Abstract
BACKGROUND
Androgen-deprivation therapy (ADT) has been the backbone of treatment for metastatic prostate cancer since the 1940s. We assessed whether concomitant treatment with ADT plus docetaxel would result in longer overall survival than that with ADT alone.
METHODS
We assigned men with metastatic, hormone-sensitive prostate cancer to receive either ADT plus docetaxel (at a dose of 75 mg per square meter of body-surface area every 3 weeks for six cycles) or ADT alone. The primary objective was to test the hypothesis that the median overall survival would be 33.3% longer among patients receiving docetaxel added to ADT early during therapy than among patients receiving ADT alone.
RESULTS
A total of 790 patients (median age, 63 years) underwent randomization. After a median follow-up of 28.9 months, the median overall survival was 13.6 months longer with ADT plus docetaxel (combination therapy) than with ADT alone (57.6 months vs. 44.0 months; hazard ratio for death in the combination group, 0.61; 95% confidence interval [CI], 0.47 to 0.80; P<0.001). The median time to biochemical, symptomatic, or radiographic progression was 20.2 months in the combination group, as compared with 11.7 months in the ADT-alone group (hazard ratio, 0.61; 95% CI, 0.51 to 0.72; P<0.001). The rate of a prostate-specific antigen level of less than 0.2 ng per milliliter at 12 months was 27.7% in the combination group versus 16.8% in the ADT-alone group (P<0.001). In the combination group, the rate of grade 3 or 4 febrile neutropenia was 6.2%, the rate of grade 3 or 4 infection with neutropenia was 2.3%, and the rate of grade 3 sensory neuropathy and of grade 3 motor neuropathy was 0.5%.
CONCLUSIONS
Six cycles of docetaxel at the beginning of ADT for metastatic prostate cancer resulted in significantly longer overall survival than that with ADT alone. (Funded by the National Cancer Institute and others; ClinicalTrials.gov number, NCT00309985.)
Regressions of metastatic prostate cancer were first documented in the 1940s and were achieved with surgical castration; subsequently, androgen-deprivation therapy (ADT) became the mainstay of therapy.1 Attempts to improve the efficacy or decrease the treatment burden of ADT have included the use of anti-androgens alone, intermittent dosing of ADT, and the use of an antiandrogen combined with medical or surgical castration.2–4 A meta-analysis revealed an increase in survival of 3 percentage points at 5 years with concurrent use of a non-steroidal antiandrogen at the time of initiation of ADT.2 However, resistance to ADT occurs in most patients, with the result that the median survival among patients with metastatic prostate cancer is approximately 3 years.5,6 In patients with resistance to ADT, docetaxel therapy resulted in a median survival that was approximately 2.5 months longer than that with mitoxantrone and prednisone.7,8
Prior studies of chemotherapy plus ADT, which did not show a benefit, were small studies that involved primarily patients with a relatively low tumor burden.9,10 Definitions of a high burden of disease have included the presence of visceral metastases, a bone-metastasis burden categorized by site (beyond the axial skeleton) or by a high number of lesions, or a combination of these.9,11,12 In this study, the E3805 study, patients received ADT alone or ADT plus docetaxel at the beginning of ADT for metastatic hormone-sensitive prostate cancer, and stratification was performed prospectively according to high or low burden of metastatic disease.
METHODS
STUDY OVERSIGHT
The primary objective of the E3805 study was to determine whether docetaxel therapy at the beginning of ADT for metastatic hormone-sensitive prostate cancer would result in longer overall survival than that with ADT alone. The study was designed in 2005 by the Eastern Cooperative Oncology Group (ECOG; now part of ECOG-ACRIN) and was approved by the institutional review board at each participating institution. The study was coordinated by the ECOG-ACRIN Cancer Research Group. The ECOG-ACRIN Statistical Center collected the data and was the leading cooperative group and data coordinating center. The first two authors attest that the study was conducted and monitored as specified by the protocol. The first author wrote the first draft of the manuscript, with subsequent contributions by all the coauthors. The authors vouch for the accuracy and completeness of the data presented. The protocol with the statistical analysis plan is available with the full text of this article at NEJM.org. Sanofi donated the docetaxel for early use (i.e., before progression during ADT) and provided a grant to ECOG-ACRIN but had no role in the design of the protocol, the collection or analysis of the data, or the preparation of the manuscript.
