Abstract
A novel avian-origin influenza A H7N9 virus emerged in China in 2013 and continues to cause sporadic human infections with mortality rates approaching 35%. Currently there are no approved human vaccines for H7N9 virus. Recombinant approaches including hemagglutinin (HA) and virus-like particles (VLPs) have resulted in experimental vaccines with advantageous safety and manufacturing characteristics. While high immunogenicity of VLP vaccines has been attributed to the native conformation of HA arranged in the regular repeated patterns within virus-like structures, there is limited data regarding molecular organization of HA within recombinant HA vaccine preparations. In this study, the full-length recombinant H7 protein (rH7) of A/Anhui/1/2013 (H7N9) virus was expressed in Sf9 cells. We showed that purified full-length rH7 retained functional ability to agglutinate red blood cells and formed oligomeric pleomorphic subviral particles (SVPs) of ~20 nm in diameter composed of approximately 10 HA0 molecules. No significant quantities of free monomeric HA0 were observed in rH7 preparation by size exclusion chromatography. Immunogenicity and protective efficacy of rH7 SVPs was confirmed in the mouse and ferret challenge models suggesting that SVPs can be used for vaccination against H7N9 virus.
Keywords: Influenza, H7N9, Influenza vaccine, Virus-like particles, VLP
1. Introduction
Cases of human infection caused by an avian-origin influenza A H7N9 virus emerged in eastern China in 2013 and have continued into 2015 [1,2]. As of February 23, 2015, the World Health Organization (WHO) had reported 571 infections and 212 deaths, mostly in East China [3]. Cases of H7N9 infection in travelers have also been reported [4,5]. Because humans are immunologically naïve to this subtype, the emergence of a transmissible H7N9 virus presents a significant public health concern. There are currently no approved human vaccines for H7N9 viruses. Experimental vaccines have been previously developed for H7 subtype viruses including live attenuated H7N7 and H7N3 vaccines [6–8], inactivated H7N7 vaccine [9], cell-based H7N1 split virus vaccine [10], and viral vector vaccines [11,12].
Recombinant approaches have resulted in novel influenza vaccines with advantages in safety and manufacturing, and they have been shown to be efficacious against influenza [13–20]. For example, H7N9 recombinant virus-like particle (VLP) vaccine induced protective immunity against H7N9 virus in mice and ferrets [21,22]. Recombinant hemagglutinin (HA) H7 vaccine has also been prepared [23]. HA, the major protein of influenza virus envelope, contains virus-neutralizing epitopes and is included in all currently approved human influenza vaccines, as well as in the majority of experimental vaccines. The observed high immunogenicity of influenza VLP vaccines has been attributed to the organization of HA into regular, highly repeated patterns in the virus resembling structures that favor activation of immune effector functions [14,24]. However, recombinant full-length HA alone is also capable of protecting against infection with influenza viruses [25,26]. Recombinant subunit HA FluBlok vaccine has been approved for human vaccination [27]. Studies have shown that immune response can depend on the molecular structure of recombinant HA protein [28]. A previous study has shown that purified rH3 HA forms rosette-shaped subviral particles (SVPs) of approximately 30–50 nm in diameter [29]. However, it is not clear if such SVPs are also formed by rH7 HA and if rH7 HA SVPs are capable of inducing protective immunity in animal models.
In this study, the influenza H7N9 virus H7 gene was cloned into a baculovirus expression vector and expressed in Spodoptera frugiperda (Sf9) insect cells. We demonstrate that rH7 protein from Sf9 cells forms high-molecular weight SVPs that elicit protective immunity in mouse and ferret challenge models. These results have implications for understanding immunogenicity of rH7 HA protein and vaccination against H7N9 influenza virus.
2. Materials and methods
2.1. Expression of H7 gene and preparation of rH7 protein
The sequence of influenza H7 HA gene of A/Anhui/1/2013 (H7N9) virus was obtained from the GISAID EpiFlu database (www.gisaid.org), accession number EPI439507. The HA gene was codon-optimized for high-level expression in Sf9 insect cells (Life Technologies, Carlsbad, CA) and synthesized biochemically (Gen-script, Piscataway, NJ). For expression, full-length H7 HA gene was cloned into baculovirus (BV) pFastBac1 transfer vector between BamHI–HindIII sites downstream from a polyhedrin promoter, as described elsewhere [17,21]. Recombinant BV expressing H7 HA gene was generated by using a Bac-to-Bac baculovirus expression system (Life Technologies). The presence of BV during virus stock preparation was monitored by Virus Counter (ViroCyt, Boulder, CO). For infection of Sf9 cells, the titer was determined by plaque assay in Sf9 cells and expressed as plaque forming units (PFU)/ml. Recombinant baculovirus stocks were prepared by infecting cells at a low multiplicity of infection (MOI) of 0.01 PFU per cell and harvested at 68–72 h post infection.
For expression of rH7 HA, Sf9 cells were maintained as suspension cultures in SF-900 II insect serum free medium (Life Technologies) at 27 ± 2 °C. Sf9 cells (2 × 106 cells/ml) were infected at a MOI of 3 for 70–72 h with BV expressing H7 gene. Briefly, infected Sf9 cells were incubated with continuous agitation at 27 ± 2 °C and harvested by centrifugation at 4000 × g for 15 min. To confirm expression of rH7, a 0.3 ml aliquot of infected Sf9 cells was seeded into eight-well Nunc chamber slide for immunofluorescence assay (IFA). Following 72 h incubation at 27 °C, Sf9 cells were fixed with cold acetone, and IFA was carried out as described elsewhere [30] using H7-specific chicken antiserum. Antigen-expressing cells were visualized using FITC-conjugated goat IgG (H+L) (KPL, Gaithersburg, MD).
