Abstract
Skeletal muscle injuries typically result from traumatic incidents such as combat injuries where soft-tissue extremity injuries are present in one of four cases. Further, about 4.5 million reconstructive surgical procedures are performed annually as a result of car accidents, cancer ablation, or cosmetic procedures. These combat- and trauma-induced skeletal muscle injuries are characterized by volumetric muscle loss (VML), which significantly reduces the functionality of the injured muscle. While skeletal muscle has an innate repair mechanism, it is unable to compensate for VML injuries because large amounts of tissue including connective tissue and basement membrane are removed or destroyed. This results in in a significant need to develop off-the-shelf biomimetic scaffolds to direct skeletal muscle regeneration. Here, the structure and organization of native skeletal muscle tissue is described in order to reveal clear design parameters that are necessary for scaffolds to mimic in order to successfully regenerate muscular tissue. We review the literature with respect to the materials and methodologies used to develop scaffolds for skeletal muscle tissue regeneration as well as the limitations of these materials. We further discuss the variety of cell sources and different injury models to provide some context for the multiple approaches used to evaluate these scaffold materials. Recent findings are highlighted to address the state of the field and directions are outlined for future strategies, both in scaffold design and in the use of different injury models to evaluate these materials, for regenerating functional skeletal muscle.
Keywords: tissue engineering, skeletal muscle regeneration, fibrin, microthreads, biomaterials
Graphical abstract
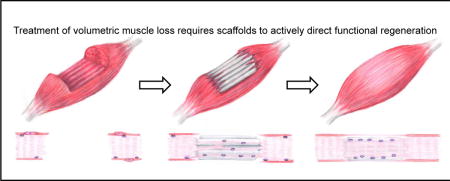
1.0 CLINICAL NEED FOR SCAFFOLDS TO REGENERATE SKELETAL MUSCLE
Large skeletal muscle defects profoundly impact the quality of life of patients by significantly reducing the functionality of the injured muscles [1]. Skeletal muscle injuries typically result from traumatic incidents such as those presented from excessive exercise, contusions, lacerations, surgical resection or reconstruction, or combat injuries [2–4]. Approximately 35–55% of all sports injuries involve skeletal muscle damage to the myofiber and/or connective tissue [5]. Further, there are about 4.5 million reconstructive surgical procedures performed annually as a result of traumatic injury, car accidents, cancer ablation, or cosmetic procedures [6, 7]. On the battlefield, extremity injuries are present in 54% of cases, with 53% of these injuries involving soft tissue damage [8, 9]. Many of these injuries involve the loss of at least 20% of a given muscle’s mass, which is termed volumetric muscle loss (VML) [1, 10]. These injuries result in billions of dollars in health care expenses, as well as the development of different surgical techniques and medical devices to repair them [11–14].
While skeletal muscle has an innate repair mechanism that can repair small wounds, the magnitude of critically sized injuries or VML defects limits these mechanisms from repairing large wounds [2]. Further, there are no treatments available that attempt to harness or direct this endogenous regenerative pathway. There are several established protocols for the treatment of small-scale skeletal muscle injuries such as RICE (resting the wound with a combination of ice, compression, and elevation) [13–15]. However, larger-scale injuries such as those presented by VML often require specialized surgical solutions [1, 16]. The current standard of care for large-scale VML injuries is an autologous tissue transfer from an uninjured site, where surgeons remove the damaged muscle tissue and graft healthy muscle from a donor site unaffected by the traumatic incident [7, 17]. Regardless of the level of success of these procedures, there is always a loss of muscle strength and function, usually due to the formation of scar tissue [10, 16, 18]. While autologous tissue transfers are the best available treatment option, as many as 10% of these surgeries result in complete graft failure due to complications such as infection and necrosis [17, 19]. In the case of extensive traumatic injuries such as those presented by military personnel, there may be limited donor site availability [18, 20]. Often these extensive injuries result in amputation, requiring the use of prosthetic devices to regain partial functionality [10]. As such, there is a significant need to develop off-the-shelf biomimetic materials that can direct skeletal muscle tissue formation within these large defect sites to facilitate functional tissue regeneration [1, 18].
2.0 SKELETAL MUSCLE ANATOMY
Skeletal muscle is one of the most abundant tissues in the human body and accounts for 40% to 45% of the total body mass [21, 22]. It coordinates locomotion through attachment points to the skeleton and its macroscopic structure consists of organized networks of myofibers, blood vessels, nerves, and connective tissue. The working contractile unit of skeletal muscle is the sarcomere which consists of interposing filaments of actin and myosin [23]. Multiple myoblasts fuse together to form myofibrils which are structures with repeating sarcomeres along the length of the myofibril. A bundle of myofibrils form the basic structural element of muscle and is called the myofiber (Figure 1). Myofibers range between 20–100 μm in size and are the conglomeration of multiple myofibrils [21, 23]. Ultimately, the nuclei of these myoblasts translocate to the periphery of the myofibers residing just beneath the sarcolemma, or cell membrane of myofibers. Similarly, myofibers within a muscle organize into larger structures referred to as fascicles [21] and in general, the anatomical structure of skeletal muscle can be thought of as a series of self-contracting cables that organize into a complex hierarchical structure. Functionally, myofibers are classified as slow or fast twitch fibers, which in part is due to differences in the metabolic activity of these fibers. Broadly, slow twitch fibers utilize oxidative metabolic pathways and fast twitch fibers utilize glycolytic metabolic pathways, and also differ physically by varying myosin heavy chain proteins within the contractile apparatus of the fibers (for a review, please see [24]).
Figure 1.
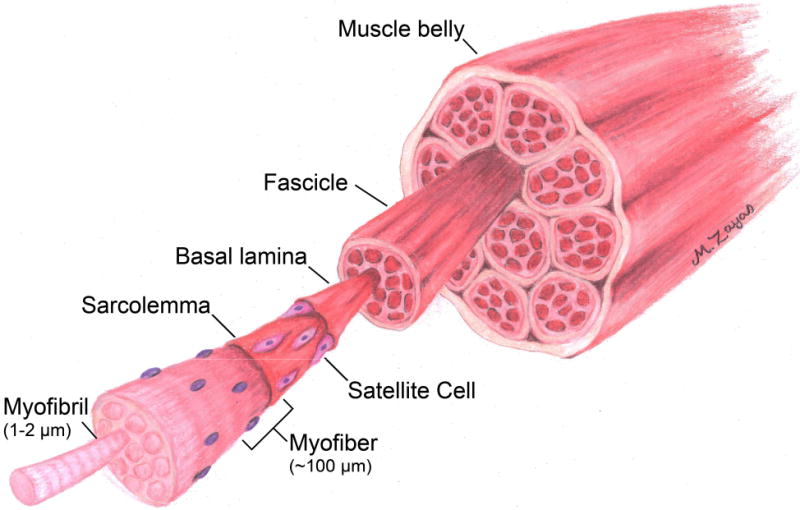
Schematic illustration showing the hierarchical arrangement of skeletal muscle tissue. Skeletal muscle tissue is a highly organized series of cable like structures, with the smallest functional unit referred to as a myofibril. Bundles of myofibrils form myofibers, which in turn are grouped together to form fascicles, which are bundled to form the muscle belly.
Each myofiber is surrounded by a basement membrane referred to as the basal lamina (Figure 1). This dense structure is composed of a variety of basement membrane proteins such as type IV collagen, laminin-2, and fibronectin and is essential for guiding the endogenous regenerative pathway of skeletal muscle [25, 26]. In addition to these basement membrane proteins, the extracellular matrix is also composed of sulfated glycosaminoglycans and proteoglycans, such as heparan sulfate, as well as type I collagen, which assist in maintaining the structural integrity of skeletal muscle [27, 28]. Skeletal muscle progenitor cells, termed satellite cells (SCs), reside in the interstitial space between the sarcolemma of myofibers and the basal lamina. These cells are identified by expressing paired box gene 7 (Pax7) and are typically quiescent in mature, healthy tissue [2, 29–31]. Satellite cells account for 2–7% of all myofiber nuclei, but they are the primary cell type responsible for the regeneration of skeletal muscle tissue [32].
3.0 NATIVE REGENERATIVE PATHWAY OF SKELETAL MUSCLE
Skeletal muscle regeneration is a complex process that involves an inflammatory response, growth factor signaling, SC mediated repair of the injury, and fibroblast infiltration, ultimately leading to either functional regeneration or scar tissue formation. These processes have been loosely classified into three phases: (1) the destruction/inflammatory phase, (2) the repair phase, and (3) the remodeling phase (Figure 2) [10, 13, 21, 33, 34].
Figure 2.
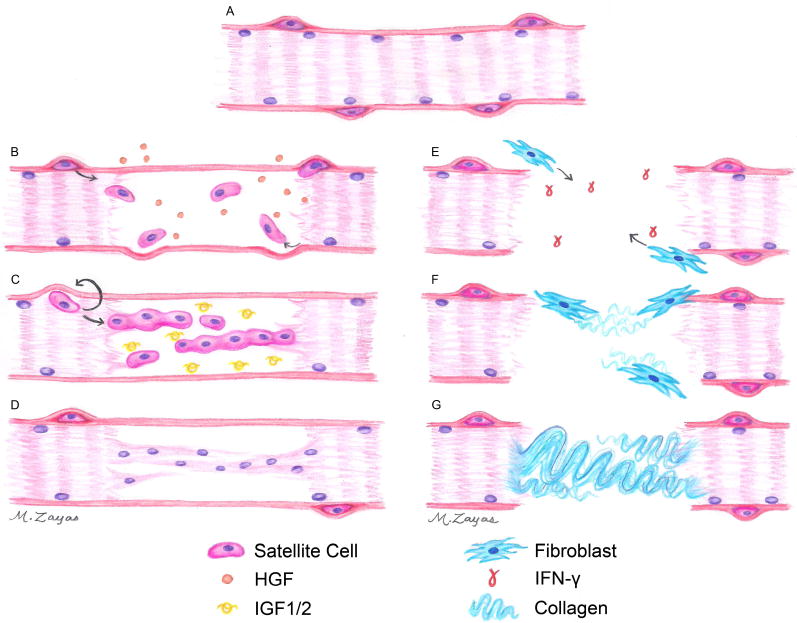
Schematic representation of native skeletal muscle regeneration. (A) SCs can be identified by their position between the sarcolemma and basal lamina of mature skeletal muscle. (B) Upon injury, SCs are recruited to the wound site and begin to proliferate. (C) These proliferating SCs will eventually fuse together to form immature myofibers, (D) which will fuse with the existing healthy myofibers, and will then mature by expressing more contractile proteins to regenerate functional tissue. In VML injuries, (E) the basal lamina is destroyed or removed, facilitating the migration of fibroblasts into the wound site. (F) In the absence of proper signaling cues, fibroblasts begin to deposit collagen, (G) ultimately leading to massive scar tissue formation.
3.1 Destruction/Inflammatory Phase
Traumatic injury to skeletal muscle leads to the rupture of the sarcolemma which exposes the intracellular contents of the myofiber to large amounts of extracellular calcium. This triggers the activation of calcium-dependent proteases and initiates myofiber necrosis [21, 34, 35]. Necrosis occurs along the length of the damaged myofiber until it reaches a contraction band, a dense band of cytoskeletal material that is designed to halt the necrosis of injured myofibers [13, 36, 37]. Almost immediately after injury, neutrophils migrate to the injury site and assist in the degradation of damaged myofibers. Macrophages quickly migrate to the injury site and become the predominant cell type present in as little as two days post-injury [38]. One of the most critical cytokines secreted from the inflammatory cells is nitric oxide, whose functions include: (1) the facilitation of further degradation of myofibers present in the wound environment [33, 38], (2) the release of growth factors such as hepatocyte growth factor (HGF) from the surrounding matrix [33, 39–42], (3) the induction of vasodilation of nearby blood vessels to recruit additional inflammatory cells [13, 43, 44], and (4) the inhibition of macrophage polarization into an anti-inflammatory, fibrosis promoting M2a macrophage [45]. While macrophages migrate to the wound site within two days of injury, they also modulate the cytokines they release over time. Initially, pro-inflammatory M1 phenotype macrophages assist in the phagocytosis of necrotic myofibers and also support satellite cell survival and proliferation [33, 38]. Over the next 3–7 days, M1 macrophages are replaced by M2 macrophages, which are believed to enhance the regenerative response of tissues by promoting myoblast proliferation, growth, and differentiation [10, 33, 38]. Persistence of M1 phenotype macrophages will continue to secrete cytokines such as interferon gamma (IFN-γ), which, in addition to stimulating macrophage phagocytosis, also inhibits the recruitment of M2 macrophages, or the differentiation of naïve macrophages to the M2 phenotype [46]. One of the key factors that M2 macrophages secrete is interleukin-10 (IL-10), which increases myoblast proliferation and overall skeletal muscle regeneration [47, 48].
