Abstract
Endothelial dysfunction reflects pathophysiological changes in the phenotype and functions of endothelial cells that result from and/or contribute to a plethora of cardiovascular diseases. Here we review the role of hydrogen sulfide (H2S) in the pathogenesis of endothelial dysfunction, one of the fastest advanced and hottest research topics. Conventionally treated as an environment pollutant, H2S is also produced in endothelial cells and participates in the fine regulation of endothelial integrity and functions. Disturbed H2S bioavailability has been suggested to be a novel indicator of the progress and prognosis of endothelial dysfunction. Endothelial dysfunction appears to exhibit in different forms in different pathologies but therapeutics aimed at remedying the altered H2S bioavailability may benefit all.
Keywords: Cystathionine gamma-lyase, Endothelium-derived hyperpolarising factor, Gasotransmitters, Heme oxygenase-1, Hydrogen sulfide, Nitric oxide
From the endothelium and for the endothelium
H2S is a pungent colourless gas with distinctive rotten-egg smell, often regarded as an environmental pollutant and a toxin. Yet H2S can be produced in eukaryotic cells. H2S can be made in the endothelium by the enzymatic action of cystathionine γ-lase (CSE) with cysteine as the substrate (Glossary Box). There is no solid evidence for the involvement of cystathioine β-synthase (CBS) in endothelial production of H2S. By contrast, the engagement of 3-mercaptopyruvate sulfurtransferase (MST), as a sulfur-carrying enzyme, and cysteine aminotransferase in endothelial production of H2S has been reported [1,2].
Over the last decade, the study on the roles of H2S in the homeostasis of endothelial function and in the pathogenesis of EDF has grown exponentially. This research has deepened our understanding of the regulation of endothelial function in health and facilitated the development of preventive and therapeutic strategies for EDF in cardiovascular diseases. This review provides a succinct update on the related progresses and describes the challenges and future directions for the field, with a focus on the metabolism and functions of H2S in different types of EDF.
Endothelium function and its regulation by H2S
The functional importance of the endothelium is realised by its wide coverage of the inner surface of the cardiovascular system, polarised architecture in blood vessels, and heterogeneity in its morphology, structure, and gene expression profile at different locations of different types of blood vessels [3].
The endothelium protects vasculature from inflammatory damage and provides a permeability barrier to control blood volume and its electrolyte content. The endothelium is usually where vascular inflammation starts and propagates. Pro-inflammatory cytokines upregulate the expression of adhesion molecules in endothelial cells. Leukocyte adhesion and rolling on endothelial cells ensue. H2S inhibits vascular inflammation [3,4] via different signalling pathways, including the inhibition of p38 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), the activation of KATP and BKCa channels as well as HO-1 expression [5,6]. Moreover, H2S decreases reactive oxygen species (ROS) levels in endothelial cells [7-10], which is achieved partially by scavenging ROS [11] and partially by upgrading antioxidant defence machineries. Many antioxidant enzymes, such as catalase, superoxide dismutase, glutathione peroxidase, and glutathione-S-transferase, are upregulated by H2S [12]. Increased production of reduced glutathione (GSH) also accounts for the cytoprotective effect of H2S in endothelial cells [10].
The endothelium offers an anti-coagulation and anti-platelet boundary to inhibit aggregation and to maintain blood fluidity and fibrinolysis. H2S donors have been shown to prevent the aggregation of platelets [13] and thrombus formation in venules [14].
The endothelium also orchestrates angiogenesis and the vascular remodelling process. H2S inhibits vascular smooth muscle cell (VSMC) proliferation and phenotypic change at one hand, but on the other hand, it stimulates endothelial replication and migration, conditioning endothelial cells toward angiogenesis and self-reparation [15]. Pharmacological inhibition and genetic deletion of CSE in the endothelium reduces migration and sprouting of endothelial cells. It has also been shown that the vascular endothelial growth factor (VEGF)-induced angiogenesis ex vivo was markedly suppressed in aortic rings from CSE-KO mice [15].
The endothelium regulates vascular contraction and dilation. Vascular tone is regulated by H2S in both endothelium-dependent and –independent manners. Generated from VSMCs or delivered by exogenous H2S donors, H2S can directly, independent of the presence of the endothelium, open KATP channels in VSMCs to cause vasorelaxation [16]. The elimination of CSE expression in mouse endothelia abolished endothelial production of H2S as well as acetylcholine-induced endothelium-dependent vasorelaxation [17]. This original observation has been confirmed by numerous other studies, demonstrating that H2S is indeed an endothelium-derived relaxing factor (EDRF) [18]. Furthermore, the endothelium-dependent vasorelaxing effect of H2S is more prominent in peripheral resistance arteries than in large conduit arteries, requires membrane hyperplorisation of both endothelial cells and VSMCs, and is abolished by the blockade of small to medium conductance KCa channels. With the support of other lines of evidence, a characteristic identity of endothelium-derived hyperpolarising factor (EDHF) emerges for H2S [19, 20] (Box 1).
Box 1. Hydrogen sulfide is an endothelium-derived hyperpolarising factor (EDHF).
Endothelium-dependent vasorelaxation is mediated by endothelium-derived relaxing factors (EDRF), including nitric oxide (NO), prostacyclin (PGI2) and endothelium-derived hyperpolarising factor (EDHF).
EDHF has the following characteristics [18, 21]. 1) It is produced in and released from endothelial cells to hyperlolarise and relax vascular smooth muscle cells (VSMCs). 2) Its vasorelaxant effect is independent of NO/PGI2 pathways. 3) It increases the activities of small (SKCa, <10 pS) and intermediate (IKCa channels, 20∼50 pS) conductance calcium-dependent K+ channels, which are barred by the co-application of charybdotoxin (ChTX) and apamin. 4) It has more profound vasorelaxant effect on peripheral resistance arteries than conduit arteries. 5) Its vasorelaxant effect may be more potent in females than males.
Among nominated EDHF candidates over the last 25 years are hydrogen peroxide, arachidonic acid metabolites (such as THETAs and EETs), K+ ion per se, and C-type natriuretic peptide [18-22]. However, none of these candidates fully fulfill the role of EDHF. Recent studies have provided evidence that H2S is one of the most qualified EDHFs.
Endothelium-dependent, but NO/PGI2-independent, relaxation of mesenteric artery from rats or mice is mediated by H2S [23,24]. Deficiency in CSE expression eliminated methacholine-induced endothelium-dependent relaxation of mouse mesenteric arteries, but not that of aorta [20]. VSMCs from CSE-KO mice have lower resting membrane potential than that of WT mice [19], indicating the depolarising effect of endogenous H2S on VSMCs. Furthermore, methacholine hyperpolarised VMSCs of mesenteric artery from WT mice, but not those from CSE-KO mice. This effect of methacholine was abolished by co-applied ChTX/apamin. In contrast, methacholine did not alter membrane potential of VSMCs of aortae from WT mice or CSE-KO mice. Both methacholine and H2S induced greater VSMC hyperpolarisation of female mesenteric arteries of WT mice than that of male WT mice [20].
The mechanisms underlying the EDHF role of H2S have been explored. In an autocrine mode, endothelial produced H2S activates endothelium-located SKCa and IKCa channels. The resulting endothelial hyperpolarisation can evoke VSMC hyperpolarisation by electrical coupling through myoendothelial gap junction or by the increased K+ efflux that activates VSMC Kir channel and/or Na+/K+-ATPase. In a paracrine mode, endothelium-generated H2S is directly released to VSMCs to induce hyperpolarisation of VSMC by opening KATP channels in these cells.