The rH7 protein was prepared from the 2 l Sf9 culture. Sf9 cells were harvested and solubilized using treatment with Triton X-100 or Tergitol-NP9 non-ionic surfactant for 1 h at 20 °C, essentially as described elsewhere [29,31]. After solubilization, the cell lysate was clarified by centrifugation and the rH7 protein was purified from the lysate by lectin affinity chromatography. The purified rH7 protein was dialyzed against buffer containing 20 mM Tris–HCl pH 7.4, 150 mM NaCl and 0.01% Polysorbate 80 (PS80) non-ionic surfactant. This procedure resulted in a highly purified rH7 protein preparation. Finally, purified rH7 protein was filter-sterilized using a 0.2 μm filter and stored at −80 °C.
2.2. Characterization of rH7 protein
Protein expression was examined by SDS-PAGE using 4–12% polyacrylamide gels followed by staining with GelCode Blue stain (Pierce, Rockford, IL). The content of rH7 protein was quantified by Qubit 2.0 fluorometer (Life Technologies). Western blot was performed using chicken antibody specific to avian H7N3 virus (a gift from Darrell Kapczynski). Cross-linking studies were done using treatment of rH7 protein with 0.025% glutaraldehyde for 5 min at 37 °C. Before cross-linking reaction, rH7 protein sample was diluted to reduce the effects of free amine in the reaction. Furthermore, due to the dilution, cross-linking is expected to occur within the trimers, rather than between different trimers. The cross-linking reaction was stopped by adding 0.1 volume of 1 M Tris–HCl pH 8.0, and the rH7 preparation was as examined by SDS-PAGE and western blot.
The hemagglutination titer was determined by serially diluting rH7 protein at twofold increments in 50 μl in phosphate buffered saline (PBS) in a 96-well plate. To each dilution, 50 μl of 1% turkey red blood cell (RBC, Lampire, Pipersville, PA) working solution was added, mixtures of rH7 and RBCs were gently agitated and the plate was incubated at 25 °C for 30–60 min before visual examination as recommended by the WHO [32]. Negative hemagglutination results appeared as dots in the center of the wells and activity was expressed in hemagglutination units (HAU). The titer was determined as the highest dilution factor that produced a positive reading.
Purified rH7 preparations were also evaluated by transmission electron microscopy (TEM) to demonstrate presence of SVPs. Purified rH7 was adsorbed at concentrations 50–200 μg/ml onto freshly discharged 400 mesh carbon parlodion-coated copper grids (Poly-Sciences, Warrington, PA). The grids were rinsed with buffer containing 20 mM Tris–HCl, pH 7.4, and 120 mM KCl, negatively stained with 1% phosphotungstic acid, and dried by aspiration. SVPs were visualized on a Hitachi H-7600 transmission electron microscope (Hitachi High Technologies America, Schaumburg, IL) operating at 80 kV and digitally captured with a CCD camera at 1k × 1k resolution (Advanced Microscopy Techniques Corp., Danvers, MA).
2.3. Analytical chromatography
A size exclusion chromatography (SEC) column XK16/40 containing S300 resin (GE Healthcare Bio-Sciences, Pittsburgh, PA) was calibrated essentially as described previously [33] using molecular weight standards (Bio-Rad Laboratories, Hercules, CA), which included thyroglobulin (670 kDa), γ-globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa) and vitamin B12 (1.35 kDa). Then, rH7 preparation was subjected to chromatography. Because of the presence of PS80 surfactant in the rH7 preparation, optical density readings were unreliable, and the chromatography profile was determined by western blot. Fractions (2 ml each) from the S300 column were collected and chromatography peaks were determined by SDS-PAGE and western blot for the presence of rH7 antigen.
2.4. Vaccinations and challenge of mice
All animal experiments were performed under the guidance of the Institutional Animal Care and Use Committees and were conducted in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facilities. For vaccination studies, 8–9 week old female BALB/c mice (Jackson Laboratories, Bar Harbor, ME) (five per group) received two vaccinations 3 weeks apart with 5 μg of rH7 SVP in PBS in 50 μl volume intranasally (i.n) with, or without, 5 μg of Monophosphoryl Lipid A (MPL) adjuvant (Invivogen, San Diego, CA). Three weeks following boost, mice were challenged i.n. with 10 mouse 50% lethal dose (10 × MLD50) of A/Anhui/1/2013 (H7N9) virus and morbidity (weight loss) and mortality were determined. Survival and body weight were monitored for 14 days and mice were humanely euthanized if more than 25% of initial body weight was lost. Before challenge, serum collected from blood samples was taken and immune response was determined using hemagglutination inhibition (HI) assay as described elsewhere [17,34].
2.5. Vaccinations and challenge of ferrets
Adult male Fitch ferrets, 4–5 months of age (Triple F Farms, Sayre, PA), serologically negative by HI assay for currently circulating influenza viruses, were vaccinated intramuscularly (i.m.) on days 0, 28, and 56 using 15 μg of rH7 SVP vaccine without adjuvant in 0.5 ml volume per dose. Controls similarly received PBS. The dose and the route were chosen to mimic human inactivated vaccines. Serum collected from blood samples were tested by HI assay for the presence of antibodies against H7N9 virus. Sera from day 56 (post second boost) were also analyzed by HI assay for the presence of cross-reactive antibodies against H5 and H9 subtypes with A/Vietnam/1203/2004 (H5N1) virus, and H9 SVPs derived from A/Hong Kong/33982/2009 (H9N2), respectively. For HI assay, H9 SVPs, prepared as described above, served as virus antigen. Serum taken before vaccinations serves as a negative control for the HI assays.
Four ferrets per group were challenged on day 70 with 106 PFU of Anhui/1/2013 (H7N9) virus, which is non-lethal in this species. Animals were euthanized on day 5 post-challenge for collection of nasal washes, nasal turbinates, and lung tissues as previously described [35]. All samples were immediately frozen at −80 °C until use. Tissues were homogenized in 1 ml cold PBS and titrated by standard plaque assay in MDCK cells as previously described [36]. The limit of virus detection was 10 PFU.
The statistical significance of differences in the virus titers between vaccinated and PBS-control animals was determined by Mann–Whitney nonparametric t test.
