Abstract
Maternal immune activation (MIA) results in the development of autism in the offspring via hyperactivation of IL-6 signaling. Furthermore, experimental studies showed that the MIA-associated activation of interleukin-1β (IL-1β) concurrently with IL-6 increases the rate and the severity of hippocampal kindling in mice, thus offering an explanation for autism-epilepsy comorbidity. We examined whether epileptic phenotype triggered by prenatal exposure to IL-6 and IL-1β combination is restricted to kindling or whether it is reproducible in another model of epilepsy, whereby spontaneous seizures develop following kainic acid (KA)- induced status epilepticus. We also examined whether in mice prenatally exposed to IL-6 and IL-6+IL-1β, the presence of spontaneous seizures would exacerbate autism-like features. Between days 12 and 16 of pregnancy, C57bl/6j mice received daily injections of IL-6, IL-1β or IL-6+IL-1β combination. At postnatal day 40, male offspring was examined for the presence of social behavioral deficit and status epilepticus was induced by intrahippocampal KA injection. After six weeks of monitoring for spontaneous seizures, sociability was tested again. Both IL-6 and IL-6+IL-1β offspring presented with social behavioral deficit. Prenatal exposure to IL-6 alleviated, while such exposure to IL-6+IL-1β exacerbated the severity of KA-induced epilepsy. Increased severity of epilepsy in the IL-6+IL-1β mice correlated with the improvement of autism-like behavior. We conclude that complex and not necessarily agonistic relationships exist between epileptic and autism-like phenotypes in an animal model of MIA coupled with KA-induced epilepsy, and that the nature of these relationships depends on components of MIA involved.
Keywords: Autism, epilepsy, comorbidity, maternal immune activation, cytokines, interleukin-6, interleukin-1β
1. Introduction
Common bi-directional connection between autism and epilepsy [1-3] has prompted numerous studies of autism-epilepsy comorbidity using animal models. Not unlike in clinics, the association between autism-like and epileptic phenotypes varies significantly in experimental systems. For example, SCN1a haploinsufficient mice, which serve as a model of Dravet's syndrome, present with both autism-like behavior and spontaneous seizures [4]. Conversely, mice which carry a missense mutation of a gene encoding Neuroligin-3 (a mutation that has been implicated in autism [5]) and display autism-like behavior [6], have increased resistance to primary generalized seizures [7]. At the same time, inbred BTBR mice which are characterized by many behavioral and anatomical abnormalities consistent with autism [8], show no epileptic phenotype [9]. Identifying animal models appropriate for exploring autism-epilepsy connections, and reflecting variety of etiologies and mechanisms of both disorders is important for understanding the mechanisms of the comorbidity, and for the development of its effective therapies.
Maternal immune activation (MIA) may represent a system suitable for exploring autism-epilepsy comorbidity. Epidemiological studies suggest that MIA, particularly when triggered by viral infections, represents a risk factor for the development of autism in the offspring [10, 11]. Congruent with clinical findings, the offspring of mice in which viral infection has been mimicked during pregnancy by means of polyinosinic–polycytidylic acid (Poly I:C), present with a spectrum of behavioral, anatomical and physiological perturbations consistent with autism [12, 13]. Concurrently, these animals show increased susceptibility to epilepsy in the hippocampal rapid kindling paradigm [14]. Furthermore, components of MIA liable for the evolvement of autism-like and epileptic phenotypes have been identified: while autism-like impairments stem solely from the activation of interleukin-6 (IL-6) [15], parallel propensity to epilepsy requires simultaneous induction of IL-6 and interleukin-1β (IL-1β) [14].
In the present study we pursued to further examine autism-epilepsy connection in the MIA system. We deemed it important to establish that epileptogenicity in the MIA offspring is not model-specific, that is not restricted to rapid kindling. Such studies appear even more warranted considering somewhat limited relevance of the rapid kindling model, in which no spontaneous recurrent seizures are observed. We chose a model of chronic epilepsy where spontaneous recurrent seizures develop following status epilepticus (SE) induced by intrahippocampal administration kainic acid (KA) [16, 17]. Further, in order to expand on our earlier findings [14], we examined whether the autism-epilepsy connection in the MIA offspring is bidirectional, that is whether the presence of spontaneous seizures in KA-injected mice would exacerbate the severity of autism-like impairments.
2. Material and methods
2.1. Animals
The experiments were performed in C57BL/6J mice. Breeding pairs were obtained from The Jackson Laboratory (Sacramento, CA). Breeding was performed at the UCLA Department of Laboratory Medicine. The procedures complied with the policies of the National Institutes of Health and were approved by the UCLA Office of Research Administration.
The presence of vaginal plug was considered as embryonic day (E) 0. The offspring was weaned at postnatal day (P) 28. Considering the higher prevalence of autism among males [18], and that in the MIA model autism-like behavior is reserved for male offspring exclusively [19], the experiments proper were conducted in male mice. Animals were maintained at 12 hours light-dark cycle, with free access to food and water. Mice were house individually, which was necessary for monitoring spontaneous seizures.
