Abstract
Ligands from the B7 family bind to receptors of the CD28 family, which regulate early T cell activation in lymphoid organs and control inflammation and autoimmunity in peripheral tissues. PD-1, a member of the CD28 family, is an inhibitory receptor on T cells and is responsible for their dysfunction in infectious diseases and cancers. The complex mechanisms controlling expression and signaling of PD-1 and PD-L1 are emerging. Recently completed and ongoing clinical trials that target these molecules have shown remarkable success by generating durable clinical responses in some cancer patients. In chronic viral infections, preclinical data reveal that targeting PD-1 and its ligands can improve T cell responses and viral clearance. There is also promise in stimulating this pathway for the treatment of autoimmune and inflammatory disorders.
Keywords: PD-1, PD-L1, PD-L2, immunotherapy, cancer, viral infection
Expression of PD-1 and its ligands PD-L1 and PD-L2
Programmed death-1 (PD-1, CD279) is an inhibitory receptor from the CD28 family that is expressed on various immune cells including T and B lymphocytes, dendritic cells (DCs), monocytes, and macrophages [1, 2, 3, 4]. While PD-1 is not expressed on naïve T cells, it is upregulated following T cell receptor (TCR)-mediated activation and readily observed on both activated and exhausted T cells [5, 6]. These “exhausted” T cells have a dysfunctional phenotype and are unable to appropriately respond to stimuli. Although there is a relatively wide expression pattern for PD-1, its most important role is likely as a coinhibitory receptor on T cells. Current therapeutic approaches focus on blocking the interaction of this receptor with its ligands to enhance T cell responses.
PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273) are both B7 family members and are currently the only known ligands for PD-1 [3, 7, 8]. However, their effects are not exclusively mediated through PD-1 as PD-L1 interacts with B7-1 and PD-L2 can bind to another receptor RGMb [9, 10]. Although both PD-L1 and PD-L2 bind to PD-1 and deliver coinhibitory signals to T cells, their expression differs significantly. PD-L2 is expressed in relatively few cells and tissues but is upregulated on activated antigen-presenting cells (APCs) including monocytes, macrophage, and DCs [4].
PD-L1 expression is much more diverse. PD-L1 can be seen on T cells, B cells, monocytes, macrophage, DCs and is typically upregulated with activation. Unlike the classic B7 family members, B7-1 and B7-2, which are mainly restricted to expression on APCs, PD-L1 is expressed in a number of non-hematopoietic tissues including the heart, pancreas, placenta, vascular endothelium, liver, lung, and skin [2, 7]. This tissue expression plays an important role in regulating immune responses in the periphery [11, 12]. In addition to these normal tissues, PD-L1 is often overexpressed on cancers as a mechanism for the cancerous cells to avoid immune surveillance. It is most likely that PD-L1/L2 expression on APCs and non-hematopoietic tissue (including tumors) is the most important from a therapeutic standpoint.
Beginning with the observation that PD-1 knockout mice develop spontaneous autoimmunity, it has since been demonstrated in numerous studies that the PD-1/PD-L1/L2 pathway is important for T cell regulation in a variety of infectious, autoimmune, and cancer models in mice [13]. These studies largely demonstrate an important role for these molecules in regulating T cell responses which is the basis for the development of a new generation of targeted therapies against PD-1 and PD-L1.
In this review, we will begin by covering the important roles of these molecules, and their mechanisms of expression and signaling. This is an exciting time to review these molecules because we are just now beginning to see patients benefiting from over two decades of basic research focused on this pathway. We will review the therapeutic potential of this pathway and summarize the latest clinical trial results of drugs targeting PD-1 and PD-L1.
PD-1 signaling
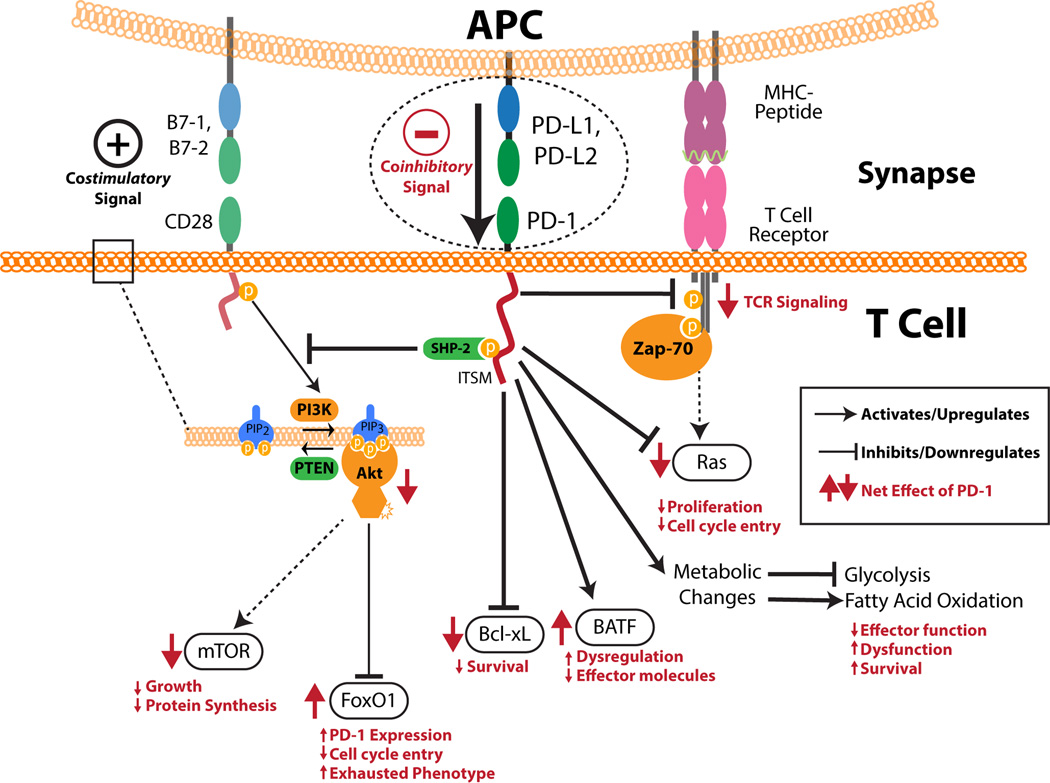