3. Results
3.1. Expression and characterization of recombinant rH7 HA protein
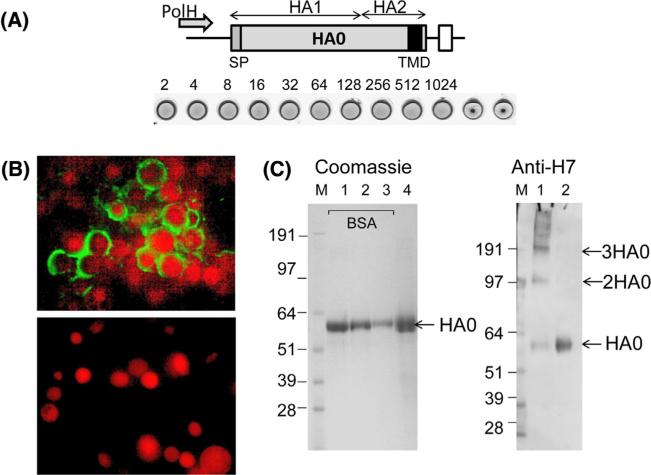
Influenza HA gene derived from A/Anhui/1/2013 (H7N9) was expressed in Sf9 cells from the polyhedrin promoter using a recombinant baculovirus (rBV) expression system. The gene encoded the full-length HA0 protein including HA1 with signal peptide (SP), and HA2 with transmembrane domain (TMD) and carboxy terminal domain (Fig. 1A). The expression of rH7 was initially monitored by IFA. As expected, baculovirus-infected Sf9 cells had larger nuclei as compared to uninfected control Sf9 cells, with expression of rH7 detected in the cell cytoplasm and cell membranes, with no detectable nuclear staining (Fig. 1B). At 72 h after infection with recombinant baculovirus vector Sf9 cells were harvested. For rH7 HA purification, Sf9 cells were lysed using non-ionic surfactant [31] and rH7 was purified using affinity chromatography. The rH7 HA protein was detected by hemagglutination assay (Fig. 1A), SDS-PAGE and western blot (Fig. 1C). The rH7 exhibited hemagglutination activity (HAU = 1024 per 50 μl of 0.4 mg/ml total rH7 protein), as determined with turkey RBCs, thus confirming that the HA protein retained its function of RBC binding ability (Fig. 1A). As expected for Sf9 cells, the majority of expressed rH7 HA represented uncleaved HA0 polypeptide of 62–64 kilodaltons (kDa). No significant proteolytic processing of HA0 into HA1 and HA2 was detected by SDS-PAGE. By western blot, the HA0 protein was recognized by H7-specific antiserum. Next, partial cross-linking experiment of rH7 using glutaraldehyde was carried out, followed by the SDS sample buffer treatment and western blot. In addition to the HA0 band of 62–64 kDa, a ~120 kDa minor band was detected, which corresponded to the expected size of HA0 dimer, as well as the major band of approximately 200 kDa, which corresponded to the expected size of HA0 trimer (Fig. 1C). Furthermore, bands of >200 kDa were also detected by western blot using H7-specific antiserum. The prominent band corresponding to the rH7 trimer, as well as the presence of bands corresponding to the dimeric HA0 and higher molecular weight complexes suggested that at least part of the purified full-length rH7 protein forms oligomeric structures.
Fig. 1.
Expression and characterization of rH7 SVP. (A) Expression cassette for rH7 gene. Indicated are locations of baculovirus polyhedrin promoter (PolH), locations of HA1 and HA2 within rH7 HA0 protein, RNA transcription termination signal (open box), signal peptide (SP) and transmembrane domain (TMD) within rH7 (filled box). Hemagglutination assay of purified rH7 protein using 1% turkey red blood cells is also shown. Dilutions are indicated. (B) Immunofluorescence assay (IFA) of Sf9 cells infected with rH7-expressing recombinant baculovirus (upper panel), and of uninfected control Sf9 cells (bottom panel). Cell nuclei were counterstained using propidium iodide. (C) Left panel: SDS-PAGE stained with GelCode Blue stain; lanes 1, 2, 3, and 4 show 1.5, 1.0 and 0.5 μg BSA, as well as 3.0 μg rH7, respectively. Right panel: western blot using H7 antiserum; lane 1 shows rH7 after cross-linking with glutaraldehyde; lane 2 shows purified rH7. M, SeeBlue Plus2 marker (Life Technologies). Western blot was done using chicken H7N3 virus-specific antibody at 1:100 dilution.
3.2. Analysis of rH7 SVP by chromatography and electron microscopy
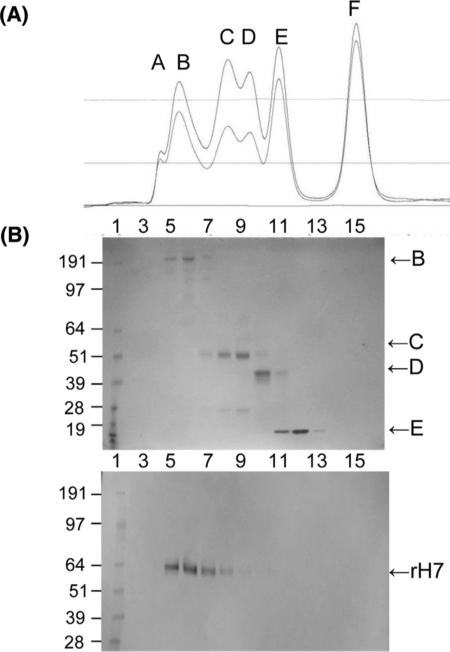
We further evaluated rH7 by the analytical size-exclusion chromatography (SEC) (Fig. 2). The SEC is designed to separate proteins according to their size, although other factors such as shape of a macromolecule can affect separation. The S300 SEC column was initially calibrated using chromatography standards including thyroglobulin (670 kDa), γ-globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa) and vitamin B12 (1.35 kDa) (Fig. 2A). Then, the chromatography profile of the purified rH7 was examined. The majority of rH7 migrated in the same retention volume as the thyroglobulin (670 kDa) (Fig. 2B). Assuming that rH7 migrated on S300 as a standard spherical macromolecule, this result indicated that the molecular weight of the rH7 was equivalent to approximately 10–11 monomeric HA0 (62 kDa) or 3–4 of trimeric HA0 (186 kDa). The rH7 protein was also detectable in the peak fraction of 158 kDa γ-globulin, which indicated presence of trimeric HA0. No significant quantities of monomeric HA0 were detected by SEC under these conditions (Fig. 2). Thus, analytical chromatography strongly suggested that the majority of rH7 represents high molecular weight oligomeric SVPs.
