Abstract
Background
Peripheral neuropathy is the dose limiting toxicity of bortezomib in patients with multiple myeloma (MM).
Objectives
To examine the safety, feasibility and efficacy of acupuncture in reducing bortezomib-induced peripheral neuropathy (BIPN) symptoms.
Methods
Patients with MM experiencing persistent BIPN ≥grade 2 despite adequate medical intervention and discontinuation of bortezomib received 10 acupuncture treatments for 10 weeks (2×/week for 2 weeks, 1×/week for 4 weeks, and then biweekly for 4 weeks). Responses were assessed by the Clinical Total Neuropathy Score (TNSc), Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity (FACT/GOG-Ntx) questionnaire, and the Neuropathy Pain Scale (NPS). Repeated-measures analysis of variance was used to test for monotonic decline in scores on each of the measures. Serial serum levels of proinflammatory and neurotrophic cytokines were obtained at baseline and weeks 1, 2, 4, 8, and 14.
Results
Twenty-seven patients with MM were enrolled in the trial. There were no adverse events associated with the acupuncture treatments. TNSc data were deemed invalid and therefore were not reported. At weeks 10 and 14, FACT/GOG-Ntx and NPS showed significant reduction suggesting decreased pain, and improved function (P values were <.0001 for both FACT/GOG-Ntx and NPS at weeks 10 and 14). However, nerve conduction studies did not significantly change between baseline assessment and end of study. There was no correlation in serum cytokines for responders versus none responders.
Conclusions
Acupuncture is safe, feasible and produces subjective improvements in patients’ symptoms. A follow-up randomized controlled trial is warranted.
Keywords: acupuncture, bortezomib, peripheral neuropathy, multiple myeloma
Introduction
Bortezomib is an effective therapy for relapsed and newly diagnosed multiple myeloma (MM) patients.1 Bortezomib-induced peripheral neuropathy (BIPN) is a common and debilitating side effect of bortezomib therapy, affecting 37% to 44% of patients.2 BIPN occurs gradually within the first 5 cycles of treatment and typically requires dose modification or interruption of therapy, thus negatively affecting the therapeutic benefits. In most patients, BIPN is reversible, with symptom improvement or resolution at a median of 1.5 to 4 months (range 0.1–12.5 months) after bortezomib discontinuation.3–5 In some patients, BIPN can persist for years after discontinuation and may significantly impact patients’ quality of life.6 Painful BIPN accounts for 5% to 10% of BIPN and usually occurs suddenly within the first 3 cycles of bortezomib. It tends to be more severe, and takes longer to resolve after drug discontinuation.4,7
The clinical characteristics of BIPN include paresthesia (tingling, burning sensation), hyperalgesia (increased sensitivity to noxious stimulation), allodynia (pain that results from a normally innocuous stimulation), and decreased physical activity.8 Some patients may experience neuropathic pain in the “stocking and glove” distribution, with an average pain score of 7.8 out of 10.9 Other signs and symptoms include distal sensory loss (with greater loss in lower extremities than the upper), decreased deep tendon reflexes, and changes in proprioception affecting daily activities. Up to 10% of patients with BIPN experience grades 1 to 3 motor neuropathy.2 Affected areas tend to have increased sharpness detection thresholds and lower skin temperature than unaffected areas.9 Electrophysiology studies show that patients with BIPN have a number of measurable alterations in nerve conduction function, including lower amplitude of sensory action potentials, prolonged latency of H-reflex, decreased amplitude of compound muscle action potentials, and slowing of sensory and motor conduction velocities.2
Risk factors for BIPN include multiple myeloma diagnosis, higher cumulative dose of bortezomib (with a threshold of 30 mg/m2), preexisting neuropathy, age greater than 75 years, and renal or hepatic dysfunction.2,4,7 Treatment for BIPN has been limited to symptomatic management with narcotics, antidepressants, anticonvulsants, and vitamins, although data supporting their efficacy are limited.2 Many of these drugs are themselves associated with problematic and unpleasant side effects such as dizziness, sedation, dry mouth, and constipation, adding to patients’ poor quality of life.2,7,9
Acupuncture, a traditional Chinese medicine technique, is safe and has shown promise for reducing the symptoms of chemotherapy-induced peripheral neuropathy (CIPN) in several studies.10–14 A few studies have examined the effect of acupuncture in reducing neuropathic pain in cancer patients. In a blinded, randomized controlled trial patients treated with acupuncture had a 36% reduction in pain intensity by the end of 2 months, whereas placebo treated patients only had only a 2% reduction (P < .0001).15 Similar findings were described in other reports.12,16 In one study, a positive correlation between the improvement in symptoms and nerve conduction function was observed, suggesting that acupuncture may accelerate nerve regeneration.16
To date, there have been no clinical trials conducted to study the effect of acupuncture in alleviating BIPN symptoms in patients with MM.8 Our prior studies included a case report and case series of 5 MM patients with BIPN who improved significantly after acupuncture treatment.17,18 Here, we report the results of a single arm prospective clinical trial of MM patients suffering from BIPN treated after maximizing medical intervention. Patients were treated with acupuncture according to a standard protocol and the acupuncture regimen was assessed for safety, feasibility, and efficacy in reducing BIPN symptoms. In addition, possible mechanisms of action were explored, including changes in serum proinflammatory cytokines, β-endorphin, and neurotrophic factors.