Signaling through PD-1 is triggered by engagement with its known ligands, PD-L1 and PD-L2. Despite the name of the receptor, cell death is not the primary result of engagement. Instead, the primary effect of this signaling is to inhibit TCR and essential costimulatory signals (Figure 1). Upon engagement, PD-1 clusters and localizes to the TCR complex [14]. PD-1 can inhibit the phosphorylation of the TCR CD3ζ chains and Zap-70, which are early steps following TCR engagement [14, 15, 16]. Downstream activation of Ras, an enhancer of survival and proliferation, is also inhibited by PD-1 [17]. Along with the direct TCR signals, CD28 delivers costimulatory signals by activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. PD-1 signaling represses this pathway by blocking PI3K activation [15]. This action begins with the phosphorylation of the intracellular immunoreceptor tyrosine-based switch motif (ITSM) and immunoreceptor tyrosine-based inhibitory motif (ITIM). The ITSM appears to be the more important of these two motifs [16, 18]. The phosphorylated ITSM recruits the tyrosine phosphatase, SHP-2 [14, 15]. This phosphatase leads to the inactivation of PI3K and downstream inhibition of the Akt pathway. Of note, although both PD-1 and CTLA-4 inhibit T cells, the mechanisms of these two receptors are distinct [15].
Figure 1. PD-1 signaling.
PD-1 has both an intracellular immunoreceptor tyrosine-based switch motif (ITSM) and immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic tail. SHP-2 can bind to the phosphorylated ITSM. PD-1 ligation by ligands leads to overall inhibition of TCR signaling through inhibition of CD3ζ chain phosphorylation and Zap-70 association. PD-1 signaling causes the downregulation of both Ras and Bcl-xL which affect proliferation and cell survival, respectively. An increase in BATF can be seen which impairs the effector function of T cells. PD-1 also inhibits the phosphatidylinositol 3-kinase (PI3K)/Akt pathway by inhibiting the activation of PI3K. This has downstream effects including the downregulation of mechanistic target of rapamycin (mTOR) and an increased half-life of FoxO1. PD-1 signaling also influences the cell’s metabolism by inhibiting glycolysis and promoting fatty acid oxidation. Together, all of these effects cause T cells to become less proliferative, lose their effector functions, and take on an exhausted and dysfunctional phenotype. The net effect of PD-1 ligation on all of these processes is shown in red with arrow direction indicating upregulation and downregulation.
The downstream signaling effects through PD-1 are numerous (Figure 1). As with other coinhibitory receptors, a decrease in T cell proliferation is seen along with a decrease in several inflammatory cytokines including tumor necrosis factor α (TNF-α), interferon γ (IFN- γ), and interleukin 2 (IL-2) [2, 3, 6]. PD-1 signaling also appears to be self-reinforcing. Activation of this receptor protects the transcription factor, FoxO1, from degradation which leads to expression of more PD-1 [19].
More global effects are also seen on T cells. It has been shown that PD-L1 plays an important role in the differentiation of inducible regulatory T cells (iTregs) both in vitro and in vivo [20]. PD-L1 expression on not only APCs but also other non-hematopoietic tissues may be capable of this induction. PD-1 signaling is accompanied by a down-regulation of phospho-Akt, mechanistic target of rapamycin (mTOR), S6, and Erk2 and an upregulation of phosphatase and tensin homolog (PTEN) [20]. Earlier work demonstrated that the Akt signaling pathway is a strong inhibitor of iTreg development which supports the proposed mechanism of the generation of PD-L1-induced Tregs [21].
It was also recently shown that PD-1 signaling influences the metabolism of T cells [22]. PD-1 signaling results in the inhibition of glycolysis and metabolism of amino acids while simultaneously promoting fatty acid oxidation [22]. These effects on T cell metabolism are consistent with an inhibition or reversal of effector function and may partly explain the mechanism of impaired function seen in PD-1+ T cells.
PD-1 plays an important role in exhausted T cells. It was first noted that in chronic viral infections, PD-1 was upregulated selectively on exhausted CD8 T cells [6]. This observation has been seen in numerous chronic viral infections in both mice and humans [6, 23, 24, 25, 26, 27]. PD-1 expression by T cells in the tumor microenvironment is also associated with an exhausted and dysfunctional phenotype [28]. Most importantly, blockade of the PD-1 signaling is able to restore CD8 T cell function and allows recovery of cytotoxic capabilities from the exhausted phenotype [29]. This treatment results in improved control of viral infection in several animal models and is the basis for future clinical trials manipulating PD-1 signaling in infectious disease.