PATIENTS
Patients were enrolled by ECOG-ACRIN, the Southwest Oncology Group, the Alliance for Clinical Trials in Oncology, and NRG Oncology (a merged group that includes the National Surgical Adjuvant Breast and Bowel Project, the Radiation Therapy Oncology Group, and the Gynecologic Oncology Group) and through the Clinical Trials Support Unit. Eligible patients had a pathological diagnosis of prostate cancer or a clinical scenario consistent with prostate cancer with an elevated prostate-specific antigen (PSA) level; radiologic evidence of metastatic disease; and an ECOG performance-status score of 0, 1, or 2 (on a scale from 0 to 5, with higher scores indicating greater disability; patients with a score of 2 were eligible if the decrement in functioning was due to prostate cancer). Prior adjuvant ADT was allowed if the duration of therapy was 24 months or less and progression had occurred more than 12 months after completion of therapy. Patients who were receiving ADT for meta-static disease were eligible if there was no evidence of progression and treatment had commenced within 120 days before randomization. Organ function that was adequate for docetaxel treatment was required (details are provided in the protocol). All patients provided written informed consent in accordance with institutional and federal guidelines.
TREATMENT PLAN, STRATIFICATION, AND RANDOMIZATION
Patients were randomly assigned to ADT alone or to combination therapy with ADT plus docetaxel at a dose of 75 mg per square meter of body-surface area given every 3 weeks for six cycles, with premedication with 8 mg of oral dexamethasone at 12 hours, 3 hours, and 1 hour before docetaxel infusion. Daily prednisone was not required. Patients were stratified according to age (<70 years vs. ≥70 years), ECOG performance-status score (0 or 1 vs. 2), and planned use of combined androgen blockade for more than 30 days (yes vs. no) or agents approved for prevention of skeletal-related events in castration-resistant disease (zoledronic acid or denosumab) (yes vs. no). Patients were also stratified according to the duration of prior adjuvant ADT (<12 months vs. ≥12 months) and the extent of metastases (high volume [defined as the presence of visceral metastases or ≥4 bone lesions with ≥1 beyond the vertebral bodies and pelvis] vs. low volume). Patients were required to take at least 500 mg of oral calcium carbonate and at least 400 IU of vitamin D per day.
DOSE MODIFICATIONS
No dose modifications of ADT were allowed, and the use of a nonsteroidal antiandrogen with castration (medical or surgical) at the time of initiation of therapy was at the discretion of the investigator. Intermittent hormonal therapy was not allowed. For docetaxel, no more than two dose modifications (decreases to 65 mg per square meter and 55 mg per square meter) were allowed. Dose adjustments were made according to the organ system that showed the greatest degree of toxic effects, which were graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE; version 3.0 until September 2011 and version 4.0 thereafter). Details on dosing are provided in the protocol. If a dose was reduced owing to toxic effects, it was not increased subsequently, and docetaxel was discontinued if administration was delayed longer than 3 weeks from the scheduled day of dosing. The use of growth factors was at the discretion of the investigator.
MONITORING OF TOXIC EFFECTS AND EFFICACY
Patients assigned to combination therapy were seen every 3 weeks during the period of docetaxel administration and then every 3 months. Patients assigned to ADT alone were seen every 3 months. For the reporting of serious adverse events to the NCI and to guide dose modifications, CTCAE version 3.0 was used until September 2011, at which time the study began using version 4.0. To ensure consistency, case-report forms for toxic effects that were recorded in the study database retained the use of version 3.0. All grade 3 or higher toxic effects in the combination group were captured, and an attribution of relatedness to study therapy was made by the local investigators. Adverse events among patients assigned to ADT alone were not routinely documented, although major adverse events were to be reported.
