Abstract
The use of extracorporeal carbon dioxide removal (ECCO2R) is well established as a therapy for patients suffering from acute respiratory failure. Development of next generation low blood flow (< 500 mL/min) ECCO2R devices necessitates more efficient gas exchange devices. Since over 90% of blood CO2 is transported as bicarbonate (HCO3−), we previously reported development of a carbonic anhydrase (CA) immobilized bioactive hollow fiber membrane (HFM) which significantly accelerates CO2 removal from blood in model gas exchange devices by converting bicarbonate to CO2 directly at the HFM surface. This present study tested the hypothesis that dilute sulfur dioxide (SO2) in oxygen sweep gas could further increase CO2 removal by creating an acidic microenvironment within the diffusional boundary layer adjacent to the HFM surface, facilitating dehydration of bicarbonate to CO2. CA was covalently immobilized onto poly (methyl pentene) (PMP) HFMs through glutaraldehyde activated chitosan spacers, potted in model gas exchange devices (0.0151m2) and tested for CO2 removal rate with oxygen (O2) sweep gas and a 2.2% SO2 in oxygen sweep gas mixture. Using pure O2 sweep gas, CA-PMP increased CO2 removal by 31% (258 mL/min/m2) compared to PMP (197 mL/min/m2) (P < 0.05). Using 2.2% SO2 acidic sweep gas increased PMP CO2 removal by 17% (230 mL/min/m2) compared to pure oxygen sweep gas control (P < 0.05); device outlet blood pH was 7.38 units. When employing both CA-PMP and 2.2% SO2 sweep gas, CO2 removal increased by 109% (411 mL/min/m2) (P < 0.05); device outlet blood pH was 7.35 units. Dilute acidic sweep gas increases CO2 removal, and when used in combination with bioactive CA-HFMs has a synergistic effect to more than double CO2 removal while maintaining physiologic pH. Through these technologies the next generation of intravascular and paracorporeal respiratory assist devices can remove more CO2 with smaller blood contacting surface areas.
Keywords: Carbonic anhydrase, enzyme immobilization, CO2 removal, hollow fiber membrane, respiratory dialysis, ECCO2R
Graphical abstract
1. Introduction
In patients suffering from acute respiratory failure, extracorporeal carbon dioxide removal (ECCO2R) is a powerful alternative or adjuvant therapy to avoid mechanical ventilation (MV) induced lung injury. High tidal volume MV can initiate and often exacerbate lung injury, increasing patient morbidity and mortality [1]–[3]. Delivery of low tidal volumes and airway pressures mitigates these deleterious effects, as demonstrated by the acute respiratory distress syndrome (ARDS) Network trial where low tidal volume MV at 6 mL/kg vs 12 mL/kg reduced lung injury and improved survival [4]. Recent data suggests even more ultra-protective MV settings may further improve outcomes, as alveolar over-distention is still observed at 6 mL/kg [3], [5], [6]. Clinicians are often unable to apply lung protective ventilation (LPV) strategies, reporting hypercapnia and acidosis as significant barriers to implementation [7]. In consequence, mortality rates remain between 40 and 45% for ARDS ICU patients [8]. For the chronic obstructive pulmonary disease (COPD) population, concerns over hypercapnia and severe acidosis can be mitigated through ECCO2R, enabling LPV, weaning of patients off MV, or avoiding intubation altogether [9]–[12]. ECCO2R in combination with LPV has not seen widespread application as current devices require surgical placement of cannula 19 Fr or larger to facilitate blood flow rates up to 1 L/minute or higher, in order to remove a significant fraction (50%) of total adult CO2 production [6], [13]. Large diameter arterial cannulation systems have shown complication rates as high as 24% comprising of vein tearing, limb ischemia, compartment syndrome and intracranial hemorrhage, in part due to their demand for approximately 25% of cardiac output [14], [15]. A clinical need exists for low blood flow ECCO2R devices (< 500 ml/min) that require less invasive cannulation and can regulate blood CO2 independent of alveolar ventilation in patients suffering from acute lung failure [3], [16], [17].
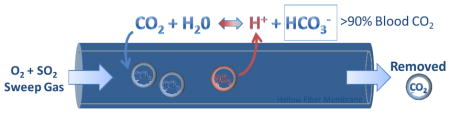
A clinically desirable low flow ECCO2R device would encompass minimally invasive vascular access (11–15 Fr cannula) with low blood flow rates (200–500 mL/min) [6], [18] and eliminate up to 100 mL/min of CO2 to meet 50% of the metabolic needs of an adult patient [19]. Current devices including the Quadrox D (Maquet, Rastatt, Germany), Hilite 7000LT (Medos Medizintechnik AG, Stolberg, Germany) and Affinity NT (Medtronic, Eden Praire, USA) require an external blood pump, blood flow rates greater than 1L/min and large surface areas greater than 1.3 m2 [20]. The PALP (Maquet, Rastatt, Germany), iLA Active (Novalung, Baden- Württemberg, Germany) and Hemolung RAS® (ALung Technologies, Pittsburgh, USA) devices have taken steps towards low blood flow CO2 removal, enabling partial CO2 removal support at blood flow rates less than 1 L/min [21]–[23]. Achieving clinically significant CO2 removal at blood flow rates less than 500 mL/min remains a challenge. New technologies such as active blood mixing within gas exchange fiber bundles have improved CO2 removal efficiency at low blood flow rates [23]–[25], but gas transport in ECCO2R devices is ultimately limited by the blood CO2 partial pressure (PCO2) gradient across hollow fiber membranes (HFMs) [26]. Our lung tissues face the same diffusional challenges as HFMs, however they employ the enzyme carbonic anhydrase (CA) within red blood cells and on the endothelial surfaces of lung capillaries to accelerate diffusion by catalyzing the reversible dehydration of HCO3− (bicarbonate) to gaseous carbon dioxide: . We reported development of CA immobilized bioactive HFMs which converts bicarbonate to CO2 directly at the HFM surface, restoring the trans-HFM CO2 gradient as it is depleted in the diffusional boundary layer, and increasing CO2 removal rates from blood by 36% in model gas exchange devices [27]–[29]. The main impediment to CO2 removal by bioactive HFMs is diffusional boundary layer resistance which restricts transport of CO2 and bicarbonate from the bulk fluid to the HFM surface, not the CA catalyzed conversion of bicarbonate to CO2 [28]. Further improvements in the trans-HFM CO2 gradient and exploitation of CA coating activity could be realized through blood acidification, chemically shifting equilibrium from bicarbonate to CO2.