2.2. Modelling MIA
Based on earlier findings [14, 15], we used recombinant cytokines IL-6 and IL-1β to mimic MIA in pregnant mice. This offered a cleaner approach as compared with the use Poly I:C, as the MIA system was limited to factors specifically responsible for the evolvement of autism-like and epileptic phenotypes in the offspring and thus allowed avoiding wider variability on both seizure and behavioral responses inherent to the Poly I:C protocol. Between E12 and E16, mice received daily intraperitoneal injections of saline (n=8), recombinant IL-6 (20 ug/kg, n=11), recombinant IL-1β (20 ug/kg, n=10), or recombinant IL-6+IL-1β (10+10 mg/kg, n=13). Both cytokines were manufactured by R&D systems (Minneapolis, MN) [14].
Cytokine treatment had no observable effects on pregnant mice. Body weight gain was similar to those in saline-treated animals (across all experimental groups body weight was 30-33 g at E12 and 42-45 g on E16, with no differences among the groups). We did not measure core temperature in these animals, as the insertion of rectal probe may lead to premature termination of pregnancy. However, in our earlier studies [14] we reported that the applied treatment regimens did not induce hyperthermia, when the temperature was measured in specially allocated group of mice. After giving birth, cytokine-treated dams did not reject pups at a rate higher than saline-treated ones, and did not refuse nursing (occasional rejections and subsequent offspring death occur even in the absence of any manipulations and handling). From each saline-treated mouse between 1 and 3 male offspring reached P28. For cytokine treatments, the number of male offspring reaching P28 was 0-3. Survivors from dams subjected to different cytokine treatments showed no noticeable differences in weight gain and general behavior as compared with offspring of saline-treated mice.
2.3. First behavioral testing
Impaired social interaction is a hallmark of autism [20] and is commonly used as a key parameter in characterizing autism-like impairments in animal models [8, 21]. We employed a widely used three chamber sociability test [21] adapted in our lab [14] in order to quantify impairments of social behavior in mice. The test was performed between P35 and P40. The apparatus (Noldus, Leesburg, VA) was a 60 x 40 cm Plexiglas box divided into three connected compartments. Each of the end compartments contained wired cylindrical enclosure (11 cm high, 10 cm diameter, bar space 1 cm apart). First, a test mouse was placed inside the apparatus, was allowed to explore it for 10 minutes, and was then removed. An unfamiliar age- and sex-matched mouse (conspecific) was placed inside one enclosure, and an unfamiliar object (cube) – inside another enclosure. The placements of the conspecific and of the object were randomized between the two compartments for different test mice. Test mouse was reintroduced into the apparatus and was allowed to explore for 10 minutes. Behavior was recorded on video and was analyzed off-line by investigators blind to treatments. Total time of direct exploration (i.e. sniffing) by the test mouse of the conspecific (tconspecific) and of the object (tobject) was counted. Sociability index was calculated using the formula [tconspecific / tconspecific + tobject] X 100 – 50. The index spans from −50 (complete avoidance of the conspecific) to 0 (indifference) to +50 (full preference for the conspecific) [12, 14].
2.4. Induction and monitoring of chronic epilepsy
Between P40 and P45, animals were anesthetized with Isoflurane and placed in the stereotaxic apparatus. KA (Sigma, St. Louis, MO) was stereotaxically injected in the amount of 50 ng in 0.5 ul of saline into the left ventral hippocampus (coordinates from Bregma: posterior – 2.9 mm, lateral- 2.8 mm, down- 4.0 mm [22]). Control animals received intrahippocampal injection of saline.
In order to avoid litter effect (i.e. that is that offspring from the same litter are identical and thus represent a virtual n=1 [23, 24]), no subjects belonging to the same litter underwent same postnatal procedure. For example, if an IL-6- treated mouse produced 3 male offspring reaching P28, one of them received intrahippocampal injection of saline, another – intrahippocampal injection of kainic acid, and the third one was not used in the experiments. This principle was applied to all groups.
KA injection resulted in the development of limbic SE, the latter consisting of repeated clonic-tonic generalized seizures (rearing and/or rearing and falling; stages 4-5 on the Racine scale [25]) intermittently with focal seizures (motor arrest and/or facial clonus; stage 1 on the Racine scale). SE lasted between 3 and 5 hours. Only those animals which developed repeated stage 4-5 convulsions were used for further studies.
Three-four weeks after intrahippocampal KA or saline injection the animals were prepared for seizure monitoring. Under Isoflurane anesthesia, wireless transmitter model ETA-F10 (Data Science International, DSI, St. Paul, MN) was placed inside a subcutaneous pocket on the back; its two leads were fed under the skin to the skull surface and fixed with skull screws. The leads were fixed to the skull with dental cement.