PSA levels were measured at each scheduled visit. Imaging (computed tomography [CT] of the abdomen and pelvis, technetium-99m bone scanning, and radiography or CT of the chest) was performed at baseline and at the time of documented castration resistance or as clinically indicated. Disease progression on imaging was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.013 (the criteria are summarized in the protocol). A complete serologic response was defined as a PSA level of less than 0.2 ng per milliliter on two consecutive measurements at least 4 weeks apart. Serologic progression was defined as an increase in the PSA level of more than 50% above the nadir reached after the initiation of ADT, with two consecutive increases at least 2 weeks apart. The date of a first recorded increase of more than 50% above the nadir was deemed the date of progression. If the nadir level was less than 2 ng per milliliter, a minimum increase of more than 2 ng per milliliter was required.
All time-to-event end points were determined from the time of randomization. Overall survival was defined as the time until death from any cause. The time to castration-resistant prostate cancer was defined as the time until documented clinical or serologic progression with a testosterone level of less than 50 ng per deciliter (or source documentation of medical castration or surgical castration). The time to clinical progression was defined as the time until increasing symptoms of bone metastases; progression according to RECIST, version 1.0; or clinical deterioration due to cancer according to the investigator’s opinion.
STATISTICAL ANALYSIS
The study underwent two major amendments (details are provided in the protocol); in all versions, an intention-to-treat analysis plan was used. With each amendment, the sample size was adjusted so that the study would have 80% power to detect a 33.3% difference in median overall survival between the combination group and the ADT-alone group, with the use of a stratified log-rank test at a one-sided alpha level of 2.5%. At study inception, only patients with high-volume disease were to be enrolled, and the sample size was to be 568 patients. After completion of enrollment in the NCI-sponsored S9346 trial,4 an amendment to the E3805 study was made in July 2008, after 53 patients had been enrolled, to include patients with low-volume disease. A prospective stratification of high volume versus low volume of metastatic disease was added, and the sample size was increased to 600 patients. The final amendment was made in December 2011, after 579 patients had been enrolled, to reflect new data documenting an increase in median overall survival owing to the use of PSA in the detection and monitoring of disease activity5 (initial projections were based on studies from the pre-PSA era) and to address the September 2011 report of the data and safety monitoring committee, which noted that 70% of enrolled patients had high-volume disease. The final design required the enrollment of 780 patients, with projections of median overall survival with ADT alone of 33 months among patients with high-volume disease and 67 months among patients with low-volume disease.
Interim analyses were to be performed before all semiannual meetings of the data and safety monitoring committee starting when approximately 25% of the planned full information was obtained and continuing until either the criteria for early stopping were met or full information was obtained (after 399 deaths). The study was monitored for early stopping for futility with the use of repeated-confidence-interval methods.14 At each interim analysis, the nominal (1 – [2 × alpha]) confidence interval for the hazard ratio for death in the comparison of the combination-therapy group with the ADT-alone group was computed. For a given analysis time (information fraction), alpha was set at the nominal one-sided significance level of the use function boundary. Information from interim analyses was reviewed by members of the independent data and safety monitoring committee, who determined whether efficacy or futility had been demonstrated and who decided whether the study should be stopped and the results reported early.
Descriptive statistics were used to characterize patients at study entry. Kaplan–Meier estimates15 were used for event-time distributions. Cox proportional-hazard models,16 stratified according to the factors described above, were used to estimate hazard ratios for time-to-event end points. Stratified log-rank tests17 were used to compare event-time distributions between the two groups. Response rates were compared with the use of Fisher’s exact test.18 An intention-to-treat analysis was conducted that included all randomly assigned patients regardless of eligibility and treatment status. P values are two-sided, and confidence intervals are at the 95% level.
