tokyo, Japan), with a 6.5-mm insertion tube, 2.0-mm channel and a working length of 1,030 mm was used during FEES.
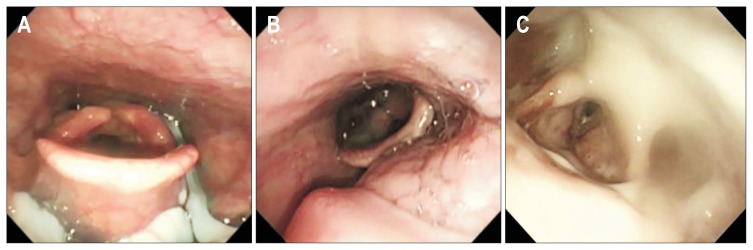
The endoscope was inserted through the nostril and placed between the end of the soft palate and the epiglottis. The patient was then allowed a 1-minute rest period to adapt to the presence of the laryngoscope and prepare for testing. The examination consisted of anatomic-physiologic assessment (including velar and laryngopharyngeal anatomy, movement, and sensation) and direct examination of swallowing test diets. When clinically indicated, an entire anatomic assessment of esophagus and stomach was performed. For the FEES test diets, we first used a 5-mL yogurt for viscous food followed by 5-mL indigocarmine dye-mixed water for liquid food (for ease of visualization as part of the FEES protocol adopted in the hospital). To minimize the possibility of aspiration during FEES, patients who had a compromised ability to swallow their own saliva and aspiration during viscous food swallowing were not given liquid food. The entire clinical procedure was recorded on video, and the videotape of the procedure was analyzed by the endoscopist (T.H.L.). The FEES measures included penetration, aspiration, and pharyngeal residue. Eight-point PAS was documented in all subjects. To facilitate statistical analysis, we reconstructed the scale and redefined level 1 as normal, levels 2–5 as penetration (food material entered into the laryngeal vestibule but not below the true vocal cord) (Fig. 1A), and levels 6–8 as aspiration (food material entered the airway below the true vocal cord) (Fig. 1B). Pharyngeal residue was defined as retention of the entire given material in the valleculae or pyriform sinuses after the swallow. However, double swallows are common in normal subjects, as well as in OPD patients. A previous study suggested that a small amount of residue, estimated at no more than 10% to 15% of the entire bolus, after first swallow should be considered a normal finding.15 Therefore, we defined pharyngeal residue in valleculae or pyriform sinuses as follows (Fig. 1C): (1) medium to large amount of residue after first swallow; (2) small amount of residue even after a double swallow.
Fig. 1.
Penetration, aspiration, and pharyngeal residue detected on endoscopist-directed flexible endoscopic evaluation of swallowing. (A) Penetration was defined when food material entered the laryngeal vestibule but did not pass below the true vocal cords. (B) Aspiration was defined as food material entering the airway below the true vocal cords. (C) Pharyngeal residue was defined as retention of >15% of a given entire material in valleculae or the pyriform sinuses.
3. Adverse events
To determine the safety of endoscopist-directed FEES, patients were monitored throughout the FEES examination for epistaxis, vasovagal syncope, airway compromise (oxygen saturation level below 90% or complaint of dyspnea), and/or significant change in cardiovascular function (either decrease or increase of 20 mm Hg in blood pressure or 20 beats per minute in heart rate). Discomfort level during the examination was rated as “none,” “mild,” “moderate,” or “severe” as an indicator of aversion to the procedure.
4. Statistical analysis
We used the κ-value with 95% confidence interval (CI) to analyze the agreement of penetration, aspiration, and pharyngeal residue for overall results and diets between the two tests. Interpretation of κ-values was performed according to Landis and Koch.16 McNemar test was used to determine whether FEES was superior to detect presence of penetration, aspiration, and pharyngeal residue compared with VFSS, and whether the simultaneous VFSS with FEES improved the detection rate compared to VFSS alone.17 The significance level was set at 0.05 and SPSS 14.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
RESULTS
The clinical characteristics of all patients are listed in Table 1. There were 31 males (62%) and 19 females (38%). The patients’ age ranged from 26 to 88 years (mean age, 67.8 years). The most common cause of dysphagia was ischemic stroke (38%).