The interaction between H2S and NO
The interaction of H2S with nitric oxide (NO) can affect each other's fate and endothelial function to different extents (Figure 1). NO inhibits CSE activity by inducing S-nitrosation of the enzyme [19], whereas it may induce CSE expression [16, 30]. These seemingly opposite effects of NO actually offer more precise control over H2S production by NO at different levels. NO may also increase the cellular uptake of cystine, indirectly increasing H2S production [25].
Figure 1.
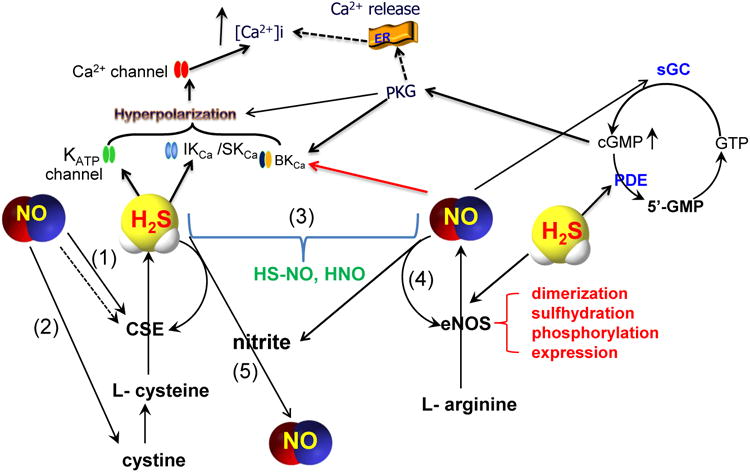
The interaction and convergence of H2S and NO signaling pathways in endothelial function regulation. (1) NO may increase the expression of CSE [16], but inhibit the activity of CSE [19]. (2) NO may increase the cellular uptake of cystine [25]. (3) NO and H2S may form new molecules [26-28]. (4) NO makes its targets resistant to H2S so that H2S cannot modify the same targets as it does in the absence of NO [29]. (5) Under acidic condition, H2S induces the release of NO from nitrite or other NO derivatives. The solid arrow lines depict the stimulatory interactions whereas the dotted arrow lines indicate the inhibitory interactions.
Inversely, H2S affects NO production. An earlier study showed that H2S-gassed solution or NaHS decreased NO formation, eNOS activity and expression, and L-arginine uptake in isolated rat aortas and cultured human umbilical vein endothelial cells. These inhibitory effects of H2S could be indirectly mediated by the activation of KATP channels [31]. However, more consistent observations in recent years support the stimulatory effect of H2S on NO signalling pathway [14, 29, 32-35]. When mice were treated with Na2S, immunohistochemistry of ear venule walls showed a significant up-regulation of eNOS expression [14]. In vitro treatment of rat corpus cavernosum with NaHS increased eNOS mRNA and protein levels and enhanced NO production [35]. The phosphorylation and S-sulfhydration of eNOS [29, 34, 36] and the dimer formation of eNOS [29] are facilitated by H2S and so is NO production [29]. The lack of CSE in CSE-KO mice led to elevated oxidative stress, dysfunctional eNOS, diminished NO levels, and exacerbated myocardial and hepatic I/R injury, which were restored by acute administration of Na2S [32].
H2S and NO act on many common downstream signalling pathways, and the net outcome depends on the integration of the individual effect. NO increases cGMP production via the stimulation of soluble guanylyl cyclase. H2S potentiates cGMP accumulation via the inhibition of phosphodiesterase [37, 38]. Inhibition of eNOS attenuated H2S-stimulated vasorelaxation, and silencing CSE abolishes NO-stimulated cGMP accumulation and angiogenesis [36]. Different from this synergistic effect of H2S and NO, NO-induced S-nitrosation of eNOS decreases NO production and H2S-induced S-sulfhydration of the same increases NO formation [29].
Do NO and H2S have a direct reaction when presented simultaneously in cellular milieu? Earlier study predicted this being likely and an S-nitrosothiol intermediate could be formed [26]. At physiological pH, H2S may react with NO to form S-nitrosothiols, thereby limiting the vasorelaxing (and perhaps pro-angiogenic) activity of NO. Under acidic conditions (pH<7.0), H2S/HS- was capable of inducing the release of NO from oxidised nitrogen species (such as NaNO2) or NO derivatives (such as SNP) [27]. Whether such an interaction of H2S and NO derivatives, however, occurs under physiological in vivo condition has not been directly demonstrated. An unstable molecule thionitrous acid (HS–NO) was proposed as the product of the interaction between H2S and S-nitrosothils [27, 28]. Another study showed that an anaerobic reaction of NO and H2S led to the oxidation of H2S, depletion of NO, and generation of nitroxyl (HNO) [39]. The actual chemical reactions by which H2S facilitates the transformation of NO to HNO has been unclear, and other intermediates, such as HS-NO, may be involved.
Not being a separate entity of disease, EDF reflects pathophysiological changes in the phenotype and functions of endothelial cells that result from and/or contribute to various cardiovascular diseases. Also, EDF is not necessarily a generalised pathologic condition and may be limited to certain blood vessels in different situations. Decreased NO bioavailability has been used as a hallmark of EDF but, depending on the types of blood vessels and the pathogenic conditions of EF, the changes in NO bioavailability may not be causatively linked to all subtypes of EDF. For example, EDF due to decreased EDHF may be indicated by endothelial H2S bioavailability, rather than endothelial NO bioavailability. In the cases where NO-dependent endothelial functions, such as acetylcholine-dependent vasodilation of conduit artery, remain normal, the change in H2S-dependent endothelium-dependent vasorelaxation causes abnormality in EDF [17]. Moreover, as aforementioned, endothelial NO metabolism can be significantly affected by H2S (Figure 1). Therefore, disturbed H2S bioavailability and physiological functions may serve as another novel indicator of the progress and prognosis of EDF. With different pathogenic causes and consequences but a common underlying pathology, EDF in various cardiovascular diseases may be differently related to H2S metabolism but all benefit from strategies that aim at correcting the altered H2S bioavailability (Figure 2).
Figure 2.
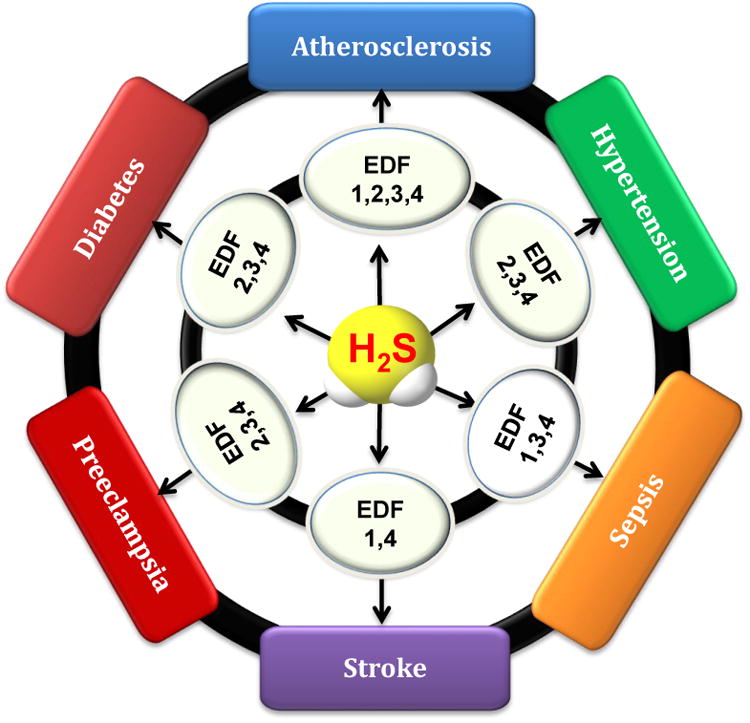
H2S signaling and endothelial dysfunction in cardiovascular diseases. Decreased endothelial H2S bioavailability contributes to the pathogenesis of all subtypes of EDF discussed. The phenotypes of EDF are numbered to facilitate the description. 1 - Barrier and anti-coagulation. 2 - Angiogenesis and self-repair. 3 - Synthesis and secretion. 4 -Endothelium-dependent vasorelaxation.