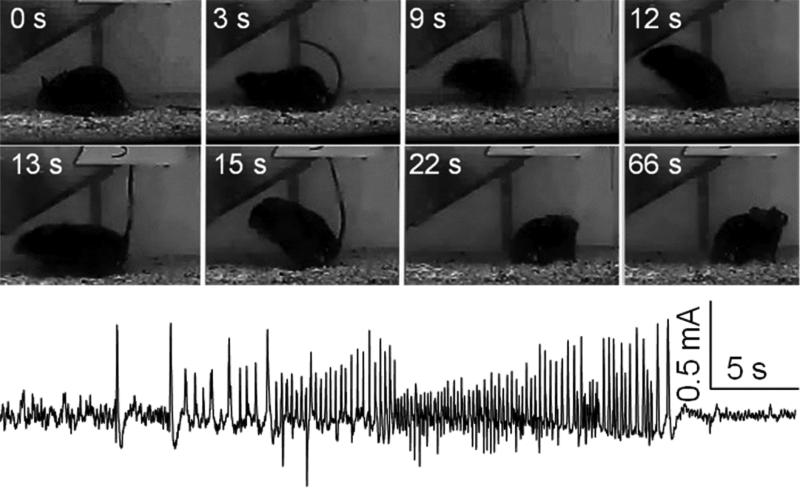
Video and EEG monitoring of spontaneous seizures began one week after surgery and continued for six weeks. Behavioral seizures were recorded using digital cameras focusing on individual cages; data were saved on the digital video recorder. For the acquisition of electrographic seizures, home cages with individually housed animals were placed on top of wireless receivers RPC-1 (DSI) connected to a computer equipped with Harmonie acquisition software (Stellate Systems, Montreal, QC). In order to unambiguously establish the presence of chronic epilepsy, the only seizures considered were secondary generalized complex partial seizures (stage 4-5 on the Racine scale [25]) with clearly identifiable electrographic features (Fig. 1). Average number of seizures per day was calculated.
Fig. 1. An example of spontaneous seizure after kainic acid (KA) - induced status epilepticus.
Spontaneous seizure was captured in a 12 week-old mouse five weeks after intrahippocampal KA injection (50 ng). Top two rows. Snapshots of a stage 4 spontaneous seizure. Time is indicated from the seizure onset. Note the progression from tonic phase (3 s) to clonic-tonic phase (“kangaroo” posture, 9-15 s). Also, note postictal depression lasting for over 40 s (22 s and 66 s samples), evident as retaining same position without movements. Bottom row. Electrographic seizure from the same mouse. Note postictal depression.
2.5. Second behavioral testing
After the end of seizure monitoring (i.e. at the age of 16 weeks), animals underwent second sociability test, following the procedure described under 2.3. First behavioral testing.
2.6. Histology
After the second behavioral test, the animals were anesthetized with Pentobarbital, and transcardially perfused with 4% paraformaldehyde. Brains were removed, post-fixed in 4% paraformaldehyde and embedded in paraffin. Coronal 10 micron-thick sections of the hippocampus were cut for immunohistochemistry. In order to characterize neurodegeneration, primary rabbit polyclonal anti-NeuN antibodies (1:300, Abcam, Cambridge MA) were used in conjunction with secondary biotinylated goat anti-rabbit antibodies (1:200, Abcam). Immunostaining was visualized using 3,3-diaminobenzidine (Sigma) with nickel intensification. The numbers of NeuN-positive cells (i.e. neurons) in the CA1 and CA3 areas of both left and right hippocampi were counted in 6 consecutive sections, using an eyepiece graticule with an indexed grid under 20X magnification on a Leica DLM microscope (McBain Instruments, Simi Valley, CA). The infusion of KA was visually verified by locating the injection cannula track.
2.7. Data analysis
Data were analyzed using Prism 6 software (Graphpad, San Diego, CA). Sample sizes, treatments and group names are described in Table 1. Statistical tests are noted in Figure legends.
Table 1.
Experimental groups.
| Pregnant mouse treatment | Offspring treatment | Group name | Number of offspring used for the experiments |
|---|---|---|---|
| Saline | Saline | Saline/Saline | 5 |
| Saline | Kainic acid (KA) | Saline/KA | 8 |
| IL-6 | Saline | IL-6/Saline | 6 |
| IL-6 | KA | IL-6/KA | 5 |
| IL-1β | Saline | IL-1β/Saline | 4 |
| IL-1β | KA | IL-1β/KA | 6 |
| IL-6+IL-1β | Saline | IL-6+IL-1β/Saline | 5 |
| IL-6+IL-1β | KA | IL-6+IL-1β/KA | 9 |
3. Results
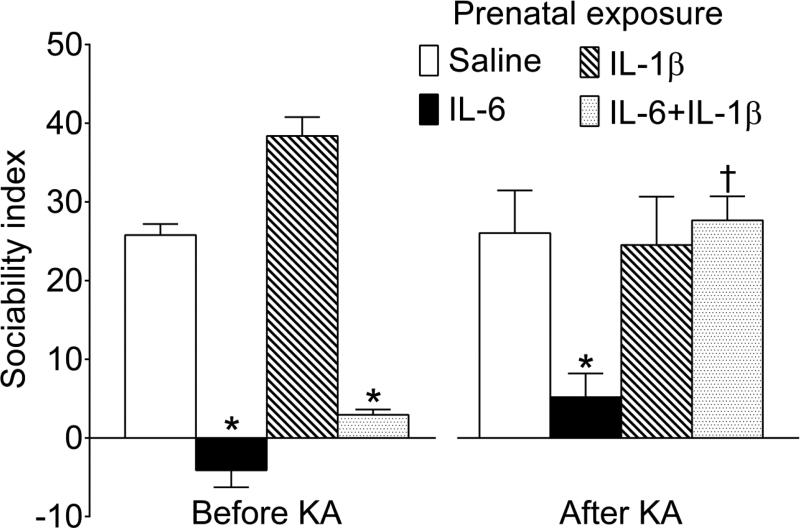
3.1. First sociability test (Fig. 2, Before KA)
Fig. 2. Performance in the three-chamber sociability test.