Fig. 2.
Characterization of rH7 by size exclusion chromatography (SEC). (A) Size exclusion chromatography (SEC) of standards using Sephadex S300. SEC standards included thyroglobulin (B, 670 kDa), γ-globulin (C, 158 kDa), ovalbumin (D, 44 kDa), myoglobin (E, 17 kDa) and vitamin B12 (F, 1.35 kDa). Absorbance at 280 nm (upper curve) and 260 nm (lower curve) are shown. Peak A indicates protein aggregates (void peak), according to the Gel Filtration Standard kit (Bio-Rad). (B) SDS-PAGE of standards from SEC (upper panel) and western blot of rH7 from SEC (lower panel).
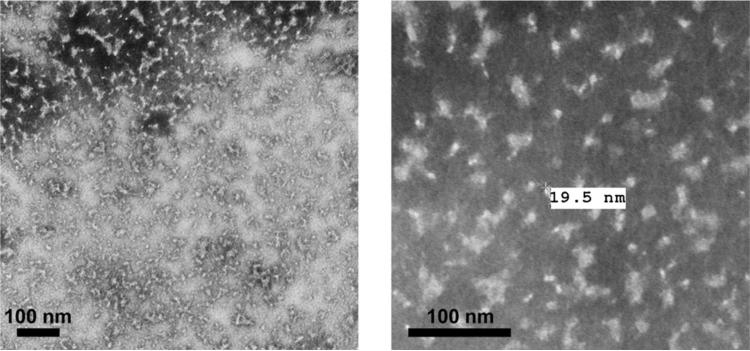
Finally, the sizes and morphology of rH7 SVP were examined by negative-staining transmission electron microscopy (TEM) (Fig. 3). The rH7 SVP were identified as pleomorphic spheroid nanoparticles with average diameter of approximately 20 nm, which is consistent with large (>670 kDa) molecular weight as determined by the SEC. Pleomorphic shapes likely reflect irregular structures of the rHA oligomers. The size and morphology of rH7 SVP differed from the 30–50 nm regular rosette-shaped previously reported for rH3 protein [29]. In summary, these results suggested that purified rH7 protein from Sf9 cells is arranged into high-molecular weight SVP particles.
Fig. 3.
Characterization of rH7 by transmission electron microscopy (TEM). TEM of rH7 preparation was negatively stained with 1% phosphotungstic acid and visualized on a Hitachi H-7600 transmission electron microscope. Bar, 100 nm.
3.3. Immunogenicity and protective efficacy of rH7 SVPs in mice
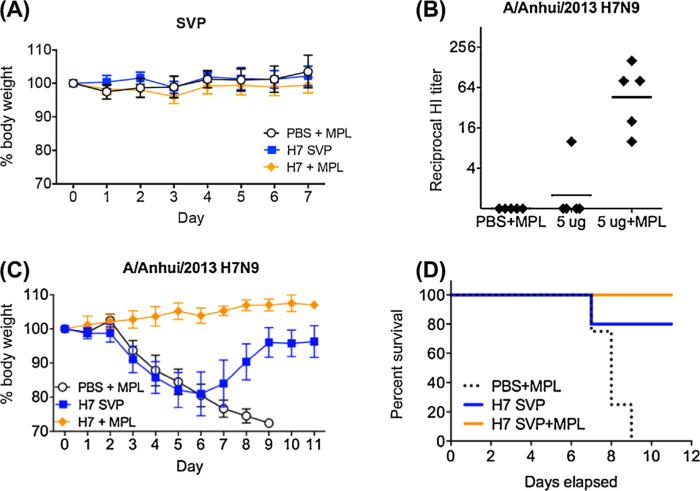
Mice received two intranasal (i.n.) vaccinations of rH7 SVPs in the presence or absence of MPL adjuvant three weeks apart, while control animals received PBS with MPL in place of vaccine. Following immunizations, no adverse effects were observed in any of the mice suggesting that H7 SVP vaccine was well tolerated by the i.n. route (Fig. 4A). The HI antibody responses to H7N9 virus were measured among individual serum samples collected prior to virus challenge at 6 weeks following initial vaccination. As expected, no HI antibody to A/Anhui/1/2013 (H7N9) virus was detected in the serum of control PBS/MPL animals. A 5 μg H7 SVP vaccine dose elicited a detectable HI antibody response in one out of five mice in the vaccinated group (Fig. 4B). The addition of MPL to the SVP vaccine substantially increased the response and antibody titers to H7N9 virus, as the pre-challenge HI titers were highest among the SVP/MPL-vaccinated mice compared to mice in the group without adjuvant. These data indicate that the SVP vaccine when given mucosally elicited systemic HI antibodies to the H7N9 virus. The addition of MPL adjuvant augmented the antibody response to vaccination.
Fig. 4.
Immunogenicity and efficacy of rH7 SVP in mice. BALB/c mice received two intranasal (i.n.) vaccinations of 5 μg rH7 SVPs in the presence or absence of MPL adjuvant three weeks apart. The control animals received PBS with MPL in place of vaccine. Mice were challenged with 10 MLD50 of A/Anhui/1/2013 (H7N9) virus, and morbidity (reduction in weight loss) and mortality (survival) were determined. (A) Weights of mice after vaccination. (B) Hemagglutination inhibition (HI) titers in sera of mice before challenge. (C) Weight loss in all mice after challenge with 10 LD50 of A/Anhui/1/2013 (H7N9) virus. (D) Survival of mice after challenge with A/Anhui/1/2013 (H7N9) virus.