Blood acidification was first described by Snider et al. in 1987, in which infusion 2–8 mEq/min of lactic acid infusion was used to chemically increase trans-HFM CO2 pressure gradients by acidifying the blood entering the ECCO2R devices, shifting equilibrium from bicarbonate to favor gaseous CO2 and increasing CO2 removal by 120–170%, however visible hemolysis was present [30]. More recently, Zanella et al. have refined this approach to mitigate hemolysis concerns [31]–[34]. The resulting acidified blood increased PCO2 from 56 to 136 mmHg, decreased pH from 7.39 to 6.91, and increased CO2 removal up to 70% [31]. The pH drop is similar to the pH values measured in human capillaries during heavy exercise [35]. Additionally, blood acidification offsets respiratory alkalosis as the blood leaving ECCO2R devices without acidification have an increased pH [31].
In this study we hypothesized local blood acidification at the HFM surface would increase CO2 removal while minimizing perturbations in whole blood pH. Since HFM CO2 removal is driven by trans-HFM pressure gradients, it should not be necessary to acidify the bulk fluid, but instead only the diffusional boundary layer adjacent to the HFM surface. While lactic acid infusions increase blood PCO2, this approach acidifies the entire blood volume passing through the device. By mixing dilute concentrations of acidic sulfur dioxide (SO2) gas into the oxygen (O2) sweep gas, we created an acidic HFM boundary layer, synergistically working with CA-HFMs to increase trans-HFM CO2 gradients and accelerate CO2 blood removal while preserving whole blood pH. The acidic byproduct sulfite naturally occurs in mammalian systems, and has been shown safe in animal models at doses similar to those which would be seen in clinical use of an acidic sweep gas device [36].
2. Methods
2.1. Materials
Allylamine, Glutaraldehyde, chitosan (MW= 50–190kD, based on viscosity) and glacial acetic acid were purchased from Sigma-Aldrich (St. Louis, MO). Commercial poly (methyl pentene) (PMP) hollow fiber membranes (HFMs) (Oxyplus™; OD: 380 μm, ID: 200 μm) were obtained from Membrana GmbH (Wuppertal, Germany). Bovine blood with Na-heparin anticoagulation (1:100 dilution of 1000U/mL) for gas exchange was purchased from Lampire Biological Laboratories (Pipersville, PA). Purified recombinant human carbonic anhydrase II was provided by Dr. Silverman and Dr. McKenna from University of Florida (Gainesville, Fl) [37]. Sulfur dioxide was purchased from Matheson Gas (Pittsburgh, PA). Sulfite assay kit was obtained from R-Biopharm (Darmstadt, Germany). All other reagents were purchased from Sigma-Aldrich and were of analytical grade or purer.
2.2. PMP amination
Allylamine was polymerized onto unmodified PMP through plasma enhanced chemical vapor deposition (PECVD) with the PVA TePla Ion 40 system to create amine functional groups for covalent CA immobilization. PMP HFMs samples (238 cm2 surface area) were placed on the second shelf from the top. The chamber was evacuated to a pressure of 50 mTorr and then allylamine was continuously introduced to the chamber through a mass flow controller at 180 mL/min for a final chamber pressure of 350 mTorr. Pulsed power was applied for 5 minutes at 300 watts, with a 20% duty cycle and a 150 Hz frequency. After deposition the samples were immediately rinsed with 100mM Phosphate Buffer (PB) at pH 8.5, 3 times for 15 minutes each. This treatment results in a 5.6 nmol/cm2 amine density as quantified through colorimetric technique [38].
2.3. Carbonic anhydrase immobilization
Carbonic anhydrase (CA) was immobilized onto PMP HFMs (CA-PMP) by secondary amine linkage through reaction of surface amine and glutaraldehyde crosslinkers, as follows. Aminated PMP (238 cm2 surface area) was folded into a 60mL test tube and incubated under constant inversion by orbital mixer with 50mL of 5% GA + 120mM Sodium Cyanoborohydride in PB at pH 8.5 for 15 minutes at 25C°. The fibers were rinsed under constant inversion by orbital mixer with 100mM PB pH 8.5, 3 times for 15 minutes each to remove residual unreacted GA. Then chitosan spacers were immobilized by reacting HFMs with 50mL of 1% (w/v) chitosan + 120mM Sodium Cyanoborohydride suspended in 1% (v/v) acetic acid in DI water for 15 minutes at 25C°, and rinsed with PB as previously described. A second GA reaction and rinse was performed to activate chitosan amine groups for enzyme immobilization. Finally CA was immobilized to the HFMs under constant inversion by orbital mixer with 50mL of 1mg/mL hCAII in 100mM PB at pH 8.5 for 12 hours, followed by 15 minute 120mM Sodium Cyanoborohydride incubation in PB at pH 8.5. Any non-covalently bound CA was removed through three, 15 minute PB rinse sessions. Immobilizing CA by physical adsorption yielded fibers with no detectable activity after 3+ rinses (data not shown).