Left. The first test was performed between P35 and P40 prior to KA administration. The offspring of saline-treated and IL-1β- treated mice showed strong preference towards the conspecific vs. the unanimated object. In the IL-6 and IL-6+IL-1β offspring, sociability was significantly diminished to the levels of indifference. Right. The second test was performed ten weeks after KA SE induction, upon the establishment of spontaneous seizures at the age of 16 weeks. In Saline/KA and IL-1β/KA animals the sociability remained intact (i.e. was similar to the one prior to the KA injection). In IL-6/KA mice, social behavior was diminished, but the impairment was not further exacerbated by recurrent seizures. In IL-6+IL-1β/KA animals, sociability was significantly improved as compared with pre-epilepsy level, and was statistically similar to the one in normal (i.e. Saline/Saline) mice. Data are shown as Mean±SEM. *-p<0.05 vs respective Saline; †- p p<0.05 “After KA” vs. “Before KA”. 2-way ANOVA followed by Sidak's multiple comparisons test. Interaction F=11.52 (p<0.05); effect of seizures F=4.46, p<0.05; effects of prenatal treatment F=28.26, p<0.05.
Consistent with earlier findings [12, 14, 15], the offspring of mice injected during pregnancy with saline showed high degree of sociability, which was evident as preferential exploration of the conspecific as opposed to the unanimated object (e.g. sociability index 25 translates into 3:1 conspecific vs. object ratio). Also in agreement with reported data [14, 15], the offspring of IL-6 and of IL-6+IL-1β- treated animals exhibited diminished sociability, as it displayed no preference towards either the conspecific, or the object. Prenatal exposure to IL-1β alone did not impair social behavior.
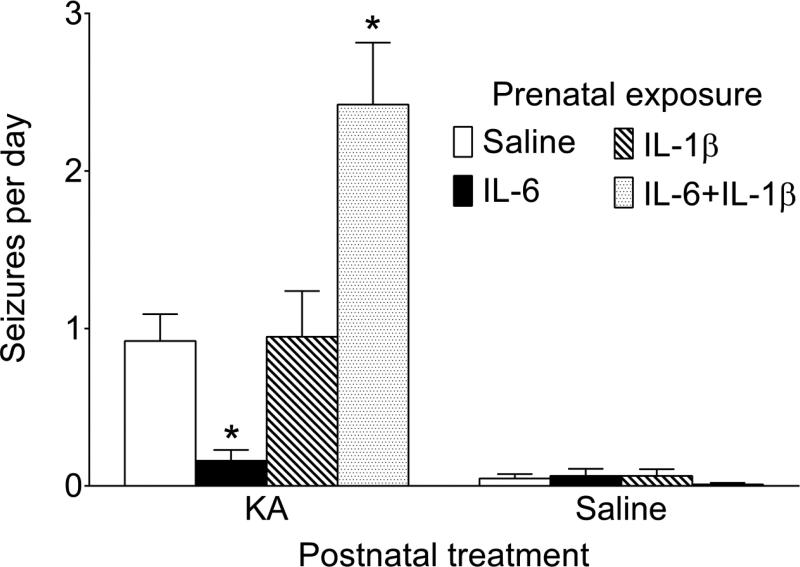
3.2. Spontaneous seizures (Fig. 3)
Fig. 3. Spontaneous seizures.
Average daily count of spontaneous seizures was derived from 6 weeks-long seizure monitoring. IL-6/KA mice developed significantly fewer, and IL-6+IL-1β/KA mice- significantly more spontaneous seizures than Saline/KA animals. Occasional rare spontaneous seizures were observed after intrahippocampal saline injection in animals of all groups. Data are shown as Mean±SEM. *-p<0.05 vs Saline+KA. Kruskal-Wallis followed by Mann-Whitney test.
Over the observation period, all animals of all KA-treated groups displayed spontaneous seizures. Saline/KA mice developed on average one seizure per day (minimal one seizure over two days, and maximal 3 seizures per day). In IL-6/KA animals the occurrence of spontaneous seizures was significantly less frequent than in Saline/KA mice, and did not exceed one seizure per two days. In contrast, animals of IL-6+IL-1β/KA group developed significantly more frequent seizures than the Saline/KA counterparts (at least two seizures and up to 5 seizures per day). Prenatal exposure to IL-1β did not affect the frequency of seizures in KA- injected mice. Occasional spontaneous seizures were observed in mice which were injected with Saline in lieu of KA, with daily seizure frequency not exceeding one seizure over 10 days of observation.
3.3. Second sociability test (Fig. 2, After KA)
After the establishment of chronic epilepsy, Saline/KA and IL-1β/KA mice displayed normal levels of sociability, with the sociability indices statistically similar to those before intrahippocampal KA injection. IL-6/KA mice remained impaired; sociability deficit was not further exacerbated in the presence of spontaneous seizures, as the sociability index was statistically similar to the one before the induction of epilepsy. At the same time, IL-6+IL-1β/KA mice showed significant improvement in social behavior: in these animals sociability index was significantly higher than that prior to the induction of epilepsy, and was comparable to this parameter in Saline/Saline mice. Animals that had been injected with saline instead of KA (i.e. belonging to the Saline/Saline, IL-6/Saline, IL-1β/Saline and IL-6+IL-1β/Saline groups) showed same levels of sociability as before intrahippocampal saline administration (data not shown).