H2S-related EDF in atherosclerosis
Atherosclerosis is a systemic and chronic vascular disease of large- and medium-sized arteries. Pro-atherosclerotic factors, such as high blood pressure, inflammatory factors, lipid accumulation, and hyperhomocysteinemia, cause focal endothelial dysfunction. This early event triggers artery inflammatory responses, platelet deposition, macrophage differentiation, and foam cell formation. Smooth muscle cell proliferation and migration, extracellular matrix protein synthesis, and thrombus formation ensue. As such, to prevent or correct EDF would be a primary target for atherosclerosis management. Deficiency in CSE expression and H2S bioavailability are causatively linked to the development of atherosclerosis. Feeding CSE-KO mice with atherogenic paigen-type diet elicited early development of fatty streak lesions in the aortic root and increased aortic intimal proliferation [40].
Decreased endothelial H2S bioavailability disarms the endothelium from H2S protection against EDF in atherosclerosis with multiple mechanisms. For example, H2S has a role in maintaining normal lipid metabolism. The fat-fed CSE-KO mice with decreased H2S production showed elevated plasma levels of total cholesterol and low-density lipoprotein (LDL)-cholesterol and hyperhomocysteinemia, which were corrected by NaHS treatment regime [40, 41]. H2S is also involved in decreasing leukocyte adhesion and infiltration into the vessel. H2S-generating compounds (NaHS or GYY4137) inhibited NF-κB-mediated intercellular adhesion molecule-1 (ICAM-1) expression [42] or CX3CR1 and CX3CL1 expression in atherosclerosis [43]. Vascular endothelial cells from CSE-KO mice had increased expression of adhesion molecules (P-selectin and E-selectin) and integrins (ICAM-1 and VCAM-1) [40].
H2S also acts to regulate sheer stress and blood viscosity. Atherosclerosis is unevenly developed along the arterial vascular tree partially due to changed patterns of blood flow and sheer stress. The branches and curvatures of vasculatures are athero-susceptible regions where laminar sheer stress transforms into irregular and swiveled one and these locations are mostly prone to develop EDF and atherosclerosis [44]. Oscillatory shear stress-mediated monocyte binding to endothelial cells and vasodilation were inhibited by H2S in the presence of functional eNOS. On the other hand, H2S increased sheer stress-dependent eNOS expression and decreased expression of ICAM-1 [45, 46]. It appears that both H2S and NO are involved in the sheer stress-mediated development of atherosclerosis. Increased platelet aggregation due to decreased endothelial H2S bioavailability may also contribute to changed blood viscosity in atherosclerosis. H2S may also protect the endothelium through antioxidant modulation. During atherosclerosis development, monocytes-derived macrophages are recruited into the nascent atheromatous lesion and ingest oxidised low density lipoproteins (ox-LDL) to become foam cells and to form fatty streaks within artery wall. CSE-KO mice fed with atherogenic paigen-type diet had clearly enhanced oxidative stress and increased level of ox-LDL in the sub-endothelial space [40]. Supplementations of NaHS or GYY4137 attenuated H2O2 and ox-LDL-mediated endothelial cytotoxicity [47, 48].
H2S-related EDF in diabetic vascular complications
EDF is one of the most important underlying factors for diabetic micro-and macroangiopathy. Increased polyol pathway flux, diacylglycerol formation, protein kinase C activation, and the production of advanced glycation end-products are putative mechanisms for diabetic EDF [49].
Circulating H2S levels are lower in animal models of diabetes, such as streptozotocin-induced diabetic rats [50, 51] and non-obese diabetic mice [52], and in type 2 diabetic patients [50, 53]. As discussed below, the accuracy of measuring circulating H2S level is a significant challenge. Therefore, the correlation of circulating H2S levels with diabetic EDF cannot be concluded yet. However, CSE mRNA is unaltered in the thoracic aorta of diabetic rats [54]. There are also no changes in the expression of CSE, CBS or MST in endothelial cells exposed to elevated extracellular glucose, or in the thoracic aorta of streptozotocin-diabetic rats [50, 55]. In fact, CSE expression in cerebral microvessels was increased in type I diabetes [56].
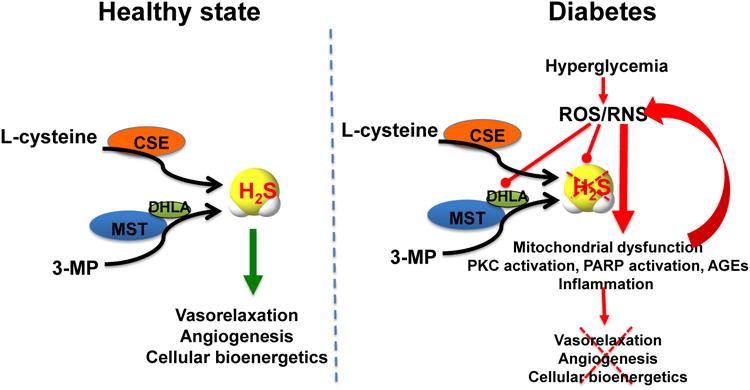
What, then, are the molecular mechanisms for the lower circulating H2S levels in diabetes with increased or unaltered expression of H2S-generating enzymes? Recent data indicate that the endothelial MST was inhibited or inactivated during hyperglycemia, leading to impaired endothelial cell H2S production, suppressed angiogenesis and attenuation of mitochondrial function [55]. Moreover, cellular levels of H2S are determined by the balance of its production and consumption/elimination [57]. When endothelial cells are placed in elevated extracellular glucose, H2S consumption is increased [50]. This increase can be attenuated by either treatment of the cells with ROS scavengers or with mitochondrial uncoupling agents, pointing to the importance of mitochondria-derived ROS in the process of increased H2S consumption in hyperglycemia [51]. It is likely, therefore, that lower circulating H2S level in diabetes is partially related to the oxidative inactivation of endothelial MST, and partially to an increased H2S consumption in hyperglycemic endothelial cell (Figure 3).
Figure 3.
H2S-related pathomechanisms of diabetic endothelial dysfunction. Left side: Vascular production of H2S in normal blood vessels is largely due to the physiological activity of CSE and MST. Right side: When endothelial cells are placed in elevated extracellular glucose, they respond with increased ROS production (from the mitochondrial electron transport chain, and other sources, not shown). The increased ROS inhibits the MST pathway and (directly and indirectly) enhances the consumption of H2S, leading to a H2S-deficient cellular state. This, in turn, creates additional mitochondrial dysfunction, which produces additional ROS in a positive feedback cycle. DHLA, dihydrolipoic acid; AGE, advanved glycation endproducts; PKC, protein kinase C; PARP, poly(ADP-ribose) polymerase; 3-MP, 3-mercaptopyruvate; ROS, reactive oxygen species; RNS, reactive nitrogen species.
Inhibition of endothelial H2S production exacerbates ROS production in response to hyperglycemia [51], which was reversed by supplementation of NaHS [50]. Increased ROS consumes intracellular H2S, which then creates additional mitochondrial dysfunction. Such a positive-feed-forward cycle may play an important role in the development of EDF by activation of mitochondrial cell death signalling (e.g. caspase activation, increased Bax, and decreased Bcl-2 protein expression [58], upregulation of endothelial cell adhesion molecules (e.g. ICAM-1) [59] and downregulation of gap junction proteins (e.g. Cx43 and Cx40) [60]. H2S deficiency may also play a role in the upregulation of the production of endothelin by hyperglycemic endothelial cells [61].