Table 1.
Baseline Characteristics of Subjects
| Characteristic | Value |
|---|---|
| Gender | |
| Male | 31 (62) |
| Female | 19 (38) |
| Age, yr | 67.8±14.7 |
| Causes of dysphagia | |
| Ischemic stroke | 19 (38) |
| Hemorrhagic stroke | 13 (26) |
| Malignancy | 5 (10) |
| Dementia | 4 (8) |
| Deconditioned state | 4 (8) |
| Traumatic brain injury | 3 (6) |
| Parkinson’s disease | 1 (2) |
| Neuromuscular disease | 1 (2) |
Data are presented as number (%) or mean±SD.
1. Agreement between VFSS and FEES
Overall, there was moderate agreement between VFSS and FEES results in detecting the presence of penetration and aspiration (κ=0.49; 95% CI, 0.30 to 0.67). Ten patients who did not have an examination with liquid food via VFSS or FEES were excluded. Fifty and 40 patients were evaluated for swallowing tests with viscous or liquid food, respectively. There was fair agreement between VFSS and FEES with viscous food (κ=0.34; 95% CI, 0.15 to 0.53) and liquid food as shown in Table 2 (κ=0.22; 95% CI, 0.02 to 0.42). Overall, the agreement in pharyngeal residue between VFSS and FEES was moderate (κ=0.53; 95% CI, 0.29 to 0.77). In addition, there was strong agreement in detection of pharyngeal residue between the two tests with viscous food (κ=0.63; 95% CI, 0.41 to 0.94) and fair agreement with liquid food, as shown in Table 3 (κ=0.37; 95% CI, 0.10 to 0.63).
Table 2.
Agreement in Terms of Penetration, Aspiration of Liquid, and Viscous Swallows between a Videofluoroscopic Swallowing Study and Endoscopist-Directed Flexible Endoscopic Evaluation of Swallowing
| Variable | VFSS | Total | ||
|---|---|---|---|---|
|
| ||||
| None | Penetration | Aspiration | ||
| Viscous food* | ||||
| FEES | ||||
| None | 25 | 0 | 0 | 25 |
| Penetration | 8 | 2 | 0 | 10 |
| Aspiration | 8 | 2 | 5 | 15 |
| Total | 41 | 4 | 5 | 50 |
| Liquid food† | ||||
| FEES | ||||
| None | 13 | 2 | 1 | 16 |
| Penetration | 5 | 2 | 0 | 7 |
| Aspiration | 8 | 4 | 5 | 17 |
| Total | 26 | 8 | 6 | 40 |
VFSS, videofluoroscopic swallowing study; FEES, flexible endoscopic evaluation of swallowing.
There was “fair” agreement between VFSS and FEES on viscous food (κ=0.34; 95% confidence interval [CI], 0.15 to 0.53);
There was “fair” agreement between VFSS and FEES on liquid food (κ=0.22; 95% CI, 0.02 to 0.42).
Table 3.
Agreement in Terms of Pharyngeal Residue during Liquid and Viscous Swallows between a Videofluoroscopic Swallowing Study and Endoscopist-Directed Flexible Endoscopic Evaluation of Swallowing
| Variable | VFSS residue | Total | |
|---|---|---|---|
|
| |||
| Negative | Positive | ||
| Viscous food* | |||
| FEES residue | |||
| Negative | 16 | 2 | 18 |
| Positive | 7 | 24 | 31 |
| Total | 23 | 26 | 49 |
| Liquid food† | |||
| FEES residue | |||
| Negative | 18 | 1 | 19 |
| Positive | 10 | 7 | 17 |
| Total | 28 | 8 | 36 |
VFSS, videofluoroscopic swallowing study; FEES, flexible endoscopic evaluation of swallowing.
There was “substantial” agreement between VFSS and FEES on viscous food (κ=0.63; 95% confidence interval [CI], 0.41 to 0.84);
There was “fair” agreement between VFSS and FEES on liquid food (κ=0.37; 95% CI, 0.10 to 0.63).