Methods
Patients
Patients with MM who have been treated with bortezomib in the past with persistent BIPN (grade ≥2) were eligible using National Cancer Institute–Common Toxicity Criteria (NCI-CTC) 4.0. The grading system is as follows: grade 1—paresthesias or areflexia without pain or loss of function; grade 2—symptomatic, interferes with function but not daily living activities; grade 3—symptomatic, interferes with daily living activities; and grade 4—sensorimotor neuropathy that significantly interferes with daily living activities. All patients were treated at the University of Maryland Greenebaum Cancer Center (UMGCC). Patients were excluded from the study if they had acupuncture treatment within the previous month. Participants were recruited through the multiple myeloma clinic at UMGCC. The co-investigator identified patients and determined their BIPN severity and time course on bortezomib. All patients provided a written informed consent prior to enrollment. This clinical trial was approved by the institutional review board of the University of Maryland, Baltimore and registered at clinicaltrials.gov (NCT01541644).
Interventions
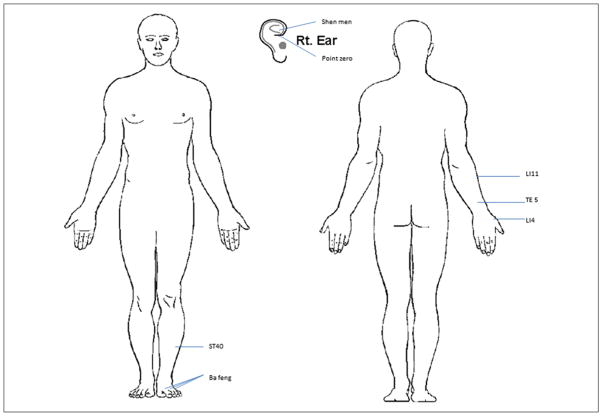
Patients had 10 acupuncture treatment sessions as follows: twice weekly for 2 weeks, weekly for 4 weeks, and then biweekly for 4 weeks. All patients continued their prescribed peripheral neuropathy medications and were encouraged not to change the type or dosage of such medications during the study. Acupuncture treatment was provided by an experienced acupuncturist according to a standard protocol that included preselected based on our clinical experience and prior research in using acupuncture to treat pain and neuropathy among cancer patients.12,15,16 The acupuncture treatments were delivered by a licensed acupuncturist who was trained at the Helm Medical Acupuncture Institute and had practiced continuously for 8 years. The points were selected for their analgesic characteristics and effectiveness in treating pain, swelling, and numbness (Yin Yang House, 2013). Points included bilateral ear points (shen men, point zero, and 2 additional auricular acupuncture points where electro-dermal signal was detected), bilateral body acupuncture points LI4, TE5, LI11, ST40, and Ba Feng located in upper and lower extremities (Figure 1). For auricular points, the electrodermal signal was detected through a Pointer-Excell II device (Lhasa OMS, Inc, Weymouth, MA), a handheld auricular acupoint finder that sounds an alert when an electrodermal signal is detected. Acupuncture procedures were performed in a quiet room, using a comfortable bed or massage table. During the treatment session, the patient was placed in supine position, and the skin at the site of acupoint was disinfected with an alcohol swab. Disposable sterilized Seirin acupuncture needles (Seirin Co, Shizuoka, Japan) were used; filiform 0.16 mm × 15 mm needles were used for the ear points and 0.25 mm × 40 mm needles were used for body points. The acupuncture needles were inserted 0.5 inches into the skin and allowed to remain in the skin for 20 minutes after de qi sensation was achieved. The de qi sensation is a feeling of soreness, numbness, and distention, and is an indicator of a stimulation level considered to be sufficient for the therapeutic effect of acupuncture to take place.19,20
Figure 1.
Acupuncture point location map. Acupuncture needles were inserted 0.5 to 2 inches into the skin to reach de qi sensation and remained in the skin for 20 minutes.
Outcome Measures
Safety Assessments
All patients in this trial were assessed for safety after each acupuncture session. Safety assessments consisted of the research nurse observing and interviewing each individual for signs and/or reports of excessive bruising, local persistent pain, or evidence of bleeding beyond approximately one drop of blood at the needle placement point.
Peripheral Neuropathy Assessments
Patients were assessed for both objective and self-reported signs and symptoms of neuropathy using the Clinical Total Neuropathy Score (TNSc), the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity questionnaire (FACT/GOG-NTx), and the Neuropathy Pain Scale (NPS).