Mechanisms controlling PD-1 expression
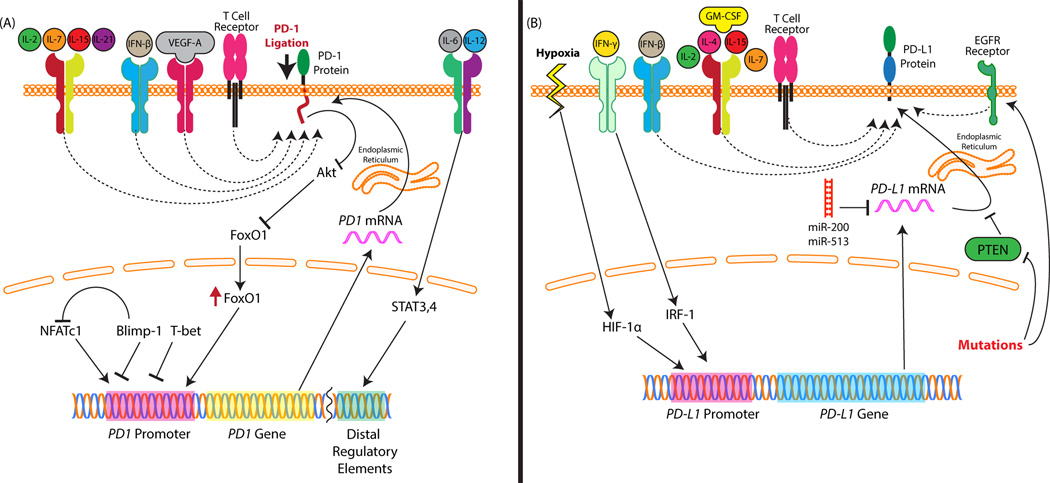
Considering the clinical importance of these molecules, there is great interest in understanding the mechanisms behind their expression. PD-1 is upregulated on T cells following TCR ligation [5](Figure 2A). Cytokine signals are important for the regulation of this molecule too. Signaling through the common gamma chain appears to be important. The common gamma chain ligands, IL-2, IL-7, IL-15, and IL-21 can upregulate PD-1 expression on T cells [30].
Figure 2. Regulation of PD-1 and PD-L1 expression.
PD-1 and its ligands are regulated by a complex network of factors. (A) PD-1 expression on T cells can be upregulated by numerous cytokines. Many of the common gamma chain cytokines (interleukin-2, IL-7, IL-15, IL-21) can upregulate PD-1. IL-6 and IL-12 through signal transducer and activator of transcription 3 (STAT3) and STAT4, respectively, enhance expression of PD-1 through distal regulatory elements. Of particular relevance to the tumor microenvironment, vascular endothelial growth factor A (VEGF-A) can upregulate PD-1 through a VEGF receptor found on T cells. The nuclear factors FoxO1 and NFATc1 upregulate PD-1 through its promoter. Blimp-1 and T-bet also interact with the promoter but block its expression. Blimp-1 also functions by inhibiting NFATc1 promoter-binding. (B) PD-L1 expression is also regulated by numerous mechanisms. Like PD-1, several of the common gamma chain cytokines upregulate it. IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) are also strong upregulators of both PD-L1 and PD-L2. In IFN-γ signaling, IRF-1 can bind to interferon response elements in the promoter of PD-L1. Hypoxia can lead to upregulation of HIF-α which binds to PD-L1’s promoter and stimulates expression. Mutations of the EGFR receptor and loss of PTEN in tumors can upregulate PD-L1. Another post-transcriptional mechanism of regulation is through micro RNAs. miR-200 suppression leads not only to cancer stage progression but also simultaneous upregulation of PD-L1. miR-513 can similarly regulate PD-L1 expression in biliary epithelial cells.
Several more direct transcriptional mechanisms have been found as well. The transcription factor, T-bet, directly and actively represses PD-1 expression [25]. After repeated antigenic stimulation, T-bet is downregulated which leads to PD-1 expression and exhaustion. IL-6 and IL-12 (via STAT3 and STAT4, respectively) can also induce PD-1 in activated T cells through distal regulatory elements that interact with the PD-1 promoter [31]. NFATc1 is a transcription factor that directly activates PD-1 expression [31, 32]. Blimp-1 inhibits PD-1 expression in viral infections by not only repressing NFATc1 but also generating suppressive chromatin changes in the PD-1 locus [27]. Other epigenetic modifications have been described including regulation of PD-1 by DNA methylation. Viral infection leads to a loss of this methylation in CD8 T cells which then allows for transcription of PD-1 [26, 33]. This demethylation is directly related to the strength and duration of TCR signaling [26]. FoxO1 is another important transcription factor that promotes an exhausted cytotoxic T cell profile and upregulates PD-1 [19]. FoxO1 is of particular importance because PD-1 signaling prevents FoxO1 degradation and thus defines a positive feedback loop where PD-1 signaling promotes expression of more PD-1 [19].
PD-1 expression on T cells within the tumor microenvironment is a highly important factor in the use of immunotherapy for the treatment of cancers. PD-1 expression on T cells is predictive of response to therapies targeting this signaling pathway [34]. Beyond general T cell activation and local cytokines promoting expression of PD-1, it has been shown that vascular endothelial growth factor A (VEGF-A) can promote PD-1 expression on CD8 T cells through a VEGF receptor on these cells [35]. From all of these studies we can see that there is a complex network of many distinct mechanisms that influence the expression of PD-1.
Mechanisms regulating expression of PD-L1 and PD-L2
While PD-L1 and PD-L2 share some similarity in the molecules that induce them, there are some clear differences as well. Relatively little is known about the mechanisms regulating PD-L2 expression compared to PD-L1.
Several of the common gamma chain cytokines, IL-2, IL-7, and IL-15, upregulate PD-L1 on monocytes and macrophages as well as on T cells (Figure 2B). IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-4 upregulate both PD-L1 and PD-L2 on macrophages [3, 4]. IL-4 and GM-CSF appear to have the most profound effect on expression of PD-L2. Downstream IFN-γ signaling specifically results in binding of interferon regulatory factor-1(IRF-1) to the PD-L1 promoter [36].