We next determined the level of protection against homologous virus challenge measured by morbidity (reduction in weight loss) and mortality (survival) following challenge with 10 × MLD50 of A/Anhui/1/2013 (H7N9) virus. Mock-immunized control mice that were challenged with the virus displayed considerable weight loss (Fig. 4C) and all control mice died by day 9 post challenge (Fig. 4D). Mice that were vaccinated with H7 SVP without adjuvant also lost weight, but the majority of mice recovered resulting in 80% survival following challenge. Interestingly enough, mice in this group showed low systemic HI response (Fig. 4B) suggesting that other factors may have also contributed to protection, such as mucosal response following vaccine administration. Finally, mice vaccinated with SVP in the presence of MPL adjuvant did not lose any weight, and all survived H7N9 virus challenge (Fig. 4D). Taken together, these data demonstrate that the rH7 SVPs, especially when adjuvanted, provide protection against morbidity and mortality following H7N9 virus challenge.
3.4. Immunogenicity and protective efficacy of rH7 SVPs in ferrets
Ferrets currently represent a well-accepted small animal model for influenza [15]. Ferrets were vaccinated i.m. on days 0, 28, and 56 using 15 μg of rH7 SVP vaccine without adjuvant. Presence of antibodies to A/Anhui/1/2013 (H7N9) virus was determined by HI assay. Additionally, we evaluated cross-reactivity to A/Vietnam/1203/2004 (H5N1) and A/Hong Kong/33982/2009 (H9N2) antigens. HI data among individual animals are shown in Table 1. As expected, none of the mock-vaccinated PBS control animals developed serum HI antibody to influenza virus. After first vaccination, only one out of four animals showed detectable HI titer against H7N9 virus. After second vaccination (boost), two out of four ferrets had HI titers of 80. Finally, after second boost, all animals seroconverted with HI titers ranging from 40 to 160 (Table 1). Interestingly, these sera showed a degree of cross-reactivity with H9, but not H5 antigen (Table 1).
Table 1.
HI results in individual ferrets vaccinated with rH7 SVP or mock-vaccinated (PBS) controls.
| Pre Boost | Post Boost | Post Second Boost |
|||
|---|---|---|---|---|---|
| HI titer against A/Anhui/H7N9* | HI titer against Anhui/H7N9 | HI titer against Anhui/H7N9 | HI titer against H9 SVP*** | HI titer against VN/H5N1** | |
| H7 SVP | |||||
| 8166 | <10 | <10 | 40 | 10 | <10 |
| 8167 | <10 | 80 | 80 | <10 | <10 |
| 8168 | <10 | <10 | 160 | 10 | <10 |
| 8169 | 40 | 80 | 80 | 10 | <10 |
| PBS | |||||
| 8170 | <10 | <10 | <10 | <10 | <10 |
| 8171 | <10 | <10 | <10 | <10 | <10 |
| 8173 | <10 | <10 | <10 | <10 | <10 |
| 8174 | <10 | <10 | <10 | <10 | <10 |
A/Anhui/1/2013 (H7N9) virus;
H9 SVP derived from A/HongKong/33982/2009 (H9N2) virus;
A/VietNam/1203/2004 (H5N1) virus. Controls included sera from mock-vaccinated (PBS) animals, as well as pre-vaccination sera.
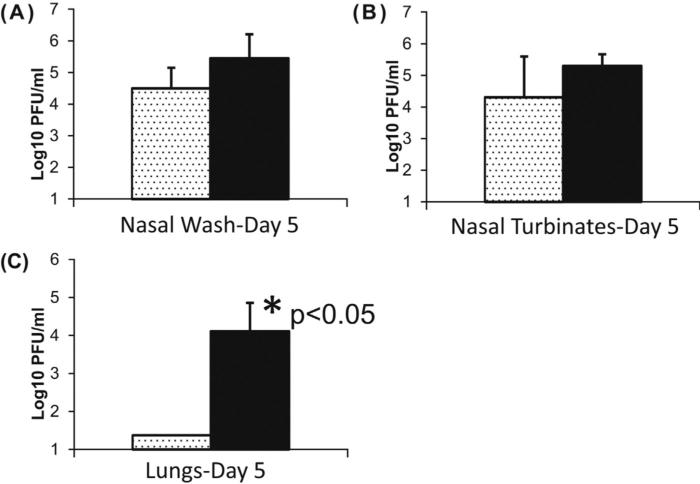
Ferrets were challenged i.n. with a stringent (106 PFU) dose of A/Anhui/1/2013 (H7N9) virus. The virus does not cause lethal disease in ferrets [37]; therefore, we measured the titer of replicating H7N9 virus in the nasal washes, nasal turbinates and lung tissues after challenge. On day 5 post challenge, the H7N9 virus titer in the nasal wash and nasal turbinates appeared to be lower as compared to the control unvaccinated animals but the difference was not statistically significant (Fig. 5A and B). However, the infectious virus titer in the lungs of animals that received rH7 SVP vaccine was reduced to undetectable levels on day 5 post challenge (Fig. 5C). In the Mann–Whitney nonparametric test, p value was 0.0159 and the difference between those two groups was statistically significant (p < 0.05).
Fig. 5.
Efficacy of rH7 SVP in ferrets on day 5 after challenge with A/Anhui/1/2013 (H7N9) virus. Ferrets were vaccinated i.m. on days 0, 28, and 56 using 45 μg of rH7 SVP vaccine without adjuvant. Animals were challenged on day 70 with 106 PFU of Anhui/1/2013 (H7N9) virus. Since this virus does not cause lethal disease in ferrets, animals were euthanized on day 5 post-challenge for detection of replicating virus in nasal washes, nasal turbinates and lung tissues. (A) Titer of replicating H7N9 virus in nasal washes. (B) Titer of replicating H7N9 virus in nasal turbinates. (C) Titer of replicating H7N9 virus in lungs. Asterisk indicates statistical significance. The statistical significance of differences in the virus titers between vaccinated and control animals was determined by the Mann–Whitney nonparametric test (p < 0.05).