3.2 Repair Phase
The repair phase of skeletal muscle regeneration is comprised of two sub-phases: the recruitment and proliferation of SCs (Figure 2B) and the differentiation of SCs into mature muscle tissue (Figure 2C). The recruitment/proliferation phase is initiated upon mechanical injury of muscle fibers when HGF is rapidly released from the extracellular matrix (ECM) of the muscle fiber itself, stimulating the activation and proliferation of SCs as well as the chemotaxis of SCs to migrate to the injury site [40, 41, 49, 50]. HGF has been found to be the sole growth factor capable of bringing SCs back into the cell cycle [51–53]. Other factors, such as fibroblast growth factor 2 (FGF2) and fibroblast growth factor 6 (FGF6) are also released from the ECM, which also stimulate SC proliferation to gather enough progenitor cells to repair the injury [13, 50, 54–58]. Because of their location between the sarcolemma and the basal lamina, SCs typically migrate longitudinally from the same myofiber to the injury site rather than laterally from other intact fibers [2, 29, 59]. However, once injuries result in the rupture of the basal lamina, lateral migration of SCs to the injury site does occur [60, 61]. SCs are extremely efficient at repairing muscle and are capable of generating thousands of myoblasts from a small number of satellite cells [62, 63]. Once SCs reenter the cell cycle (i.e. activate), they begin to express myogenic factor 5 (Myf5) and will express myogenic differentiation factor-1 (MyoD) when they are committed to myogenic differentiation [2, 64–67]. Additionally, once SCs are committed to differentiation they are referred to as myoblasts, and begin to lose Pax7 expression [22, 68].
The second sub-phase of regeneration, the differentiation phase, occurs when myoblasts begin to express myogenin, fuse with other myoblasts, then mature into myofibers (Figure 2C) [65, 68]. This process is inhibited by the growth factors associated with the proliferation phase of muscle regeneration, mainly HGF, FGF2, and FGF6, highlighting the importance of the temporal expression of growth factors in skeletal muscle regeneration [2, 50, 69, 70]. Specifically, there is evidence that prolonged systemic supplementation of HGF will inhibit healing, showing a need for the growth factor to be present for no longer than 48–72 hours [50, 71, 72]. Instead, the process of myoblast differentiation is stimulated by the insulin-like growth factor (IGF) family [73, 74]. This family (consisting of members IGF1 and IGF2) is unique in that each growth factor will stimulate both the proliferation and subsequent differentiation of myoblasts [75–78]. Both in vitro and in vivo studies have suggested that IGF1 is more potent in skeletal muscle regeneration than IGF2, either due to growth factor activity or the increased availability of its receptor, insulin-like growth factor receptor-I (IGFR1) [79, 80]. Neutralizing the function of IGF1 was found to inhibit skeletal muscle regeneration, highlighting the importance of this factor in the regenerative response [81]. Injections of exogenous IGF1 into skeletal muscle injuries in aged animals restores the proliferative capacity of SCs, making it a potent factor in skeletal muscle regeneration, and a necessary factor for enhancing the endogenous regenerative response of skeletal muscle [82–84]. Further, the expression of IGF1 in the macrophage population appears to drive macrophages towards an M2 phenotype, in addition to stimulating myoblast fusion [85]. IGF-mediated cell-fusion events occur after the initiation of myogenin expression which also terminates MyoD/Myf5 expression [2, 30, 68, 86]. Nascent myofibers are characterized by centrally aligned nuclei and as these cells mature and generate more contractile proteins the nuclei gradually translocate to the periphery of the myofiber [2, 13, 30, 66].
3.3 Remodeling Phase
The final stage of muscle regeneration is the remodeling phase and is a balance between the culmination of the differentiation sub-phase of muscle regeneration and the infiltration of fibroblasts into the injury site. Regenerating myoblasts will fuse with the existing musculature at the interface of the injury or to the ECM (Figure 2D) [10]. The infiltration of fibroblasts can begin as early as the inflammatory phase and supports regeneration by repairing and replacing connective tissue that was damaged by the injury [13, 87]. While the connective tissue synthesized by fibroblasts is important in providing stability for the regenerating tissue and replacing damaged or lost ECM, collagen deposition often remodels into scar tissue and limits the organization of the regenerated tissue (Figure 2 E–G) [10, 13, 87, 88]. The balance between these two antagonistic responses dictates the functional outcome from the regenerative pathway. In general, small injuries remodel without the presence of scar tissue, but as the injury becomes larger the fibroblast infiltration increases such that there begins to be a significant amount of collagen deposition, ultimately impeding the functional regeneration of skeletal muscle tissue.
In injuries resulting from VML, there are no regenerative cues present due to the complete destruction or removal of the basal lamina and surrounding connective tissue (Figure 2E). This results in a loss of traditional signaling that indicates the need for SCs to migrate into the injury site. Therefore, there does not appear to be a large influx of myoblasts into these injuries [13, 89]. Because of the lack of SC infiltration into the injury site, the infiltrating fibroblasts dominate this stage of regeneration, resulting in large amounts of collagen deposition [90, 91]. Ultimately, the deposition of collagen I into the wound site results in the formation of scar tissue (Figure 2G), limiting the ability of the muscle to uniformly contract, and resulting in a loss of function, as observed from the patient [10, 88, 92]. In addition to the loss of mechanical function observed in patients with VML injuries, these injuries also result in significant losses of muscle volume, which can generate physical deformities that may impact the psychological health of the patient.
3.4 Cell Types Involved in Skeletal Muscle Regeneration
Concurrent with the repair phase of skeletal muscle regeneration, blood vessels, nerves, and fibroblasts infiltrate the injury site [2, 10, 34]. Skeletal muscle is a highly vascularized tissue and as such, its revascularization is vital for successful regeneration [93, 94]. The close proximity of SCs and endothelial cells in skeletal muscle facilitates paracrine signaling between these cell types to support both angiogenesis and myogenesis [95, 96]. Muscle is not only highly vascularized, but also heavily innervated to facilitate the cooperative contraction of myofibers to conduct uniaxial force [34]. Factors such as IGF1 have been shown to induce nerve sprouting and HGF and FGF2 have been shown to be potent angiogenic factors, suggesting that the growth factor cascade in skeletal muscle regeneration affects a variety of cell types in addition to myoblasts [97–102]. In addition to mediating the deposition of collagen, scar tissue formation and functionally impaired regeneration [12, 13, 36, 103], fibroblasts infiltrate during the early in the inflammatory stages of skeletal muscle regeneration along with SC recruitment [13, 87]. The interactions between these two cell types are critical for proper regeneration. It is believed that fibroblasts mediate regeneration through repairing and replacing connective tissue that was damaged by the injury. Finally, as reviewed in 3.1, macrophage signaling plays a critical role in skeletal muscle regeneration. Inflammatory M1 macrophages secrete cytokines such as IFN-γ and tumor necrosis factor α (TNF-α), which support the phagocytosis of damaged myofibers, in addition to stimulating the in vivo survival and proliferation of myoblasts. However, these same factors inhibit myoblast differentiation, so it is essential that the spectrum of macrophage polarization shifts to an M2 dominate phenotype. These M2 macrophages secrete several cytokines, including IL-10, which is an extremely potent factor that stimulates the regeneration of skeletal muscle tissue [47].
4.0 CURRENT TISSUE ENGINEERING STRATEGIES AND LIMITATIONS
There are two main approaches to develop constructs for skeletal muscle regeneration: i) the in vitro development of engineered skeletal muscle constructs to augment repair, or ii) the development of scaffolds to regenerate damaged skeletal muscle tissue in vivo [104–107]. Materials designed to guide regeneration of skeletal muscle can be further classified as either acellular or cellular; with the latter incorporating myoblasts or SCs into their matrix. The utilization of the scaffolding material as a delivery vehicle for progenitor cells allows for an exogenous regenerative response to complement endogenous regeneration by augmenting the number of cells available to regenerate large scale injures. Conversely, acellular strategies invoke the endogenous regenerative response by delivering sustained amounts of growth factors to stimulate the recruitment and proliferation local progenitor cells into the injury site.
To direct skeletal muscle growth, biomimetic scaffolds must facilitate cell alignment, promote skeletal muscle formation, and stimulate vascularization and innervation. Cell alignment is one of the most critical factors in skeletal muscle regeneration due to the unique geometric architecture of muscle fibers [21, 108–111]. Fibrous elements within scaffolding materials have been shown to direct cell alignment along the length of the scaffold’s surface [112–115]. Strategies to promote skeletal muscle formation and angiogenesis include the incorporation of growth factors and peptide sequences within the scaffolding material [93, 116, 117]. Sustained delivery of growth factors improves skeletal muscle regeneration compared to single bolus dosing, suggesting that the controlled release of growth factors from scaffold materials is an efficient delivery method for these molecules [73, 83, 93]. Finally, scaffolds need to balance degradation with structural and mechanical support of the injury site such that enough structural stability is present within the scaffold material to support mechanical loading until the regenerated tissue is stable enough to withstand loading independently [4, 91, 118].
4.1 In Vitro Skeletal Muscle Development
Engineered skeletal muscle constructs have been developed to study skeletal muscle formation in vitro to develop high content models for drug screening applications and ultimately to replace or augment damaged tissue in vivo [21, 107, 108]. These tissues are typically prepared by seeding myoblasts onto a protein surface, commonly fibrin, which is remodeled into muscle tissue [80, 119–124]. Skeletal muscle constructs engineered in vitro can spontaneously contract and have been electrically stimulated to induce tetanic contractions, demonstrating that these constructs can accurately mimic skeletal muscle function [122]. Vandenburgh and colleagues developed a platform technology that generates skeletal muscle constructs in the bottom of 96 well plates between two polydimethylsiloxane (PDMS) posts as a high content model for drug screening applications. Each individual well is capable of being electrically stimulated and assessed for tetanic force production [125–127]. One of the limitations of these skeletal muscle constructs is the inability of nutrients to diffuse to the innermost layers of the tissue. While this is not a problem for small skeletal muscle constructs used in drug screening applications, constructs approaching 0.4 mm in thickness result in the formation of a necrotic core making it unsuitable for implantation [122, 128]. Recently, a study using a heterogeneous population of cells isolated from the muscles in mice hind limbs demonstrated that vascular networks could be formed in vitro to support the survival of these constructs for at least 40 days in culture [129]. Another recent study investigated myoblasts seeded into fibrin hydrogels to produce bundles of biomimetic engineered muscle components up to 1 cm in length. These constructs generated functional skeletal muscle tissues after 2–4 weeks in culture [124]. Remarkably, these bundles also repaired themselves after cardiotoxin insult in vitro, demonstrating that the SC niche was present within these constructs. Vascularization of these constructs was achieved in vivo by implanting the scaffolds perpendicular to the intact panniculus carnosis muscle. However, it is currently unclear whether these bundles can engraft into existing musculature and contribute to their overall force production.