The TNSc is a shorter version of TNS that combines information from symptoms and signs of neuropathy and generates a single score to quantify neuropathy. TNS has demonstrated reliability and validity, with inter- and intra-reliability being .97 and .99, respectively.21,22 TNSc has been shown to be sensitive and accurate in assessing the severity and change in patients’ CIPN.23 It results in a cumulative score ranging from 0 to 28, with the higher TNSc scores reflecting worsening neuropathy symptoms and signs.22 TNSc has 7 components that combine evaluation of subjective symptoms (sensory, motor, and autonomic symptoms) and objective symptoms (pin sensibility, vibration sensibility, strength, and deep tendon reflex). Before the start of the study, the neurologist trained the research nurse to perform these evaluations of the TNSc. The research nurse subsequently performed all evaluations on the subjective and objective symptoms on all patients.
The FACT/GOG-Ntx is an 11-item neurotoxcity sub-scale covering sensory neuropathy, motor neuropathy, hearing neuropathy, and dysfunction associated with neuropathy to assess the details of neurotoxicity symptoms in the patient. The FACT/GOG-Ntx has demonstrated reliability and validity, with a Cronbach’s α of .81 for the neurotoxicity subscale and an overall Cronbach’s α of .84.24 Furthermore, the FACT/GOG-Ntx has demonstrated sensitivity to meaningful clinical distinctions and change over time.24 The FACT/GOG-Ntx has been validated for self, interviewer, and computer administration.24 It results in a cumulative score ranging from 0 to 44, with the higher scores reflecting worse neuropathy symptoms.
The NPS is a multidimensional tool that uses self-report visual analogue scales to quantify, on a scale of 0 to 10, global pain intensity and unpleasantness, and 8 other descriptive qualities of neuropathic pain. The NPS also includes one semistructured question about temporal sequence.25,26 The NPS is capable of distinguishing neuropathic pain from nonneuropathic pain.27 The 10 items demonstrate weak correlations with one another, supporting their discriminate validity.25 It results in a cumulative score ranging from 0 to 100, with increasing scores reflecting worsening symptoms of neuropathic pain.
Neuropathy assessments were performed at baseline (before the first acupuncture session), after the first acupuncture session (week 1) and before the third (week 2), fifth (week 3), sixth (week 4), seventh (week 5), eighth (week 6), ninth (week 8), tenth (week 10) acupuncture sessions and at week 14 follow-up. The research nurse conducted TNSc testing, and the patients filled out the FACT/GOG-Ntx and NPS questionnaires before being collected by the research nurse.
Biomarker Collection and Testing
Proinflammatory cytokines measured included the following: interleukin-6 (IL-6), IL-8, IL-10, IL-17a, macrophage inflammatory protein-1α (MIP-1α), tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), and insulin-like growth factor-1 (IGF-1). Neurotrophic factors measured included nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and β-endorphin (β-EP). These markers were selected because studies indicate that peripheral neuropathy is associated with elevated levels of inflammatory markers,28 altered cytokine profiles29 and reduced NGFs in peripheral nerves.30 Furthermore, acupuncture has been associated with significant changes in cytokines, including IL-1β, IL-6, IL-17a, and TNF-α,31–36 and improvements in brain NGF availability and utilization,37 and these factors may represent a pathway through which acupuncture exerts its antineuropathic effects. In addition, a recent study showed that the severity of CIPN was associated with serum MIP-1α level.38
Blood draws were performed at baseline (before the first acupuncture session), right after first acupuncture session (week 1), before the third (week 2), sixth (week 4), ninth (week 8) acupuncture session and at week 14 follow-up. The serum was isolated according to the manufacturer’s protocol, aliquoted and stored at −80°C until cytokine analysis, that is, the levels of IL-6, IL-8, IL-10, IL-17a, MIP-1α, TNF-α, VEGF, and IGF-1. Neurotrophic factors measured included NGF and were measured in the serum samples using a Multiplex system (Millipore, Billerica, MA) according the manufacturer’s instructions. The levels of β-EP, BDNF, and neurotrophic factor were measured via enzyme-linked immunosorbent assay kit from Wuhan EIAab Science Co (Wuhan, China). All laboratory procedures were performed by trained personnel in the Cytokine Core Laboratory at the University of Maryland, School of Medicine.
Nerve Conduction Studies
After enrollment, but prior to receiving the intervention, and at week 12, subjects who consented to electrodiagnostic testing underwent nerve conduction studies. Motor nerve conduction studies were performed on both peroneal and tibial nerves. Motor amplitudes, motor conduction velocities, distal latencies, and F-wave latencies were recorded from each nerve in standard fashion. Similarly, sensory nerve conduction studies were performed on both sural nerves. Sensory amplitudes and conduction velocities were recorded on each nerve.
Statistical Considerations
The primary objectives of this single-arm clinical cohort study were to assess the feasibility and safety of acupuncture in treatment of patients with MM who suffered from moderate to severe BIPN. The acupuncture administered to patients with MM was considered a feasible intervention if at least 80% patients completed 4 or more acupuncture sessions.