H2S-related EDF in sepsis
While the precise mechanisms responsible for EDF in sepsis are incompletely understood, inflammatory mediators including cytokines, chemokines, and NO have been implicated. Probably due to the presumed similarities of H2S and NO, impact of H2S in sepsis has gained significant attention [62,63]. Although early studies reported that sepsis increases endogenous H2S production in human patients and experimental models of sepsis [65], more recent studies showed depressed levels of free H2S in experimental sepsis. [62, 64]. Time and context-dependent changes in the levels of H2S and its metabolites as well as that in H2S-generating enzymes during sepsis remain to be clarified [65].
As in the case of NO, H2S likely exerts a wide spectrum of effects during inflammation in a concentration-dependent fashion; low sulfide concentrations are anti-inflammatory [65] while high sulfide levels are pro-inflammatory [66]. Along these lines, a recent study showed that breathing H2S gas exerted beneficial effects in endotoxemic mice by maintaining physiological levels of sulfide and thiosulfate, a major oxidation metabolite of H2S [62]. The protective effects of H2S inhalation were associated with inhibition of LPS-induced inflammatory cytokine induction and marked upregulation of anti-inflammatory cytokine IL-10 in the liver.
Acute lung injury (ALI) is characterized by lung inflammation and increased pulmonary vascular permeability. Sepsis is a major cause of ALI. Studies have revealed that vascular endothelium plays a crucial role in mediating inflammatory response in the lung [67]. Therefore, pulmonary vascular endothelium represents one of the major therapeutic targets. Since the beneficial effects of H2S inhalation during sepsis was associated with increased thiosulfate levels and administration of sodium thiosulfate (NaS2O3, STS) per se prevented septic shock and acute liver failure in mice [62], we hypothesised that thiosulfate may be a “carrier molecule” of H2S bioactivity (Figure 4). In a recent study, administration of STS markedly prevented the lipopolysaccharide (LPS)-induced ALI in mice [68]. STS inhibited sepsis-induced production of inflammatory cytokines, lung permeability, histological lung injury, and NFκB activation in the lung. In endothelial cells, STS increased intracellular levels of sulfide and sulfane sulfur, inhibited LPS or TNFα-induced increase in endothelial permeability and production of cytokines and ROS. Considering the clinical availability and established safety track record of STS, these observations may have clinically relevance.
Figure 4.
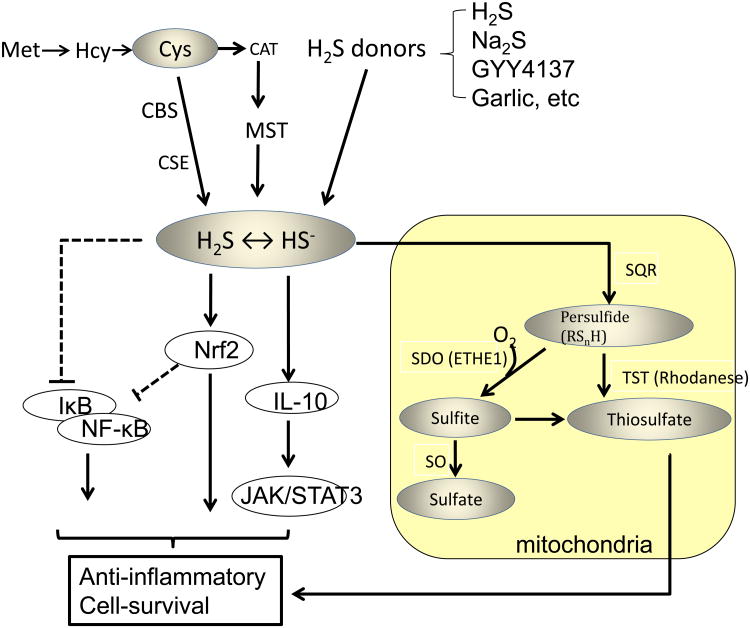
Hypothetical mechanisms responsible for anti-inflammatory effects of endogenous or exogenous H2S. H2S may exert its anti-inflammatory effects via modulating a variety of signaling mechanisms including NfKB, Nrf-2, or IL-10/JAK/STAT3-dependent signaling. Thiosulfate may also exert anti-inflammatory effects. The solid arrow lines depict the stimulatory interactions whereas the dotted barbed lines indicate the inhibitory interactions. Met, methionine; Hcy, homocysteine; Cys, cysteine; CAT, cysteine aminotransferase; CBS, cystathionine beta-synthase; CSE, cystathionine gamma-lyase; MST, 3-mercaptopyruvate sulfurtransferase; Nrf2, Nuclear factor (erythroid-derived)-like 2; JAK, janus kinase; STAT, signal transducer and activator of transcription; SQR, sulfide quinone oxidoreductase; SDO, sulfide dioxigenase; SO, sulfite oxidase; TST, thiosulfate sulfurtransferase.
H2S-related EDF in stroke
EDF is a risk factor for stroke, a cerebrovascular accident, being a leading cause of death worldwide and the primary cause of disability in the western world [69]. While the role of altered brain H2S metabolism in stroke-induced neuronal injury is controversial, this review will focus on H2S-related EDF in stroke.
The disruption of the blood-brain barrier (BBB), formed by capillary endothelial cells, is a hallmark of stroke and contributes significantly to ischaemic brain damage. The protective effects of H2S for cerebrovascular endothelial cell function was shown in a study in which H2S donors, such as 5-(4-methoxyphenyl)-3H-1,2-dithiole-3-thione (ADT), robustly protected BBB integrity and suppressed local neuroinflammation following middle cerebral artery occlusion (MCAO), mediated at least in part through inhibition of NF-κB activation [70]. In another study, NaHS treatment significantly increased neurovascular endothelial cell synthesis and cellular expression of VEGF and angiopoietin-1 around the ischaemic lesion post-MCAO and induced capillary-like tube formation and endothelial cell migration in vitro, through AKT and ERK-dependent mechanisms [71]. These studies suggest that H2S-based therapeutic strategies may help maintain BBB integrity and decrease EDF-related stroke damage. In the same line, studies on human cerebral microvascular endothelial cells, used frequently as an in vitro model of the BBB, showed that slow-release and mitochondria-targeted H2S donors such as GYY4137 and AP39, respectively, prevented cellular and mitochondrial oxidative damage [72, 73].
The effects of exogenous H2S donors and endogenously produced H2S seem to be different on stroke-related EDF without clear mechanistic explanations. For example, CSE-KO mice and the mice treated topically with inhibitors of CSE and MST were resistant to post-ischaemic cerebral vasodilation, hyperaemia and early BBB disruption following transient focal cerebral ischaemia. The hypothetic explanation of this phenomenon is that there was excessive and detrimental vascular overproduction of H2S during reperfusion, leading to hyperaemia-induced BBB damage [74].
H2S-related EDF in hypertension
H2S-related EDF plays a critical role in the pathogenesis of essential hypertension. The expression of CSE in blood vessels of spontaneously hypertensive rats was lower and correlated with blood pressure levels [75, 76]. Treatment of hypertensive rats with zofenopril, a sulfur containing ACE inhibitor that also acts as a H2S donor, restored endothelium-dependent relaxation [75]. Salt-sensitive hypertensive rats exhibited a down-regulation of CBS in renal tissues [77]. In dexamethasone-induced hypertension model, CSE expression was downregulated in resistance vessels [78]. Changes in the expression of H2S-generating enzymes in all of the above animal experiments were paralleled with lower H2S bioavailability. Clinically, grade-2 and grade-3 hypertension patients [79] and diabetic patients with hypertension [80] have lower plasma H2S concentrations. Beyond the aforementioned correlative studies, a causative relationship between H2S-related EDF and hypertension was established by the observation that genetically eliminating CSE expression resulted in age-dependent development of hypertension in mice [17]. In these CSE-KO mice, endothelium-dependent relaxation of resistance mesenteric arteries was essentially abolished [17].