2. Detection rate
FEES had a superior detection rate of penetration compared to VFSS with viscous food (p<0.001) and liquid food (p=0.021). However, FEES had the same detection rate of penetration in the overall results (p=0.065). In addition, FEES had a superior detection rate of aspiration compared to VFSS with viscous food (p<0.001), liquid food (p<0.001) and in the overall results (p<0.001).
The detection rate of pharyngeal residue between the two tests was similar for both viscous food (p=0.180) and the overall results (p=0.227). However, FEES had a superior detection rate of pharyngeal residue with liquid food (p=0.012).
For all diets, adding FEES to VFSS significantly increased the detection rate compared to VFSS alone (Table 4). In addition, there was a significant increase in the detection rate in the overall results of pharyngeal residue between VFSS and adding FEES to VFSS (Table 4).
Table 4.
Rates of Detection of Penetration, Aspiration, and Pharyngeal Residue by Videofluoroscopic Swallowing Study and Endoscopist-Directed Flexible Endoscopic Evaluation of Swallowing
| Variable | Penetration† | Aspiration | Pharyngeal residue | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| VFSS | VFSS+FEES | p-value | VFSS | VFSS+FEES | p-value | VFSS | VFSS+FEES | p-value | |
| Viscous food | 9/50 (0.18) | 25/50 (0.50) | <0.001* | 5/50 (0.10) | 15/50 (0.30) | <0.001* | 26/49 (0.53) | 33/49 (0.67) | <0.016* |
| Liquid food | 14/40 (0.35) | 27/40 (0.68) | <0.001* | 6/40 (0.15) | 18/40 (0.45) | <0.001* | 8/36 (0.22) | 18/36 (0.50) | <0.001* |
VFSS, videofluoroscopic swallowing study; FEES, flexible endoscopic evaluation of swallowing.
p<0.05 by exact McNemar test;
This also included aspiration.
3. Adverse events
There were no occurrences of vasovagal syncope, airway compromise or significant change in cardiovascular function during FEES examination. There were two cases of epistaxis (4%) that resolved spontaneously without requiring any packing. Most of the patients (94%) had “none” or “mild” discomfort level during FEES.
DISCUSSION
This study is the first to demonstrate that endoscopist-directed FEES is not inferior to VFSS in detecting the presence of penetration, aspiration, and pharyngeal residue, and can be performed safely in OPD patients. Endoscopist-directed FEES can additionally provide anatomical and functional information other than swallowing function.
Results of the present study are in agreement with earlier reports11,12,18–21 that FEES is more sensitive than VFSS in the assessment of penetration, aspiration, and pharyngeal residue. The addition of FEES increased the detection rate as compared to VFSS alone. Recently a systematic comparative review of FEES and VFSS showed that FEES had better sensitivity for penetration (97% vs 83%), aspiration (88% vs 77%), and pharyngeal residue (97% vs 80%).22 However, the differences in the assessment of penetration, aspiration, and pharyngeal residue between VFSS and FEES are due not to their definitions but the instruments used. However, the present study demonstrated agreement between VFSS and FEES regarding penetration, aspiration, and liquid residue. This indicates that the results of VFSS and FEES may not be interpreted in the same way and are not mutually interchangeable.
FEES could identify penetration or aspiration appropriately in certain cases, whereas VFSS could not. Similarly, VFSS was found to identify penetration or aspiration appropriately in certain cases, whereas FEES did not. Furthermore, FEES was more sensitive than VFSS for the detection of pharyngeal residue in certain cases. These findings indicate that the use of both VFSS and FEES may reduce the risk of aspiration and therefore that the two methods should be used together.
The sensitivity of FEES was unchanged when performed by an endoscopist inexperienced in the field of endoscopic dysphagia assessment. Warnecke et al.10 reported that FEES following a simplified protocol can be performed reliably with minimal experience. These results suggest that FEES can be widely used in the practice of gastroenterology even after training comprising only a short lecture on FEES.