Secondary objectives included assessing efficacy of the intervention as measured by the TNSc, the FACT/GOG-Ntx, and the NPS. An average reduction in subjects’ TNSc over 10 weeks of treatment of at least 10% was considered clinically meaningful. Intention-to treat analysis was performed on 27 patients who entered the study. Average TNSc, FACT/GOG-Ntx, and NPS scores were estimated and compared between the baseline and preselected time-points during treatment. We used a single-group repeated-measures analysis of variance approach with a .05 significance level to test for plausible differences in means across the 3 levels of the parameter. In addition, to explore the effect of time since bortezomib discontinuation on study outcomes, we conducted a post hoc analysis of neuropathy score changes excluding 8 participants who had painful BIPN and whose PN symptoms had lasted for less than 6 months.
Exploratory outcomes included changes in serum cytokines over the length of the trial. Plausible changes in multiple cytokines over time were estimated and compared using the doubly repeated measures approach (multiple markers and repeated time points).
Fisher’s exact test and logistic regression model were used to assess differences in demographic and clinical characteristics between responders and nonresponders to acupuncture. A patient was defined as responder if the reduction in NPS score from baseline to the end of acupuncture treatment was at least 30%, which is an end point used often in other pain research to define response.39 The statistical tests were 2-sided and done at the .05 level of significance. Statistical analysis was conducted using SAS (v.9.3, SAS Institute, Inc, Cary, NC) and Splus (v.8.2, TIBCO, Boston, MA).
Results
From May 17, 2011 to February 28, 2012, 46 patients with MM were screened and 27 patients who met the eligibility criteria and agreed to participate were enrolled in the study. Patients’ baseline characteristics are summarized in Table 1. All patients had persistent PN after discontinuation of bortezomib for a median of 19 months (range 1–83 months). Eight patients were enrolled within 6 months of stopping bortezomib; all of them had grades 3 to 4 painful PN. Median time from diagnosis of MM to study entry was 30 months (range 5–178 months). Nineteen patients were in remission, with 12 on maintenance lenalidomide, and 7 not on any therapy. Eight patients had progressive disease and were receiving salvage therapy. Therapy of PN included narcotics (n = 13), gabapentin (n = 12), amitriptyline (n = 3), pregabalin (n = 2), and duloxetine (n = 2). Eight patients had diabetes (a known risk factor for PN in bortezomib-treated patients).
Table 1.
Patients’ Baseline Characteristics.
| Characteristics | No. of Patients (N = 27) |
|---|---|
| Age in years, median (range) | 63 (49–77) |
| Race, n (%) | |
| Caucasian | 17 (63) |
| African American | 9 (33) |
| Body mass index in kg/m2, median (range) | 32 (24–49) |
| Grade of BIPN, n (%) | |
| 2 | 12 (44) |
| 3 | 14 (52) |
| 4 | 1 (4) |
| Acute painful PN | 8 (29) |
| Month after discontinuation of bortezomib, median (range) | 19 (1–83) |
| MM disease status at the time of enrollment, n (%) | |
| Remission | 19 (70) |
| Progression | 8 (30) |
Abbreviations: BIPN, bortezomib-induced peripheral neuropathy; PN, peripheral neuropathy; MM, multiple myeloma.
There were no significant adverse events associated with the acupuncture treatment. No excessive bruising, local persistent pain and evidence of bleeding beyond potentially a drop of blood at the point of needle placement were reported.
One patient withdrew consent after 3 ear needles were placed because of fear of pain. The patient did not complete the first treatment and was therefore deemed not evaluable. One patient discontinued the study after 1 acupuncture treatment because of transportation issues. Twenty-five patients (93%) completed 4 acupuncture sessions, and 20 patients (77%) completed all 10 sessions of acupuncture treatment. Trial schema is summarized in Figure 2.
Figure 2.
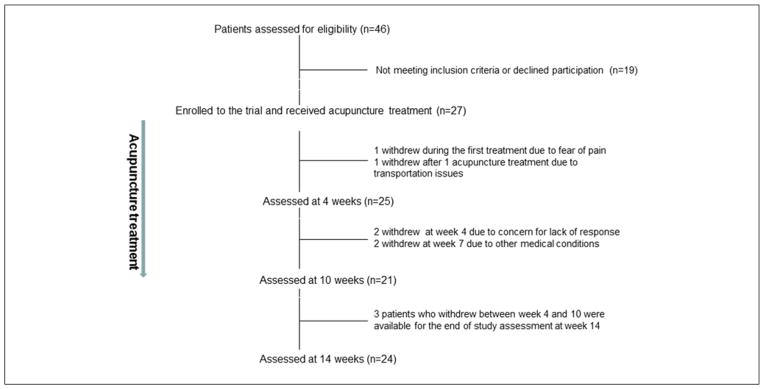
Trial schema.
Twenty-two patients maintained the same dose of pain medications throughout the study; 3 patients increased their pain medication and 2 decreased the use of pain medication.
The TNSc results in our trial were deemed invalid because of the fact that the original validation of TNSc was performed by 2 neuromuscular trained physicians and the TNSc in our trial was performed by a research nurse. A study has suggested that neurologists and nurses may not do TNSc equally (unpublished data, personal communication with Dr Cornblath at the Johns Hopkins Hospital). The reliability and validity of the research nurse’s TNSc measurement was not established before this clinical trial began. Therefore, TNSc data were not reported.