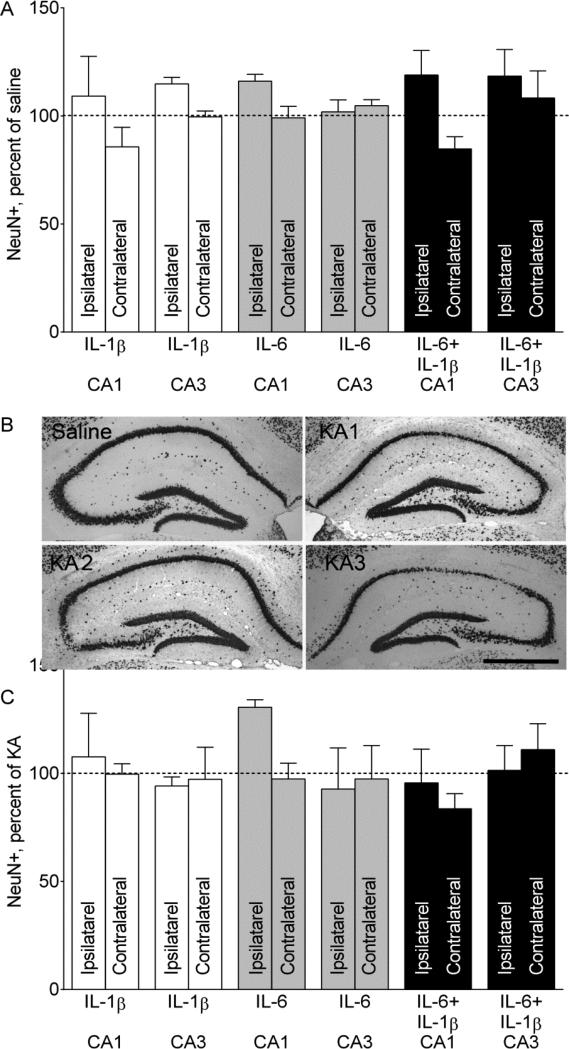
3.4. NeuN immunostaining (Fig. 4)
Fig. 4. NeuN- positive cell counts in the hippocampus.
A. NeuN positive cell counts after prenatal cytokine exposure only. Administration of neither of the cytokines, nor of their combination produced neurodegeneration in the hippocampus. Ipsilateral and Contralateral refer to the site of saline injection. Data are shown as Mean±SEM per cent of NeuN-positive cell counts in the offspring of saline-treated mice. 2-Way ANOVA+Sidak's multiple comparison test. Interaction F=1.346, p>0.05; effects of prenatal treatment F=0.703, p>0.05. B. Examples of NeuN immunostaining a Saline/Saline (noted as Saline) and three different Saline/KA (noted as KAx) mice. In Saline and KA2 animals, left (injected) hippocampi, and in KA1 and KA3- hippocampi contralateral to the injection are shown. Various degrees of neurodegeneration in the CA1, CA3 and hilar areas are observed. KA1- very mild CA1 and CA3 cell loss; KA2- moderate CA3 injury; KA3- moderate CA1 and hilus injury. Scale bar- 500 uM. C. NeuN-positive cell counts after intrahippocampal KA administration. Administration of neither of the cytokines, nor of their combination exacerbated KA-induced neuronal cell loss in the hippocampus. Ipsilateral and Contralateral refer to the site of KA injection. Data are shown as Mean±SEM per cent of NeuN-positive cell counts in the offspring of Saline/KA mice. 2-Way ANOVA+Sidak's multiple comparison test. Interaction F=0.69, p>0.05; effects of prenatal treatment F=1.02, p>0.05; effects of seizures F=0.41, p>0.05.
Consistent with our earlier observations [14], prenatal exposure to IL-6, IL-1β and IL-6+IL-1β produced no discernable neuronal degeneration in the hippocampus (Fig. 4A). Unilateral intrahippocampal injection of KA produced bilateral neuronal hippocampal degeneration, the extent of which widely varied from animal to animal from very mild to moderate in all KA-treated groups (examples are on Fig. 4B). We failed to find any connection between prenatal exposure to a specific cytokine or cytokine combination and the extent of KA-produced neuronal cell loss (Fig. 4C).
4. Discussion
Our studies show that complex and sometimes unexpected relationships exist between epileptic and autism-like phenotypes in an animal model of MIA coupled with KA-induced chronic epilepsy, and that the nature of these relationships depends on components of MIA involved. Firstly, it should be noted that mechanisms directly responsible for developmental deficits in the offspring following MIA by and large remain unknown. It has been suggested that cytokines induced by in infectious agent can cross the blood-placental barrier and activate the fetal immune system, which in turn precipitates behavioral impairments later in life [26]. It has been also shown that MIA (specifically prenatal exposure to Poly I:C) results in the overexpression of various cytokines in the brain of the offspring possibly throughout the life-span [27].