RESULTS
PATIENTS
From July 2006 through December 2012, a total of 790 patients were enrolled and underwent randomization. Ten patients were ineligible, 7 had incomplete information to assess eligibility, and 6 patients in the combination group did not start the assigned therapy (Fig. S1 in the Supplementary Appendix, available at NEJM.org). All randomly assigned patients were followed and included in the primary analysis of their assigned group. At the planned interim analysis in October 2013, a total of 53% of the planned full information had been obtained, prespecified criteria for significance had been met, and the data were released by the data and safety monitoring committee. This report represents data with a cutoff date for survival of December 23, 2013; the median follow-up was 28.9 months, with 136 deaths in the ADT-alone group and 101 deaths in the combination group. All other data reflect the database as of December 23, 2014.
Patient characteristics were well balanced between the two groups (Table 1). The median age was 64 years (range, 36 to 88) in the combination group and 63 years (range, 39 to 91) in the ADT-alone group. In both groups, approximately 85% of the patients were white, approximately 70% had an ECOG performance-status score of 0, approximately 65% had high-volume disease, and approximately 60% had a Gleason score of 8 or higher (on a scale from 2 to 10, with higher scores indicating a more aggressive form of prostate cancer and a worse prognosis). In both groups, 73% of the patients had received no prior local therapy for prostate cancer with curative (rather than palliative) intent. Among patients who started ADT before randomization, the median time from the start of ADT to randomization was 1.2 months (range, 0 to 3.9) in the combination group and 1.3 months (range, 0 to 3.9) in the ADT-alone group.
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | ADT plus Docetaxel (N = 397) | ADT Alone (N = 393) |
|---|---|---|
| Age — yr | ||
| Median | 64 | 63 |
| Range | 36–88 | 39–91 |
| Race — no. (%)† | ||
| White | 344 (86.6) | 330 (84.0) |
| Black | 39 (9.8) | 37 (9.4) |
| Other | 4 (1.0) | 6 (1.5) |
| Unknown | 10 (2.5) | 20 (5.1) |
| ECOG performance status — no. (%)‡ | ||
| 0 | 277 (69.8) | 272 (69.2) |
| 1 | 114 (28.7) | 115 (29.3) |
| 2 | 6 (1.5) | 6 (1.5) |
| Volume of metastases — no. (%)§ | ||
| Low | 134 (33.8) | 143 (36.4) |
| High | 263 (66.2) | 250 (63.6) |
| Visceral metastases — no. (%) | 57 (14.4) | 66 (16.8) |
| Gleason score — no. (%)¶ | ||
| 4–6 | 21 (5.3) | 21 (5.3) |
| 7 | 96 (24.2) | 83 (21.1) |
| 8–10 | 241 (60.7) | 243 (61.8) |
| Unknown | 39 (9.8) | 46 (11.7) |
| PSA level at start of ADT — ng/ml | ||
| Median | 50.9 | 52.1 |
| Range | 0.2–8540.1 | 0.1–8056.0 |
| Prior treatment for prostate cancer — no. (%) | ||
| No local therapy | 289 (72.8) | 286 (72.8) |
| Primary radiation | 27 (6.8) | 33 (8.4) |
| Prostatectomy | 81 (20.4) | 73 (18.6) |
| Missing data | 0 | 1 (0.3) |
| Adjuvant ADT — no. (%) | 18 (4.5) | 16 (4.1) |
| Time from start of ADT to randomization — mo|| | ||
| Median | 1.2 | 1.3 |
| Range | 0.03–3.9 | 0.03–3.9 |
| No ADT before randomization — no. (%) | 51 (12.8) | 52 (13.2) |
There were no significant differences in characteristics between the two groups when analyzed with the use of the Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables, with the categories for “unknown” or “missing data” excluded. ADT denotes androgen-deprivation therapy, and PSA prostate-specific antigen.
Race was self-reported.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability. One patient assigned to ADT alone did not have an on-study form submitted, so the ECOG performance-status score was unknown, but the stratification by the site placed the patient into the stratum of a performance-status score of 2 at randomization.
A high volume of metastases was defined by the presence of visceral metastases or four or more bone lesions with at least one beyond the vertebral bodies and pelvis. One patient assigned to ADT alone did not have an on-study form submitted, so the volume of metastases was unknown, but the stratification by the site placed the patient into the high-volume stratum at randomization.