Mean FACT/GOG-Ntx scores decreased from 20.1 (standard deviation [SD] = 6.5) at baseline to 13.2 at week 10 (8.2), P < .0001. FACT/GOG-Ntx scores remained low at week 14, with a mean of 13.3 (8.6) and P < .0001 (Figure 3A). Mean NPS scores also decreased from 41 (SD = 25) at baseline to 29 (21) after the first acupuncture treatment, and to 16 (18) after 10 weeks of treatment, with P < .0001. At week 14 (4 weeks after the last acupuncture treatment), a significant reduction in mean NPS score was maintained with a mean of 18 (17) and P < .0001 (Figure 3B). Among the 19 patients who were enrolled 6 or more months after bortezomib discontinuation, FACT/GOG-Ntx scores were significantly reduced from 19.9 at baseline (SD = 6.6) to 14.3 at week 10 (8.9), P = .003, and remained low at week 14 with a mean of 13.7 (8.9) and P = .001. Also among this subgroup, NPS scores were significantly reduced from 40 (SD = 26) at baseline to 20 (20) at week 10, P = .003, and remained low at week 14 with mean 20 (19) and P = .001.
Figure 3.
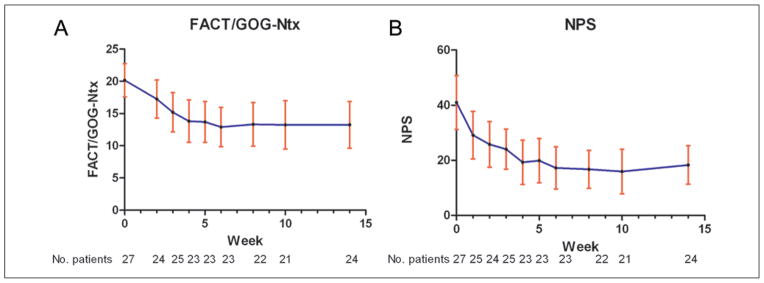
(A) Change in FACT/GOG-Ntx scores over 14 weeks. FACT/GOG-Ntx scores at each time point (means and 95% confidence intervals). (B) Change in NPS scores over 14 weeks. NPS scores at each time point (means and 95% confidence intervals).
Abbreviations: FACT/GOG-Ntx, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity; NPS, Neuropathy Pain Scale.
Among the 25 patients who completed at least 4 acupuncture treatments, by the end of acupuncture treatment, 14 (56%) reported improved daily functions (eg, walking and coordination); 10 (40%) reported a greater than 50% decrease in average NPS, and 7 (28%) reported a greater than 50% reduction in FACT/GOG-Ntx total scores.
Improvements in the FACT/GOG-Ntx scores during the study were reported in walking, hand function (buttoning buttons, trouble feeling objects), and ear functions (ears ringing or buzzing, trouble hearing). However, overall function (joint pain or muscle cramps, and weakness) did not improve (data not shown). Improvements of multiple components of neuropathic pain were reported during the study (data not shown). Patients also reported reductions in unpleasant hot/cold sensations, which are usually not alleviated by opioid.26
Six patients refused nerve conduction studies, and 4 patients had baseline measurement only. Of the 15 patients who had nerve conduction studies before and after acupuncture treatments, 5 patients (33%) showed a greater than 10% increase in motor nerve amplitude, 8 (53%) showed no significant changes, and 2 (13%) showed a greater than 10% decrease in motor nerve amplitude. At baseline, the majority of patients (87%) had severe sensory nerve deficits, with no measurable sural nerve sensory responses. Two patients (13%) had a greater than 10% increase in sensory nerve amplitude, 12 (80%) showed no changes and 1 (7%) showed a greater than 10% decrease in sensory nerve amplitude. There were no significant correlations observed between symptoms/functional improvements and results of the nerve conduction studies.
There were no significant changes in any of the 12 cytokines at any time points (Table 2). There was no association found between the severity of BIPN measured by NPS, FACT-Ntx, or BIPN grade with serum MIP-1α level.
Table 2.
Summary Statistics for Serum Concentrations of 10 Biomarkers Over 14 Weeks.