PD-L1 overexpression on tumors has also been studied. While many of the mechanisms upregulating expression may be similar to those seen in leukocytes, several tumor-specific triggers have also been identified. Loss of PTEN is a common mutation in tumors and leads to over-activation of the PI3K/Akt pathway. This mutation and the ensuing downstream signaling can lead to overexpression of PD-L1 [37]. This overexpression mechanism is largely posttranscriptionally mediated. Similarly, there is evidence that overstimulation of the epidermal growth factor receptor (EGFR) pathway which is often found in cancers with EGFR mutations can lead to upregulation of PD-L1 in human cancer cells [38]. Another study showed a trend toward NRAS mutations being associated with higher PD-L1 levels [39]. Non-mutagenic mechanisms have been established too. It has been shown that a number of important signaling pathways including the PI3K and mitogen-activated protein kinase (MAPK) pathways can modify PD-L1 expression [40]. They also showed that pharmacologically manipulating these pathways may be a possible strategy to modify PD-L1 expression in tumors. Another group has shown specific evidence that treatment of melanoma patients with MAPK inhibitors will likely be beneficial in patients whose tumors express PD-L1 and contain tumor infiltrating lymphocytes (TILs) prior to treatment [41]. A feature common to nearly all solid tumors is hypoxia, which can lead to induction of the transcription factor, hypoxia-inducible factor-1α (HIF-1α). HIF-1α can bind to a hypoxia response element in the PD-L1 promoter and lead to expression of PD-L1 on not only tumor cells, but also myeloid derived suppressor cells (MDSCs), macrophages, and DCs within the tumor microenvironment [42]. Micro RNAs also play a role in regulating tumorexpressed PD-L1. Downregulation of miR-200 in tumors leads not only to metastasis but also a simultaneous enhancement of expression of PD-L1 [43]. In other tissues, miR-513 similarly targets degradation of PD-L1 transcript [44].
Immunotherapy targeting PD-1 in chronic infection
Chronic infection results in a sustained high level of antigen exposure, which ultimately leads to T cell exhaustion [45]. In a mouse model of chronic lymphocytic choriomeningitis virus (LCMV) infection, blocking PD-1 and lymphocyte-activation gene (LAG-3) simultaneously reversed the exhausted phenotype and led to the clearance of viral infection [46]. T cell exhaustion is also found in chronic infections such as HIV [47], and hepatitis B and C virus (HBV, HCV) infections in humans [48, 49]. Reversal of the exhausted phenotype can be achieved by blocking PD-1, and this leads to clearance of the virus.
The proof of principle of this approach was demonstrated when the CTLA-4 inhibitor, tremelimumab, was tested in a Phase I trial in hepatocellular carcinoma and chronic HCV infection. Tremelimumab (15 mg/kg IV every 90 days) was administered until cancer progression. In this study, HCV viral loads declined in most patients and there was an increase in virus-specific IFN-γ producing lymphocytes post-treatment [50]. Nivolumab, an anti-PD-1 monoclonal antibody, was tested in interferon-refractory (n=42) and -naïve (n=12) patients with chronic HCV infection [51]. Patients were randomized 5:1 to receive a single infusion of nivolumab in a dose escalation protocol or of placebo (n=7). Five patients in the nivolumab arm had a significant reduction in HCV RNA; 3 achieved a >4 log reduction, 2 patients achieved RNA below the lower limit of quantitation, and one remained RNA-undetectable 1 year poststudy. Nivolumab was well tolerated and one patient had an asymptomatic alanine transaminase (ALT) elevation. Nivolumab and anti-PD-L1 treatments are being tested in HIV patients on antiretroviral therapy to eliminate the undetectable reservoir of viral infection. These studies show that reversing T cell exhaustion can be one strategy to control chronic viral infections.
Anti-PD-1 inhibitors in cancer therapy
The success of inhibiting the central immune check point, CTLA-4, in melanoma [52, 53] led to the development of peripheral checkpoint inhibitors targeting the PD-1/ PD-L1 pathway. PD-1 inhibitors block the interaction of the ligands, PD-L1 and PD-L2, with T cells and increase T cell proliferation and function [54]. The PD-1 inhibitors currently in clinical trials are nivolumab (MDX-1106/BMS-936558 – Bristol Meyers Squibb), pembrolizumab (MK-3475 - Merck) and pidilizumab (CT-011 – Cure Tech) and they have some differences.
Nivolumab and pembrolizumab are fully human IgG4 and humanized IgG4 monoclonal antibodies (mAbs), respectively. Unlike the IgG1 and IgG3 subtypes, IgG4 has markedly decreased antibody dependent cell-mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) activity which prevents depletion of activated T cells [55]. Below we present an overview of selected trials of PD-1 inhibitors in solid tumors and hematological malignancies (Table 1).
Table 1.