Gleason scores range from 2 to 10, with higher scores indicating a more aggressive form of prostate cancer and a worse prognosis.
Time from start of ADT to randomization is among patients who started ADT before randomization.
SURVIVAL
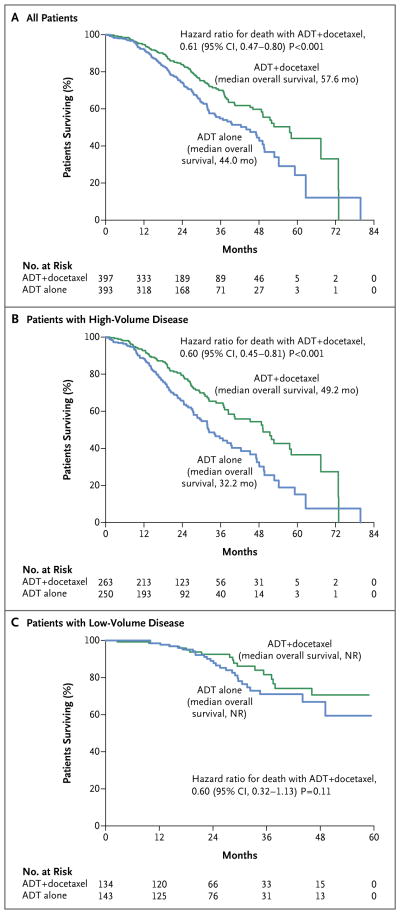
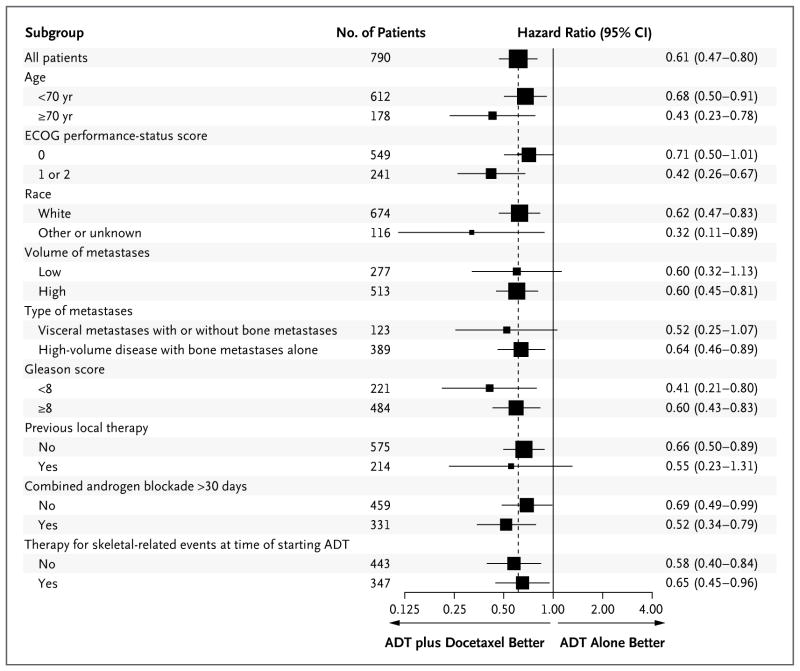
The median overall survival was 13.6 months longer with the addition to ADT of early docetax-el than with ADT alone (57.6 months vs. 44.0 months; hazard ratio for death in the combination group, 0.61; 95% confidence interval [CI], 0.47 to 0.80; P<0.001) (Fig. 1A). There were 85 prostate-cancer deaths in the combination group and 114 prostate-cancer deaths in the ADT-alone group (Table S1 in the Supplementary Appendix). The benefit at the last analysis was more apparent in the subgroup with high-volume disease than in the overall study population (Fig. 1B), with a median overall survival that was 17.0 months longer in the combination group than in the ADT-alone group (49.2 months vs. 32.2 months; hazard ratio for death, 0.60; 95% CI, 0.45 to 0.81; P<0.001). The median survival at the time of the analysis had not been reached in the subgroup with low-volume disease in either study group (Fig. 1C). A benefit of docetaxel treatment was detected in all the subgroups analyzed (Fig. 2).