2.4. CO2 Removal with SO2 Sweep Gas
A scaled-down gas exchange module was fabricated by inserting HFMs (114 fibers, 18 cm, 0.0238 m2) into a 1/4 in. ID polycarbonate-tubing (McMaster Carr, Elmhurst, IL) to which single luer locks were UV-glued 0.75 in. from each end in opposing directions. Both ends of the HFMs were secured to the tubing using a 5 minute epoxy adhesive (Devcon, Danvers, MA) and then trimmed to the length of the module to expose the HFM lumens, yielding 11 cm of HFM uncovered within the module for a total active surface area of 0.0151 m2.
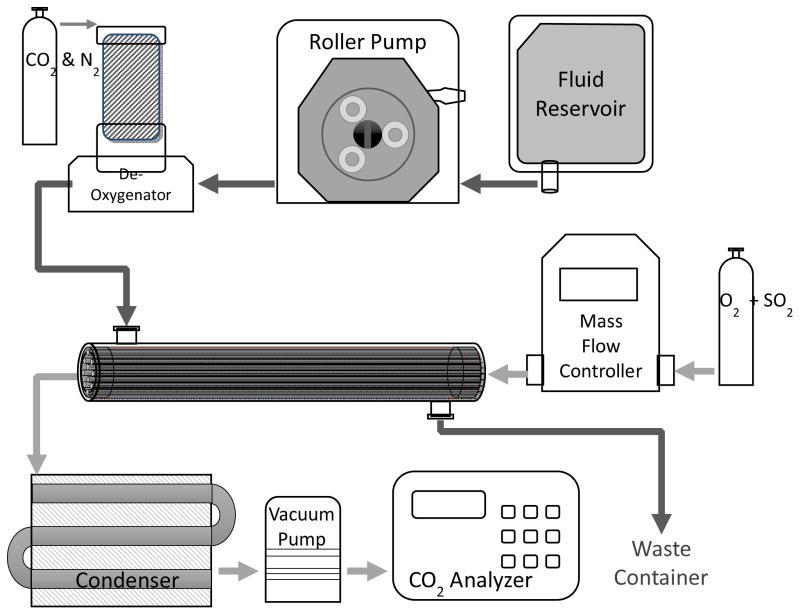
An in-vitro gas exchange test loop (Figure 1) was setup under a fume hood and used to assess CO2 removal rates of PMP and CA-PMP HFMs with and without SO2 acidic sweep gas (N = 3 for each group). Blood flowed in a single pass loop from a 3 L reservoir, through a peristaltic pump, into a Medtronic Minimax Plus Pediatric Oxygenator (Minneapolis, MN) to balance the fluid gasses, then into the model oxygenator testing module and finally into a waste container. The partial pressure of CO2 at the model oxygenator blood inlet was adjusted to 50 mmHg. PCO2 and pH were measured with a RAPIDLAB 248 Blood-Gas analyzer (Siemens, Deerfield, IL). Custom mixtures of acidic sweep gas up to 2.2% SO2 balanced in oxygen were created and pulled under vacuum from independent O2 and SO2 gas tanks and regulated through two separate GR Series mass flow controllers (Fathom Technologies, Georgetown, TX). The gasses were then mixed into a single line which flowed through the scaled-down oxygenator HFM lumens, moisture trap condenser and finally into an infrared CO2 analyzer WMA-4 CO2 Analyzer (PP Systems, Amesbury, MA). The following testing conditions were controlled ensured that the HFM CO2 removal efficiency (ml/min/m2) matches the commercially available Hemolung device. The fluid flow rate through the model oxygenator was set to 45 mL/min. The sweep gas flow rate was adjusted to maintain a constant CO2 concentration in the sweep gas exiting the model oxygenator at approximately 3000 ppm to avoid sweep gas flow limitation of gas exchange [28]. The sweep gas flow rate for PMP HFMs ranged from 0.90 L/min (O2) to 1.02 L/min (O2 + 2.2% SO2), while CA-PMP ranged from 1.35 L/min (O2) to 2.20 L/min (O2 + 2.2% SO2). The fluid temperature was maintained at 37C° by heat bath. The rate of CO2 removal (V̇CO2) for each model oxygenator device was calculated using the sweep gas flow rate ( ) and CO2 fraction (FCO2) exiting the scaled-down gas exchange module and then normalized to 50mmHg to correct for small deviations in the inlet . The V̇CO2 for each model gas exchange group is reported as an average of three individual devices.
Figure 1.
Experimental setup for the in vitro CO2 gas exchange assessment. Heparinized bovine blood was deoxygenated to a physiological inlet of 50 mmHg and perfused over the HFMs of a mini respiratory device while SO2 + O2 sweep gas was passed through the fiber lumens in the opposite direction. Both the blood reservoir and de-oxygenator employed the use of a heat exchanger to maintain blood temperature at 37C°.