Clinical trials of antibodies to PD-1
| Drug | NCT | Phase | Design | Population | N | Key findings /Conclusions |
|---|---|---|---|---|---|---|
| Melanoma | ||||||
| Nivolumab | NCT00730639 | 1 | Dose finding and dose expansion | Advanced melanoma | 107 | OS =43% at 2 years which compares favorably with historical population |
| NCT01176461 | 1 | Nivolumab +/− peptide vaccine (NY-ESO-1, gp100, MART-1) | Advance melanoma, Ipilimumab naïve and refractory | 90 | ORR=25.1%. No difference in response between Ipilimumab naïve and refractory or addition of vaccine to nivolumab. PD-L1 negative patients also responded. | |
| NCT01721772 | 3 | Nivolumab vs Dacarbazine | Metastatic melanoma without BRAF mutation | 418 | Significantly better 1 yr OS in nivolumab arm (72.9% vs 42.1%). Relatively well tolerated | |
| NCT01721746 | 3 | Nivolumab vs investigators choice | Metastatic melanoma after CTLA-4 or BRAF inhibitor therapy | 370 | Results in 167 patients showed higher ORR in nivolumab arm and durable tumor regression as well | |
| Pembrolizumab | NCT01295827 | 1 | Dose finding and dose expansion | Advanced melanoma including CTLA4 treated patients | 135 | ORR =38%. No difference in response between ipilimumab naïve and refractory. Acceptable safety profile and slightly better responses in the higher dose-10mg/kg arm |
| NCT01295827 | 1 | Nivolumab 2 mg/kg vs 10 mg/kg | Advanced melanoma whose disease progressed on ipilimumab | 173 | ORR=26% after ipilimumab therapy. No difference between the two drug doses. | |
| Pidilizumab | NCT01435369 | 2 | Pidilizumab 1.5 vs 6 mg/kg | Advanced melanoma | 103 | Low response rate of 5.9% |
| Non-small cell lung cancer | ||||||
| Nivolumab | NCT00730639 | 1 | Dose finding and dose escalation | Advanced malignancies | 296 | ORR=18.4% in NSCLC cohort. Low rate of Grade 3 SAE =14% |
| NCT01454102 | 1 | Nivolumab + Erlotinib | Stage IIB/IV NSCLC in EGFR mutated patients naïve or post progression | 21 | ORR=19% with an acceptable safety profile | |
| NCT01642004 | 3 | Open label randomized nivolumab vs docetaxel | Metastatic squamous cell lung cancer after 1 line of platinum based therapy | 272 | Superior OS in the nivolumab arm at 1 year (9.2 vs 6 mos.) with durable responses | |
| Pembrolizumab | NCT01295827 | 1 | Pembrolizumab at 2mg/kg or 10 mg/kg | Advanced NSCLC | 282 | ORR=21%, PD-L1+ tumors had higher response rates than negative tumors |
| Genitourinary malignancies | ||||||
| Nivolumab | NCT01354431 | 2 | Nivolumab at 0.3 vs 2 vs 10 mg/kg doses | Previously treated RCC with VEGF inhibitors | 168 | ORR =21% across all 3 arms with a tolerable safety profile. No dose response relationship was response |
| Pembrolizumab | NCT01848834 | 1b | Dose finding study | PD-L1+ >1% and advanced urothelial cancer | 33 | ORR=24%, with CR in 10% with an acceptable safety profile |
| Other cancers | ||||||
| Nivolumab | NCT01592370 | 1 | Dose escalation and dose expansion | Relapsed, refractory Hodgkin lymphoma | 23 | ORR=87% with a PFS at 24 weeks of 86% with an acceptable safety profile. |
| Nivolumab | NCT01876511 | 2 | Pembrolizumab 10 mg/kg q2 weeks | Advanced malignancies with or without mismatch deficiency | 41 | IrPFS = 78% vs 11% for mismatch deficient vs proficient colorectal cancers. |
ORR- Objective response rate; PFS - Progression free survival; irPFS – immune related Progression Free survival; OS - Overall Survival, CR - Complete response; NSCLC - Non-small cell lung cancer; SAE - Serious Adverse Events
Melanoma
Melanoma is a known immunogenic tumor and TILs in melanoma have been shown to colocalize with melanocytes expressing PD-L1. This interaction of T cells with tumor-expressed PD-L1 contributes to immune evasion in melanoma [56]. Ipilimumab (a monoclonal antibody against CTLA-4) demonstrated an overall survival (OS) (See Glossary) benefit in two Phase III trials in metastatic melanoma [53, 57]. However, approximately only 20% of patients with metastatic melanoma survive after 3 years even after ipilimumab, leaving marked room for improvement.
In a Phase I study of refractory melanoma patients, nivolumab had an objective response rate (ORR) of 31% with grade 3/4 serious adverse events (SAEs) in 22% of patients [58]. These results demonstrated both efficacy and acceptable safety of nivolumab in melanoma patients. In another Phase I study, prior treatment with ipilimumab or the addition of a peptide vaccine to melanoma antigens did not affect responses to nivolumab [59]. These results support basic research data showing that the immune checkpoints, CTLA-4 and PD-1, signal through mechanistically distinct pathways [15]. A randomized Phase III trial (n=418) compared nivolumab at 3 mg/kg every 2 weeks (n=210) with dacarbazine (chemotherapy) in BRAF-negative previously untreated metastatic melanoma [60]. The ORR (40% vs. 13.9%), progression-free survival (PFS) (5.1 vs. 2.2 months) and OS at 1 year (72.9% vs. 42.1%) were significantly better in the nivolumab arm when compared with dacarbazine. Moreover, grade 3/4 adverse effects were slightly reduced in the nivolumab arm (11.7 vs. 17.6%) and immunological adverse events occurred in 1–2% of patients. In another open label Phase III study, patients with metastatic melanoma who progressed on ipilimumab were randomized to nivolumab or to the investigators’ choice of chemotherapy. The ORR was higher in the nivolumab arm (32% vs. 11%) with durable tumor regression in responders [61] Based on these results, nivolumab received FDA approval in December 2014 for patients with melanoma who were previously treated with ipilimumab or a BRAF inhibitor. Recently in a Phase 1 study the combination of two immune check point inhibitors, ipilimumab and nivolumab, was safe and produced superior responses than ipilimumab alone for the upfront treatment of metastatic melanoma [62].
Pembrolizumab was studied in a dose escalation study with a dose range of 1–10 mg/kg in 135 patients with refractory melanoma, some of whom received prior ipilimumab treatment [63]. The ORR was 38% and grade 3/4 adverse events were present in 13% of patients and there was no difference between ipilimumab-naïve and refractory patients. Based on these safety data KEYNOTE-001, an open label trial, tested pembrolizumab in two doses at 2mg/kg or 10 mg/kg after progression on ipilimumab and BRAF or MEK inhibitors, in BRAF-mutant tumors [64]. 173 patients with metastatic melanoma received pembrolizumab and the ORR was 26% in both groups and grade 3 to 4 SAEs were reported as 12%. The safety and efficacy of the 2 mg/kg and the 10 mg/kg doses were comparable with no significant benefit of the increased dose. Pembrolizumab was granted breakthrough status by the FDA for the treatment of ipilimumab- or BRAF inhibitor-refractory metastatic melanoma patients.