Larger skeletal muscle constructs ranging between 1 and 4 cm in length have been implanted to observe engraftment, vascularization, and functional recovery of VML injuries. Implanting in vitro skeletal muscle constructs near existing blood vessels greatly enhances their vascularization [130]. Successful in vivo engraftment significantly increases the force output of these constructs, demonstrating that these constructs are capable of integrating with the surrounding tissue to remain viable and enhance their force production [131]. Finally, in vitro skeletal muscle constructs approximately 4 cm in length, termed “skeletal muscle units” (SMUs), were implanted into a rat VML model where 33% of the tibialis anterior (TA) muscle was removed. While these SMU constructs supported some vascular and nervous tissue integration as well as a significant increase in TA force production after 28 days, the forces generated in these muscles remained significantly below baseline force values [132].
The major limitation of these methods is the lack of vascularization of in vitro skeletal muscle constructs. Dennis and Kosnik showed that myoblasts need to be within 150 μm of a source of oxygen to proliferate or differentiate, limiting the size of viable constructs without a functional vascular network [128]. Further, these constructs are generated by seeding myoblasts and fibroblasts on protein surfaces and then they are differentiated into functional tissue. This requires 3–4 weeks of culture time for the formation of a robust ECM to support construct contractility [107]. The ability to generate contractile constructs from a patient’s own cells is a promising option [107, 127], and likely the only cell source for such regenerative strategies; however, nearly a month would pass between obtaining the biopsy and implantation. Therefore, these constructs might be better suited for reconstruction of chronic conditions rather than for the repair of acute VML injuries.
4.2 Biomaterials for Skeletal Muscle Regeneration
Scaffolds for skeletal muscle regeneration have been made from a variety of materials, ranging from synthetic polymers such as poly(glycolic acid) (PGA)[133] and poly-ε-caprolactone (PCL) [109]; to decellularized ECM materials [134]; to natural polymers such as alginate [93], collagen [113], and fibrin [91]. In addition to the use of a variety of materials as acellular scaffolds, strategies for skeletal muscle regeneration have utilized a variety of cell sources [105, 108]. The incorporation of cells into scaffolds has significantly increased the survival of implanted cells in the injury site, which was previously below 5% [135, 136]. While the source of these cells can vary with different scaffolds, in general SCs or myoblasts derived from SCs in culture have been investigated to regenerate skeletal muscle injuries [105, 137]. The cell sources used, in vivo functional outcomes, and the limitations of each material are summarized in Table 1. The sections below discuss recent advances in the design of scaffolds for the regeneration of VML injuries and are organized based on the composition of their scaffolding materials.
Table 1.
A summary of the scaffold materials and cellular components used in skeletal muscle regeneration.
| Scaffold Material | Cellular Component | Functional Outcomes | Limitations | References |
|---|---|---|---|---|
| Poly(glycolic acid) (PGA) | Rat myoblasts | Formation of multinucleated myotubes and some capillary | Insufficient vascularization for tissue formation | [133, 138, 158] |
| Poly-ε-caprolactone (PCL) | Human and mouse myoblasts | Protein coatings facilitated myoblast proliferation and fusion | Scaffold alone does not support myofiber growth | [109, 142] |
| Decellularized scaffolds | Acellular | Support cell infiltration, nascent myofiber formation, and functional recovery | Long incubation times required to observe functional recovery | [18, 28, 90, 134, 172-175, 177, 180] |
| Decellularized scaffolds | Rat myoblasts and human mesenchymal stem cells | Shorter time points to functional outcomes in acellular scaffolds | Not all cell types integrate into muscle defect. Cell sourcing may become a limiting factor | [16, 89, 171, 176, 178, 179, 181] |
| Minced skeletal muscle | Native tissue | Support functional recovery of injury | Does not support robust alignment of nascent myofibers | [182] |
| Alginate | Rat and mice myoblasts | Reduced wound size 1 month after injury, supporting enhanced angiogenesis | Scaffold did not replace damaged tissue | [183, 196, 199] |
| Alginate | Acellular | Support functional recovery and vascularization of nascent myofibers | Utilized ischemia-induced skeletal muscle injury. Scaffold did not replace damaged tissue | [93, 197] |
| Collagen | Rat and mice myoblasts | Scaffolds support cell growth and integration with host tissue | Disorganized muscle regeneration with little data collected on functional recovery | [113, 202–204] |
| Collagen | Acellular | Scaffolds support myoblast infiltration | Little information obtained from long term (> 1 month) implantation studies | [205, 206, 208, 209] |
| Fibrin | Rat and mice myoblasts | Scaffolds promote angiogenesis and myoblast survival, differentiation, and integration with host tissue | Scaffolds rapidly degrade and support disorganized muscle regeneration with limited data on functional recovery | [20, 130, 140, 214, 215] |
| Fibrin | Human myoblasts | Longitudinal topography of scaffolds facilitate myoblast integration with host tissue to stimulated aligned regeneration | Rapid scaffold degradation may attenuate muscle regeneration | [91] |
4.2.1 Synthetic Polymers
A variety of synthetic materials have been used as scaffolds for skeletal muscle regeneration such as PGA [133, 138], poly(lactic acid) (PLA) [139], their copolymers (PLLA/PLGA) [140], and PCL [109, 141, 142]. Synthetic materials offer several advantages to natural polymers such as their ability to have precisely tuned mechanical and structural properties that can be tailored to each tissue engineering application [115, 143, 144]. Synthetic polymers can be readily fabricated into a variety of geometries such as individual fibers or electrospun meshes with aligned or random nanofiber orientation [145–147]. Myoblasts seeded onto electrospun meshes with aligned nanofiber orientation generate highly aligned cultures that, when cultured in differentiation medium, readily fuse into aligned myotubes [109, 148]. Incorporating electrically conductive materials within synthetic scaffolding materials facilitated more efficient electrical stimulation of myoblast cultures, further improving myoblast differentiation compared to aligned fibers without stimulation [149–151]. Synthetic scaffolds can easily be fabricated with multiple growth factors incorporated into their bulk structure to facilitate the controlled release of these factors into a potential injury site [152].
The mechanical stability of synthetic polymer scaffolds that have been used for skeletal muscle applications [153–155] have been used previously with successful outcomes as a structural support material with which to repair abdominal hernias [156, 157]. In these applications, several studies noted the beginning of a vascular network forming in as little as 6 weeks after implantation of PGA meshes, suggesting that these scaffold materials have a modest ability to stimulate angiogenesis [138, 158]. However, a common criticism of synthetic materials is that their surfaces do not always readily support cell attachment [142]. Due to their inherent suboptimal bioactivity, many strategies exist to functionalize the surfaces of synthetic polymer scaffolds such as the addition of collagen or decellularized ECM to modulate tissue responses [109, 153]. Another limitation of synthetic materials is that they typically stimulate a foreign body response as characterized by an increase in the number of foreign body giant cells [159]. This foreign body response induces a prolonged inflammatory response, as well as a sustained M1 macrophage polarization; ultimately resulting in attenuated regeneration [160, 161]. The limited bioactivity of scaffolds made from synthetic polymers limits their utility for treating VML injuries because of the need for these scaffolds to guide functional tissue regeneration, rather than to trigger a sustained inflammatory response.
An alternate approach to the suboptimal bioactivity of synthetic scaffolds has been to use them as a sacrificial layer to support ECM deposition from myoblasts. Using this approach, a polyurethane (PU) foam is generated from a hardened sugar template, and the ECM is synthesized from rat myoblasts [162]. While the PU foam was coated with fibronectin to facilitate myoblast attachment, type I collagen was detected via immunostaining; and laminin and type IV collagen were detected via tandem mass spectroscopy after 4 weeks of culture. The mechanical strength of this engineered ECM is currently reported to be approximately one third of decellularized skeletal muscle derived ECM (M-ECM), however changes to the growth medium or the inclusion of fibroblasts may facilitate an increase in ECM production to increase the mechanical properties as well as the morphological similarities between the engineered ECM and native M-ECM.
4.2.2 Decellularized Scaffolds
Decellularized ECM has been harvested from a variety of tissues such as the small intestine, urinary bladder, dermis, and pericardium and has been used as a biomaterial because it contains many matrix proteins and growth factors thought to be critical for tissue regeneration in their native configuration [163–165]. While ECM-derived scaffolds are thought to contain all of the proteins and growth factors necessary to direct tissue regeneration, care needs to be taken with the decellularization process, since the detergents used can significantly affect the protein and growth factor content within the scaffold [166–169]. Further, incomplete decellularization can result in the persistence of DNA fragments in the material which have the potential to induce an inflammatory response [170]. Interestingly, there does not appear to be site-specific advantages between the decellularized ECM source site and the site of implantation in skeletal muscle applications. The regenerative response in a rodent partial thickness abdominal wall defect due to M-ECM was equivalent to the regenerative response using a small intestine submucosa (SIS) scaffold [28]. One advantage of using ECM derived scaffolds is that they are angiogenic and serve to promote vascularization of skeletal muscle injury sites [16, 171]. However, there are conflicting reports regarding the efficacy of a variety of acellular ECM scaffolds, including M-ECM, dermal ECM, and SIS to enhance the functional recovery of a large muscle defect. Investigations looking at remodeling events 1–2 months post-injury reported modest myoblast infiltration into the injury site, although it appears to be somewhat disorganized [172–175]. There is no consensus whether these additional myofibers contribute to the functional recovery of the muscle 2 months post-injury. Some studies report no improvements in force generation [176], while others report a significant improvement in functional recovery [177]. The addition of bone-marrow derived mesenchymal stem cells (MSCs) to ECM scaffolds significantly improved functional recovery and blood vessel formation compared to acellular ECM scaffolds (approximately 85% and 75% of the force produced in the contralateral leg, respectively) [178]. However, MSCs have not been shown to engraft into skeletal muscle tissue so the exact mechanism of MSC-mediated increases in recovery are not clear [178]. Decellularized bladder matrices seeded with muscle derived cells that were differentiated prior to implantation also significantly increased the functional recovery of VML injuries 2 months post-injury two fold by engrafting into the uninjured tissue [89, 179]. Long term remodeling, between 3–6 months post-injury is required for acellular urinary bladder matrix (UBM) and SIS scaffold mediated functional recovery [180, 181]. Interestingly, Corona et al. observed an increase in the force production of their untreated injury control groups over time (18.6 ± 0.2 N/kg body weight at 2 months compared to 19.8 ± 0.6 N/kg body weight at 6 months) [90], suggesting that the scar tissue deposited in the injury site may be reorganizing over time to facilitate some amount of contractility [90, 181]. In human clinical trials, there have been several accounts of successful partial restoration of mechanical function in four of six patients using SIS and UBM ECM scaffolds to treat VML defects [18, 134]. While ECM scaffolds have been shown to significantly enhance skeletal muscle repair in VML defects, there remains a significant reduction in mechanical function compared to uninjured muscle. As ECM based scaffolds are derived from complex tissue environments, the exact composition of these materials is not well characterized. While these materials have the ability to direct a regenerative response after longer time points, their mechanism of action remains an active area of research.
An alternative strategy to the use of decellularized matrix could be to utilize minced skeletal muscle tissue that has not been decellularized. In this approach, morselized muscle tissue was implanted within a VML defect, resulting in an approximate 55% restoration of the functional deficit of unrepaired muscle controls [182]. These materials may be advantageous for use in traumatic injury where the damaged tissue is still present, but they would not be a viable therapy for injuries where the tissue was completely destroyed or lost. Extended recovery times of 4 months did not appreciably increase the contractility of injured muscles relative to 2 months post injury, suggesting a shorter window of remodeling for cellular constructs than acellular scaffolds. Improvements to this promising therapy may require that the materials actively direct the reorganization of muscle fibers to facilitate their alignment with the surrounding healthy tissue.