2.5. Carbonic Anhydrase Esterase Activity Assay
The CA enzyme activity on HFMs was assayed using the substrate p-nitrophenyl acetate (p-NPA) before and after exposure to SO2 [39]. CA-PMP fibers were fabricated into model oxygenators as described in section 2.3, assayed for activity, exposed to 2.2% SO2 acidic sweep gas for 30 minutes as described in section 2.4, and then assayed for activity again. Enzyme activity was measured spectrophotometrically by monitoring the hydrolysis of p-nitrophenyl acetate (p-NPA) to p-nitrophenol (p-NP) at 412 nm. A recirculating loop of 15 mL, 100 mM phosphate buffer pH 7.5, 80 μM p-NPA was setup to measure the esterase activity. Absorbance measurements at 412nm were recorded every minute over a 5 minute period, and plotted as a function of time. One activity unit was defined as the amount of enzyme required to generate 1 μmol pNP per minute.
2.6. Sulfite Assay
The total fluid SO2 content was assessed using PBS in place of bovine blood, removing potential for interaction between sulfites and plasma proteins. An enzymatic sulfite assay kit (r-Biopharm) was used according to manufacturer’s instructions. Briefly, sulfite is oxidized to sulfate by sulfite oxidase, yielding hydrogen peroxide which is reduced by NADH-peroxidase in the presence of NADH. The amount of sulfite is equivalent to the amount of NADH oxidized, which is determined by spectrophotometric absorbance at 340 nm.
2.7. Statistical Analyses
All data are presented as a mean with standard deviation. Statistical significance between sample groups was determined using ANOVA followed by post hoc Tukey testing of specific differences. All other data was compared using a Student’s t-test assuming equal sample variance. Differences were considered statistically significant for P < 0.05.
3. Results
3.1. CO2 Removal with SO2 Sweep Gas
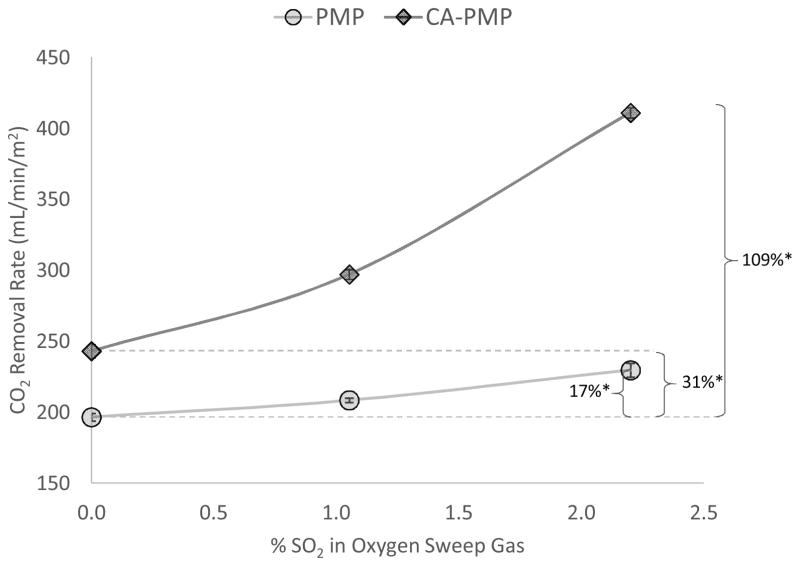
PMP and CA-PMP blood CO2 removal rates were measured using oxygen sweep gas with up to 2.2% SO2 (Figure 2). With pure O2 sweep gas, bioactive CA-PMP HFMs increased CO2 removal by 31% (258 ± 10 mL/min/m2) compared to control PMP (197 ± 3 mL/min/m2) (P < 0.05). Using SO2 acidic sweep gas through PMP fibers, CO2 removal increased by 17% (230 ± 5 mL/min/m2), compared to pure oxygen sweep gas through the same fibers (P < 0.05). When employing both CA-PMP and SO2 acidic sweep gas, CO2 removal increased by 109% (411 ± 4 mL/min/m2), compared to unmodified HFMs with pure oxygen sweep gas (P < 0.05).
Figure 2.
CO2 removal by PMP and CA-PMP HFMs from bovine blood using pure O2 and 2.2% SO2 acidic sweep gas. (N = 3) *P < 0.05
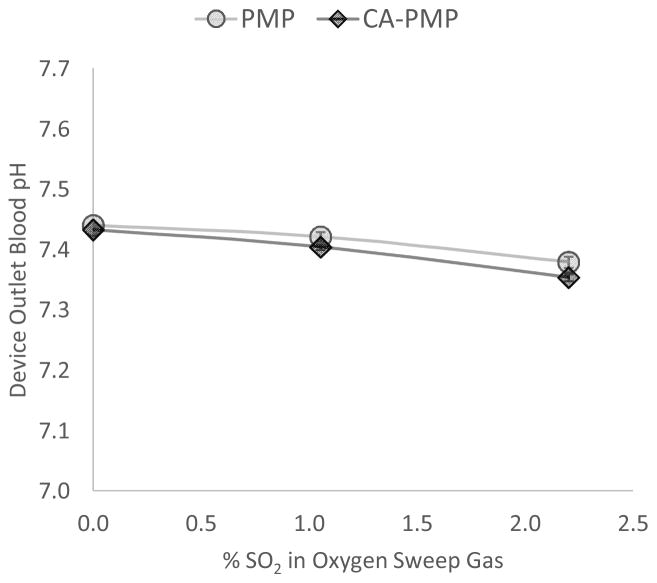
The blood pH and PCO2 exiting the device were measured to establish the effects of SO2 acidic sweep gas on the bulk blood. For PMP and CA-PMP devices using pure O2 sweep gas, blood pH exiting the device increased from 7.37 ± 0.006 at the inlet to 7.44 ± 0.004 and 7.43 ± 0.006 respectively. However when using 2.2% SO2 acidic sweep gas, blood pH exiting the device was nearly unchanged from the inlet, with an outlet pH of 7.38 ± 0.009 for PMP and 7.35 ± 0.006 for CA-PMP (Figure 3). For blood PCO2 exiting the device, pressure decreased from 50mmHg inlet to 37 ± 0.5 mmHg outlet for PMP and 35 ± 0.4 mmHg for CA-PMP with pure O2 sweep gas. When using 2.2% acidic sweep gas, PCO2 exiting the device was 40 ± 0.3 mmHg for PMP and 38 ± 0.5 mmHg for CA-PMP. The addition of dilute SO2 to O2 sweep gas maintained near physiologic pH and PCO2 for blood exiting the device.