Non-small cell lung cancer
In non-small cell lung cancer (NSCLC), PD-1 is expressed in 35% of TILs and PD-L1 is expressed 20–25% of lung cancer specimens. Constitutive oncogenic signaling through the PI3K or EGFR pathway [37, 38] or cytokine secretion by lymphocytes leads to activation of the PD- 1/PD-L1 pathway in NSCLC [65].
Nivolumab was first tested in a dose escalation Phase I trial of refractory malignancies of whom 129 had metastatic NSCLC [66, 67]. The ORR in NSCLC was 18% with 33% of squamous and 12% of non-squamous cancers responding. The OS at 1 year was 42% and the median duration of response was 74 weeks and a sustained response of >24 weeks was seen in 57% of patients. Grade 3/4 toxicities were present in only 6% of patients and pneumonitis occurred in 7% of patients. Pneumonitis is a concern in these patients as they already can have poor lung reserve. Nivolumab was approved by the FDA for treatment of squamous NSCLC after progression on a platinum-based chemotherapy regimen. This approval was based on the results of an open-label, multicenter, randomized trial of 272 patients with metastatic squamous NSCLC who were randomized to docetaxel or nivolumab at 3 mg/kg every 2 weeks [68]. There was a significant improvement in median OS of 9.2 (nivolumab) vs. 6 months (docetaxel) seen for patients receiving nivolumab. This represents a significant improvement for patients with squamous NSCLC whose treatment options are limited.
Pembrolizumab, in a pooled analysis of 262 relapsed NSCLC patients (KEYNOTE-001), had an ORR of 21% as a single agent, and results were similar in patients with squamous or nonsquamous histology [69]. In patients with strong PD-L1 expression (>50%) the ORR was 39% and 16% in weak/negative expression suggesting that PD-L1 alone cannot be used as a biomarker to select patients. The FDA granted breakthrough status for pembrolizumab in lung cancer in October 2014.
Genitourinary malignancies
In renal cell cancer, increased TILs along with high PD-L1 expression in the initial biopsy is associated with shorter survival in patients treated with tyrosine kinase inhibitors (TKIs) for metastatic disease [70]. Similarly, high PD-L1 expression is associated with failure of response to Bacillus Calmette Guerin (BCG) for localized bladder cancer by neutralizing the T cell response to BCG immunotherapy [71]. These data suggest that the PD-1 axis contributes to resistant disease in urothelial malignancies.
Nivolumab was tested in a dose escalation Phase I trial of patients with refractory malignancies of whom 33 had metastatic renal cell cancer with an ORR of 27% [66]. In a Phase II trial of 168 clear cell renal cell cancer (RCC) patients, nivolumab was tested at three doses of 0.3, 2, or 10 mg/kg and the median OS was 18.2, 25.5, and 24.7 months, respectively, which was higher than the historical OS rates of 11–16.5 months in this cancer [72]. As a result, a Phase III randomized study evaluating nivolumab and everolimus as a second-line therapy for metastatic RCC is underway.
Pembrolizumab was similarly tested in the KEYNOTE-012 study in 33 patients with metastatic urothelial cancer at 10 mg/kg and the ORR was 24.1% with a median OS of 9.3 months [73]. These studies show favorable efficacy and acceptable safety of PD-1 inhibitors in bladder and renal cancers and are highly likely to move forward in clinical trials.
Other tumors: Colon cancer, Hodgkin lymphoma
Hodgkin lymphoma is a B cell tumor in which the PD-1/PD-L1 axis is activated by JAK signaling and chromosomal amplifications in the 9p24.1 region which codes for the PD-L1/PD-L2 ligands. In an ongoing Phase I study of 23 patients with relapsed Hodgkin lymphoma, the ORR was 87% with a 17% complete response rate [74]. A recent Phase 2 study in colon cancer showed that immune-related progression-free survival rates were superior in mismatch-deficient compared to mismatch-proficient colon cancers (78 vs 11%) [46]. These studies show that these agents are likely to be effective across a wide variety of malignancies.
Anti-PD-L1 inhibitors in cancer therapy
Antibodies against PD-L1 act by blocking the interaction of PD-L1 with PD-1 but do not block the interaction of PD-1 with PD-L2. This may help to decrease toxicity since the PD-1/PD-L2 pathway still plays a role in peripheral tolerance. The three therapeutic monoclonal antibodies against PD-L1 are BMS-986559 (MDX-1105), MPDL3280A, and MEDI4736 and are in various phases of clinical trials. Here we briefly discuss the clinical trials with these agents (Table 2).
Table 2.