Figure 1. Kaplan–Meier Estimates of Overall Survival.
The median duration of follow-up was 28.9 months among all patients (Panel A), 29.2 months among patients with high-volume disease (Panel B), and 27.6 months among patients with low-volume disease (Panel C). ADT denotes androgen-deprivation therapy, and NR not reached.
Figure 2. Hazard Ratios for Death in Subgroups.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability. Patients were stratified on the basis of an ECOG performance-status score of 0 or 1 versus 2, but because there were so few patients with a score of 2, the analysis was performed on the basis of a score of 0 versus 1 or 2. Race was self-reported; other or unknown race includes black (76 patients), Asian (8), Native American (2), and unknown (30). A high volume of metastases was defined by the presence of visceral metastases or four or more bone lesions with at least one beyond the vertebral bodies and pelvis. Gleason scores range from 2 to 10, with higher scores indicating a more aggressive form of prostate cancer and a worse prognosis. The x axis of the forest plot is scaled according to the natural logarithm of the hazard ratio. The size of the squares is proportional to the inverse of the variance of the log hazard ratio (small squares correspond to large variances).
SECONDARY END POINTS AND TOXIC EFFECTS
The proportion of patients who had a decrease in the PSA level to less than 0.2 ng per milliliter at 12 months was 27.7% in the combination group, as compared with 16.8% in the ADT-alone group (P<0.001) (Table 2). The median time to the development of castration-resistant prostate cancer (biochemical, symptomatic, or radiographic) was 20.2 months with combination therapy, as compared with 11.7 months with ADT alone (hazard ratio in the combination group, 0.61; 95% CI, 0.51 to 0.72; P<0.001) (Table 2, and Fig. S2A in the Supplementary Appendix). The median time to clinical progression was 33.0 months with combination therapy, as compared with 19.8 months with ADT alone (hazard ratio, 0.61; 95% CI, 0.50 to 0.75; P<0.001) (Table 2, and Fig. S2B in the Supplementary Appendix).
Table 2.
Secondary End Points.
| End Point | ADT plus Docetaxel (N = 397) | ADT Alone (N = 393) | P Value | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| PSA level <0.2 ng/ml at 6 mo — no. (%) | 127 (32.0) | 77 (19.6) | <0.001 | |
| PSA level <0.2 ng/ml at 12 mo — no. (%) | 110 (27.7) | 66 (16.8) | <0.001 | |
| Time to castration-resistant prostate cancer — mo* | ||||
| Median | 20.2 | 11.7 | <0.001 | 0.61 (0.51–0.72) |
| 95% CI | 17.2–23.6 | 10.8–14.7 | ||
| Time to clinical progression — mo† | ||||
| Median | 33.0 | 19.8 | <0.001 | 0.61 (0.50–0.75) |
| 95% CI | 27.3–41.2 | 17.9–22.8 | ||
The time to castration-resistant prostate cancer was the time until documented clinical or serologic progression with a testosterone level of less than 50 ng per deciliter (or source documentation of medical castration or surgical castration).
Clinical progression was defined by increasing symptoms of bone metastases; progression according to the Response Evaluation Criteria in Solid Tumors, version 1.0; or clinical deterioration due to cancer according to the investigator’s opinion.
Among the patients who received combination therapy, approximately 2% had a treatment-related grade 3 or 4 allergic reaction; grade 3 fatigue occurred in 4% of the patients, and grade 3 diarrhea, stomatitis, motor neuropathy, and sensory neuropathy each occurred at a rate of 1% or less (Table 3). Approximately 1% of the patients in the combination group had a thromboembolic event. One patient died suddenly at home of an unknown cause during the course of docetaxel therapy; the death was considered to be possibly related to docetaxel according to the Adverse Event Expedited Reporting System. Approximately 6% of the patients in the combination group had neutropenic fever, and approximately 2% had grade 3 or 4 infection with neutropenia.