| Biomarker | Baseline (N = 27), Mean (SD) | Week 1 (n = 26), Mean (SD) | Week 2 (n = 24), Mean (SD) | Week 4 (n = 23), Mean (SD) | Week 8 (n = 22), Mean (SD) | Week 14 (n = 24), Mean (SD) |
|---|---|---|---|---|---|---|
| IL-6 (pg/mL) | 4.04 (6.81) | 3.54 (5.45) | 2.58 (2.74) | 2.58 (2.61) | 7.21 (21.52) | 3.16 (3.29) |
| IL-8 (pg/mL) | 1.14 (0.13) | 1.13 (0.10) | 1.15 (0.13) | 1.14 (0.12) | 1.14 (0.13) | 1.09 (0.20) |
| IL-10 (pg/mL) | 3.81 (3.78) | 3.73 (3.34) | 3.76 (2.79) | 3.62 (3.01) | 11.35 (32.60) | 6.71 (10.76) |
| TNF-α (pg/mL) | 1.93 (1.12) | 1.99 (1.31) | 2.60 (2.80) | 2.43 (2.97) | 3.26 (3.44) | 2.08 (1.33) |
| VEGF (pg/mL) | 72.51 (43.91) | 73.19 (43.63) | 87.73 (75.73) | 95.89 (86.48) | 91.28 (59.30) | 92.18 (81.98) |
| IGF-1 (pg/mL) | 4839.67 (3241.82) | 4811.01 (3423.22) | 4928.70 (3517.36) | 4822.97 (3138.45) | 4763.74 (3219.96) | 5565.86 (4338.65) |
| NGF (pg/mL) | 1.08 (0.79) | 1.13 (0.86) | 1.54 (2.29) | 1.98 (4.09) | 1.18 (1.01) | 0.98 (0.65) |
| BDNF (pg/mL) | 952.60 (873.50) | 756.90 (626.98) | 926.83 (818.60) | 1023.37 (947.13) | 717.19 (513.33) | 705.10 (579.02) |
| NT-3 (pg/mL) | 30.85 (111.40) | 31.58 (116.22) | 29.37 (107.57) | 30.22 (102.75) | 36.42 (127.93) | 24.77 (85.28) |
| β-EP (ng/mL) | 1.14 (0.13) | 1.13 (0.10) | 1.15 (0.13) | 1.14 (0.12) | 1.14 (0.13) | 1.09 (0.20) |
| IL-17a (pg/mL) | N/Aa | N/Aa | N/Aa | N/Aa | N/Aa | N/Aa |
| MIP-1α (pg/mL) | 15.4 (12.9) | 18.4 (16.0) | Not tested | Not tested | 39.2 (45.0) | 14.7 (12.3) |
Abbreviations: SD, standard deviation; IL, interleukin; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; IGF-1, insulin-like growth factor-1; NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; NT-3, neurotrophin-3; β-EP, β-endorphin; MIP-1α, macrophage inflammatory protein-1α; N/A, not applicable.
All but one patient had IL-17a below detectable levels.
Eighteen out of 26 patients (69%) had at least a 30% reduction in NPS scores from baseline to the end of acupuncture treatments, and were considered responsive to the acupuncture treatment. Neither race, age, body mass index, diabetes status, grade of BIPN, duration of BIPN, or the presence of painful PN were predictive of response or lack of to acupuncture treatment. NPS score improvement after the first acupuncture treatment was positively correlated with continued improvement of the NPS score at week 10 (r = 0.82, P < .0001).
Discussion
This is the first prospective study, to the best of our knowledge, on the use of acupuncture in treating multiple myeloma patients with moderate to severe BIPN. This is also the first study to use reliable and valid measures of symptom severity, including the FACT/GOG-NTx and NPS to evaluate treatment response, and the first study to explore the associations of changes in cytokines and neurotrophic factors during acupuncture treatments for BIPN patients. We established that acupuncture is a safe and feasible treatment option for patients experiencing BIPN, with no safety issues being reported, and with greater than 90% of participants completing treatments. Furthermore, patients treated with acupuncture showed significantly reduced neuropathic pain and improved function, giving preliminary evidence of the potential efficacy of the treatment, and supporting the need for further research.
Our results are consistent with those from other studies of acupuncture among patients with CIPN, although the study populations acupuncture treatments, and outcome measures differed somewhat. Our study participants consisted of patients with MM who were suffering from BIPN, whereas the study by Alimi et al15 included patients with chronic peripheral or central neuropathic pain after cancer treatment, and the study by Wong and Sagar12 included patients with advanced gynecological cancers who developed peripheral neuropathy after treatment with carboplatin and paclitaxel. Our study used a combination of ear points and body points that are used most often in community acupuncture clinics, whereas the study by Alimi et al15 used ear points only, and the case series by Wong and Sagar12 used body points only. Lastly, both prior studies focused on the reduction of pain, whereas our study assessed the changes in neuropathy symptoms (ie, tingling, numbness, and functional disturbance) in addition to pain.
A possible explanation for the lack of improvement in nerve conduction studies could be the short duration of the treatment (10 weeks) and follow-up period (14 weeks). Shorter follow-up periods would make it easier to observe subjective symptoms changes, rather than objective changes that require reversal of the neuropathy process (such as nerve regeneration).
It is notable that at the week 14 follow-up, improvements in FACT/GOG-Ntx and NPS scores were maintained. This raises the possibility that acupuncture could provide not only short-term relief (that persists while patients continue receiving the acupuncture sessions) but may also provide a longer term benefit for BIPN after the treatment sessions are over. Furthermore, these reductions were observed among subjects whose BIPN symptoms had persisted for a median of 19 months after bortezomib discontinuation, making spontaneous recovery an unlikely explanation.