4.1. Spontaneous seizures
Our earlier studies suggested that prenatal exposure to the recombinant IL-6+IL-1β combination, while increasing the propensity to hippocampal kindling, by itself produced no spontaneous seizures in the offspring [14]. This finding was by and large confirmed in the present experiments: while rare occasional seizures were documented following intrahippocampal saline administration, their occurrence was comparable among animals of Saline/Saline and Cytokines/Saline groups, and was far lower than in all KA-injected mice. These seizures may be attributed to mechanical trauma inflicted to the hippocampus by the saline infusion. In terms of KA-induced epilepsy which in itself produced measurable spontaneous recurrent seizures, in animals of the IL-6+IL-1β/KA group the frequency of seizures was significantly higher than in Saline/KA mice. This observation is in line with the earlier established pro-epileptic effects of prenatal exposure to the IL-6 and IL-1β combination, which accelerated the rate and the severity of hippocampal rapid kindling in the offspring [14], and therefore confirms that the reported effects were not model-specific. Also in agreement with earlier report is the lack of effects of prenatal treatment with IL-1β alone, which again suggests the specificity of IL-6+IL-1β combination in producing the epileptic phenotype.
At the same time, the offspring of IL-6- treated mice developed significantly fewer seizures than Saline/KA animals. This was an unexpected finding, as the IL-6 offspring did not show increased resistance to kindling epileptogenesis [14]. More importantly, this observation appears to contradict the concept of autism-epilepsy comorbidity although, as it has been discussed in the Introduction, it is not unprecedented [7] .
The role and the involvement of IL-6 in epilepsy have not been firmly established. Some studies implicate IL-6 signaling in epilepsy. Thus, increased plasma levels of IL-6 has been reported in epilepsy patients [28, 29], and following status epilepticus in rats [30]; kindling of basolateral amygdala increased IL-6 mRNA in the rat hippocampus [31]; IL-6 knockout mice showed decreased incidence of seizures induced by Theiler's murine encephalomyelitis virus [32]. However it not certain whether IL-6 contributes to epileptogenesis; represents a mere surrogate marker of epileptic process; or serves as a compensatory anticonvulsant mechanism as our findings seem to suggest. Indeed several studies have found potential antiepileptic effects of IL-6. IL-6 knockout mice showed increased seizure susceptibility to a variety of convulsant stimuli, including pentyleneterazole, KA, NMDA and AMPA, as compared with normal B6 mice [33], as well as increased susceptibility to audiogenic seizures [34], thus inferring anticonvulsant effects of the cytokine. IL-6 exerted neuroprotective effects under conditions of hypoxia in hippocampal cell cultures, and mitigated the severity of pentyleneterazole convulsions in mice, via increasing the expression and function of adenosine A1 receptor [35].
Taken together with the discussed data, differential modulation of seizure phenotype by prenatal IL-6 exposure in our kindling experiments [14] vs. present studies performed in the KA model show that the cytokine involvement in epilepsy depends strongly on the employed epilepsy model.
It should be also mentioned that the observed exacerbation of chronic epilepsy in the IL-6+IL-1β/KA mice, as well as the mitigation of epilepsy in the IL-6 offspring was not apparently related to the increased neurodegeneration and neuroprotection respectively, as the extent of neuronal cell loss was similar across all KA-treated groups. Nor did preexisting hippocampal neurodegeneration contributed to more severe epilepsy in the IL-6+IL-1β offspring, which showed no discernable cell loss in the hippocampus in this and earlier [14] studies.
4.2. Social interaction
The present experiments have confirmed earlier reports that prenatal exposure to IL-6 produces autism-like behavioral impairments in the offspring [13, 15, 26, 36, 37], and that the addition of IL-1β to IL-6 does not further exacerbate autism-like abnormalities [14] . Increased IL-6 signaling, and specifically MIA-triggered IL-6 activation have been strongly implicated in autism [13, 15, 26, 38, 39]. Mechanisms remain poorly understood, but dysfunctional neuronal circuitries in somatosensory cortex and the hippocampus [37], hyperactivation of STAT3 signaling pathway [26], and disrupted long-range connectivity [36] have been contemplated. In addition to looking into effects of the cytokines proper, in order to address known bidirectional connection between epilepsy and autism [3], we examined whether and how chronic epilepsy, once established, affects animals’ social behavior. Here, we found that in animals of the Saline/KA group sociability was not impaired. Not only KA alone did not produce autism-like phenotype, but pre-existing autism-like impairments in the IL-6 offspring were not further exacerbated by the KA-induced chronic epilepsy. These observations seemingly contradict other reports on the evolvement of autism-like behavioral abnormalities, including impaired social interaction following epileptogenic insults. For example, single episode of KA-induced seizures in P7-P10 rats [40], seizures induced by the injection bicuculline into prefrontal cortex in P21 rats [41], as well as hypoxia- induced convulsions in P10 rats [42], all resulted in impaired social behavior later in life. An obvious difference between the cited and our study is the age of epileptogenic insults, whereby in our experiments KA SE was induced in young adults, and in other studies- between neonatal and pre-adolescent ages. Thus a reasonable explanation may be that seizure-induced mal-plasticity leads to autism only when the insult occurs during a critical window of brain maturation. However, social behavioral deficits were reported in rats, in which pilocarpine SE was induced during the adulthood [43]; the difference between this and out study is a mode of seizure induction (i.e. systemic pilocarpine vs. intrahippocampal KA). Another difference between all cited studies on the one hand, and our experiments on the other hand is species (i.e. rats vs. mice), which may also contribute to the observed differences.