In a model of Ang-II-induced hypertension, administration of NaHS reversed deficits in EDF and NO bioavailability, limited endothelial ROS production, and attenuated the increase in systolic blood pressure. Inhibition of endogenous production of H2S exacerbated the above-mentioned parameters [81]. In other studies, administration of H2S donors to hypertensive animals lowers mean arterial blood pressure and reverses vascular remodeling by inhibiting VSMC proliferation and collagen accumulation in the vessel wall [1, 16, 82-84].
H2S-related EDF in preeclampsia
One third of all maternal deaths and premature delivery worldwide are due to preeclampsia [85]. The long-term risk of premature death increases by almost 3-fold if the mother has had severe preeclampsia [86]. Preeclampsia is a pregnancy-specific multi-organ syndrome characterised by widespread endothelial damage and the onset of new hypertension with proteinuria after 20 weeks of gestation. The disruption of endothelial homeostasis due to dysregulation of cytoprotective pathways and loss of VEGF activity due to increase in anti-angiogenic factors, soluble Flt-1 (sFlt-1) and soluble endoglin (sEng), are increasingly recognized as fundamental features of preeclampsia [87, 88].
CSE and CBS are expressed in the utero-placental unit [89]. A recent study showed that the CSE pathway inhibits release of sFlt-1 and sEng from endothelial cells and human placenta [90]. Endothelial siRNA knockdown of CSE increased, whereas adenoviral-mediated CSE over-expression inhibited, the release of sFlt-1 and sEng from endothelial cells. Furthermore, inhibition of CSE activity increased blood pressure and sFlt-1 and sEng levels, and decreased fetal growth in pregnant mice. These symptoms were reversed by GYY4137, demonstrating that the effects were due to inhibition of H2S production [90]. A subsequent study showed that NaHS attenuated sFlt-1-induced hypertension and renal damage in non-pregnant Sprague-Dawley rats [91].
One of the mechanisms for H2S-offered protection against acute myocardial ischaemia/reperfusion injury is the upregulation of the VEGF–Akt–NOS3–NO pathway [92]. Interestingly, human placental explants subjected to ischaemia-reperfusion showed down-regulated CSE expression [93]. Recently, VEGF receptor-2 was reported as the direct target of H2S, and VEGF receptor inhibitor suppressed angiogenesis induced by H2S [94]. These findings indicate that H2S promotes angiogenesis via VEGF receptor activation. The lowered plasma H2S level in pregnant women with preeclampsia coincides with decreased circulating placental growth factor levels in women with preeclampsia as both are linked to dysregulation of CSE/H2S signaling pathway [90]. H2S supplementation not only restored placental vasculature in CSE-inhibited pregnant mice but also improved the lagging fetal growth [90]. Thus, endogenous H2S is required for healthy placental vasculature and a decrease in CSE/H2S activity may contribute to the pathogenesis of preeclampsia and fetal growth restriction.
Besides CSE, the only other enzyme identified to inhibit sFlt-1 and sEng is heme oxygenase-1 (HO-1) through its products, carbon monoxide (CO) and bilirubin [95, 96]. When both CSE and HO-1fail, the negative feedback loop is lost, and the anti-angiogenic factors (sFlt-1 and sEng) go into overdrive, leading to preeclampsia (Figure 5). The relationship between CSE and HO-1 remains unexplored and is a subject of great interest.
Figure 5.
Defective H2S-CO pathways leading to preeclampsia. Decreased production of hydrogen sulfide (H2S) and carbon monoxide (CO) due to the downregulation of cystathionine γ-lyase (CSE) and heme oxygenase-1 (HO-1), respectively, lower the levels of soluble Flt-1 (sFlt-1) and soluble Endoglin (sEng) and increase placenta growth factor (PlGF) production. These alterations constitute pathogenic factors endothelial dysfunction in preeclampsia.
Concluding Remarks
We have come a long way in understanding the pivotal importance of H2S bioavailability in the endothelium. H2S regulates endothelial proliferation and endothelium-dependent vascular functions. Decreased H2S bioavailability has been consistently reported in different subtypes of EDF. The correlation has been made based on the changes in endothelial functions due to reduced gene expression or activity of H2S-generating enzymes in the endothelium [9, 17, 40], the relative changes in H2S levels before and after the occurrence of EDF using the same measurement method under the same experiment conditions, and the actual levels of H2S in the blood and the endothelium. Can we confidently conclude that H2S bioavailability is a novel hallmark of EDF? In contrast to the well documented role of NO in EDF, there is still a long way to go to establish the similar role of H2S in EDF before we can successfully address multi-faceted challenges we are facing.
The concentration of H2S in the circulation and in specific vasculature beds has been one of the important parameters used in the literature for correlating the changes in endothelial H2S bioavailability with the development of EDF. However, the use of this parameter, especially the blood H2S level, is problematic in two aspects. One is that H2S produced in non-endothelial cells may contribute to its circulation level. As such, the blood H2S level does not necessarily reflect actual endothelium H2S bioavailability. The same is true when the blood level of NO is used as a biomarker for EDF. The other problem is the accuracy and interpretation of H2S measurement. Since the appropriate and reliable methodology for measuring H2S levels in aquatic milieu is still in the developmental stage, there is no universally accepted method to assess H2S level and there is no consensus on the physiological range for H2S in the blood. In any rate, more recent studies report blood H2S levels in the higher nanomolar to lower micromolar range under physiological conditions [99, 100]. It also becomes generally acknowledged that blood H2S levels above the higher micromolar range will be either toxic or artifact, and would certainly not be physiologically relevant. For these considerations, one should be cautious in linking the reported values of blood H2S levels to EDF and, in the same reasoning, to many other types of diseases. The changes in endothelial expression and activities of H2S-generating enzymes and the detection of relative changes in H2S levels in endothelial cells appear to be more meaningful in evaluating the role of endothelial H2S bioavailability in EDF.
The therapeutic intervention to restore H2S bioavailability may effectively remedy certain types of EDF but also take the risk of perturbing endothelial integrity and function of the otherwise normal vasculature in nearby or more remote normal organs. For example, supplementation of H2S may promote angiogenesis in the ischaemia-damaged tissues but it may spillover to induce vascularisation in other healthy organs and systems. The pathogenesis mechanisms of different types of EDF, the specifically affected vasculature in EDF, and the progress of the vascular disease (chronic versus acute) should be all taken into consideration when designing and applying H2S-releasing compounds and their delivery tools and paths. Monitoring the concentrations of H2S in the circulation and in specific vasculature beds is also critical for the therapeutic purpose. The readers are referred to some recent articles for more detailed discussion on our current understanding on the physiological levels of H2S and the H2S-based therapeutic approaches [97, 99, 100]. Other major challenges in the field are briefly described in Box 2.
Box 2. Outstanding questions.
What is the physiological activator for CSE to acutely produce H2S from endothelial cells? One of the mechanisms underlying endothelial production of H2S is the activation of cholinergic receptors on endothelial cells, which leads to increase in [Ca2+]i and the activation of calcium-calmodulin complex. The latter stimulates endothelial CSE to produce H2S in mice [17]. Does acetylcholine/muscarinic receptor exist in rat or mouse vascular endothelium? Two key enzymes are expressed in rat vascular tissues and endothelial cells for in situ synthesis in and secretion from endothelial cells, choline acetyltransferase and vesicular acetylcholine transporter [98]. It is also known that the endothelium of rats or mice produces acetylcholine [98]. Both nicotinic acetylcholine receptor [98] and M3 muscarinic acetylcholine receptors [101, 102] are expressed in rat or mouse vascular endothelia. Are there other endogenous factors that can increase [Ca2+]i in endothelial cells to activate CSE? Previous studies have shown that only a prolonged increase in [Ca2+]i can induce CSE translocation from the cytosol to mitochondria in vascular smooth muscle cells [103]. It is rationalized that, therefore, the calcium/calmodulin activation of CSE would depend on specific types of stimuli and the kinetics of intracellular calcium change.