Table 3.
Adverse Events of Grade 3 or Higher among the 390 Patients Who Received the Docetaxel-Containing Regimen and Had Follow-up Data Available.*
| Event | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|
| no. of patients (%) | |||
| Allergic reaction | 7 (1.8) | 1 (0.3) | 0 |
|
| |||
| Fatigue | 16 (4.1) | 0 | 0 |
|
| |||
| Diarrhea | 4 (1.0) | 0 | 0 |
|
| |||
| Stomatitis | 2 (0.5) | 0 | 0 |
|
| |||
| Neuropathy, motor | 2 (0.5) | 0 | 0 |
|
| |||
| Neuropathy, sensory | 2 (0.5) | 0 | 0 |
|
| |||
| Thromboembolism | 1 (0.3) | 2 (0.5) | 0 |
|
| |||
| Sudden death | 0 | 0 | 1 (0.3) |
|
| |||
| Anemia | 4 (1.0) | 1 (0.3) | 0 |
|
| |||
| Thrombocytopenia | 0 | 1 (0.3) | 0 |
|
| |||
| Neutropenia | 12 (3.1) | 35 (9.0) | 0 |
|
| |||
| Febrile neutropenia | 15 (3.8) | 9 (2.3) | 0 |
|
| |||
| Infection with neutropenia | 5 (1.3) | 4 (1.0) | 0 |
|
| |||
| Any event | 65 (16.7) | 49 (12.6) | 1 (0.3) |
Patients were classified according to the worst grade reported across all body systems. Patients assigned to ADT plus docetaxel were monitored every 3 weeks during the time docetaxel was administered and then every 3 months, whereas patients assigned to the ADT-alone group were seen every 3 months after randomization. Toxic effects in the group that received ADT plus docetaxel were captured at this frequency to ascertain the adverse-event profile of chemotherapy. The adverse-event profile of ADT was assumed to be common to the two groups. The potential risk of ascertainment bias for adverse events and early progression in the ADT-plus-docetaxel group was recognized, but such bias, if it existed, would have favored the ADT-alone group.
ASSIGNED THERAPY ADMINISTERED AND SUBSEQUENT THERAPY
Approximately 86% of the 390 patients in the combination group who started the assigned therapy completed six cycles of docetaxel therapy (Table S2 in the Supplementary Appendix), and approximately 74% of all the treated patients received all planned cycles without dose modifications. At the time of this analysis, castration-resistant prostate cancer with at least biochemical progression had developed in 287 patients assigned to ADT alone; 137 patients of these patients had received docetaxel for castration-resistant prostate cancer, and another 10 patients had received docetaxel before castration resistance was confirmed. In addition, 104 patients had received abiraterone or enzalutamide after confirmed castration resistance (9 of these patients may have received placebo as part of a trial). Table S3 in the Supplementary Appendix documents the use of other therapies that have been shown to prolong overall survival in patients with castration-resistant prostate cancer.
DISCUSSION
Improvements in outcomes in men with metastatic castration-resistant prostate cancer have been achieved with the use of cytotoxic chemotherapy, next-generation hormonal therapies, immunotherapy, and therapy with radiopharmaceutical agents.19–25 This study showed that docetaxel given at the time ADT was initiated for hormone-sensitive disease resulted in better cancer control than that with ADT alone, with a longer time to the development of castration resistance, a higher rate of decrease of the PSA level to less than 0.2 ng per milliliter at 12 months, a lower number of prostate-cancer deaths, and substantially longer overall survival. The longer overall survival than that with ADT alone was achieved despite the fact that almost half the 287 patients who received ADT alone and then met the criteria for castration resistance received docetaxel at the time of disease progression along with other therapies that prolong overall survival in patients with metastatic castration-resistant prostate cancer.