Figure 3.
Increasing the % SO2 within oxygen sweep gas decreases blood pH exiting the device. (N = 3)
3.2. Carbonic Anhydrase Esterase Activity Assay
Immobilized carbonic anhydrase activity was measured before and after exposure to SO2. Activity remained unchanged with 9.3 ± 0.8 U before and 9.5 ± 0.9 U after exposure to 2.2% SO2 acidic sweep gas, indicating CA coating stability to the acidic microenvironment over this time course.
3.3. Blood Sulfite Assay
Total sulfite was measured in PBS fluid exiting the scaled-down gas exchange module with 2.2% SO2 sweep gas. The sulfite concentration leaving the device was 0.08 ± 0.001 mMol/L for PMP and 0.28 ± 0.002 mMol/L, or 3.5 times more, for bioactive CA-PMP HFMs (P < 0.05) (Figure 4).
Figure 4.
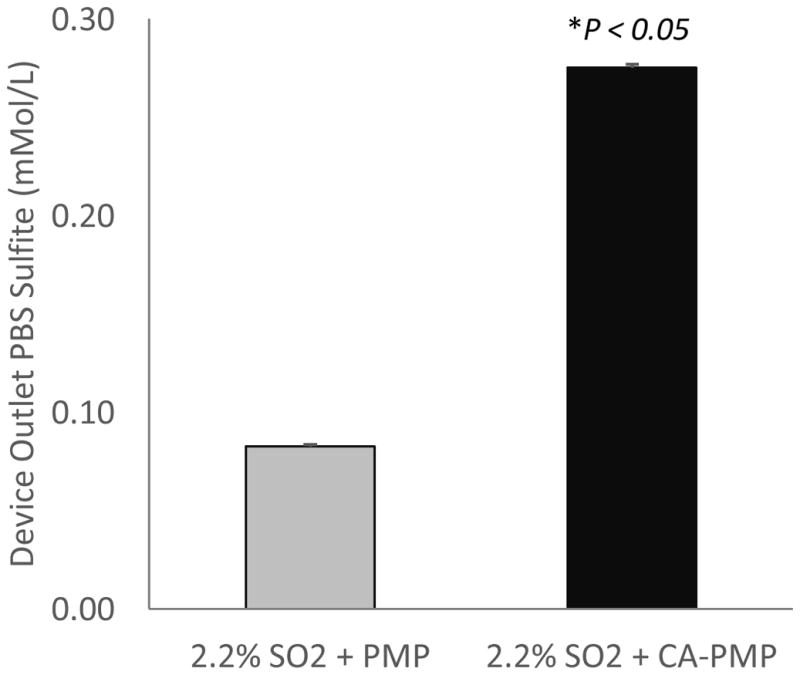
Total PBS sulfite concentration of fluid exiting the device with 2.2% SO2 acidic sweep gas. (N = 3)
4. Discussion
The goal of this study was to quantify how local blood acidification using acidified sweep gas could increase CO2 removal in HFM devices while minimizing effects on whole blood pH. Previous work by our group to chemically increase trans-HFM CO2 pressure utilized bioactive CA-HFMs for a 31–37% improvement in blood CO2 removal efficiency [27], [28]. In this work, the addition of SO2 to an oxygen sweep gas increased CO2 removal of unmodified PMP HFMs up to 17%, and for bioactive CA-PMP HFMs up to 109% (Figure 2). Synergy between CA coatings and SO2 acidic sweep gas dramatically increases CO2 removal efficiency of HFMs. To our knowledge, this is the first report assessing the potential of an acidic sweep gas to increase CO2 removal from blood using HFM devices like blood oxygenators.
Zanella et al. have utilized lactic acidic (LA) infusions for increasing HFM CO2 removal [31]–[33]. In their work, LA was mixed with bulk extracorporeal blood via direct infusion or dialysis, upon which acidified blood flowed into a HFM device for CO2 removal. When directly infusing LA with 500 mL/min extracorporeal blood flow rate, pH dropped to 6.91 while lactate concentration increased from 1.2 to 15.1 mM, for a 70% increase in CO2 removal from 58 mL/min/m2 to 95 mL/min/m2 [31]. When using a dialysis membrane to acidify blood with 250 mL/min blood flow rate, pH dropped to 6.99 while lactate concentration increased from 0.7 to 12.3 mM, for a 62–78% increase in CO2 removal from 99 mL/min/m2 to 160 mL/min/m2 [33]. In contrast to bulk LA acidification, we demonstrated HFM CO2 removal efficiency can be significantly increased through localized blood acidification with SO2 acidic sweep gas, consequently introducing 50 times less acidic anions to blood, while mitigating transient acidosis (Table 1). Recently Zanella et al. described a unique approach utilizing acidified dialysis to transport bicarbonate and CO2 from blood into a dialysate using a commercial dialysis filter. The dialysate was then acidified with LA and flowed into a HFM commercial oxygenator device for dialysate CO2 removal [34]. Using blood flow rates of 250 mL/min, blood pH remained nearly unchanged at 7.43, blood lactate concentration increased from 0.6 to 14.1 mM, and dialysate CO2 removal increased 157 % from 18 mL/min/m2 to 48 mL/min/m2. A drawback of this approach is the requirement of two devices for CO2 removal from blood, a membrane oxygenator and a dialysis filter to remove bicarbonate/CO2 from blood. In contrast, our approach suggests that 100 mL/min of CO2 removal could be accomplished with one HFM based device, with a surface area of 0.25 to 0.5 m2. In all approaches used by Zanella et. al, blood hyperlactatemia (2–5mM lactate) persists (see Table 1). Clinically, lactate is monitored as a biomarker of organ dysfunction, shock and has been correlated with increased mortality in critically ill patients [40]–[45]. Consequently addition of exogenous LA may limit the diagnostic potential of blood lactate levels as a predictor of adverse outcomes.