4.2.3 Natural Polymers
Naturally occurring polymers such as alginate [93, 98, 183], collagen [113, 184, 185], and fibrin [20, 91, 140] have been used extensively in skeletal muscle engineering. Unlike synthetic polymers, natural polymers possess intrinsic bioactive signaling cues and can form complexes with carbohydrates to form molecules such as heparan sulfate proteoglycan which bind growth factors to enhance cell migration, proliferation, or differentiation [186–189]. In addition, growth factors can adsorb directly to some natural polymers. Alginate is derived from seaweed and was originally used as an encapsulation agent for a variety of cell types [190] and due to its amenability to chemical alterations to promote cell adhesion [191], as well as its tunable mechanical and structural properties [192–194], it has become increasingly used as a scaffold for skeletal muscle regeneration. Alginate gels with stiffnesses between 13 and 45 kPa were found to maximize myoblast proliferation and differentiation [195]. Additionally, alginate facilitates the controlled release of a variety of growth factors important to skeletal muscle regeneration, such as HGF [136, 183], FGF2 [136, 183], IGF1 [93, 196], and vascular endothelial growth factor (VEGF) [93, 196–198]. Alginate gels loaded with HGF and FGF2 significantly increased myoblast viability within the construct, as well as the migration of myoblasts out of these scaffolds, demonstrating that alginate can deliver myoblasts to skeletal muscle [136]. When translated to a mouse TA laceration model, myoblast-seeded gels loaded with HGF and FGF2 significantly reduced the wound size 30 days after injury with an increased number of regenerating myofibers. It was found that the combination of growth factors and the myoblasts produced this effect, but individually each growth factor only supported a modest regenerative response [183]. More recently [199], alginate gels incorporating VEGF and IGF1 have been implanted into ischemia-induced skeletal muscle injuries to address skeletal muscle regeneration by improving the early vascularization and myoblast survival within the injury sites [93, 197]. Functionally, these studies demonstrated that the delivery of VEGF and IGF1 increased the force production 4–5 fold compared to blank alginate gels 7 weeks after injury, approximating the force production of contralateral leg controls [93]. While this model of injury is not as severe as VML injuries, bulk alginate scaffolds loaded with multiple growth factors can significantly improve the regeneration of skeletal muscle injuries by improving the overall vascularization and myoblast differentiation in situ. However, alginate gels have only been implanted on the surface of injury sites [199], and their ability to direct skeletal muscle regeneration without the presence of the existing skeletal muscle ECM is not known.
Collagen, particularly type I, belongs to a family of ECM proteins found in connective tissues such as dermis, tendons, and blood vessels [200]. Scaffolds made from type I collagen have been used in a wide variety of tissue engineering applications including skin, cartilage, bone, tendon, skeletal muscle, and nerve [188, 201]. Collagen gels have been used in skeletal muscle regeneration as a cell delivery vehicle since intramuscular injections of myoblasts result in the rapid death of the delivered cells [135]. Myoblasts implanted within a collagen gel have been shown to migrate out of the scaffold and fuse with the existing myofibers present at the periphery of the injury site [202, 203]. Disorganized myofibers form as a result of the amorphous polymerization process of the collagen lattices, resulting in less optimal healing of muscle injuries [204]. Acellular collagen sponges also supported disorganized repair of large muscle defects. However, a large amount of adipose tissue was observed 12 to 24 weeks post-injury, which further limited the amount of organized functional recovery [205]. To improve the alignment of regenerating myofibers, collagen gels can be fabricated with aligned pore structures through controlled freeze-drying processes. These modified scaffolds facilitated aligned myotubes along the direction of the pores, which when integrated into a large muscle defect, were capable of producing force upon electrical stimulation [113]. However, because of the lack of a robust regenerative response observed with implanted collagen scaffolds, collagen materials were customized with molecules such as heparin to achieve controlled release of growth factors into the injury site [206, 207]. In fact, the functional recovery of muscles damaged by acute ischemia was significantly increased 1 month after injury by adding a VEGF-loaded collagen scaffold, demonstrating that scaffolds can contribute to regeneration by supplying necessary growth factors to the wound site (53% vs. 75% of the force produced before VML defects) [208]. Other growth factors such as HGF, IGF1, and FGF2 have been added to denatured collagen gels to increase SC recruitment into the injury site [209]; however, no functional measurements were performed on these regenerating muscles and data was obtained only at early time points.
Fibrin has been used extensively as a scaffold material in tissue engineering because of its intrinsic bioactivity and role as a provisional matrix during the initial stages of wound healing [189, 210]. Fibrin is a branched microfibrillar polymer formed when activated thrombin cleaves two small peptides from fibrinogen allowing fibrinogen to self-assemble into a complex fibrillar network. Fibrin gels have been used as the provisional seeding matrix for many in vitro skeletal muscle constructs as described above [211–213]. Despite completely degrading within 3 weeks post-injury, fibrin gels promote myoblast survival and differentiation into myofibers that can integrate with uninjured tissue [20, 214, 215]. Implanting a myoblast populated fibrin gel near an existing blood vessel greatly enhances the vascular perfusion of these constructs. These primary myoblasts formed vascularized functional mature skeletal muscle tissue three weeks after implantation [130]. In a different approach to vascularization of skeletal muscle constructs, endothelial cells were seeded into fibrin-PLLA/PLGA constructs in addition to fibroblasts and myoblasts. This tri-culture supported a complex vascular network, suggesting the need for multiple cell types to be present in cell constructs for skeletal muscle regeneration [140]. The addition of PLLA/PLGA to these tri-culture scaffolds was done in part to augment the stability of fibrin constructs, as uncrosslinked fibrin is rapidly remodeled. Another strategy to decrease the rate of proteolytic degradation of fibrin has been to add polyethylene glycol (PEG) groups to the bulk structure of fibrin. In addition to modulating the structural properties of the material, PEGylation can be used as a strategic loading mechanism for a variety of growth factors such as IGF1, to facilitate controlled release of therapeutic molecules to enhance skeletal muscle regeneration [216].
To increase the guided organization of skeletal muscle regeneration, our laboratory pioneered the use of fibrin microthreads, which are morphologically similar to the hierarchal cable-like structure of skeletal muscle. Fibrin microthreads facilitate cell alignment along the longitudinal axis of the material [217] and can be loaded with growth factors such as FGF2 [218] to create discrete structures that can be combined with microthreads loaded with other factors to generate customized composite scaffolds to enhance tissue regeneration. The combination of a bioinspired scaffold material and a highly myogenic human cell line allowed for more complete endogenous muscle regeneration in a mouse model of VML injury (55% vs. 90% of the force produced in the contralateral leg) [91]. While we observed a reduction in the deposition of scar tissue 3–4 months post-injury, histological analyses of tissue harvested at early time points suggested that the microthreads were largely degraded within 2 weeks of implantation and many of the regenerated myofibers in the wound site at later time points exhibited some degree of misalignment with respect to the native muscle tissue [91]. We developed a method to attenuate the degradation rate of fibrin microthreads using carbodiimide crosslinking chemistry to enhance the in vitro persistence of these scaffolds [219]. Interestingly, discrete structural and mechanical properties were developed by modifying the pH environment of the carbodiimide crosslinking reaction, suggesting that the efficiency of carbodiimide crosslinking can be regulated both through controlling the pH environment as well as the total crosslinking time [219].
4.3 Limitations
There have been many significant successes in developing materials to guide skeletal muscle regeneration. One of the strengths of synthetic materials is the ease in which they are fabricated, as well as the flexibility to construct scaffolds in a variety of controlled geometries to control myoblast growth and differentiation [109], despite needing functionalization with natural materials to improve myoblast attachment or regenerative outcomes [153]. Care must also be taken during the fabrication process to avoid making materials that induce a foreign body response [159, 220]. Decellularized ECM materials have been shown to significantly improve functional outcomes in VML defects. However, they do not appear to achieve complete alignment between healthy and regenerating tissue, likely due to the volume of defect space. While natural scaffolds are typically more bioactive than unmodified synthetic materials, bulk scaffolds that form through random protein polymerization do not generate uniform cell alignment (Figure 3B). Applying static axial stretching techniques to natural polymers may facilitate the alignment of protein fibrils within the bulk scaffold, which will direct uniaxial alignment of cells. However to date, these materials have not yet been applied to VML injuries [219, 221]. Fibrin microthreads have been shown to direct aligned muscle regeneration (Figure 3C), but these microthreads degrade rapidly in vivo [91]. While crosslinked fibrin microthreads should attenuate this degradation rate, they have not yet been implanted to assess their in vivo degradation kinetics.
Figure 3.
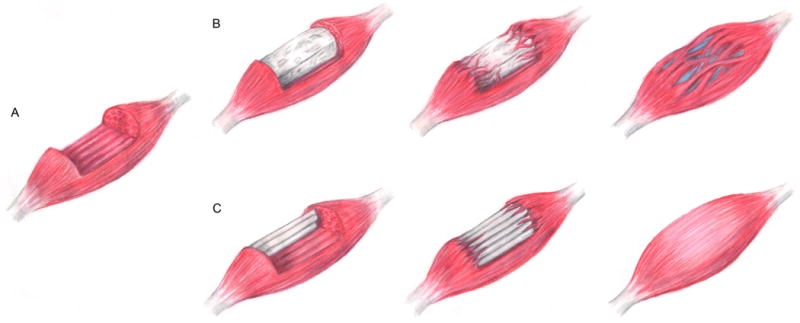
Schematic representation of scaffold mediated repair of volumetric muscle loss (VML). (A) VML injuries span large portions of the muscle belly, and (B) can be repaired using bulk scaffolds to fill this void space. These scaffolds support tissue repair, but functional regeneration is limited by disorganized myofiber and scar tissue formation. (C) Scaffolds with precisely engineered topographic cues to direct aligned tissue growth, such as microthreads, will guide organized myofiber formation for more robust functional tissue regeneration.
Further, many of the models of skeletal muscle injury utilize ischemia [93, 216], toxin [196], or crush induced [222] injuries. These methods of muscle injury leave the basal lamina intact which allows SC recruitment to the injury site due to chemotactic factors being released by the basal lamina [1, 54, 91]. The combination of the early recruitment of SCs to the injury site, as well as the persistence of the regenerative template of the basal lamina, allows these injuries to completely regenerate within 3–4 weeks [2, 13, 21, 25]. In contrast, VML injuries result in the complete destruction or removal of the basal lamina, eliminating the template for SCs to regenerate organized functional skeletal muscle tissue [1, 91, 172, 223]. Table 2 briefly summarizes the methods and limitations of each of these animal models.
Table 2.
A summary of the different animal models used to study skeletal muscle regeneration.
| Injury Model | Mechanism of Injury | Limitations | References |
|---|---|---|---|
| Laceration | Laceration of the belly of the skeletal muscle and opposing stumps are sutured together | Small size of injury may encourage spontaneous regeneration | [183] |
| Ischemia | Ligation of artery that vascularizes the skeletal muscle | Muscle matrix remains intact after injury and may serve as a template for endogenous regeneration | [93, 197, 216] |
| Cardiotoxin | Support cell infiltration, nascent myofiber formation, and functional recovery | Long incubation times required to observe functional recovery | [196] |
| Crush induced | Blunt trauma to crush skeletal muscle without inducing any injury due to laceration | Muscle matrix remains intact and in place after injury and may serve as a template for endogenous regeneration | [222] |
| Volumetric muscle loss | Large amount of tissue is completely removed from skeletal muscle | Variability exists in the size, as well as location, of the defect, making direct comparisons between studies difficult | [89, 91, 172, 177, 178, 180, 223] |
Finally, implanted cell-seeded constructs have utilized a variety of cell types, sourced from several different species. Myoblasts, or expanded SCs, are a promising cell type for cell-based therapies as they can functionally integrate into the existing musculature in the host. Further, they seem to be the most likely cell source for autologous therapies, which would ultimately be required for many of these injuries. Generally, studies have either involved human myoblasts in immune compromised animals [91], or allogeneic myoblast sources from litter mates to limit rejection responses [179]. However, expanding human myoblasts to sufficient numbers for therapeutic benefit in vitro is nontrivial [59], so other cell types such as MSCs have been investigated [178]. While the paracrine effects of MSCs have been well characterized in other systems [224], the ability of these cells to differentiate in situ into skeletal muscle tissue has not been established, which is critical for the repair of large skeletal muscle defects. Establishing cell culture methods and techniques to facilitate the long-term in vitro expansion of myoblasts would increase the clinical feasibility of these cells. However, the ability of acellular scaffolds to repair and regenerate skeletal muscle tissue should not be overlooked when considering clinical therapy options. The positive results seen to date clinically suggest that acellular scaffolds can also improve force production outcomes [134]. Ultimately, tissue engineering solutions will likely include a combination of biomimetic scaffold materials to guide tissue growth along with a cell source to enhance the repair of large scale defects such as those presented in VML injuries.