Clinical trials of antibodies to PD-L1
| Drug | NCT | Phase | Design | Population | N | Key findings /Conclusions |
|---|---|---|---|---|---|---|
| Unselected | ||||||
| BMS-936559 | NCT00729664 | 1 | Dose escalation and dose expansion | Advanced refractory malignancies | 207 | ORR – 6 to 17% and PFS – 12 to 41% at 24 weeks. Acceptable safety profile. Positive signal in Melanoma, renal cell cancer, NSCLC and ovarian cancer with durable responses |
| MPDL3280A | NCT01375842 | 1 | Dose escalation and dose expansion | Advanced solid tumor cancers | 171 | ORR=21% with an acceptable safety profile. PDL1+ status resulted in higher responses. No pneumonitis related deaths |
| Genitourinary malignancies | ||||||
| MPDL3280A | NCT01375842 | 1 | Dose escalation and dose expansion | Metastatic urothelial bladder cancer | 31 | ORR=50% with treatment response showing increase in CD8+Ki67+ T cells |
| Non-small cell lung cancer | ||||||
| MEDI4736 | NCT01693562 | 1 | Dose escalation and dose expansion | Advanced NSCLC | 13 | ORR =5/13 patients responded. No Grade 3 pneumonitis observed. |
ORR - Objective response rate; PFS - Progression free survival; OS - Overall Survival; CR - Complete response; NSCLC –Non-small cell lung cancer; SAE - Serious Adverse Events
BMS-936559 was first tested in a multicenter Phase I dose escalation trial (0.3 to 10 mg/kg every 14 days in 6 week cycles) in patients with refractory malignancies [75], including melanoma, NSCLC, colorectal, renal cell, ovarian, pancreatic, and breast cancer (n=207). The median duration of therapy was 12 weeks (range, 2 to 111) and SAEs occurred in 9% of patients. Patients with melanoma (9/52), renal cell (2/17), NSCLC (5/49), and ovarian cancer (1/17) had responses and half of these responses were sustained for more than 1 year. It is currently not being developed as a clinical agent in malignancies despite its initial promise.
MPDL3280A is a bioengineered anti-PD-L1 antibody with minimal ADCC and CDC activity. In a Phase I dose escalation trial of advanced solid tumors, no maximum tolerated dose (MTD) was defined at escalating doses [76]. The ORR was 21%, 24 week PFS was 44% and patients with PD-L1 positive tumors had a higher ORR (39%) than those with negative tumors (13%). Interestingly, there was no grade 3–5 pneumonitis or diarrhea in this small study suggesting that the PD-L2 pathway (not inhibited by the anti-PD-L1 antibodies) could be important in minimizing toxicity. Further, in a Phase I study of urothelial cancer MPDL3280A showed significant activity (ORR 26%) with a good duration of response [77]. Based on these data MPDL3208A received breakthrough status for bladder and NSCLC.
MEDI4736 is an IgG1 monoclonal antibody against PD-L1 that is being tested in an ongoing Phase I trial in NSCLC patients and shows preliminary clinical activity with a favorable toxicity profile [78]. Based on the Phase I results, a Phase III trial in patients with locally advanced NSCLC is being planned.
Concluding remarks
Under physiological conditions the PD-1 pathway is important for maintaining peripheral immune tolerance. This pathway represents one of the many redundant pathways to prevent inappropriate immune responses. Such redundant coinhibitory pathways are exploited by tumors and chronic viral infections to cause T cell exhaustion, which results in tumor immune evasion and decreased viral clearance. Recent therapeutic advances targeting this pathway have met with good success in human cancers. More importantly, these treatments can provide durable responses. It remains to be seen whether combinatorial approaches with radiation, chemotherapy, other coinhibitory antibodies, or vaccines can improve the response rate in cancers. Predictive biomarkers need to be developed to identify short and long term responders to immunotherapy. Different cancers may result in different mechanisms of PD-1/PD-L1 expression and hence a single biomarker may not be useful across all tumor types. Tumor related factors include specific oncogenic pathway activations, mutational burden, and PD-L1 expression, while host factors could be the presence of prior infections or vaccinations. Bioinformatics and immunogenetic approaches will be needed to identify relevant tumor associated antigens to which cytotoxic T cells respond or maintain response after immune checkpoint inhibitors.
Highlights.
PD-1 is a coinhibitory receptor found on T cells and other immune cells
PD-L1 and PD-L2 are ligands for PD-1
PD-1 ligation leads to impaired T cell function in cancer and chronic infections
Blocking PD-1 and PD-L1 successfully treats some human cancers
Blocking PD-1 can treat chronic viral infections
Outstanding Questions Box.
Biomarkers of response
Although therapies targeting PD-1 and PD-L1 are highly effective when they work, their current response rates leave much to be desired. Understanding what factors determine if a patient will respond is a critical next step to advancing the use of these therapies. Predictors of response to immune checkpoint inhibitors could be related to tumor-associated factors or host factors. Tumor expression of PD-L1, specific mutations in the tumor, and the presence of tumor antigen-specific T cells are all examples of potential biomarkers currently being assessed. For example, PD-L1 expression will likely be a useful biomarker as patients with PD-L1 expression appear to have a higher response to anti-PD-1 therapy than those without [34]. In a retrospective analysis, NRAS mutant melanoma had a higher response rate to anti-PD-1 therapy [39]. CD8+, PD-1+, and PDL1+ cells in the tumor margins correlate with response to anti-PD-1 therapy in melanoma [34]. A highly restricted TCR repertoire also correlated positively with responses. In NSCLC, higher nonsynonymous mutational burden leading to increased neoantigens was associated with better responses to pembrolizumab [79]. Similarly, mismatch repair-deficient colon cancers which have a higher somatic mutational burden responded better to anti-PD-1 therapy [46]. In summary, there are clearly a variety of factors that control whether a patient will respond well to these therapies. Current and future work will address what these markers are and their relative importance. This work will be important not only for guiding therapeutic choices in patient treatment but also for finding strategies to enhance responses in patients treated with these drugs.
Acknowledgements
J.M.C. is supported by National Institutes of Health (NIH) T32GM007288. X.Z. is supported by NIH R01CA175495, Department of Defense Established Investigator Idea Development Award PC131008 and Dr. Louis Sklarow Memorial Trust.