4. Discussion
Recombinant technologies resulted in novel influenza vaccines that have safety and manufacturing advantages over traditional live attenuated and inactivated vaccines. Recombinant influenza vaccines are produced by using cell culture methods and do not require egg-based technology for the production. Two types of recombinant vaccines have been made in Sf9 insect cells using baculovirus expression system. The first type is recombinant HA vaccine FluBlok that was recently been approved by the Food and Drug (FDA) administration for human use [27]. Another type of recombinant influenza vaccine is recombinant VLP comprised of HA and additional influenza proteins such as NA and/or M1 [14,15,17,34,38]. VLPs have been shown to elicit highly efficient protective immune responses, which in some cases exceeded immune responses elicited by recombinant HA [16,39]. In influenza VLPs, HA and other proteins are arranged into ~120 nm pleomorphic particles that morphologically resemble influenza virions, which is viewed as advantageous for immunogenicity and efficacy of VLP vaccines [14,24,40]. However, rHA is also immunogenic and protective in animal models and in humans [20,27,41]. Currently, limited studies are available regarding molecular organization of the of rHA vaccine. Previously, it has been shown that rH3 was forming rosette-shaped regular structures of 30–50 nm in diameter [29]. In the current study, we have shown that rH7 isolated from Sf9 cells represents high-molecular-weight SVP nanoparticles of approximately 20 nm likely comprised 3–4 HA trimers. These SVP nanoparticles are composed of the full-length recombinant HA protein, similarly to the FluBlok vaccine. Recently, composition of the recombinant rHA FluBlok vaccine has been studied by the high-resolution TEM, which suggested that 5–7 HA molecules are needed to form an oligomeric complex [42]. Hypothetically, the difference in the size and morphology of rH7 can be explained by subtype-specific variations in the HA or differences in the protein purification and formulation. Nevertheless, data suggest that the majority of rH7 forms oligomeric SVPs, which may be important for immunogenicity. Previously, comparative efficacy of neutralizing antibodies elicited by recombinant HA proteins from avian H5N1 influenza virus has been studied [28]. A high-molecular-weight HA oligomer elicited the strongest antibody response, followed by the HA trimer, while the monomer showed minimal efficacy [28]. Interestingly enough, immunogenicity of a plant-made monomeric HA could be improved by chemical conjugation to the surface of the Tobacco mosaic virus (TMV) that is not infectious in mammals [43]. In another study, recombinant H7 that contained a T4 trimerization domain to ensure trimer formation has induced protection in mice [20]. Thus, formation of oligomeric structures appears important for rH7 immunogenicity. It is likely that formation of rH7 SVP in our study mimics to some extent the conformational epitopes and high immunogenicity of influenza virions and VLPs. Notably, the size of rH7 SVP observed in the current study resembles the 22 nm particles of hepatitis B virus (HBV) surface antigen (HBsAg), an effective recombinant vaccine for HBV [24,44]. Potentially, efficacy of rH7 can be further improved by mutagenesis of the four cysteine residues in the transmembrane and carboxy terminal domains that have been shown to be associated with the recombinant HA vaccine potency loss [29].
The mechanism of rHA SVP formation remains to be elucidated. The full-length HA polypeptide includes the carboxy terminal TMD, that inserts itself into lipid bilayers. In the virus, HA molecules are arranged into the homotrimers, which are embedded into the outer envelope of the virus via TM region and are easily identifiable by TEM as characteristic spikes on the virion. HA assembles into a homotrimer in the endoplasmic reticulum and is transported by the secretory pathway to the plasma membrane, where virus assembly and budding take place [45,46]. In the plasma membrane, HA is localized in detergent-resistant rafts that in some conditions resist solubilization by nonionic detergents such as Triton X-100 [46]. Based on the previous observations and the current study, our working hypothesis is that SVPs are comprised of the HA trimers that are associated into SVPs via TMD regions. The observed ~20 nm size of SVP is consistent with the previous observation of influenza glycoprotein ectodomains protruding ~13 nm from the lipid bilayer surface [47]. Additional studies are needed to determine the composition of SVPs and to elucidate the process of their formation.
The protective potential of H7 SVP was initially confirmed in mice. Mucosal vaccination with H7 SVP was well tolerated and protected mice against challenge with homologous H7N9 virus. The presence of MPL adjuvant has improved immunogenicity and protection. The follow-up studies are needed to elucidate the role of mucosal immunity, such as IgA in protection. Additionally, we used ferrets because they are considered to be the most suitable animal model for influenza vaccine efficacy studies [15,48–50]. Using this animal model, experimental influenza VLP vaccines have previously shown protective efficacy against potentially pandemic avian influenza H5N1 and H9N2 subtype viruses [17,51]. In this study, we addressed the effect of H7 SVP vaccination on viral replication in the ferret respiratory tract. H7N9 virus can efficiently replicate in the lung and potentially decrease lung function [37], a feature found in the highly pathogenic avian H5N1 viruses [52]. We found that H7 SVP vaccine provided significant protection in the ferret lung. Taken together, data suggest that the formation of SVP nanoparticles may represent the important feature that contributes to the immunogenicity of rHA vaccines and that rH7 SVP can be employed as a vaccine against H7N9 influenza virus.
Highlights.
Recombinant full-length H7 hemagglutinin (rH7) was expressed in Sf9 cells.
Purified rH7 forms subviral particles (SVP) of approximately 20 nm in diameter.
SVP structures are composed of approximately 3–4 trimers of rH7.
Purified rH7 SVP elicit protective immune response in mice and ferrets.
Acknowledgments
We thank Darrell Kapczynski (SEPRL, USDA) for H7-specific antiserum. We also thank Noah Horn, Raphael Prather, Brian Nickols, Xia Lei, and Xiaohui Li for valuable contributions and discussions. LMP current address is The American Association of Immunologists, 9650 Rockville Pike, Bethesda, MD, USA. This research was supported in part by grants 1R01AI111532 (NIH NIAID) and 2013-33610-21041 (USDA NIFA) to P.P., and in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank the Comparative Medicine Branch (NIAID, NIH) for assistance with the mouse studies. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agencies.