Table 1.
Comparison of blood acidification approaches by pH, CO2 removal % increase, and total lactate or sulfite anion infused.
We hypothesize SO2 sweep gas acidifies blood in or near the diffusional boundary layer, which provides the main resistance to CO2 removal in HFM devices [26]. By reacting with water SO2 creates sulfurous acid, which rapidly dissociates into bisulfite and sulfite ions: SO2 + H2O ⇔ HSO3− + H+ ⇔ SO32− + H+. SO2 was selected for its high solubility and acid dissociation constant in water, enabling dilute concentrations (1–2%) within the oxygen sweep gas [46]. The synergistic activity of CA-PMP with SO2 sweep gas indicates acidification of blood hydrating the immobilized CA layer, adjacent to the HFM surface. Additionally the minimal impact of acidic sweep gas on bulk blood pH demonstrates a majority of acidic protons are consumed in bicarbonate dehydration. By consuming acidic protons during catalysis, immobilized CA facilitates diffusion of SO2 from the sweep gas into the fluid boundary layer. Consequently, 3.5 times more sulfite was measured in the bulk fluid for CA-PMP compared to PMP, 0.28 mMol/L versus 0.08 mMol/L respectively (Figure 4). Despite the sulfite infusion, blood pH remained near physiologic from inlet of 7.37 to outlet 7.38 for PMP and 7.35 for CA-PMP. Calculation of base excess (BE) demonstrates metabolic acidosis of the blood occurred which cannot be fully described by the measured sulfite content, as drop in BE was 3.46 mEq/L for PMP and 5.75 mEq/L CA-PMP (Supplemental Table S2). Variations in the plasma electrolyte concentration, PCO2 and total amount of weak acids can regulate blood pH [47]. The release of chloride ions from RBCs and albumin have reported in the literature, as a PCO2 dependent change in the strong ion difference, which acidify the plasma to limit pH increase due to CO2 removal [47], [48]. This buffer and the polyprotic nature of sulfurous acid account for a portion of the observed drop in BE, but future work will quantify blood electrolytes to fully describe anions which impact drop in BE. Mixtures beyond 2.2% SO2 were not possible as we were limited by the mass flow controllers, however the data suggests CO2 removal could be further improved by increasing SO2 concentration. No hemolysis due to the acidic sweep gas was observed by spectrophotometric assay of plasma free hemoglobin (data not shown).
At physiologic pH and temperature, the dissociated byproducts of SO2 introduced into blood are in the sulfite and bisulfite ion form. These species are endogenous to biological systems from metabolism of sulfur amino acids. Sulfites are commonly used as antioxidant preservatives in cosmetics, food and pharmaceutical products [49]. Sulfite oxidase, found in the mitochondria of most mammalian tissues, catalyzes sulfur detoxification by oxidizing sulfite to sulfate for excretion through urine [50], [51]. Various review articles have surveyed the literature for toxicology and safety of sulfites, finding no serious adverse effects as a result of chronically administered sulfite [36], [50], [52]. Roughly 1 – 5% of the population does present sulfite sensitivity, due to impaired sulfite oxidase activity, though most reactions are mild [49], [51]. Additionally, recovery of sulfite oxidase activity has been reported in an infant with molybdenum cofactor deficiency through dosage of cyclic pyranopterin monophosphate [53]. Invitro toxicology assessment of sulfite indicates potential to react with DNA causing mutagenesis, however these effects have not been replicated in-vivo at physiologic concentrations [36], [52], [54]. As a potentially protective mechanism, blood plasma proteins have been shown to reversibly react with sulfite to form s-sulfonate groups, mitigating insult of high sulfite concentrations on tissues [36], [55], [56]. Typical daily dietary sulfite intake can be up to 0.14 mmol/kg body weight and normal human serum sulfite can range from 0 – .01 mM [36], [57]. Based upon urinary sulfate excretion, daily endogenous human sulfite generation is estimated at 0.3 – 0.4 mmol/kg [58]. Scaling the 0.28 mM sulfite concentration we observe with acidic sweep gas to a low blood flow CO2 removal device at 500 mL/min, yields a theoretical sulfite infusion rate of 0.12 mmol/kg/hour or 2.88 mmol/kg/day for an average 70 kg adult. Studies have demonstrated the capacity of mammalian sulfite oxidase is extremely high compared with normal endogenous and exogenous sulfite loads [59]. Perfused dog livers tested for up to 3 hours oxidized sulfite at rates of at least 19 mmol/kg/day (559% more sulfite than the daily acidic sweep gas rate) [36], [60]. In rhesus monkeys, Gunnison et al. estimated the biological half-life of sulfite at 10 minutes after intravenous doses of 0.3 – 0.6 mmol/kg sulfite (at least 208% more sulfite than the hourly acidic sweep gas rate), and the capacity to metabolize orally administered sulfite at 2.74 mmol/kg/day for 11 consecutive days (within 5% of the daily acidic sweep gas rate) [36], [59]. In healthy human subjects, consumption of 0.21 mmol/kg sulfite (75% more sulfite than hourly acidic sweep gas rate) elevated serum sulfite concentration up to 0.112 mM within 30 minutes, which then returned to basal levels within 3 hours without any adverse reactions [61]. This data suggests the acidic sweep gas sulfite levels seen here (up to 2.88 mmol/kg/day) are tolerable, and could be oxidized and excreted by the human body without serious adverse reaction. We conclude use of SO2 acidic sweep gas has potential as a clinically viable approach to increasing HFM CO2 removal efficiency.