The results of the nerve conduction studies confirm that the majority of patient population in our study suffered from significant sensory deficit with no measurable nerve sensory responses. This differs from the prior PN nerve conduction study as their patients had a mean value of 27.05 ± 19.75 m/s in the sural nerve conduction velocities at baseline.16 As such, it might be difficult to detect any significant changes in the already significantly damaged sensory nerves in our study. The fact that 5 out of 15 patients had greater than 10% increase in motor nerve amplitude is interesting and warrants further study.
Although studies have not been able to fully explain the mechanism of acupuncture, it has been proposed that acupuncture works through its effect on inflammatory cytokines, or through its effect on neurotransmitters and neurohormones. However, we did not observe any significant changes in any of the cytokines or neurotrophic factors measured in serum during the 10 weeks of acupuncture treatments. There were also no correlations between serum biomarkers and overall observed responses. However, cytokines in patients with MM may be affected by disease status and treatment, thus making it is difficult to detect potential changes due to acupuncture treatment. Furthermore, although neurotrophic factors can be detected in the serum, the correlation between cerebrospinal fluid/peripheral tissue level and blood has not been established.
In summary, acupuncture was found to be safe and feasible in this group of patients with MM suffering from BIPN. Furthermore, we observed statistically and clinically significant reductions in subjective measurements of BIPN that were sustained after the end of acupuncture. These results provide preliminary evidence of efficacy and support the need for further research. A randomized controlled trial of acupuncture to treat peripheral neuropathy among cancer patients is planned and further exploration of the mechanisms of action is warranted.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants NCI K12 CA126849 and R21 CA173263 (TB) and NINR P30NR011396 (SGD).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor ps-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 2.Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008;112:1593–1599. doi: 10.1182/blood-2008-04-149385. [DOI] [PubMed] [Google Scholar]
- 3.Arnulf B, Pylypenko H, Grosicki S, et al. Updated survival analysis of a randomized phase iii study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica. 2012;97:1925–1928. doi: 10.3324/haematol.2012.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III apex trial in relapsed multiple myeloma: Impact of a dose-modification guideline. Br J Haematol. 2009;144:895–903. doi: 10.1111/j.1365-2141.2008.07573.x. [DOI] [PubMed] [Google Scholar]
- 6.Cavaletti G, Jakubowiak AJ. Peripheral neuropathy during bortezomib treatment of multiple myeloma: a review of recent studies. Leuk Lymphoma. 2010;51:1178–1187. doi: 10.3109/10428194.2010.483303. [DOI] [PubMed] [Google Scholar]
- 7.Badros A, Goloubeva O, Dalal JS, et al. Neurotoxicity of bortezomib therapy in multiple myeloma: a single-center experience and review of the literature. Cancer. 2007;110:1042–1049. doi: 10.1002/cncr.22921. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Garcia MK, Chang DZ, et al. Multiple myeloma, painful neuropathy, acupuncture? Am J Clin Oncol. 2009;32:319–325. doi: 10.1097/COC.0b013e318173a520. [DOI] [PubMed] [Google Scholar]
- 9.Cata JP, Weng HR, Burton AW, Villareal H, Giralt S, Dougherty PM. Quantitative sensory findings in patients with bortezomib-induced pain. J Pain. 2007;8:296–306. doi: 10.1016/j.jpain.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Vincent C. The safety of acupuncture. BMJ. 2001;323:467–468. doi: 10.1136/bmj.323.7311.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abuaisha BB, Costanzi JB, Boulton AJ. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: a long-term study. Diabetes Res Clin Pract. 1998;39:115–121. doi: 10.1016/s0168-8227(97)00123-x. [DOI] [PubMed] [Google Scholar]
- 12.Wong R, Sagar S. Acupuncture treatment for chemotherapy-induced peripheral neuropathy—a case series. Acupunct Med. 2006;24:87–91. doi: 10.1136/aim.24.2.87. [DOI] [PubMed] [Google Scholar]
- 13.Galantino ML, Eke-Okoro ST, Findley TW, Condoluci D. Use of noninvasive electroacupuncture for the treatment of hiv-related peripheral neuropathy: a pilot study. J Altern Complement Med. 1999;5:135–142. doi: 10.1089/acm.1999.5.135. [DOI] [PubMed] [Google Scholar]
- 14.Ahn AC, Bennani T, Freeman R, Hamdy O, Kaptchuk TJ. Two styles of acupuncture for treating painful diabetic neuropathy—a pilot randomised control trial. Acupunct Med. 2007;25:11–17. doi: 10.1136/aim.25.1-2.11. [DOI] [PubMed] [Google Scholar]