What is the relationship of the changes in blood sheer stress and endothelial H2S production? Alteration in the pattern and force of sheer stress has significant effect on [Ca2+] i in endothelial cells and, as such, it may offer another endogenous regulation mechanism for the activation of endothelial CSE.
What are the regulatory mechanisms for endothelial production of H2S by MST/CAT pathway? The physiological stimuli for MST activation have not been identified. The endothelial expression of MST should also be verified in other species from mice up to humans. Moreover, the relative contributions of MST and CSE on endothelial H2S production under physiological condition and with endothelial dysfunction are not clear.
What is the impact of the interaction between H2S and vascular endothelial growth factor (VEGF) on endothelial dysfunction? Papapetropoulos et al. demonstrated that VEGF stimulates endothelial production of H2S, and both H2S and VEGF produced angiogenesis [15]. Conversely, daily injection of NaHS into rats for 7 days increased free plasma VEGF level and upregulated renal expression of VEGF-A mRNA. The upregulated VEGF pathway was believed responsible for the protective effects of H2S against soluble fms-like tyrosine kinase-1 (sFLT1)-induced hypertension, proteinuria, and glomerular endotheliosis in rats [91]. By upregulating iNOS expression and NO production in human keratinocytes, H2S indirectly down-regulates ERK1/2 activation thereby resulting in the decrease of VEGF release [104]. The interaction of endothelium-derived endogenous substances such as H2S, CO and NO with VEGF in endothelium dysfunction is unknown.
How do H2S and CO suppress sFlt-1 and sEng release and protect pregnancy [90, 95]? What intermediary pathways are involved in H2S-mediated protection against hypertension and kidney and liver injury when VEGF or placenta growth factor (PlGF) activity is compromised [90, 91].
What is the physiological concentration of endogenous H2S in the endothelium? Both the production and removal of H2S need to be better quantitated in the endothelium as well as in circulation. Unfortunately, many currently available H2S measurement techniques are problematic due to their insufficient accuracy, sensitivity, and reliability in detecting H2S levels in the circulation and inside the cells. Consequently, there is no consensus on the physiological levels of H2S in endothelial cells or in the blood. Another closely related outstanding question is the detection of endothelial H2S bioavailability in humans under physiological condition and in diseases. Furthermore, the advance in H2S biomedical research has been impeded by the lack of suitable pharmacological tools – specific inhibitors and activators of H2S-generating enzymes as well as optimized H2S donors.
It is anticipated that a better understanding of the regulatory mechanisms of endothelial H2S bioavailability will help accelerate the translation of advances in H2S biology to clinical management of the EDF-related cardiovascular diseases. For now, endothelial dysfunction (EDF) remains the Achilles heel in cardiovascular diseases. “Should his heel have been shielded from the poisonous Trojan arrow, Achilles would have been immortal.”
Highlights.
Endothelial H2S bioavailability regulates endothelial proliferation and function
H2S is an endothelium-derived hyperpolarising factor
Altered H2S bioavailability is a novel hallmark of endothelial dysfunction.
H2S bioavailability is a therapeutic target for remedying endothelial dysfunction.
Acknowledgments
This work has been supported by a Discovery Grant from Natural Sciences and Engineering Research council of Canada to RW. CS has been supported by the American Diabetes Association, the National Institutes of Health of USA and the Shriners Hospitals for Children. FI has been supported by the National Institutes of Health of USA. MW has been supported by the Medical Research Council of UK. AA has been supported by programme grants from British Heart Foundation (RG/09/001/25940), Medical Research Council (G0700288), Royal Society and European Union. AP has been supported through an Aristeia grant (1436) that is co-financed by the European Union (ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning”. MW and AP are supported by the COST Action BM1005 (ENOG: European network on gasotransmitters).
Glossary Box
- AP39
A mitochondria-targeted H2S donor shown to prevent endothelial cell (and mitochondrial) toxicity induced by oxidative stress at low nM concentrations. It also stimulates cellular bioenergentics and lowers heart rate and blood pressure in hypertensive animals
- Cystathionine γ-lase (CSE)
An enzyme in the reverse-transsulfuration pathway which uses L-cysteine as the substrate to produce H2S
- Endothelium-derived hyperpolarising factor (EDHF)
The endogenous substances produced in the endothelium which can cause the hyperpolarisation of juxtaposed vascular smooth muscle cells and vasorelaxation
- Endothelium-derived relaxing factor (EDRF)
The endogenous substances produced in the endothelium which can cause the relaxation of juxtaposed vascular smooth muscle cells
- Endothelial dysfunction (EDF)
A pathophysiological status in the phenotype and functions of endothelial cells that results from and contributes to a plethora of cardiovascular diseases
- Endothelial NO synthase (eNOS)
An endothelium-located enzyme that catalyzes the production of nitric oxide from L-arginine
- Gasotransmitters
A class of endogenously produced gaseous molecules with importantsignaling functions for cellular homeostasis. These include nitric oxide, carbon monoxide, hydrogen sulfide, and ammonium
- GYY4137
A water soluble slow release H2S donor shown to lower systemic blood pressure in rats and mice and induced blood vessel relaxation ex vivo by endothelium- and KATP channel-dependent mechanisms. It also inhibits oxidative stress-induced mitochondrial and cellular injury in vitro and in vivo
- Heme oxygenase-1 (HO-1)
An inducible enzyme that catalyses the catabolism of heme to produce carbon monoxide, iron, and biliverdin. The latter is rapidly converted to bilirubin by biliverdin reductase
- 3-mercaptopyruvate sulfurtransferase (MST)
An enzyme that transfers the sulfane sulfur from 3-mercaptopyruvate to other sulfur acceptors. Eventually, the bound sulfur is released or reduced to liberate H2S
- Oxidised low-density lipoprotein (ox-LDL)
Low-density lipoprotein transfers cholesterol and triglycerides through the bloodstream to be used by various cells. Its lipid component and/or the protein component can be oxidized to become ox-LDL. Ox-LDL is a risk factor for vascular inflammation, macrophage infiltration, platelet adhesion, and atherosclerosis
- Placenta growth factor (PlGF)
PlGF shares a 53% amino acid sequence homology with VEGF and signals exclusively via VEGF receptor-1 and stimulates NO and promotes monocyte migration
- Reactive oxygen species (ROS)
A group of oxygen-containing reactive molecules, which participate in cellular signal transduction under physiological conditions and become detrimental under pathological conditions when ROS are over-produced
- Soluble Endoglin (sEng)
A soluble, cleaved form of endoglin (CD105; the co-receptor for the transforming growth factor-β) produced by the proteolytic cleaving action of metalloproteinase MMP-14 in the extracellular domain of endothelial cell membrane. It is elevated in the serum of preeclamptic women 8-12 weeks prior to clinical onset of the disease and like sFlt-1, inhibits capillary morphogenesis
- Soluble fms-like tyrosine kinase-1 (sFlt-1 or sVEGFR-1)
sFlt-1 is a splice variant of the fit gene. It is a tyrosine kinase protein that deactivates VEGF receptor signaling by mopping up VEGF as well as acting as a ‘dominant negative receptor inhibitor’ to prevent VEGF receptor dimerization for cell signaling
- Vascular endothelial growth factor (VEGF)
Being a member of the platelet-derived growth factor family of cystine-knot growth factors, VEGF stimulates the growth and proliferation of vascular endothelial cells, a critical process for both vasculogenesis and angiogenesis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang R. Physiological implications of hydrogen sulfide – A whiff exploration that blossomed. Physiol Rev. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 2.Shibuya N, et al. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biolchem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 3.Altaany Z, et al. Hydrogen sulfide and endothelial dysfunction: Relationship with nitric oxide. Curr Med Chem. 2014;21(32):3646–3661. doi: 10.2174/0929867321666140706142930. [DOI] [PubMed] [Google Scholar]