Application of the acidic sweep gas and bioactive CA-HFMs to current ECCO2R devices yields a CO2 removal device capable of removing a clinically significant 100 mL/min (50% of the metabolic needs of an adult patient [19]) with smaller surface areas. Model devices in this study utilized surface area (0.0151 m2) and liquid flow rates (45 mL/min) appropriately scaled to mimic the mass transport environment of comparable human HFM devices under clinically relevant conditions (197 mL/min/m2). We would expect similar performance by our approaches when translated to full scale commercial devices. For example, highly efficient low blood flow (< 500 mL/min) CO2 removal devices such as the Hemolung RAS® (ALung Technologies, Pittsburgh PA) reported peak CO2 removal rates of 121 mL/min with 0.6 m2, for a comparable efficiency of 201 mL/min/m2 [23]. Incorporation of an acidic sweep gas and bioactive CA-HFMs into this device could improve average efficiency up to 283 mL/min/m2, thereby requiring just 0.35m2 for 100 mL/min CO2 removal support, at blood flow rates less than 500 mL/min.
In conclusion, development of highly efficient ECCO2R devices will facilitate minimally invasive vascular access (11–15 Fr cannula) for partial respiratory support (up to 50%) at low blood flow rates (200–500 mL/min). By mixing dilute concentrations of acidic SO2 gas into the oxygen sweep gas, we acidified the blood in and around diffusional boundary layer, increasing the trans-HFM CO2 pressure gradient to more than double CO2 removal while maintaining near physiologic pH. Future work will focus on assessing the enzymatic coating stability over time and validating the SO2 acidic sweep gas technique and sulfite byproduct safety in an animal model, elucidating the difference in BE and sulfite concentration and explore means of removing exogenous sulfite if necessary. Bioactive CA coatings in combination with SO2 acidic sweep gas could lead to next generation highly efficient CO2 removal devices for the treatment of acute and acute-on-chronic lung failure.
Supplementary Material
Acknowledgments
This publication was made possible by Grant Number R01 HL70051 and R01 HL117637 from the National Institutes of Health, National Heart, Lung and Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. This work was also funded by research grants from the Pennsylvania Department of Health (SAP #4100030667, #4100035341, and #4100041556). The author would like to thank Drs. Christopher D. Boone and Robert McKenna (Department of Biochemistry and Molecular Biology, College of Medicine, University of Florida, Gainesville, FL 32610) for supplying the wild-type hCA II used in these experiments, that was funded in part from the National Institutes of Health (GM25154) award. We would like to recognize the University of Pittsburgh’s McGowan Institute for Regenerative Medicine for support of this study. Primary funding for David Arazawa was provided by the NIH training grant (T32-HL076124) at the University of Pittsburgh: Cardiovascular Bioengineering Training Program (CBTP).
Footnotes
Disclosure
W.J. Federspiel has an equity interest in Alung Technologies which has licensed portions of the technology described in this manuscript. For all other authors, there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tremblay LN, Slutsky AS. Applied Physiology in Intensive Care Medicine. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. Ventilator-induced lung injury: from the bench to the bedside; pp. 357–366. [DOI] [PubMed] [Google Scholar]
- 2.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Medicine. 2005 Oct;32(1):24–33. doi: 10.1007/s00134-005-2817-8. [DOI] [PubMed] [Google Scholar]
- 3.Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L, Ranieri VM. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009 Oct;111(4):826–835. doi: 10.1097/ALN.0b013e3181b764d2. [DOI] [PubMed] [Google Scholar]
- 4.Brower R, Matthay MA. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2000 May;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM. Tidal Hyperinflation During Low Tidal Volume Ventilation in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2006 Oct;:200607–915OC. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 6.Pesenti A, Patroniti N, Fumagalli R. Carbon dioxide dialysis will save the lung. Critical Care Medicine. 2010 Oct;38:S549–S554. doi: 10.1097/CCM.0b013e3181f1fe0c. [DOI] [PubMed] [Google Scholar]
- 7.Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Critical Care Medicine. 2004 Jun;32(6):1289–1293. doi: 10.1097/01.ccm.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 8.on behalf of the ALIEN Network. Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa S, Fernández RL, Kacmarek RM. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Medicine. 2011 Dec;37(12):1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 9.Lund LW, Federspiel WJ. Removing extra CO2 in COPD patients. Curr Respir Care Rep. :1–8. doi: 10.1007/s13665-013-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calverley PMA. Respiratory failure in chronic obstructive pulmonary disease. European Respiratory Journal. 2003 Nov;22(Supplement 47):26s–30s. doi: 10.1183/09031936.03.00030103. [DOI] [PubMed] [Google Scholar]