The definition of high-volume disease used for this protocol was a combination of features from prior classifications. All the definitions included the presence of nonnodal, soft-tissue visceral disease as a predictor of poor prognosis. This protocol made use of sites of bone metastases and the number of metastases to avoid classifying patients with three or fewer sites as having high-volume disease even if one lesion was beyond the vertebrae and pelvis (any lesion beyond the vertebrae and pelvis, irrespective of total lesion count, would be classified as “extensive” according to the definition of the Southwest Oncology Group4). In the subgroup of patients with high-volume disease, the median overall survival was 17.0 months longer in the combination group than in the ADT-alone group (49.2 months vs. 32.2 months). On the basis of an unpublished analysis of data from the S9346 trial5 we had projected a median survival of 33 months among patients with high-volume disease (according to the definition used in our protocol) in the ADT-alone group, and as such, the definition was reproducible across two phase 3 studies conducted in a similar time period.
A previously reported randomized study involving 380 patients (the GETUG-AFU 15 study), which had a similar design to that of the current study, did not detect longer overall survival with combination therapy.10 The median overall survival was longer than in our study — 54.2 months with ADT alone (which suggests that the study had a different case mix than that in our study) — and was first analyzed in 2011, before there was widespread access to newer therapies that have been shown to prolong overall survival among patients with castration-resistant prostate cancer. In the GETUG-AFU 15 study, the time to progression was longer, the number of prostate-cancer deaths was smaller, and the number of treatment-related deaths was larger (4 of 195 patients) with early docetaxel therapy than with ADT alone. The Medical Research Council STAMPEDE trial will add further data on the role of docetaxel in hormone-sensitive prostate cancer; to date, the overall survival in the ADT-alone group in that study is 42 months,26 a finding similar to that in our study.
In conclusion, the combination of standard ADT and six cycles of docetaxel resulted in significantly longer overall survival than that with standard ADT alone in men with hormone-sensitive metastatic prostate cancer. The clinical benefit at this early analysis was more pronounced among patients with a higher burden of disease.
Supplementary Material
Acknowledgments
Supported in part by a grant from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and by grants (CA180820, CA180794, CA180795, CA180802, CA180799, CA180790, CA180853, CA189829, CA180801, CA180888, CA31946, and CA180821) from the Public Health Service. Sanofi provided the docetaxel and a grant to ECOG-ACRIN.
We thank Judi Manola for key support from the inception and throughout the course of this study.
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Presented in part at the annual meeting of American Society of Clinical Oncology, Chicago, May 30–June 3, 2014; and the European Society of Medical Oncology meeting, Madrid, September 26–30, 2014.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7. [Google Scholar]
- 2.Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355:1491–8. [PubMed] [Google Scholar]
- 3.Samson DJ, Seidenfeld J, Schmitt B, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer. 2002;95:361–76. doi: 10.1002/cncr.10647. [DOI] [PubMed] [Google Scholar]
- 4.Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–25. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tangen CM, Hussain MH, Higano CS, et al. Improved overall survival trends of men with newly diagnosed M1 prostate cancer: a SWOG phase III trial experience (S8494, S8894 and S9346) J Urol. 2012;188:1164–9. doi: 10.1016/j.juro.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu JN, Fish KM, Evans CP, Devere White RW, Dall’Era MA. No improvement noted in overall or cause-specific survival for men presenting with metastatic prostate cancer over a 20-year period. Cancer. 2014;120:818–23. doi: 10.1002/cncr.28485. [DOI] [PubMed] [Google Scholar]
- 7.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 8.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 9.Millikan RE, Wen S, Pagliaro LC, et al. Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. J Clin Oncol. 2008;26:5936–42. doi: 10.1200/JCO.2007.15.9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–58. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 11.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–42. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Jennison CTB. Statistical approaches to interim monitoring of medical trials: a review and commentary. Stat Sci. 1990;5:299–317. [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 17.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 18.Cox D. Analysis of binary data. London: Methuen and Co; 1970. [Google Scholar]
- 19.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beer TM, strong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 23.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 24.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 25.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 26.James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control of the STAMPEDE trial (MRC PR08, CRUK/06/019) Eur Urol. 2015;67:1028–38. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.