Probably the most surprising finding was the improvement of social behavior in IL-6+IL-1β/KA mice. Prenatal exposure to the IL-6+IL-1β combination leads to autism-like impairments similar to those produced by IL-6 alone. The major difference between the outcomes of the two treatments (i.e. IL-6 vs. IL-6+IL-1β) is more severe epilepsy in the IL-6+IL-1β/KA than in IL-6/KA mice. On the surface, this finding shows that once the frequency of seizures reaches certain critical level (and indeed, the cytokine combination increased KA-induced seizure frequency 2-3 fold), autism-like impairments improve. This observation contradicts the concept of autism-epilepsy connection and requires further studies and explanation. Whether recurrent seizures have similar effects on other autism-like behaviors in the MIA system (e.g. restricted behavior and impaired social interaction [12, 15]), also remains to be explored.
4.3. Conclusions
Overall, our data show that bidirectional relationships between autism and epilepsy do not necessarily follow a straightforward path of comorbidity. Under certain circumstances, bidirectional mitigation, rather than mutual exacerbation of the comorbidity may be observed. The nature of the autism-epilepsy connection strongly depends on the system used. In our case, on the one hand prenatal exposure to IL-6 produces autism-like behavior later in life, but at the same time seems to mitigate seizure phenotype upon the introduction of postnatal epileptogenic insult. On the other hand, the presence of spontaneous recurrent seizures induced by KA reverses, rather than exacerbates pre-existing autism-like behavioral deficit. The existing literature may provide reasonable explanation for the antagonism and provide a blueprint for further studies (e.g. reported anticonvulsant effects of IL-6). In addition to highlighting the complexity of autism-epilepsy connection, our findings emphasize the importance of choosing of an appropriate system for examining the comorbidity between the two disorders.
Research highlights.
Prenatal exposure to IL-6 and to IL-6+IL-1β produces autism-like behavior in mice
Prenatal exposure to IL-6+IL-1β exacerbates kainate-induced chronic epilepsy
Proepileptic effect of IL-6+IL-1β correlates with improved autism-like behavior
Prenatal exposure to IL-6 alleviates the severity of kainate-induced epilepsy
Acknowledgements
This work was supported by the Research and Training Grant from the Division of Neonatology, Dept. of Pediatrics to JWIII; by research grants R01NS065783 and R21NS089396 from the National Institutes of Health to AM; and by research grant from the Today and Tomorrow Children's Fund to AM.
Dedicated to the memory of Paul H. Patterson.
Abbreviations
- E
embryonic day
- IL-1β
interleukin-1β
- IL-6
interleukin 6
- KA
kainic acid
- MIA
maternal immune activation
- P
postnatal day
- Poly I:C
polyinosinic–polycytidylic acid
- SE
status epilepticus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
Dr. Sankar reports receiving research support from Blurbird Bio and NIH. He has also served as a consultant and/or served on the speakers bureau for UCB, Lundbeck, Sunovian, Upsher-Smith, Cyberonics, and Supernus. He has received book royalties from Demos Medical Publishers, and the CRC Press. Dr. Mazarati reports receiving research support from the NIH and the Today and Tomorrow Children's Fund.
References
- 1.Seidenberg M, Pulsipher DT, Hermann B. Association of epilepsy and comorbid conditions. Future Neurology. 2009;4:663–668. doi: 10.2217/fnl.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurology. 2002;1:352–8. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 3.Berg AT, Plioplys S. Epilepsy and autism: is there a special relationship? Epilepsy Behav. 2012;23:193–8. doi: 10.1016/j.yebeh.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–90. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nature Genetics. 2003;34:27–9. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foldy C, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron. 2013;78:498–509. doi: 10.1016/j.neuron.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill-Yardin EL, Argyropoulos A, Hosie S, Rind G, Anderson P, Hannan AJ, O'Brien TJ. Reduced susceptibility to induced seizures in the Neuroligin-3(R451C) mouse model of autism. Neuroscience Letters. 2015;589:57–61. doi: 10.1016/j.neulet.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 8.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 9.Ruskin DN, Svedova J, Cote JL, Sandau U, Rho JM, Kawamura M, Jr., Boison D, Masino SA. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS One. 2013;8:e65021. doi: 10.1371/journal.pone.0065021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism After Infection, Febrile Episodes, and Antibiotic Use During Pregnancy: An Exploratory Study. Pediatrics. 2012;130:e1447–e1454. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40:1423–30. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 12.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–16. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson PH. Maternal infection and immune involvement in autism. Trends in Molecular Medicine. 2011;17:389–94. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pineda E, Shin D, You SJ, Auvin S, Sankar R, Mazarati A. Maternal immune activation promotes hippocampal kindling epileptogenesis in mice. Ann Neurol. 2013;74:11–9. doi: 10.1002/ana.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groticke I, Hoffmann K, Loscher W. Behavioral alterations in a mouse model of temporal lobe epilepsy induced by intrahippocampal injection of kainate. Exp Neurol. 2008;213:71–83. doi: 10.1016/j.expneurol.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Bouilleret V, Ridoux V, Depaulis A, Marescaux C, Nehlig A, Le Gal La Salle G. Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience. 1999;89:717–29. doi: 10.1016/s0306-4522(98)00401-1. [DOI] [PubMed] [Google Scholar]