References
- 1.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013 May;368(20):1888–97. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Li J, Sun B, Zhang H, Zhang R, Yuan J, et al. Isolation and characteristic analysis of a novel strain H7N9 of avian influenza virus A from a patient with influenza-like symptoms in China. Int J Infect Dis. 2015 Jan; doi: 10.1016/j.ijid.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 3.WHO [25.06.15];WHO risk assessment of human infection with avian influenza A(H7N9) virus. 2015 Available from: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/RiskAssessment_H7N9_23Feb20115.pdf?ua=1.
- 4.William T, Thevarajah B, Lee SF, Suleiman M, Jeffree MS, Menon J, et al. Avian influenza (H7N9) virus infection in Chinese tourist in Malaysia, 2014. Emerg Infect Dis. 2015;21(1):142–5. doi: 10.3201/eid2101.141092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin PH, Chao TL, Kuo SW, Wang JT, Hung CC, Lin HC, et al. Virological, serological, and antiviral studies in an imported human case of avian influenza A(H7N9) virus in Taiwan. Clin Infect Dis. 2014 Jan;58(2):242–6. doi: 10.1093/cid/cit638. [DOI] [PubMed] [Google Scholar]
- 6.Min JY, Vogel L, Matsuoka Y, Lu B, Swayne D, Jin H, et al. A live attenuated H7N7 candidate vaccine virus induces neutralizing antibody that confers protection from challenge in mice, ferrets, and monkeys. J Virol. 2010 Nov;84(22):11950–60. doi: 10.1128/JVI.01305-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a phase I trial in healthy adults. Vaccine. 2009 Jun;27(28):3744–53. doi: 10.1016/j.vaccine.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babu TM, Levine M, Fitzgerald T, Luke C, Sangster MY, Jin H, et al. Live attenuated H7N7 influenza vaccine primes for a vigorous antibody response to inactivated H7N7 influenza vaccine. Vaccine. 2014 Nov;32(50):6798–804. doi: 10.1016/j.vaccine.2014.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS ONE. 2012;7(12):e49704. doi: 10.1371/journal.pone.0049704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox RJ, Major D, Hauge S, Madhun AS, Brokstad KA, Kuhne M, et al. A cell-based H7N1 split influenza virion vaccine confers protection in mouse and ferret challenge models. Influenza Other Respir Viruses. 2009 May;3(3):107–17. doi: 10.1111/j.1750-2659.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goff PH, Krammer F, Hai R, Seibert CW, Margine I, Garcia-Sastre A, et al. Induction of cross-reactive antibodies to novel H7N9 influenza virus by recombinant Newcastle disease virus expressing a North American lineage H7 subtype hemagglutinin. J Virol. 2013 Jul;87(14):8235–40. doi: 10.1128/JVI.01085-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreijtz JH, Wiersma LC, De Gruyter HL, Vogelzang-van Trierum SE, van Amerongen G, Stittelaar KJ, et al. A single immunization with modified vaccinia virus ankara-based influenza virus H7 vaccine affords protection in the influenza A(H7N9) pneumonia ferret model. J Infect Dis. 2014 Sep; doi: 10.1093/infdis/jiu528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18(1):244–51. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- 14.Kang SM, Pushko P, Bright RA, Smith G, Compans RW. Influenza virus-like particles as pandemic vaccines. Curr Top Microbiol Immunol. 2009;333:269–89. doi: 10.1007/978-3-540-92165-3_14. [DOI] [PubMed] [Google Scholar]
- 15.Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, et al. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009 Jun;83(11):5726–34. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007 May;25(19):3871–8. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 17.Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005 Dec;23(50):5751–9. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 18.Quan FS, Vunnava A, Compans RW, Kang SM. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS ONE. 2010;5(2):e9161. doi: 10.1371/journal.pone.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross TM, Mahmood K, Crevar CJ, Schneider-Ohrum K, Heaton PM, Bright RA. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS ONE. 2009;4(6):e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krammer F, Albrecht RA, Tan GS, Margine I, Hai R, Schmolke M, et al. Divergent H7 immunogens offer protection from H7N9 virus challenge. J Virol. 2014 Apr;88(8):3976–85. doi: 10.1128/JVI.03095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith GE, Flyer DC, Raghunandan R, Liu Y, Wei Z, Wu Y, et al. Development of influenza H7N9 virus like particle (VLP) vaccine: homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine. 2013 Sep;31(40):4305–13. doi: 10.1016/j.vaccine.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 22.Liu YV, Massare MJ, Pearce MB, Sun X, Belser JA, Maines TR, et al. Recombinant virus-like particles elicit protective immunity against avian influenza A(H7N9) virus infection in ferrets. Vaccine. 2015 Mar; doi: 10.1016/j.vaccine.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckland B, Boulanger R, Fino M, Srivastava I, Holtz K, Khramtsov N, et al. Technology transfer and scale-up of the Flublok recombinant hemagglutinin (HA) influenza vaccine manufacturing process. Vaccine. 2014 Sep;32(42):5496–502. doi: 10.1016/j.vaccine.2014.07.074. [DOI] [PubMed] [Google Scholar]
- 24.Pushko P, Pumpens P, Grens E. Development of virus-like particle technology from small highly symmetric to large complex virus-like particle structures. Intervirology. 2013;56(3):141–65. doi: 10.1159/000346773. [DOI] [PubMed] [Google Scholar]
- 25.Treanor JJ, El Sahly H, King J, Graham I, Izikson R, Kohberger R, et al. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok(R)) against influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine. 2011 Oct;29(44):7733–9. doi: 10.1016/j.vaccine.2011.07.128. [DOI] [PubMed] [Google Scholar]
- 26.King Jr JC, Cox MM, Reisinger K, Hedrick J, Graham I, Patriarca P. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy children aged 6–59 months. Vaccine. 2009 Nov;27(47):6589–94. doi: 10.1016/j.vaccine.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Traynor K. First recombinant flu vaccine approved. Am J Health Syst Pharm. 2013 Mar;70(5):382. doi: 10.2146/news130016. [DOI] [PubMed] [Google Scholar]
- 28.Wei CJ, Xu L, Kong WP, Shi W, Canis K, Stevens J, et al. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J Virol. 2008 Jul;82(13):6200–8. doi: 10.1128/JVI.00187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtz KM, Robinson PS, Matthews EE, Hashimoto Y, McPherson CE, Khramtsov N, et al. Modifications of cysteine residues in the transmembrane and cytoplasmic domains of a recombinant hemagglutinin protein prevents cross-linked multimer formation and potency loss. BMC Biotechnol. 2014 Dec;14(1):1. doi: 10.1186/s12896-014-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pushko P, Geisbert J, Parker M, Jahrling P, Smith J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J Virol. 2001 Dec;75(23):11677–85. doi: 10.1128/JVI.75.23.11677-11685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K, Holtz KM, Anderson K, Chubet R, Mahmoud W, Cox MM. Expression and purification of an influenza hemagglutinin – one step closer to a recombinant protein-based influenza vaccine. Vaccine. 2006 Mar;24(12):2176–85. doi: 10.1016/j.vaccine.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO [17.02.15];WHO manual on animal influenza diagnosis and surveillance. 2015 Available from: http://www.who.int/csr/resources/publications/influenza/en/whocdscsrncs20025rev.pdf.