- 4.Zanardo RC, et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20(12):2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 5.Fiorucci S, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129(4):1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 6.Zuidema MY, et al. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of BK channels. Am J Physiol Heart Circ Physiol. 2010;299(5):H1554–1567. doi: 10.1152/ajpheart.01229.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muzaffar S, et al. H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: role of protein kinases A and G. Br J Pharmacol. 2008;155(7):984–994. doi: 10.1038/bjp.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suo R, et al. Hydrogen sulfide prevents H2O2-induced senescence in human umbilical vein endothelial cells through SIRT1 activation. Mol Med Rep. 2013;6:1865–1870. doi: 10.3892/mmr.2013.1417. [DOI] [PubMed] [Google Scholar]
- 9.Bos EM, et al. Cystathionine gamma-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol. 2013;24(5):759–770. doi: 10.1681/ASN.2012030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang G, et al. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal. 2013;18(15):1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 11.Yan SK, et al. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;351(2):485–491. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 12.Wen YD, et al. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One. 2013;8(2):e53147. doi: 10.1371/journal.pone.0053147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong L, et al. The inhibitory effect of hydrogen sulfide on platelet aggregation and the underlying mechanisms. J Cardiovasc Pharmacol. 2014;64(5):481–487. doi: 10.1097/FJC.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 14.Kram L, et al. The anti-thrombotic effect of hydrogen sulfide is partly mediated by an upregulation of nitric oxide synthases. Thromb Res. 2013;132(2):e112–7. doi: 10.1016/j.thromres.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Papapetropoulos A, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA. 2009;106(51):21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao W, et al. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R. Hydrogen sulfide: a new EDRF. Kidney Int. 2009;76:700–704. doi: 10.1038/ki.2009.221. [DOI] [PubMed] [Google Scholar]
- 19.Mustafa AK, et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109(11):1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang G, et al. H2S is an endothelium-derived hyperpolari- zing factor. Antioxid Redox Signal. 2013;19(14):1634–1646. doi: 10.1089/ars.2012.4805. [DOI] [PubMed] [Google Scholar]
- 21.Edwards G, et al. Endothelium-derived hyperpolarizing factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 22.Grgic I, et al. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF dilator responses-relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157(4):509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y, et al. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287(5):H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 24.Jamroz-Wiśniewska A, et al. Leptin-induced endothelium-dependent vasorelaxation of peripheral arteries in lean and obese rats: role of nitric oxide and hydrogen sulfide. PLoS One. 2014;9(1):e86744. doi: 10.1371/journal.pone.0086744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang R. Two's company, three's a crowd – Can H2S be the third endogenous gaseous transmitter? Faseb J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 26.Whiteman M, et al. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- 27.Grossi L, et al. Hydrogen sulfide induces nitric oxide release from nitrite. Bioorg Med Chem Lett. 2009;19(21):6092–6094. doi: 10.1016/j.bmcl.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Filipovic MR, et al. Chemical characterization of the smallest S-Nitrosothiol, HSNO; cellular cross-talk of H2S and S-Nitrosothiols. J Am Chem Soc. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altanny Z, et al. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal. 2014A;7(342):ra87. doi: 10.1126/scisignal.2005478. [DOI] [PubMed] [Google Scholar]
- 30.Wang R. Shared signaling pathways among gasotransmitters. Proc Natl Acad Sci USA. 2012;109(23):8801–8802. doi: 10.1073/pnas.1206646109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng B, et al. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1608–1618. doi: 10.1152/ajpregu.00207.2006. [DOI] [PubMed] [Google Scholar]
- 32.King AL, et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA. 2014;111(8):3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen PH, et al. Hydrogen sulfide increases nitric oxide production and subsequent S-nitrosylation in endothelial cells. Sci World J. 2014;2014:480387. doi: 10.1155/2014/480387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kida M, et al. Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur J Pharm Sci. 2013;48(1-2):211–215. doi: 10.1016/j.ejps.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Meng J, et al. Hydrogen sulfide promotes nitric oxide production in corpus cavernosum by enhancing expression of endothelial nitric oxide synthase. Int J Impot Res. 2013;25(3):86–90. doi: 10.1038/ijir.2012.39. [DOI] [PubMed] [Google Scholar]
- 36.Coletta C, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bucci M, et al. cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS One. 2012;7:e53319. doi: 10.1371/journal.pone.0053319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bucci M, et al. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 39.Eberhardt M, et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat Commun. 2014;5:4381. doi: 10.1038/ncomms5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mani S, et al. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127(25):2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 41.Mani S, et al. Deficiency of cystathionine gamma-lyase and hepatic cholesterol accumulation during mouse fatty liver development. Science Bulletin. 2015;60(3):336–347. [Google Scholar]
- 42.Wang Y, et al. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29(2):173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, et al. Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PLoS One. 2012;7(7):e41147. doi: 10.1371/journal.pone.0041147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou J, et al. Shear stress–initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34(10):2191–2198. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chai Q, et al. Hydrogen sulfide impairs shear stress-induced vasodilation in mouse coronary arteries. Pflugers Arch. 2015;467(2):329–40. doi: 10.1007/s00424-014-1526-y. [DOI] [PubMed] [Google Scholar]
- 46.Go YM, et al. H2S inhibits oscillatory shear stress-induced monocyte binding to endothelial cells via nitric oxide production. Mol Cells. 2012;34(5):449–55. doi: 10.1007/s10059-012-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeney V, et al. Suppression of hemin-mediated oxidation of low-density lipoprotein and subsequent endothelial reactions by hydrogen sulfide (H2S) Free Radic Biol Med. 2009;46(5):616–623. doi: 10.1016/j.freeradbiomed.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z, et al. Hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E-/- mice. Br J Pharmacol. 2013;169(8):1795–1809. doi: 10.1111/bph.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol. 2009;156:713–727. doi: 10.1111/j.1476-5381.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain SK, et al. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki K, et al. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci USA. 2011;108(33):13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brancaleone V, et al. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br J Pharmacol. 2008;155:673–80. doi: 10.1038/bjp.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whiteman M, et al. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denizalti M, et al. The vasorelaxant effect of hydrogen sulfide is enhanced in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:509–17. doi: 10.1007/s00210-011-0601-6. [DOI] [PubMed] [Google Scholar]
- 55.Coletta C, et al. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase / H2S pathway: functional impairment by hyperglycemia and restoration by DL-alpha-lipoic acid. Mol Med. 2015 doi: 10.2119/molmed.2015.00035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Streeter EY, et al. Effect of type 1 diabetes on the production and vasoactivity of hydrogen sulfide in rat middle cerebral arteries. Physiol Rep. 2013;1:e00111. doi: 10.1002/phy2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doeller JE, et al. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 58.Guan Q, et al. Hydrogen sulfide protects against high-glucose-induced apoptosis in endothelial cells. J Cardiovasc Pharmacol. 2012;59:188–193. doi: 10.1097/FJC.0b013e31823b4915. [DOI] [PubMed] [Google Scholar]