5.0 CONCLUSIONS AND FUTURE DIRECTIONS
Tissue engineering scaffolds for VML injuries have advanced from passive materials implanted that provide structural support to inductive scaffolds that direct endogenous tissue regeneration. In addition to developing SMUs designed to replace lost skeletal muscle tissue in vitro, skeletal muscle constructs are a powerful model system to investigate biological questions regarding skeletal muscle formation and regeneration ex vivo. Novel strategies have been developed to incorporate essential growth factors and to direct skeletal muscle regeneration and these materials have expanded our knowledge on how VML injuries repair in situ. However, future scaffolds must support more efficient regeneration by enhancing SC recruitment (i.e. migration) to the defect site to recruit more progenitor cells since the matrix cues present in most conventional muscle regeneration studies are destroyed or missing in VML injuries (Figure 3C). Additional myoblasts may be required to supplement the endogenous regenerative process, to ensure complete functional regeneration. This would require novel cell culture techniques to preserve myoblast plasticity in vitro, and to enable these cells to differentiate and fuse with the existing musculature in vivo. Regardless of the source of the cells (endogenous or exogenous), future scaffolds must also direct the alignment of myoblasts within the defect site to facilitate seamless integration between healthy and regenerating tissue (Figure 3). Additionally, care must be taken to select a clinically relevant animal model to appropriately draw conclusions on effects of a given treatment on VML injuries. Finally, controlled structural and mechanical properties are necessary to ensure that scaffolds persist long enough to support organized functional skeletal muscle regeneration.
Acknowledgments
Related work in the authors’ laboratories has been supported in part by the US Army (W81XWH-11-1-0631, GDP and RLP), NIH R01-HL115282 (GDP), and NIH F31-DE023281 (JMG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
GDP discloses that he is a co-founder and has an equity interest in Vitathreads L.L.C., a company that has licensed intellectual property associated with fibrin microthreads.
References
- 1.Grogan BF, Hsu JR. Volumetric muscle loss. The Journal of the American Academy of Orthopaedic Surgeons. 2011;19(Suppl 1):S35–7. doi: 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]
- 2.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–38. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 3.Quintero AJ, Wright VJ, Fu FH, Huard J. Stem cells for the treatment of skeletal muscle injury. Clinics in sports medicine. 2009;28:1–11. doi: 10.1016/j.csm.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bian W, Bursac N. Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE engineering in medicine and biology magazine : the quarterly magazine of the Engineering in Medicine & Biology Society. 2008;27:109–13. doi: 10.1109/MEMB.2008.928460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Counsel P, Breidahl W. Muscle injuries of the lower leg. Semin Musculoskelet Radiol. 2010;14:162–75. doi: 10.1055/s-0030-1253158. [DOI] [PubMed] [Google Scholar]
- 6.2009 report of the 2008 statistics of plastic surgery statistics. American Society of Plastic Surgeons; 2009. [Google Scholar]
- 7.Eckardt A. Microsurgical reconstruction in the head and neck region: an 18-year experience with 500 consecutive cases. Journal of Cranio-Maxillofacial Surgery. 2003;31:197–201. doi: 10.1016/s1010-5182(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 8.Owens BD, Kragh JF, Jr, Macaitis J, Svoboda SJ, Wenke JC. Characterization of extremity wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Orthop Trauma. 2007;21:254–7. doi: 10.1097/BOT.0b013e31802f78fb. [DOI] [PubMed] [Google Scholar]
- 9.Owens BD, Kragh JF, Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J Trauma. 2008;64:295–9. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- 10.Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347:759–74. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- 11.Lynch GS, Schertzer JD, Ryall JG. Anabolic agents for improving muscle regeneration and function after injury. Clinical and experimental pharmacology & physiology. 2008;35:852–8. doi: 10.1111/j.1440-1681.2008.04955.x. [DOI] [PubMed] [Google Scholar]
- 12.Jarvinen TA, Jarvinen TL, Kaariainen M, Aarimaa V, Vaittinen S, Kalimo H, et al. Muscle injuries: optimising recovery. Best Pract Res Clin Rheumatol. 2007;21:317–31. doi: 10.1016/j.berh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–64. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 14.Jarvinen TA, Kaariainen M, Jarvinen M, Kalimo H. Muscle strain injuries. Curr Opin Rheumatol. 2000;12:155–61. doi: 10.1097/00002281-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Kannus P, Parkkari J, Jarvinen TL, Jarvinen TA, Jarvinen M. Basic science and clinical studies coincide: active treatment approach is needed after a sports injury. 2003 Jun;13:150–4. doi: 10.1034/j.1600-0838.2003.02225.x. [DOI] [PubMed] [Google Scholar]
- 16.De Coppi P, Bellini S, Conconi MT, Sabatti M, Simonato E, Gamba PG, et al. Myoblastacellular skeletal muscle matrix constructs guarantee a long-term repair of experimental full-thickness abdominal wall defects. Tissue engineering. 2006;12:1929–36. doi: 10.1089/ten.2006.12.1929. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi B, Copelli C, Ferrari S, Ferri A, Sesenna E. Free flaps: outcomes and complications in head and neck reconstructions. J Craniomaxillofac Surg. 2009;37:438–42. doi: 10.1016/j.jcms.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Mase VJ, Jr, Hsu JR, Wolf SE, Wenke JC, Baer DG, Owens J, et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33:511. doi: 10.3928/01477447-20100526-24. [DOI] [PubMed] [Google Scholar]
- 19.Floriano R, Peral B, Alvarez R, Verrier A. Microvascular free flaps in head and neck reconstruction. Report of 71 cases Journal of Cranio-Maxillofacial Surgery. 2006;34:90. [Google Scholar]
- 20.Beier JP, Stern-Straeter J, Foerster VT, Kneser U, Stark GB, Bach AD. Tissue engineering of injectable muscle: three-dimensional myoblast-fibrin injection in the syngeneic rat animal model. Plast Reconstr Surg. 2006;118:1113–21. doi: 10.1097/01.prs.0000221007.97115.1d. discussion 22–4. [DOI] [PubMed] [Google Scholar]
- 21.Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. The Journal of bone and joint surgery American volume. 2002;84–A:822–32. [PubMed] [Google Scholar]
- 22.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grefte S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Skeletal muscle development and regeneration. Stem Cells Dev. 2007;16:857–68. doi: 10.1089/scd.2007.0058. [DOI] [PubMed] [Google Scholar]
- 24.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 25.Sanes JR. The basement membrane/basal lamina of skeletal muscle. The Journal of biological chemistry. 2003;278:12601–4. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 26.Cassano M, Quattrocelli M, Crippa S, Perini I, Ronzoni F, Sampaolesi M. Cellular mechanisms and local progenitor activation to regulate skeletal muscle mass. Journal of muscle research and cell motility. 2009;30:243–53. doi: 10.1007/s10974-010-9204-y. [DOI] [PubMed] [Google Scholar]
- 27.Velleman SG. The role of the extracellular matrix in skeletal muscle development. Poult Sci. 1999;78:778–84. doi: 10.1093/ps/78.5.778. [DOI] [PubMed] [Google Scholar]
- 28.Wolf MT, Daly KA, Reing JE, Badylak SF. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials. 2012;33:2916–25. doi: 10.1016/j.biomaterials.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan JE, Partridge TA. Muscle satellite cells. The International Journal of Biochemistry and Cell Biology. 2003;35:1151–6. doi: 10.1016/s1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 30.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–91. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 31.Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J Histochem Cytochem. 2011;59:33–46. doi: 10.1369/jhc.2010.956995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosgrove BD, Sacco A, Gilbert PM, Blau HM. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78:185–94. doi: 10.1016/j.diff.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tidball JG. Mechanisms of muscle injury, repair, and regeneration. Comprehensive Physiology. 2011;1:2029–62. doi: 10.1002/cphy.c100092. [DOI] [PubMed] [Google Scholar]
- 34.Bodine-Fowler S. Skeletal muscle regeneration after injury: an overview. J Voice. 1994;8:53–62. doi: 10.1016/s0892-1997(05)80319-4. [DOI] [PubMed] [Google Scholar]
- 35.Kimura N, Hirata S, Miyasaka N, Kawahata K, Kohsaka H. Injury and subsequent regeneration of muscles for activation of local innate immunity to facilitate the development and relapse of autoimmune myositis in C57BL/6 mice. Arthritis Rheumatol. 2015;67:1107–16. doi: 10.1002/art.39017. [DOI] [PubMed] [Google Scholar]
- 36.Hurme T, Kalimo H, Lehto M, Jarvinen M. Healing of skeletal muscle injury: an ultrastructural and immunohistochemical study. Medicine and science in sports and exercise. 1991;23:801–10. [PubMed] [Google Scholar]
- 37.Carpenter S, Karpati G. Segmental necrosis and its demarcation in experimental micropuncture injury of skeletal muscle fibers. Journal of neuropathology and experimental neurology. 1989;48:154–70. doi: 10.1097/00005072-198903000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–87. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatsumi R. Mechano-biology of skeletal muscle hypertrophy and regeneration: possible mechanism of stretch-induced activation of resident myogenic stem cells. Animal science journal = Nihon chikusan Gakkaiho. 2010;81:11–20. doi: 10.1111/j.1740-0929.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 40.Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell. 2002;13:2909–18. doi: 10.1091/mbc.E02-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, et al. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. American journal of physiology Cell physiology. 2006;290:C1487–94. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- 42.Hara M, Tabata K, Suzuki T, Do MK, Mizunoya W, Nakamura M, et al. Calcium influx through a possible coupling of cation channels impacts skeletal muscle satellite cell activation in response to mechanical stretch. American journal of physiology Cell physiology. 2012;302:C1741–50. doi: 10.1152/ajpcell.00068.2012. [DOI] [PubMed] [Google Scholar]
- 43.Cannon JG, St Pierre BA. Cytokines in exertion-induced skeletal muscle injury. Mol Cell Biochem. 1998;179:159–67. doi: 10.1023/a:1006828425418. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Wu G, Shi D, Zhu R, Zeng H, Cao B, et al. Effects of nitric oxide on notexin-induced muscle inflammatory responses. International journal of biological sciences. 2015;11:156–67. doi: 10.7150/ijbs.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Wehling-Henricks M, Samengo G, Tidball JG. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging cell. 2015 doi: 10.1111/acel.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villalta SA, Deng B, Rinaldi C, Wehling-Henricks M, Tidball JG. IFN-gamma promotes muscle damage in the mdx mouse model of Duchenne muscular dystrophy by suppressing M2 macrophage activation and inhibiting muscle cell proliferation. J Immunol. 2011;187:5419–28. doi: 10.4049/jimmunol.1101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189:3669–80. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villalta SA, Rinaldi C, Deng B, Liu G, Fedor B, Tidball JG. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Human molecular genetics. 2011;20:790–805. doi: 10.1093/hmg/ddq523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Experimental cell research. 2001;267:107–14. doi: 10.1006/excr.2001.5252. [DOI] [PubMed] [Google Scholar]
- 50.Gal-Levi R, Leshem Y, Aoki S, Nakamura T, Halevy O. Hepatocyte growth factor plays a dual role in regulating skeletal muscle satellite cell proliferation and differentiation. Biochimica et biophysica acta. 1998;1402:39–51. doi: 10.1016/s0167-4889(97)00124-9. [DOI] [PubMed] [Google Scholar]
- 51.Tatsumi R, Allen RE. Active hepatocyte growth factor is present in skeletal muscle extracellular matrix. Muscle & nerve. 2004;30:654–8. doi: 10.1002/mus.20114. [DOI] [PubMed] [Google Scholar]