- 33.Pushko P, Kozlovskaya T, Sominskaya I, Brede A, Stankevica E, Ose V, et al. Analysis of RNA phage fr coat protein assembly by insertion, deletion and substitution mutagenesis. Protein Eng. 1993 Nov;6(8):883–91. doi: 10.1093/protein/6.8.883. [DOI] [PubMed] [Google Scholar]
- 34.Tretyakova I, Pearce MB, Florese R, Tumpey TM, Pushko P. Intranasal vaccination with H5, H7 and H9 hemagglutinins co-localized in a virus-like particle protects ferrets from multiple avian influenza viruses. Virology. 2013 Jul;442(1):67–73. doi: 10.1016/j.virol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, et al. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol. 2005 Sep;79(18):11788–800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, et al. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type I interferon response in polarized human bronchial epithelial cells. J Virol. 2007 Nov;81(22):12439–49. doi: 10.1128/JVI.01134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belser JA, Gustin KM, Pearce MB, Maines TR, Zeng H, Pappas C, et al. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature. 2013 Sep;501(7468):556–9. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe T, Watanabe S, Neumann G, Kida H, Kawaoka Y. Immunogenicity and protective efficacy of replication-incompetent influenza virus-like particles. J Virol. 2002 Jan;76(2):767–73. doi: 10.1128/JVI.76.2.767-773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pushko P, Tumpey TM, Van Hoeven N, Belser JA, Robinson R, Nathan M, et al. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine. 2007 May;25(21):4283–90. doi: 10.1016/j.vaccine.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 40.Zabel F, Mohanan D, Bessa J, Link A, Fettelschoss A, Saudan P, et al. Viral particles drive rapid differentiation of memory B cells into secondary plasma cells producing increased levels of antibodies. J Immunol. 2014 Jun;192(12):5499–508. doi: 10.4049/jimmunol.1400065. [DOI] [PubMed] [Google Scholar]
- 41.Treanor JJ, Schiff GM, Hayden FG, Brady RC, Hay CM, Meyer AL, et al. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007 Apr;297(14):1577–82. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- 42.Harris AK, Gallagher JR, McCraw DM. Characterization of influenza virus subunit vaccines by electron microscopy. ASM Biodefense Conference Abstracts. 2015:75. [Google Scholar]
- 43.Mallajosyula JK, Hiatt E, Hume S, Johnson A, Jeevan T, Chikwamba R, et al. Single-dose monomeric HA subunit vaccine generates full protection from influenza challenge. Hum Vaccin Immunother. 2014;10(3):586–95. doi: 10.4161/hv.27567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu R, Lin Q, Sun Y, Lu X, Qiu Y, Li Y, et al. Expression, purification, and characterization of hepatitis B virus surface antigens (HBsAg) in yeast Pichia pastoris. Appl Biochem Biotechnol. 2009 Aug;158(2):432–44. doi: 10.1007/s12010-009-8527-x. [DOI] [PubMed] [Google Scholar]
- 45.Whittaker GR. Intracellular trafficking of influenza virus: clinical implications for molecular medicine. Expert Rev Mol Med. 2001;2001(February):1–13. doi: 10.1017/S1462399401002447. [DOI] [PubMed] [Google Scholar]
- 46.Veit M, Thaa B. Association of influenza virus proteins with membrane rafts. Adv Virol. 2011;2011:370606. doi: 10.1155/2011/370606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaap IA, Eghiaian F, des Georges A, Veigel C. Effect of envelope proteins on the mechanical properties of influenza virus. J Biol Chem. 2012 Nov;287(49):41078–88. doi: 10.1074/jbc.M112.412726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang SS, Banner D, Fang Y, Ng DC, Kanagasabai T, Kelvin DJ, et al. Comparative analyses of pandemic H1N1 and seasonal H1N1, H3N2, and influenza B infections depict distinct clinical pictures in ferrets. PLoS ONE. 2011;6(11):e27512. doi: 10.1371/journal.pone.0027512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng X, Eisenbraun M, Xu Q, Zhou H, Kulkarni D, Subbarao K, et al. H5N1 vaccine-specific B cell responses in ferrets primed with live attenuated seasonal influenza vaccines. PLoS ONE. 2009;4(2):e4436. doi: 10.1371/journal.pone.0004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belser JA, Szretter KJ, Katz JM, Tumpey TM. Use of animal models to understand the pandemic potential of highly pathogenic avian influenza viruses. Adv Virus Res. 2009;73:55–97. doi: 10.1016/S0065-3527(09)73002-7. [DOI] [PubMed] [Google Scholar]
- 51.Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, et al. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS ONE. 2008;3(1):e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol. 2002 May;76(9):4420–9. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]