- 18.Fombonne E. The epidemiology of autism: a review. Psychol Med. 1999;29:769–86. doi: 10.1017/s0033291799008508. [DOI] [PubMed] [Google Scholar]
- 19.Xuan IC, Hampson DR. Gender-dependent effects of maternal immune activation on the behavior of mouse offspring. PLoS One. 2014;9:e104433. doi: 10.1371/journal.pone.0104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diagnostic and Statistical Manual of Mental Disorders V. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- 21.Crawley JN. Chapter 9. Social Behaviors. In: Craige CP, editor. What's Wrong With My Mouse? Wiley Interscience; Hoboken, NJ: 2007. pp. 206–224. [Google Scholar]
- 22.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. Elsevier; 2001. [Google Scholar]
- 23.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 24.Lazic SE, Essioux L. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci. 2013;14:37. doi: 10.1186/1471-2202-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography and Clinical Neurophysiology. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 26.Parker-Athill EC, Tan J. Maternal immune activation and autism spectrum disorder: interleukin-6 signaling as a key mechanistic pathway. Neurosignals. 2010;18:113–28. doi: 10.1159/000319828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehtimaki KA, Liimatainen S, Peltola J, Arvio M. The serum level of interleukin-6 in patients with intellectual disability and refractory epilepsy. Epilepsy Res. 2011;95:184–7. doi: 10.1016/j.eplepsyres.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Uludag IF, Bilgin S, Zorlu Y, Tuna G, Kirkali G. Interleukin-6, interleukin-1 beta and interleukin-1 receptor antagonist levels in epileptic seizures. Seizure. 2013;22:457–61. doi: 10.1016/j.seizure.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Holtman L, van Vliet EA, Aronica E, Wouters D, Wadman WJ, Gorter JA. Blood plasma inflammation markers during epileptogenesis in post-status epilepticus rat model for temporal lobe epilepsy. Epilepsia. 2013;54:589–95. doi: 10.1111/epi.12112. [DOI] [PubMed] [Google Scholar]
- 31.Ye F, Chen XQ, Bao GS, Hua Y, Wang ZD, Bao YC. Effect of topiramate on interleukin 6 expression in the hippocampus of amygdala-kindled epileptic rats. Exp Ther Med. 2014;7:223–227. doi: 10.3892/etm.2013.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Once initiated, viral encephalitis-induced seizures are consistent no matter the treatment or lack of interleukin-6. J Neurovirol. 2011;17:496–9. doi: 10.1007/s13365-011-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Sarro G, Russo E, Ferreri G, Giuseppe B, Flocco MA, Di Paola ED, De Sarro A. Seizure susceptibility to various convulsant stimuli of knockout interleukin-6 mice. Pharmacol Biochem Behav. 2004;77:761–6. doi: 10.1016/j.pbb.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 34.De Luca G, Di Giorgio RM, Macaione S, Calpona PR, Costantino S, Di Paola ED, De Sarro A, Ciliberto G, De Sarro G. Susceptibility to audiogenic seizure and neurotransmitter amino acid levels in different brain areas of IL-6-deficient mice. Pharmacol Biochem Behav. 2004;78:75–81. doi: 10.1016/j.pbb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Biber K, Pinto-Duarte A, Wittendorp MC, Dolga AM, Fernandes CC, Von Frijtag Drabbe Kunzel J, Keijser JN, de Vries R, Ijzerman AP, Ribeiro JA, Eisel U, Sebastiao AM, Boddeke HW. Interleukin-6 upregulates neuronal adenosine A1 receptors: implications for neuromodulation and neuroprotection. Neuropsychopharmacology. 2008;33:2237–50. doi: 10.1038/sj.npp.1301612. [DOI] [PubMed] [Google Scholar]
- 36.Bettcher BM, Watson CL, Walsh CM, Lobach IV, Neuhaus J, Miller JW, Green R, Patel N, Dutt S, Busovaca E, Rosen HJ, Yaffe K, Miller BL, Kramer JH. Interleukin-6, age, and corpus callosum integrity. PLoS One. 2014;9:e106521. doi: 10.1371/journal.pone.0106521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei H, Chadman KK, McCloskey DP, Sheikh AM, Malik M, Brown WT, Li X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim Biophys Acta. 2012;1822:831–42. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Wei H, Alberts I, Li X. Brain IL-6 and autism. Neuroscience. 2013;252:320–5. doi: 10.1016/j.neuroscience.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Wei H, Mori S, Hua K, Li X. Alteration of brain volume in IL-6 overexpressing mice related to autism. Int J Dev Neurosci. 2012;30:554–9. doi: 10.1016/j.ijdevneu.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Bernard PB, Castano AM, Beitzel CS, Carlson VB, Benke TA. Behavioral changes following a single episode of early-life seizures support the latent development of an autistic phenotype. Epilepsy Behav. 2015;44C:78–85. doi: 10.1016/j.yebeh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernan AE, Alexander A, Jenks KR, Barry J, Lenck-Santini PP, Isaeva E, Holmes GL, Scott RC. Focal epileptiform activity in the prefrontal cortex is associated with long-term attention and sociability deficits. Neurobiol Dis. 2014;63:25–34. doi: 10.1016/j.nbd.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, Lan VJ, Joseph A, Jensen FE. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS One. 2012;7:e35885. doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marin JC, Moura PJ, Cysneiros RM, Colugnati DB, Cavalheiro EA, Scorza FA, Xavier GF, Zilbovicius M, Mercadante MT. Temporal lobe epilepsy and social behavior: an animal model for autism? Epilepsy Behav. 2008;13:43–6. doi: 10.1016/j.yebeh.2008.03.004. [DOI] [PubMed] [Google Scholar]