- 52.Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–28. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 53.Anderson JE, Wozniak AC. Satellite cell activation on fibers: modeling events in vivo–an invited review. Can J Physiol Pharmacol. 2004;82:300–10. doi: 10.1139/y04-020. [DOI] [PubMed] [Google Scholar]
- 54.Hayashi S, Aso H, Watanabe K, Nara H, Rose MT, Ohwada S, et al. Sequence of IGF-I, IGF-II, and HGF expression in regenerating skeletal muscle. Histochemistry and cell biology. 2004;122:427–34. doi: 10.1007/s00418-004-0704-y. [DOI] [PubMed] [Google Scholar]
- 55.deLapeyriere O, Ollendorff V, Planche J, Ott MO, Pizette S, Coulier F, et al. Expression of the Fgf6 gene is restricted to developing skeletal muscle in the mouse embryo. Development. 1993;118:601–11. doi: 10.1242/dev.118.2.601. [DOI] [PubMed] [Google Scholar]
- 56.Floss T, Arnold HH, Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes & development. 1997;11:2040–51. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 58.Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle & nerve. 2000;23:239–45. doi: 10.1002/(sici)1097-4598(200002)23:2<239::aid-mus15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 59.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–7. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 60.Watt DJ, Morgan JE, Clifford MA, Partridge TA. The movement of muscle precursor cells between adjacent regenerating muscles in the mouse. Anat Embryol. 1987;175:527–36. doi: 10.1007/BF00309688. [DOI] [PubMed] [Google Scholar]
- 61.Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle & nerve. 1994;17:608–13. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- 62.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–6. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne GS. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci. 1999;112(Pt 17):2895–901. doi: 10.1242/jcs.112.17.2895. [DOI] [PubMed] [Google Scholar]
- 65.Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–83. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- 66.Yablonka-Reuveni Z, Day K, Vine A, Shefer G. Defining the transcriptional signature of skeletal muscle stem cells. J Anim Sci. 2008;86:E207–16. doi: 10.2527/jas.2007-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zammit P. Kinetics of Myoblast Proliferation Show That Resident Satellite Cells Are Competent to Fully Regenerate Skeletal Muscle Fibers. Experimental cell research. 2002;281:39–49. doi: 10.1006/excr.2002.5653. [DOI] [PubMed] [Google Scholar]
- 68.Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leshem Y, Spicer DB, Gal-Levi R, Halevy O. Hepatocyte growth factor (HGF) inhibits skeletal muscle cell differentiation: a role for the bHLH protein twist and the cdk inhibitor p27. Journal of cellular physiology. 2000;184:101–9. doi: 10.1002/(SICI)1097-4652(200007)184:1<101::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 70.Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene Expression Patterns of the Fibroblast Growth Factors and Their Receptors During Myogenesis of Rat Satellite Cells. Journal of Histochemistry & Cytochemistry. 2000;48:1079–96. doi: 10.1177/002215540004800805. [DOI] [PubMed] [Google Scholar]
- 71.O’Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle & nerve. 2008;38:1434–42. doi: 10.1002/mus.21146. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki S, Yamanouchi K, Soeta C, Katakai Y, Harada R, Naito K, et al. Skeletal muscle injury induces hepatocyte growth factor expression in spleen. Biochemical and biophysical research communications. 2002;292:709–14. doi: 10.1006/bbrc.2002.6706. [DOI] [PubMed] [Google Scholar]
- 73.Menetrey J, Kasemkijwattana C, Day CS, Bosch P, Vogt M, Fu FH, et al. Growth factors improve muscle healing in vivo. The Journal of bone and joint surgery British volume. 2000;82:131–7. doi: 10.1302/0301-620x.82b1.8954. [DOI] [PubMed] [Google Scholar]
- 74.Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, et al. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. Faseb J. 2007;21:1393–402. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- 75.Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135:431–40. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 77.Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201–16. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- 78.Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. The Journal of biological chemistry. 1997;272:6653–62. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- 79.Smith CW, Klaasmeyer JG, Woods TL, Jones SJ. Effects of IGF-I, IGF-II, bFGF and PDGF on the initiation of mRNA translation in C2C12 myoblasts and differentiating myoblasts. Tissue & cell. 1999;31:403–12. doi: 10.1054/tice.1999.0033. [DOI] [PubMed] [Google Scholar]
- 80.Vandenburgh HH, Karlisch P, Shansky J, Feldstein R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. The American journal of physiology. 1991;260:C475–84. doi: 10.1152/ajpcell.1991.260.3.C475. [DOI] [PubMed] [Google Scholar]
- 81.Lefaucheur JP, Sebille A. Muscle regeneration following injury can be modified in vivo by immune neutralization of basic fibroblast growth factor, transforming growth factor beta 1 or insulin-like growth factor I. J Neuroimmunol. 1995;57:85–91. doi: 10.1016/0165-5728(94)00166-l. [DOI] [PubMed] [Google Scholar]
- 82.Keller HL, St Pierre Schneider B, Eppihimer LA, Cannon JG. Association of IGF-I and IGF-II with myofiber regeneration in vivo. Muscle & nerve. 1999;22:347–54. doi: 10.1002/(sici)1097-4598(199903)22:3<347::aid-mus7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 83.Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol. 2000;89:1365–79. doi: 10.1152/jappl.2000.89.4.1365. [DOI] [PubMed] [Google Scholar]
- 84.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–51. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 85.Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, et al. Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization. Mol Ther. 2015 doi: 10.1038/mt.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wood WM, Etemad S, Yamamoto M, Goldhamer DJ. MyoD-expressing progenitors are essential for skeletal myogenesis and satellite cell development. Dev Biol. 2013;384:114–27. doi: 10.1016/j.ydbio.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–37. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lehto M, Jarvinen M, Nelimarkka O. Scar formation after skeletal muscle injury. A histological and autoradiographical study in rats. Archives of orthopaedic and traumatic surgery Archiv fur orthopadische und Unfall-Chirurgie. 1986;104:366–70. doi: 10.1007/BF00454432. [DOI] [PubMed] [Google Scholar]
- 89.Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, Dannahower A, et al. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A. 2011;17:2291–303. doi: 10.1089/ten.tea.2010.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corona BT, Wu X, Ward CL, McDaniel JS, Rathbone CR, Walters TJ. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials. 2013;34:3324–35. doi: 10.1016/j.biomaterials.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 91.Page RL, Malcuit C, Vilner L, Vojtic I, Shaw S, Hedblom E, et al. Restoration of skeletal muscle defects with adult human cells delivered on fibrin microthreads. Tissue Eng Part A. 2011;17:2629–40. doi: 10.1089/ten.TEA.2011.0024. [DOI] [PubMed] [Google Scholar]
- 92.Lehto M, Duance VC, Restall D. Collagen and fibronectin in a healing skeletal muscle injury. An immunohistological study of the effects of physical activity on the repair of injured gastrocnemius muscle in the rat. The Journal of bone and joint surgery British volume. 1985;67:820–8. doi: 10.1302/0301-620X.67B5.3902851. [DOI] [PubMed] [Google Scholar]
- 93.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3287–92. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Molecular cell. 1998;2:549–58. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- 95.Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mounier R, Chretien F, Chazaud B. Blood vessels and the satellite cell niche. Current topics in developmental biology. 2011;96:121–38. doi: 10.1016/B978-0-12-385940-2.00005-X. [DOI] [PubMed] [Google Scholar]
- 97.Caroni P, Schneider C, Kiefer MC, Zapf J. Role of muscle insulin-like growth factors in nerve sprouting: suppression of terminal sprouting in paralyzed muscle by IGF-binding protein 4. J Cell Biol. 1994;125:893–902. doi: 10.1083/jcb.125.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu G, Pareta RA, Wu R, Shi Y, Zhou X, Liu H, et al. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials. 2013;34:1311–26. doi: 10.1016/j.biomaterials.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim MS, Bhang SH, Yang HS, Rim NG, Jun I, Kim SI, et al. Development of functional fibrous matrices for the controlled release of basic fibroblast growth factor to improve therapeutic angiogenesis. Tissue Eng Part A. 2010;16:2999–3010. doi: 10.1089/ten.TEA.2009.0828. [DOI] [PubMed] [Google Scholar]
- 100.Fathi E, Nassiri SM, Atyabi N, Ahmadi SH, Imani M, Farahzadi R, et al. Induction of angiogenesis via topical delivery of basic-fibroblast growth factor from polyvinyl alcohol-dextran blend hydrogel in an ovine model of acute myocardial infarction. J Tissue Eng Regen Med. 2013;7:697–707. doi: 10.1002/term.1460. [DOI] [PubMed] [Google Scholar]
- 101.Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proceedings of the Japan Academy Series B, Physical and biological sciences. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Madonna R, Cevik C, Nasser M, De Caterina R. Hepatocyte growth factor: molecular biomarker and player in cardioprotection and cardiovascular regeneration. Thromb Haemost. 2012;107:656–61. doi: 10.1160/TH11-10-0711. [DOI] [PubMed] [Google Scholar]
- 103.Jarvinen TA, Jarvinen M, Kalimo H. Regeneration of injured skeletal muscle after the injury. Muscles, ligaments and tendons journal. 2013;3:337–45. [PMC free article] [PubMed] [Google Scholar]
- 104.Stern-Straeter J, Riedel F, Bran G, Hormann K, Goessler UR. Advances in skeletal muscle tissue engineering. In Vivo. 2007;21:435–44. [PubMed] [Google Scholar]
- 105.Koning M, Harmsen MC, van Luyn MJ, Werker PM. Current opportunities and challenges in skeletal muscle tissue engineering. J Tissue Eng Regen Med. 2009;3:407–15. doi: 10.1002/term.190. [DOI] [PubMed] [Google Scholar]
- 106.Wolf MT, Dearth CL, Sonnenberg SB, Loboa EG, Badylak SF. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Advanced drug delivery reviews. 2015;84:208–21. doi: 10.1016/j.addr.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mertens JP, Sugg KB, Lee JD, Larkin LM. Engineering muscle constructs for the creation of functional engineered musculoskeletal tissue. Regenerative medicine. 2014;9:89–100. doi: 10.2217/rme.13.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liao H, Zhou GQ. Development and progress of engineering of skeletal muscle tissue. Tissue engineering Part B, Reviews. 2009;15:319–31. doi: 10.1089/ten.teb.2009.0092. [DOI] [PubMed] [Google Scholar]
- 109.Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29:2899–906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 110.Shah R, Sinanan AC, Knowles JC, Hunt NP, Lewis MP. Craniofacial muscle engineering using a 3-dimensional phosphate glass fibre construct. Biomaterials. 2005;26:1497–505. doi: 10.1016/j.biomaterials.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 111.Beier JP, Klumpp D, Rudisile M, Dersch R, Wendorff JH, Bleiziffer O, et al. Collagen matrices from sponge to nano: new perspectives for tissue engineering of skeletal muscle. BMC Biotechnol. 2009;9:34. doi: 10.1186/1472-6750-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cornwell KG, Downing BR, Pins GD. Characterizing fibroblast migration on discrete collagen threads for applications in tissue regeneration. J Biomed Mater Res A. 2004;71:55–62. doi: 10.1002/jbm.a.30132. [DOI] [PubMed] [Google Scholar]
- 113.Kroehne V, Heschel I, Schugner F, Lasrich D, Bartsch JW, Jockusch H. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. J Cell Mol Med. 2008;12:1640–8. doi: 10.1111/j.1582-4934.2008.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Norman JJ, Desai TA. Control of cellular organization in three dimensions using a microfabricated polydimethylsiloxane-collagen composite tissue scaffold. Tissue engineering. 2005;11:378–86. doi: 10.1089/ten.2005.11.378. [DOI] [PubMed] [Google Scholar]