Glossary Box
- Immune-related Progression Free Survival
In immune checkpoint inhibitor trials on initial treatment some lesions may progress or can worsen which by traditional standards would have been considered progressive disease when it could actually be immune mediated eradication of disease. Hence a new criteria for classification of immunological adverse events have been proposed and PFS measured according to the new criteria
- Immunological Serious Adverse Events
Adverse events which are autoimmune in nature and could have been potentially caused by immune drugs are called Immunological SAE ex: autoimmune colitis, thyroiditis, pneumonitis
- Objective Response Rate (ORR)
The proportion of patients with tumor size reduction of a predefined amount and for a minimum time period
- Overall Survival (OS)
The percentage of people in a study who are alive for a certain period of time after they were diagnosed with or started treatment for cancer
- Progression Free Survival (PFS)
The PFS is defined as the time from assignment in a clinical trial until either progression of the disease or death of the patient due to any cause.
- Serious Adverse Events (SAEs)
An unfavorable symptoms, sign or lab value which in the view of either the investigator or sponsor, it results in any of the following outcomes: Death, a life-threatening adverse event, inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions (21CFR312.32). Adverse events are graded according to the Common Terminology Criteria for Adverse Events (CTCAE) on a scale of Grade 1 to 5 where grade 1 is a mild adverse event and Grade 5 is death. In clinical trials, Grade 3 or 4 SAE usually require dose adjustment or stopping of the drug.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ishida Y, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 5.Agata Y, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 6.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 8.Tseng SY, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butte MJ, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Y, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J. Exp. Med. 2014;211:943–959. doi: 10.1084/jem.20130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scandiuzzi L, et al. Tissue-expressed B7-H1 critically controls intestinal inflammation. Cell Rep. 2014;6:625–632. doi: 10.1016/j.celrep.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francisco LM, et al. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokosuka T, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheppard KA, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 17.Patsoukis N, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chemnitz JM, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 19.Staron MM, et al. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8+ T cells during chronic infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haxhinasto S, et al. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patsoukis N, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quigley M, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofmeyer KA, et al. The PD-1/PD-L1 (B7-H1) pathway in chronic infection-induced cytotoxic T lymphocyte exhaustion. J. Biomed. Biotechnol. 2011;2011:451–694. doi: 10.1155/2011/451694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kao C, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youngblood B, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity. 2011;35:400–412. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu P, et al. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J. Exp. Med. 2014;211:515–527. doi: 10.1084/jem.20130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohaegbulam KC, et al. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015;21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauken KE, et al. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinter AL, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 31.Austin JW, et al. STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J. Immunol. 2014;192:4876–4886. doi: 10.4049/jimmunol.1302750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oestreich KJ, et al. NFATc1 regulates PD-1 expression upon T cell activation. J. Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McPherson RC, et al. Epigenetic modification of the PD-1 (Pdcd1) promoter in effector CD4+ T cells tolerized by peptide immunotherapy. eLife. 2014;3:e03416. doi: 10.7554/eLife.03416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voron T, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-γ-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 37.Parsa AT, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 38.Akbay EA, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson DB, et al. Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol Res. 2015;3:288–295. doi: 10.1158/2326-6066.CIR-14-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atefi M, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin. Cancer Res. 2014;20:3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakavand H, et al. PD-L1 expression and tumor-infiltrating lymphocytes define different subsets of MAPK inhibitor treated melanoma patients. Clin. Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-2023. [DOI] [PubMed] [Google Scholar]
- 42.Noman MZ, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong AY, et al. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-γ-induced B7-H1 expression in cholangiocytes. J. Immunol. 2009;182:1325–1333. doi: 10.4049/jimmunol.182.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 46.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kostense S, et al. High viral burden in the presence of major HIV-specific CD8+ T cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 2001;31:677–686. doi: 10.1002/1521-4141(200103)31:3<677::aid-immu677>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 48.Schlaak JF, et al. The presence of high amounts of HBV-DNA in serum is associated with suppressed costimulatory effects of interleukin 12 on HBV-induced immune response. J. Hepatol. 1999;30:353–358. doi: 10.1016/s0168-8278(99)80090-7. [DOI] [PubMed] [Google Scholar]
- 49.Wedemeyer H, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 50.Sangro B, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Gardiner D, et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS ONE. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ribas A, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J. Clin. Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 53.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong RM, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int. Immunol. 2007;19:1223–1234. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 55.Chen DS, et al. Molecular pathways: next-generation immunotherapy-inhibiting programmed death-ligand 1 and programmed death-1. Clin. Cancer Res. 2012;18:6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 56.Taube JM, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 58.Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber JS, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 2013;31:4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 61.Weber JS, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 62.Postow MA, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robert C, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 65.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brahmer J, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015 May 31; doi: 10.1056/NEJMoa1504627. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garon EB, et al. LBA43 Antitumor activity of Pembrolizumab (PEMBRO; MK-3475) and correlation with Programmed Death Ligand (PD-L1) expression in a pooled analysis of patients (pts) with advanced non small cell lung carcinoma (NSCLC) Ann. Oncol. 2014;25 [Google Scholar]
- 70.Choueiri TK, et al. Correlation of PDL1 tumor expression and treatment outcomes in patients with renal cell carcinoma (RCC) receiving tyrosine kinase inhibitors: COMPARZ study analysis. ASCO Meeting Abstracts. 2014;32:416. [Google Scholar]
- 71.Inman BA, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 72.Motzer RJ, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J. Clin. Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Plimack ER, et al. LBA23A phase 1b study of pembrolizumab (PEMBRO; MK-3475) in patients (PTS) with advanced urothelial tract cancer. Ann. Oncol. 2014;25 [Google Scholar]
- 74.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herbst RS, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. ASCO Meeting Abstracts. 2013;31:3000. [Google Scholar]
- 77.Powles T, et al. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC) ASCO Meeting Abstracts. 2014;32:5011. [Google Scholar]
- 78.Brahmer JR, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. ASCO Meeting Abstracts. 2014;32:8021. [Google Scholar]
- 79.Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]