- 59.Guan Q, et al. Hydrogen sulfide suppresses high glucose-induced expression of intercellular adhesion molecule-1 in endothelial cells. J Cardiovasc Pharmacol. 2013;62:278–284. doi: 10.1097/FJC.0b013e31829875ef. [DOI] [PubMed] [Google Scholar]
- 60.Zheng YF, et al. NaHS ameliorates diabetic vascular injury by correcting depressed connexin 43 and 40 in the vasculature in streptozotocin-injected rats. J Pharm Pharmacol. 2010;62:615–621. doi: 10.1211/jpp.62.05.0009. [DOI] [PubMed] [Google Scholar]
- 61.Guan Q, et al. High glucose induces the release of endothelin-1 through the inhibition of hydrogen sulfide production in HUVECs. Int J Mol Med. 2015;35:810–814. doi: 10.3892/ijmm.2014.2059. [DOI] [PubMed] [Google Scholar]
- 62.Tokuda K, et al. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal. 2012;17:11–21. doi: 10.1089/ars.2011.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shirozu K, et al. Cystathionine gamma-lyase deficiency protects mice from galactosamine/lipopolysaccharide-induced acute liver failure. Antioxid Redox Signal. 2014;20(2):204–216. doi: 10.1089/ars.2013.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wintner EA, et al. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol. 2010;160:941–957. doi: 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. Faseb J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, et al. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via Nf-{kappa}b. AJP - Lung Cell Mol Physiol. 2007;292:L960–L971. doi: 10.1152/ajplung.00388.2006. [DOI] [PubMed] [Google Scholar]
- 67.Reddy AT, et al. Endothelial cell peroxisome proliferator-activated receptor gamma reduces endotoxemic pulmonary inflammation and injury. J Immunol. 2012;189:5411–5420. doi: 10.4049/jimmunol.1201487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakaguchi M, et al. Sodium thiosulfate attenuates acute lung injury in mice. Anesthesiology. 2014;121(6):1248–1257. doi: 10.1097/ALN.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Della-Morte D, et al. Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments. Pharmacogenomics. 2012;13:595–613. doi: 10.2217/pgs.12.14. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, et al. Hydrogen sulfide protects blood-brain barrier integrity following cerebral ischemia. J Neurochem. 2014;129:827–838. doi: 10.1111/jnc.12695. [DOI] [PubMed] [Google Scholar]
- 71.Jang H, et al. Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J Neurosci Res. 2014;92:1520–1528. doi: 10.1002/jnr.23427. [DOI] [PubMed] [Google Scholar]
- 72.Le Trionnaire S, et al. The synthesis and functional evaluation of a mitochondria-targeted hydrogen sulfide donor, (10-oxo-10-(4-(3-thioxo-3h-1,2-dithiol-5-yl)phenoxy)decyl)triphenylphosphonium bromide (ap39) Medchemcomm. 2014;5:728–736. [Google Scholar]
- 73.Szczesny B, et al. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide. 2014 doi: 10.1016/j.niox.2014.04.008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang Z, et al. Role of hydrogen sulfide in early blood-brain barrier disruption following transient focal cerebral ischemia. PloS one. 2015;10:e0117982. doi: 10.1371/journal.pone.0117982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bucci M, et al. Hydrogen sulfide accounts for the peripheral vascular effects of zofenopril independently of ACE inhibition. Cardiovasc Res. 2014;102:138–147. doi: 10.1093/cvr/cvu026. [DOI] [PubMed] [Google Scholar]
- 76.Yan H, et al. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- 77.Huang P, et al. Down-regulated CBS/H2S pathway is involved in high-salt-induced hypertension in Dahl rats. Nitric Oxide. 2015 doi: 10.1016/j.niox.2015.01.004.. [DOI] [PubMed] [Google Scholar]
- 78.d'Emmanuele VBR, et al. Hydrogen sulfide is involved in dexamethasone-induced hypertension in rat. Nitric Oxide. 2014 doi: 10.1016/j.niox.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 79.Sun N, et al. Plasma hydrogen sulfide and homocysteine levels in hypertensive patients with different blood pressure levels and complications. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:1145–1148. [PubMed] [Google Scholar]
- 80.Whiteman M, et al. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53(8):1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 81.Al-Magableh M, et al. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertens Res. 2015;38:13–20. doi: 10.1038/hr.2014.125. [DOI] [PubMed] [Google Scholar]
- 82.Li L, et al. Characterization of a novel, water-soluble hydrogen sulfide releasing molecule (GYY4137) Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 83.Tomasova L, et al. Effects of AP39, a triphenylphosphonium derivatised anethole dithiolethione hydrogen sulfide donor, on rat haemodynamic parameters and chloride can calcium Cav3 and RyR2 channels. Nitric Oxide. 2015;46:131–44. doi: 10.1016/j.niox.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 84.Meng G, et al. Emerging role of hydrogen sulfide in hypertension and related cardiovascular diseases. Br J Pharmacol. 2014 doi: 10.1111/bph.12900.. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lisonkova S, et al. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet Gynecol. 2014;24(4):771–781. doi: 10.1097/AOG.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 86.Irgens HU, et al. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323(7323):1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goulopoulou S, Davidge ST. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol Med. 2015;21(2):88–97. doi: 10.1016/j.molmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 88.Ahmed A, Ramma W. Unraveling the theories of preeclampsia: Are the protective pathways the new paradigm? Br J Pharmacol. 2015;172:1574–1586. doi: 10.1111/bph.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patel P, et al. The endogenous production of hydrogen sulphide in intrauterine tissues. Reprod Biol Endocrinol. 2009;7:10. doi: 10.1186/1477-7827-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang K, et al. Dysregulation of the hydrogen sulfide (H2S) producing enzyme cystathionine γ-lyase (CSE) contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013;127(25):2514–2522. doi: 10.1161/CIRCULATIONAHA.113.001631. [DOI] [PubMed] [Google Scholar]
- 91.Holwerda KM, et al. Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol. 2014;25(4):717–725. doi: 10.1681/ASN.2013030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kondo K, et al. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127(10):1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cindrova-Davies T, et al. Reduced cystathionine gamma-lyase and increased microRNA-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: Hydrogen sulfide as a placental vasodilator. Am J Pathol. 2013;182(4):1448–1458. doi: 10.1016/j.ajpath.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tao BB, et al. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal. 2013;19(5):448–464. doi: 10.1089/ars.2012.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cudmore M, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115(13):1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 96.George EM, et al. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension. 2011;57(5):941–948. doi: 10.1161/HYPERTENSIONAHA.111.169755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wallace J, Wang R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Dis. 2015;14(5):329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 98.Hao C, et al. Arterial baroreflex dysfunction impairs ischemia-induced angiogenesis. J Am Heart Assoc. 2014;3(3):e000804. doi: 10.1161/JAHA.114.000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shen X, et al. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med. 2011;50:1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCook O, et al. H2S during circulatory shock: Some unresolved questions. Nitric Oxide. 2014;41:48–61. doi: 10.1016/j.niox.2014.03.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gericke A, et al. Role of the M3 muscarinic acetylcholine receptor subtype in murine ophthalmic arteries after endothelial removal. Invest Ophthalmol Vis Sci. 2014;55(1):625–631. doi: 10.1167/iovs.13-13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boittin FX, et al. Connexins and M3 muscarinic receptors contribute to heterogeneous Ca(2+) signaling in mouse aortic endothelium. Cell Physiol Biochem. 2013;31(1):166–178. doi: 10.1159/000343358. [DOI] [PubMed] [Google Scholar]
- 103.Fu M, et al. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci USA. 2012;109(8):2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merighi S, et al. Hydrogen sulfide modulates the release of nitric oxide and VEGF in human keratinocytes. Pharmacol Res. 2012;66(5):428–436. doi: 10.1016/j.phrs.2012.07.002. [DOI] [PubMed] [Google Scholar]