- 115.Williamson MR, Adams EF, Coombes AG. Gravity spun polycaprolactone fibres for soft tissue engineering: interaction with fibroblasts and myoblasts in cell culture. Biomaterials. 2006;27:1019–26. doi: 10.1016/j.biomaterials.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 116.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 117.Geer DJ, Swartz DD, Andreadis ST. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. American Journal of Pathology. 2005;167:1575–86. doi: 10.1016/S0002-9440(10)61242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues–state of the art and future perspectives. Journal of biomaterials science Polymer edition. 2001;12:107–24. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 119.Vandenburgh H, Del Tatto M, Shansky J, Lemaire J, Chang A, Payumo F, et al. Tissue-engineered skeletal muscle organoids for reversible gene therapy. Human gene therapy. 1996;7:2195–200. doi: 10.1089/hum.1996.7.17-2195. [DOI] [PubMed] [Google Scholar]
- 120.Vandenburgh HH, Hatfaludy S, Karlisch P, Shansky J. Mechanically induced alterations in cultured skeletal muscle growth. Journal of biomechanics. 1991;24(Suppl 1):91–9. doi: 10.1016/0021-9290(91)90380-6. [DOI] [PubMed] [Google Scholar]
- 121.Dennis RG, Kosnik PE, 2nd, Gilbert ME, Faulkner JA. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. American journal of physiology Cell physiology. 2001;280:C288–95. doi: 10.1152/ajpcell.2001.280.2.C288. [DOI] [PubMed] [Google Scholar]
- 122.Huang YC, Dennis RG, Larkin L, Baar K. Rapid formation of functional muscle in vitro using fibrin gels. Journal of Applied Physiology. 2005;98:706–13. doi: 10.1152/japplphysiol.00273.2004. [DOI] [PubMed] [Google Scholar]
- 123.Kosnik PE, Faulkner JA, Dennis RG. Functional development of engineered skeletal muscle from adult and neonatal rats. Tissue engineering. 2001;7:573–84. doi: 10.1089/107632701753213192. [DOI] [PubMed] [Google Scholar]
- 124.Juhas M, Engelmayr GC, Jr, Fontanella AN, Palmer GM, Bursac N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5508–13. doi: 10.1073/pnas.1402723111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vandenburgh H. High-content drug screening with engineered musculoskeletal tissues. Tissue engineering Part B, Reviews. 2010;16:55–64. doi: 10.1089/ten.teb.2009.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vandenburgh H, Shansky J, Benesch-Lee F, Barbata V, Reid J, Thorrez L, et al. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle & nerve. 2008;37:438–47. doi: 10.1002/mus.20931. [DOI] [PubMed] [Google Scholar]
- 127.Vandenburgh H, Shansky J, Benesch-Lee F, Skelly K, Spinazzola JM, Saponjian Y, et al. Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts. FASEB J. 2009;23:3325–34. doi: 10.1096/fj.09-134411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dennis RG, Kosnik PE., 2nd Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim. 2000;36:327–35. doi: 10.1290/1071-2690(2000)036<0327:EAICPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 129.Carosio S, Barberi L, Rizzuto E, Nicoletti C, Del Prete Z, Musaro A. Generation of eX vivo-vascularized Muscle Engineered Tissue (X-MET) Scientific reports. 2013;3:1420. doi: 10.1038/srep01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Borschel GH, Dow DE, Dennis RG, Brown DL. Tissue-engineered axially vascularized contractile skeletal muscle. Plastic and Reconstructive Surgery. 2006;117:2235–42. doi: 10.1097/01.prs.0000224295.54073.49. [DOI] [PubMed] [Google Scholar]
- 131.Williams ML, Kostrominova TY, Arruda EM, Larkin LM. Effect of implantation on engineered skeletal muscle constructs. J Tissue Eng Regen Med. 2013;7:434–42. doi: 10.1002/term.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.VanDusen KW, Syverud BC, Williams ML, Lee JD, Larkin LM. Engineered Skeletal Muscle Units for Repair of Volumetric Muscle Loss in the Tibialis Anterior Muscle of a Rat. Tissue Eng Part A. 2014;20:2920–30. doi: 10.1089/ten.tea.2014.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saxena AK, Marler J, Benvenuto M, Willital GH, Vacanti JP. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: Preliminary studies. Tissue engineering. 1999;5:525–31. doi: 10.1089/ten.1999.5.525. [DOI] [PubMed] [Google Scholar]
- 134.Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 2014;6:234ra58. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thorrez L, Shansky J, Wang L, Fast L, VandenDriessche T, Chuah M, et al. Growth, differentiation, transplantation and survival of human skeletal myofibers on biodegradable scaffolds. Biomaterials. 2008;29:75–84. doi: 10.1016/j.biomaterials.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hill E, Boontheekul T, Mooney DJ. Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue engineering. 2006;12:1295–304. doi: 10.1089/ten.2006.12.1295. [DOI] [PubMed] [Google Scholar]
- 137.Stern-Straeter J, Bran G, Riedel F, Sauter A, Hormann K, Goessler UR. Characterization of human myoblast cultures for tissue engineering. Int J Mol Med. 2008;21:49–56. [PubMed] [Google Scholar]
- 138.Saxena AK, Willital GH, Vacanti JP. Vascularized three-dimensional skeletal muscle tissue-engineering. Bio-medical materials and engineering. 2001;11:275–81. [PubMed] [Google Scholar]
- 139.Cronin EM, Thurmond FA, Bassel-Duby R, Williams RS, Wright WE, Nelson KD, et al. Protein-coated poly(L-lactic acid) fibers provide a substrate for differentiation of human skeletal muscle cells. J Biomed Mater Res A. 2004;69:373–81. doi: 10.1002/jbm.a.30009. [DOI] [PubMed] [Google Scholar]
- 140.Lesman A, Koffler J, Atlas R, Blinder YJ, Kam Z, Levenberg S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials. 2011;32:7856–69. doi: 10.1016/j.biomaterials.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 141.Hoque ME, San WY, Wei F, Li S, Huang MH, Vert M, et al. Processing of polycaprolactone and polycaprolactone-based copolymers into 3D scaffolds, and their cellular responses. Tissue Eng Part A. 2009;15:3013–24. doi: 10.1089/ten.TEA.2008.0355. [DOI] [PubMed] [Google Scholar]
- 142.Kim MS, Jun I, Shin YM, Jang W, Kim SI, Shin H. The development of genipin-crosslinked poly(caprolactone) (PCL)/gelatin nanofibers for tissue engineering applications. Macromol Biosci. 2010;10:91–100. doi: 10.1002/mabi.200900168. [DOI] [PubMed] [Google Scholar]
- 143.Grizzi I, Garreau H, Li S, Vert M. Hydrolytic degradation of devices based on poly(DL-lactic acid) size-dependence. Biomaterials. 1995;16:305–11. doi: 10.1016/0142-9612(95)93258-f. [DOI] [PubMed] [Google Scholar]
- 144.Li S. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J Biomed Mater Res. 1999;48:342–53. doi: 10.1002/(sici)1097-4636(1999)48:3<342::aid-jbm20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 145.Ostrovidov S, Hosseini V, Ahadian S, Fujie T, Parthiban SP, Ramalingam M, et al. Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications. Tissue engineering Part B, Reviews. 2014;20:403–36. doi: 10.1089/ten.teb.2013.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, Juncker D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnology advances. 2013;31:669–87. doi: 10.1016/j.biotechadv.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Neumann T, Hauschka SD, Sanders JE. Tissue engineering of skeletal muscle using polymer fiber arrays. Tissue engineering. 2003;9:995–1003. doi: 10.1089/107632703322495637. [DOI] [PubMed] [Google Scholar]
- 148.Guex AG, Birrer DL, Fortunato G, Tevaearai HT, Giraud MN. Anisotropically oriented electrospun matrices with an imprinted periodic micropattern: a new scaffold for engineered muscle constructs. Biomed Mater. 2013;8:021001. doi: 10.1088/1748-6041/8/2/021001. [DOI] [PubMed] [Google Scholar]
- 149.Sirivisoot S, Pareta R, Harrison BS. Protocol and cell responses in three-dimensional conductive collagen gel scaffolds with conductive polymer nanofibres for tissue regeneration. Interface focus. 2014;4:20130050. doi: 10.1098/rsfs.2013.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ku SH, Lee SH, Park CB. Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation. Biomaterials. 2012;33:6098–104. doi: 10.1016/j.biomaterials.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 151.Chen MC, Sun YC, Chen YH. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta biomaterialia. 2013;9:5562–72. doi: 10.1016/j.actbio.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 152.Nelson DM, Baraniak PR, Ma Z, Guan J, Mason NS, Wagner WR. Controlled release of IGF-1 and HGF from a biodegradable polyurethane scaffold. Pharmaceutical research. 2011;28:1282–93. doi: 10.1007/s11095-011-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wolf MT, Carruthers CA, Dearth CL, Crapo PM, Huber A, Burnsed OA, et al. Polypropylene surgical mesh coated with extracellular matrix mitigates the host foreign body response. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lam CX, Hutmacher DW, Schantz JT, Woodruff MA, Teoh SH. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J Biomed Mater Res A. 2009;90:906–19. doi: 10.1002/jbm.a.32052. [DOI] [PubMed] [Google Scholar]
- 155.Zhao W, Ju YM, Christ G, Atala A, Yoo JJ, Lee SJ. Diaphragmatic muscle reconstruction with an aligned electrospun poly(epsilon-caprolactone)/collagen hybrid scaffold. Biomaterials. 2013;34:8235–40. doi: 10.1016/j.biomaterials.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 156.Cobb WS, Kercher KW, Heniford BT. The argument for lightweight polypropylene mesh in hernia repair. Surg Innov. 2005;12:63–9. doi: 10.1177/155335060501200109. [DOI] [PubMed] [Google Scholar]
- 157.Klosterhalfen B, Junge K, Klinge U. The lightweight and large porous mesh concept for hernia repair. Expert review of medical devices. 2005;2:103–17. doi: 10.1586/17434440.2.1.103. [DOI] [PubMed] [Google Scholar]
- 158.Kamelger FS, Marksteiner R, Margreiter E, Klima G, Wechselberger G, Hering S, et al. A comparative study of three different biomaterials in the engineering of skeletal muscle using a rat animal model. Biomaterials. 2004;25:1649–55. doi: 10.1016/s0142-9612(03)00520-9. [DOI] [PubMed] [Google Scholar]
- 159.Yang J, Jao B, McNally AK, Anderson JM. In vivo quantitative and qualitative assessment of foreign body giant cell formation on biomaterials in mice deficient in natural killer lymphocyte subsets, mast cells, or the interleukin-4 receptoralpha and in severe combined immunodeficient mice. J Biomed Mater Res A. 2014;102:2017–23. doi: 10.1002/jbm.a.35152. [DOI] [PubMed] [Google Scholar]
- 160.Anderson JM. Exploiting the inflammatory response on biomaterials research and development. J Mater Sci Mater Med. 2015;26:121. doi: 10.1007/s10856-015-5423-5. [DOI] [PubMed] [Google Scholar]
- 161.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–42. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 162.Hurd SA, Bhatti NM, Walker AM, Kasukonis BM, Wolchok JC. Development of a biological scaffold engineered using the extracellular matrix secreted by skeletal muscle cells. Biomaterials. 2015;49:9–17. doi: 10.1016/j.biomaterials.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 164.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta biomaterialia. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 165.Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Translational research : the journal of laboratory and clinical medicine. 2014;163:268–85. doi: 10.1016/j.trsl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Faulk DM, Carruthers CA, Warner HJ, Kramer CR, Reing JE, Zhang L, et al. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta biomaterialia. 2014;10:183–93. doi: 10.1016/j.actbio.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, et al. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transplant. 2014;33:298–308. doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 168.Tsuchiya T, Balestrini JL, Mendez J, Calle EA, Zhao L, Niklason LE. Influence of pH on extracellular matrix preservation during lung decellularization. Tissue Eng Part C Methods. 2014;20:1028–36. doi: 10.1089/ten.tec.2013.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tsuchiya T, Sivarapatna A, Rocco K, Nanashima A, Nagayasu T, Niklason LE. Future prospects for tissue engineered lung transplantation: decellularization and recellularization-based whole lung regeneration. Organogenesis. 2014;10:196–207. doi: 10.4161/org.27846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61–7. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 171.Conconi MT, De Coppi P, Bellini S, Zara G, Sabatti M, Marzaro M, et al. Homologous muscle acellular matrix seeded with autologous myoblasts as a tissue-engineering approach to abdominal wall-defect repair. Biomaterials. 2005;26:2567–74. doi: 10.1016/j.biomaterials.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 172.Sicari BM, Agrawal V, Siu BF, Medberry CJ, Dearth CL, Turner NJ, et al. A murine model of volumetric muscle loss and a regenerative medicine approach for tissue replacement. Tissue Eng Part A. 2012;18:1941–8. doi: 10.1089/ten.tea.2012.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Perniconi B, Costa A, Aulino P, Teodori L, Adamo S, Coletti D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials. 2011;32:7870–82. doi: 10.1016/j.biomaterials.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 174.Wolf MT, Daly KA, Brennan-Pierce EP, Johnson SA, Carruthers CA, D’Amore A, et al. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33:7028–38. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang L, Johnson JA, Chang DW, Zhang Q. Decellularized musculofascial extracellular matrix for tissue engineering. Biomaterials. 2013;34:2641–54. doi: 10.1016/j.biomaterials.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Merritt EK, Hammers DW, Tierney M, Suggs LJ, Walters TJ, Farrar RP. Functional assessment of skeletal muscle regeneration utilizing homologous extracellular matrix as scaffolding. Tissue Eng Part A. 2010;16:1395–405. doi: 10.1089/ten.TEA.2009.0226. [DOI] [PubMed] [Google Scholar]