When FEES was introduced, there were reasonable concerns regarding its safety. Laryngospasm is the most serious complication that can occur during vigorous manipulation of vocal cords;23 laryngospasm was not experienced in the present study. Epistaxis is the most common complication, occurring in less than 1% of patients.23,24 The incidence of self-limiting epistaxis was somewhat higher in the present study. This might have been due to passage of the relatively large-caliber flexible endoscope. Therefore, we expected that epistaxis would be preventable using a smaller-caliber flexible endoscope. Furthermore, the present study was in agreement with earlier reports23,24 that no serious complications resulted from use of an endoscope. The results indicated that endoscopist-directed FEES could also be performed with acceptable safety.
In addition to evaluation of swallowing function by endoscopist-directed FEES, the procedure allowed us to reveal any anatomical or functional abnormalities of the larynx, pharynx, esophagus, and even the stomach. Endoscopist-directed FEES can discover additional information regarding esophageal dysphagia. A careful history may determine the location of dysphagia (i.e., OPD vs esophageal dysphagia) in over 80% of patients.25 However, the location of the sense of dysphagia may not be suggestive of the obstructive site because symptoms may be referred proximally.26 The endoscope can be used for suction of pharyngeal residue in cases where a patient can not eliminate the residue by repeated swallowing.
This study had several limitations. First, VFSS and FEES were not performed simultaneously; however FEES was performed within 24 hours of VFSS. The shortest possible time period between the two tests minimizes the recovery effect of affected swallowing over time. FEES was also performed by an endoscopist who was not blinded to the results of the VFSS. Therefore, our results might not have been subject to bias. Second, swallowing tests using different foods (such as puree or soft solid food) were not performed in either endoscopist-directed FEES or VFSS. Third, obviously both techniques were operator dependent and outcome and findings depended on the challenging substances and strategies. Finally, whether the higher detection rate associated with endoscopist-directed FEES results in better health outcomes, such as a decreased rate of pneumonia, remains unclear because of the design of this study.
In conclusion, our data suggest that the role of endoscopists can be extended to the field of OPD. Further studies should assess whether the favorable results of endoscopist-directed FEES are reproducible when performed by different endoscopists.
ACKNOWLEDGEMENTS
This work was supported by the Soonchunhyang University Research Fund.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Guyomard V, Fulcher RA, Redmayne O, Metcalf AK, Potter JF, Myint PK. Effect of dysphasia and dysphagia on inpatient mortality and hospital length of stay: a database study. J Am Geriatr Soc. 2009;57:2101–2106. doi: 10.1111/j.1532-5415.2009.02526.x. [DOI] [PubMed] [Google Scholar]
- 2.Johnson ER, McKenzie SW, Sievers A. Aspiration pneumonia in stroke. Arch Phys Med Rehabil. 1993;74:973–976. [PubMed] [Google Scholar]
- 3.Martin BJ, Corlew MM, Wood H, et al. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia. 1994;9:1–6. doi: 10.1007/BF00262751. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt J, Holas M, Halvorson K, Reding M. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia. 1994;9:7–11. doi: 10.1007/BF00262752. [DOI] [PubMed] [Google Scholar]
- 5.Logemann JA, Roa Pauloski B, Rademaker A, et al. Impact of the diagnostic procedure on outcome measures of swallowing rehabilitation in head and neck cancer patients. Dysphagia. 1992;7:179–186. doi: 10.1007/BF02493468. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Harris B, Jones B. The videofluorographic swallowing study. Phys Med Rehabil Clin N Am. 2008;19:769–785. doi: 10.1016/j.pmr.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough GH, Wertz RT, Rosenbek JC, Mills RH, Webb WG, Ross KB. Inter- and intrajudge reliability for videofluoroscopic swallowing evaluation measures. Dysphagia. 2001;16:110–118. doi: 10.1007/PL00021291. [DOI] [PubMed] [Google Scholar]