- 15.Alimi D, Rubino C, Pichard-Leandri E, Fermand-Brule S, Dubreuil-Lemaire ML, Hill C. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J Clin Oncol. 2003;21:4120–4126. doi: 10.1200/JCO.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Schroder S, Liepert J, Remppis A, Greten JH. Acupuncture treatment improves nerve conduction in peripheral neuropathy. Eur J Neurol. 2007;14:276–281. doi: 10.1111/j.1468-1331.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 17.Bao T, Zhang R, Badros A, Lao L. Acupuncture treatment for bortezomib-induced peripheral neuropathy: a case report. Pain Res Treat. 2011;2011:920807. doi: 10.1155/2011/920807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao T, Lao L, Medeiros M, Zhang R, Dorsey SG, Badros A. Improvement of painful bortezomib-induced peripheral neuropathy following acupuncture treatment in a case series of multiple myeloma patients. Med Acupunct. 2012;24:181–187. [Google Scholar]
- 19.Beijing College of Traditional Chinese Medicine. Essentials of Chinese Acupuncture. Beijing, China: Foreign Languages Press; 1979. [Google Scholar]
- 20.Qiu M-L, Li L-Y. Chinese Acupuncture and Moxibustion. Edinburgh, Scotland: Churchill Livingstone; 1993. [Google Scholar]
- 21.Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53:1660–1664. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- 22.Cavaletti G, Jann S, Pace A, et al. Multi-center assessment of the total neuropathy score for chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst. 2006;11:135–141. doi: 10.1111/j.1085-9489.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- 23.Cavaletti G, Frigeni B, Lanzani F, et al. The total neuropathy score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the national cancer institute-common toxicity scale. J Peripher Nerv Syst. 2007;12:210–215. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 24.Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity (FACT/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003;13:741–748. doi: 10.1111/j.1525-1438.2003.13603.x. [DOI] [PubMed] [Google Scholar]
- 25.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332–338. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- 26.Jensen MP, Friedman M, Bonzo D, Richards P. The validity of the Neuropathic Pain Scale for assessing diabetic neuropathic pain in a clinical trial. Clin J Pain. 2006;22:97–103. doi: 10.1097/01.ajp.0000173018.64741.62. [DOI] [PubMed] [Google Scholar]
- 27.Fishbain DA, Lewis JE, Cutler R, Cole B, Rosomoff HL, Rosomoff RS. Can the neuropathic pain scale discriminate between non-neuropathic and neuropathic pain? Pain Med. 2008;9:149–160. doi: 10.1111/j.1526-4637.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 28.Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: Immune cells and molecules. Anesth Analg. 2007;105:838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- 29.Wang XM, Lehky TJ, Brell JM, Dorsey SG. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59:3–9. doi: 10.1016/j.cyto.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arrieta O, Hernandez-Pedro N, Fernandez-Gonzalez-Aragon MC, et al. Retinoic acid reduces chemotherapy-induced neuropathy in an animal model and patients with lung cancer. Neurology. 2011;77:987–995. doi: 10.1212/WNL.0b013e31822e045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joos S, Schott C, Zou H, Daniel V, Martin E. Immunomodulatory effects of acupuncture in the treatment of allergic asthma: a randomized controlled study. J Altern Complement Med. 2000;6:519–525. doi: 10.1089/acm.2000.6.519. [DOI] [PubMed] [Google Scholar]
- 32.Petti FB, Liguori A, Ippoliti F. Study on cytokines IL-2, IL-6, IL-10 in patients of chronic allergic rhinitis treated with acupuncture. J Tradit Chin Med. 2002;22:104–111. [PubMed] [Google Scholar]
- 33.Jeong HJ, Kim BS, Oh JG, Kim KS, Kim HM. Regulatory effect of cytokine production in asthma patients by Sooji Chim (Koryo hand acupuncture therapy) Immunopharmacol Immunotoxicol. 2002;24:265–274. doi: 10.1081/iph-120003759. [DOI] [PubMed] [Google Scholar]
- 34.Wu HG, Zhou LB, Pan YY, et al. Study of the mechanisms of acupuncture and moxibustion treatment for ulcerative colitis rats in view of the gene expression of cytokines. World J Gastroenterol. 1999;5:515–517. doi: 10.3748/wjg.v5.i6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong HJ, Hong SH, Nam YC, et al. The effect of acupuncture on proinflammatory cytokine production in patients with chronic headache: a preliminary report. Am J Chin Med. 2003;31:945–954. doi: 10.1142/S0192415X03001661. [DOI] [PubMed] [Google Scholar]
- 36.Bao T, Cai L, Giles JT, et al. A dual-center randomized controlled double blind trial assessing the effect of acupuncture in reducing musculoskeletal symptoms in breast cancer patients taking aromatase inhibitors. Breast Cancer Res Treat. 2013;138:167–174. doi: 10.1007/s10549-013-2427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manni L, Rocco ML, Barbaro Paparo S, Guaragna M. Electroacupucture and nerve growth factor: Potential clinical applications. Arch Ital Biol. 2011;149:247–255. doi: 10.4449/aib.v149i2.1365. [DOI] [PubMed] [Google Scholar]
- 38.Shi Q, Wang XS, Reuben JM, et al. Chemotherapy-induced peripheral neuropathy in multiple myeloma patients undergoing maintenance therapy. J Clin Oncol. 2013;31(suppl):Abstract 9646. [Google Scholar]
- 39.Henry NL, Banerjee M, Wicha M, et al. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer. 2011;117:5469–5475. doi: 10.1002/cncr.26230. [DOI] [PubMed] [Google Scholar]