- 177.Chen XK, Walters TJ. Muscle-derived decellularised extracellular matrix improves functional recovery in a rat latissimus dorsi muscle defect model. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2013;66:1750–8. doi: 10.1016/j.bjps.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 178.Merritt EK, Cannon MV, Hammers DW, Le LN, Gokhale R, Sarathy A, et al. Repair of traumatic skeletal muscle injury with bone-marrow-derived mesenchymal stem cells seeded on extracellular matrix. Tissue Eng Part A. 2010;16:2871–81. doi: 10.1089/ten.TEA.2009.0826. [DOI] [PubMed] [Google Scholar]
- 179.Corona BT, Machingal MA, Criswell T, Vadhavkar M, Dannahower AC, Bergman C, et al. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng Part A. 2012;18:1213–28. doi: 10.1089/ten.tea.2011.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Valentin JE, Turner NJ, Gilbert TW, Badylak SF. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2010;31:7475–84. doi: 10.1016/j.biomaterials.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng Part A. 2014;20:705–15. doi: 10.1089/ten.tea.2012.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Corona BT, Garg K, Ward CL, McDaniel JS, Walters TJ, Rathbone CR. Autologous minced muscle grafts: a tissue engineering therapy for the volumetric loss of skeletal muscle. American journal of physiology Cell physiology. 2013;305:C761–75. doi: 10.1152/ajpcell.00189.2013. [DOI] [PubMed] [Google Scholar]
- 183.Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2494–9. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Moon du G, Christ G, Stitzel JD, Atala A, Yoo JJ. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng Part A. 2008;14:473–82. doi: 10.1089/tea.2007.0104. [DOI] [PubMed] [Google Scholar]
- 185.Rhim C, Lowell DA, Reedy MC, Slentz DH, Zhang SJ, Kraus WE, et al. Morphology and ultrastructure of differentiating three-dimensional mammalian skeletal muscle in a collagen gel. Muscle & nerve. 2007;36:71–80. doi: 10.1002/mus.20788. [DOI] [PubMed] [Google Scholar]
- 186.Bidarra SJ, Barrias CC, Granja PL. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta biomaterialia. 2014;10:1646–62. doi: 10.1016/j.actbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 187.Walters BD, Stegemann JP. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta biomaterialia. 2014;10:1488–501. doi: 10.1016/j.actbio.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chattopadhyay S, Raines RT. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101:821–33. doi: 10.1002/bip.22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Brown AC, Barker TH. Fibrin-based biomaterials: modulation of macroscopic properties through rational design at the molecular level. Acta biomaterialia. 2014;10:1502–14. doi: 10.1016/j.actbio.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Andrejecsk JW, Cui J, Chang WG, Devalliere J, Pober JS, Saltzman WM. Paracrine exchanges of molecular signals between alginate-encapsulated pericytes and freely suspended endothelial cells within a 3D protein gel. Biomaterials. 2013;34:8899–908. doi: 10.1016/j.biomaterials.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 192.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–65. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 193.Drury JL, Boontheekul T, Mooney DJ. Cellular cross-linking of peptide modified hydrogels. Journal of Biomechanical Engineering. 2005;127:220–8. doi: 10.1115/1.1865194. [DOI] [PubMed] [Google Scholar]
- 194.Wang L, Shansky J, Borselli C, Mooney D, Vandenburgh H. Design and fabrication of a biodegradable, covalently crosslinked shape-memory alginate scaffold for cell and growth factor delivery. Tissue Eng Part A. 2012;18:2000–7. doi: 10.1089/ten.tea.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Boontheekul T, Hill EE, Kong HJ, Mooney DJ. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue engineering. 2007;13:1431–42. doi: 10.1089/ten.2006.0356. [DOI] [PubMed] [Google Scholar]
- 196.Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H. Minimally Invasive Approach to the Repair of Injured Skeletal Muscle With a Shape-memory Scaffold. Mol Ther. 2014;22:1441–9. doi: 10.1038/mt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shvartsman D, Storrie-White H, Lee K, Kearney C, Brudno Y, Ho N, et al. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol Ther. 2014;22:1243–53. doi: 10.1038/mt.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Smith MK, Peters MC, Richardson TP, Garbern JC, Mooney DJ. Locally enhanced angiogenesis promotes transplanted cell survival. Tissue engineering. 2004;10:63–71. doi: 10.1089/107632704322791709. [DOI] [PubMed] [Google Scholar]
- 199.Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32:8905–14. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chevallay B, Herbage D. Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. 2000 Mar;38:211–8. doi: 10.1007/BF02344779. [DOI] [PubMed] [Google Scholar]
- 201.Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials. 2010;3:1863–87. [Google Scholar]
- 202.Carnio S, Serena E, Rossi CA, De Coppi P, Elvassore N, Vitiello L. Three-dimensional porous scaffold allows long-term wild-type cell delivery in dystrophic muscle. J Tissue Eng Regen Med. 2011;5:1–10. doi: 10.1002/term.282. [DOI] [PubMed] [Google Scholar]
- 203.Serena E, Flaibani M, Carnio S, Boldrin L, Vitiello L, De Coppi P, et al. Electrophysiologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurological research. 2008;30:207–14. doi: 10.1179/174313208X281109. [DOI] [PubMed] [Google Scholar]
- 204.van Wachem PB, Brouwer LA, van Luyn MJ. Absence of muscle regeneration after implantation of a collagen matrix seeded with myoblasts. Biomaterials. 1999;20:419–26. doi: 10.1016/s0142-9612(98)00185-9. [DOI] [PubMed] [Google Scholar]
- 205.Kin S, Hagiwara A, Nakase Y, Kuriu Y, Nakashima S, Yoshikawa T, et al. Regeneration of skeletal muscle using in situ tissue engineering on an acellular collagen sponge scaffold in a rabbit model. Asaio J. 2007;53:506–13. doi: 10.1097/MAT.0b013e3180d09d81. [DOI] [PubMed] [Google Scholar]
- 206.van Wachem PB, Plantinga JA, Wissink MJ, Beernink R, Poot AA, Engbers GH, et al. In vivo biocompatibility of carbodiimide-crosslinked collagen matrices: Effects of crosslink density, heparin immobilization, and bFGF loading. J Biomed Mater Res. 2001;55:368–78. doi: 10.1002/1097-4636(20010605)55:3<368::aid-jbm1025>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 207.Makridakis JL, Pins GD, Dominko T, Page RL. Design of a novel engineered muscle construct using muscle derived fibroblastic cells seeded onto braided collagen threads; Bioengineering Conference, 2009 IEEE 35th Annual Northeast; 2009. pp. 1–2. [Google Scholar]
- 208.Frey SP, Jansen H, Raschke MJ, Meffert RH, Ochman S. VEGF improves skeletal muscle regeneration after acute trauma and reconstruction of the limb in a rabbit model. Clinical orthopaedics and related research. 2012;470:3607–14. doi: 10.1007/s11999-012-2456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ju YM, Atala A, Yoo JJ, Lee SJ. In situ regeneration of skeletal muscle tissue through host cell recruitment. Acta biomaterialia. 2014;10:4332–9. doi: 10.1016/j.actbio.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 210.Clark RA. Fibrin and wound healing. Ann N Y Acad Sci. 2001;936:355–67. doi: 10.1111/j.1749-6632.2001.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 211.Chiron S, Tomczak C, Duperray A, Laine J, Bonne G, Eder A, et al. Complex Interactions between Human Myoblasts and the Surrounding 3D Fibrin-Based Matrix. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0036173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bian W, Bursac N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials. 2009;30:1401–12. doi: 10.1016/j.biomaterials.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Martin NR, Passey SL, Player DJ, Khodabukus A, Ferguson RA, Sharples AP, et al. Factors affecting the structure and maturation of human tissue engineered skeletal muscle. Biomaterials. 2013;34:5759–65. doi: 10.1016/j.biomaterials.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 214.Beier JP, Kneser U, Stern-Strater J, Stark GB, Bach AD. Y chromosome detection of three-dimensional tissue-engineered skeletal muscle constructs in a syngeneic rat animal model. Cell transplantation. 2004;13:45–53. doi: 10.3727/000000004772664888. [DOI] [PubMed] [Google Scholar]
- 215.Gerard C, Forest MA, Beauregard G, Skuk D, Tremblay JP. Fibrin gel improves the survival of transplanted myoblasts. Cell transplantation. 2012;21:127–37. doi: 10.3727/096368911X576018. [DOI] [PubMed] [Google Scholar]
- 216.Hammers DW, Sarathy A, Pham CB, Drinnan CT, Farrar RP, Suggs LJ. Controlled release of IGF-I from a biodegradable matrix improves functional recovery of skeletal muscle from ischemia/reperfusion. Biotechnol Bioeng. 2012;109:1051–9. doi: 10.1002/bit.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cornwell KG, Pins GD. Discrete crosslinked fibrin microthread scaffolds for tissue regeneration. J Biomed Mater Res A. 2007;82:104–12. doi: 10.1002/jbm.a.31057. [DOI] [PubMed] [Google Scholar]
- 218.Cornwell KG, Pins GD. Enhanced proliferation and migration of fibroblasts on the surface of fibroblast growth factor-2-loaded fibrin microthreads. Tissue Eng Part A. 2010;16:3669–77. doi: 10.1089/ten.TEA.2009.0600. [DOI] [PubMed] [Google Scholar]
- 219.Grasman JM, Pumphrey LM, Dunphy M, Perez-Rogers J, Pins GD. Static axial stretching enhances the mechanical properties and cellular responses of fibrin microthreads. Acta biomaterialia. 2014;10:4367–76. doi: 10.1016/j.actbio.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.McNally AK, Anderson JM. Phenotypic expression in human monocyte-derived interleukin-4-induced foreign body giant cells and macrophages in vitro: dependence on material surface properties. J Biomed Mater Res A. 2015;103:1380–90. doi: 10.1002/jbm.a.35280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Matsumoto T, Sasaki J, Alsberg E, Egusa H, Yatani H, Sohmura T. Three-dimensional cell and tissue patterning in a strained fibrin gel system. PLoS ONE. 2007;2:e1211. doi: 10.1371/journal.pone.0001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stratos I, Graff J, Rotter R, Mittlmeier T, Vollmar B. Open blunt crush injury of different severity determines nature and extent of local tissue regeneration and repair. J Orthop Res. 2010;28:950–7. doi: 10.1002/jor.21063. [DOI] [PubMed] [Google Scholar]
- 223.Wu X, Corona BT, Chen X, Walters TJ. A Standardized Rat Model of Volumetric Muscle Loss Injury for the Development of Tissue Engineering Therapies. BioResearch Open Access. 2012;1:280–90. doi: 10.1089/biores.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. Journal of molecular and cellular cardiology. 2011;50:280–9. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