- 8.Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia. 1988;2:216–219. doi: 10.1007/BF02414429. [DOI] [PubMed] [Google Scholar]
- 9.Leder SB, Murray JT. Fiberoptic endoscopic evaluation of swallowing. Phys Med Rehabil Clin N Am. 2008;19:787–801. doi: 10.1016/j.pmr.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Warnecke T, Teismann I, Oelenberg S, et al. Towards a basic endoscopic evaluation of swallowing in acute stroke: identification of salient findings by the inexperienced examiner. BMC Med Educ. 2009;9:13. doi: 10.1186/1472-6920-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly AM, Drinnan MJ, Leslie P. Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare? Laryngoscope. 2007;117:1723–1727. doi: 10.1097/MLG.0b013e318123ee6a. [DOI] [PubMed] [Google Scholar]
- 12.Kelly AM, Leslie P, Beale T, Payten C, Drinnan MJ. Fibreoptic endoscopic evaluation of swallowing and videofluoroscopy: does examination type influence perception of pharyngeal residue severity? Clin Otolaryngol. 2006;31:425–432. doi: 10.1111/j.1749-4486.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 14.Perlman AL, Booth BM, Grayhack JP. Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia. 1994;9:90–95. doi: 10.1007/BF00714593. [DOI] [PubMed] [Google Scholar]
- 15.Langmore SE. Scoring a FEES examination. In: Langmore SE, editor. Endoscopic evaluation and treatment of swallowing disorders. 2nd ed. New York: Thieme; 2001. pp. 101–143. [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 17.Bennett BM. On comparisons of sensitivity, specificity and predictive value of a number of diagnostic procedures. Biometrics. 1972;28:793–800. doi: 10.2307/2528763. [DOI] [PubMed] [Google Scholar]
- 18.Kaye GM, Zorowitz RD, Baredes S. Role of flexible laryngoscopy in evaluating aspiration. Ann Otol Rhinol Laryngol. 1997;106:705–709. doi: 10.1177/000348949710600817. [DOI] [PubMed] [Google Scholar]
- 19.Leder SB, Sasaki CT, Burrell MI. Fiberoptic endoscopic evaluation of dysphagia to identify silent aspiration. Dysphagia. 1998;13:19–21. doi: 10.1007/PL00009544. [DOI] [PubMed] [Google Scholar]
- 20.Périé S, Laccourreye L, Flahault A, Hazebroucq V, Chaussade S, St Guily JL. Role of videoendoscopy in assessment of pharyngeal function in oropharyngeal dysphagia: comparison with videofluoroscopy and manometry. Laryngoscope. 1998;108:1712–1716. doi: 10.1097/00005537-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Wu CH, Hsiao TY, Chen JC, Chang YC, Lee SY. Evaluation of swallowing safety with fiberoptic endoscope: comparison with videofluoroscopic technique. Laryngoscope. 1997;107:396–401. doi: 10.1097/00005537-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Giraldo LF, Leal LR, Leon GA, Bastidas AR, Garcia R, Ovalle S. Systematic review and meta-analysis of the accuracy of flexible endoscopic evaluation of swallowing (FEES) and videofluoroscopic swallowing study (VFSS) for the diagnosis of oropharyngeal dysphagia in adults. Am J Respir Crit Care Med. 2013;187:A5678. [Google Scholar]
- 23.Aviv JE, Kaplan ST, Thomson JE, Spitzer J, Diamond B, Close LG. The safety of flexible endoscopic evaluation of swallowing with sensory testing (FEESST): an analysis of 500 consecutive evaluations. Dysphagia. 2000;15:39–44. doi: 10.1007/s004559910008. [DOI] [PubMed] [Google Scholar]
- 24.Aviv JE, Murry T, Zschommler A, Cohen M, Gartner C. Flexible endoscopic evaluation of swallowing with sensory testing: patient characteristics and analysis of safety in 1,340 consecutive examinations. Ann Otol Rhinol Laryngol. 2005;114:173–176. doi: 10.1177/000348940511400301. [DOI] [PubMed] [Google Scholar]
- 25.Castell DO, Donner MW. Evaluation of dysphagia: a careful history is crucial. Dysphagia. 1987;2:65–71. doi: 10.1007/BF02408136. [DOI] [PubMed] [Google Scholar]
- 26.Lind CD. Dysphagia: evaluation and treatment. Gastroenterol Clin North Am. 2003;32:553–575. doi: 10.1016/S0889-8553(03)00024-4. [DOI] [PubMed] [Google Scholar]