- 11.Budweiser S, Jörres RA, Pfeifer M. Treatment of respiratory failure in COPD. International Journal of Chronic Obstructive Pulmonary Disease. 2008 Dec;3(4):605. doi: 10.2147/copd.s3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy RM, Guntupalli KK. Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. International journal of chronic obstructive pulmonary disease. 2007;2(4):441. [PMC free article] [PubMed] [Google Scholar]
- 13.Crotti S, Lissoni A, Tubiolo D, Azzari S, Tarsia P, Caspani L, Gattinoni L. Artificial Lung as an Alternative to Mechanical Ventilation in COPD Exacerbation. Eur Respir J. 2012 Jan;39(1):212– 215. doi: 10.1183/09031936.00021111. [DOI] [PubMed] [Google Scholar]
- 14.Bein T, Weber F, Philipp A, Prasser C, Pfeifer M, Schmid FX, Butz B, Birnbaum D, Taeger K, Schlitt HJ. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia*. Critical Care Medicine. 2006 May;34(5):1372–1377. doi: 10.1097/01.CCM.0000215111.85483.BD. [DOI] [PubMed] [Google Scholar]
- 15.BOND SJ, STEWART DL, NAGARAJ HS, WINSTON S, GROFF DB. Complicated Cannula Insertions and Cannula Dislodgments Associated With Extracorporeal Membrane Oxygenation. ASAIO Journal. 1998;44(3) doi: 10.1097/00002480-199805000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Batchinsky AI, Jordan BS, Regn D, Necsoiu C, Federspiel WJ, Morris MJ, Cancio LC. Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med. 2011 Jun;39(6):1382–1387. doi: 10.1097/CCM.0b013e31820eda45. [DOI] [PubMed] [Google Scholar]
- 17.Wearden PD, Federspiel WJ, Morley SW, Rosenberg M, Bieniek PD, Lund LW, Ochs BD. Respiratory dialysis with an active-mixing extracorporeal carbon dioxide removal system in a chronic sheep study. Intensive Care Medicine. 2012 Aug;38(10):1705–1711. doi: 10.1007/s00134-012-2651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terragni P, Maiolo G, Ranieri VM. Role and potentials of low-flow CO(2) removal system in mechanical ventilation. Curr Opin Crit Care. 2012 Feb;18(1):93–98. doi: 10.1097/MCC.0b013e32834f17ef. [DOI] [PubMed] [Google Scholar]
- 19.Galletti P, Colton C. Artificial Lungs and Blood– Gas Exchange Devices. In: Bronzino JD, editor. The Biomedical Engineering Handbook. CRC Press LLC; Boca Raton: 2000. pp. 1–19. [Google Scholar]
- 20.Cove M, MacLaren G, Federspiel W, Kellum J. Bench to bedside review: Extracorporeal carbon dioxide removal, past present and future. Critical Care. 2012 Sep;16(5):232. doi: 10.1186/cc11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gramaticopolo S, Chronopoulos A, Piccinni P, Nalesso F, Brendolan A, Zanella M, Cruz DN, Ronco C. Extracorporeal CO2 removal--a way to achieve ultraprotective mechanical ventilation and lung support: the missing piece of multiple organ support therapy. Contrib Nephrol. 2010;165:174–184. doi: 10.1159/000313757. [DOI] [PubMed] [Google Scholar]
- 22.Karagiannidis C, Kampe KA, Sipmann FS, Larsson A, Hedenstierna G, Windisch W, Mueller T. Veno-venous extracorporeal CO2 removal for the treatment of severe respiratory acidosis: pathophysiological and technical considerations. Critical Care. 2014;18(3):R124. doi: 10.1186/cc13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burki NK, Mani RK, Herth FJF, Schmidt W, Teschler H, Bonin F, Becker H, Randerath WJ, Stieglitz S, Hagmeyer L, Priegnitz C, Pfeifer M, Blaas S, Putensen C, Theuerkauf N, Quintel M, Moerer O. A novel extracorporeal CO2 removal system: Results of a pilot study in COPD patients with hypercapnic respiratory failure. CHEST. 2012 Dec; doi: 10.1378/chest.12-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffries RG, Frankowski BJ, Burgreen GW, Federspiel WJ. Effect of Impeller Design and Spacing on Gas Exchange in a Percutaneous Respiratory Assist Catheter: Impeller Testing for a Respiratory Assist Catheter. Artificial Organs. 2014 May; doi: 10.1111/aor.12308. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mihelc KM, Frankowski BJ, Lieber SC, Moore ND, Hattler BG, Federspiel WJ. Evaluation of a Respiratory Assist Catheter that Uses an Impeller Within a Hollow Fiber Membrane Bundle. ASAIO Journal. 2009 Nov;55(6):569–574. doi: 10.1097/MAT.0b013e3181bc2655. [DOI] [PubMed] [Google Scholar]
- 26.Federspiel WJ, Henchir KA. Lung, artificial: basic principles and current applications. Encyclopedia of Biomaterials and Biomedical Engineering. 2004;9:910. [Google Scholar]
- 27.Arazawa DT, Oh H-I, Ye S-H, Johnson CA, Jr, Woolley JR, Wagner WR, Federspiel WJ. Immobilized carbonic anhydrase on hollow fiber membranes accelerates CO2 removal from blood. Journal of Membrane Science. 2012 Jun;403–404:25–31. doi: 10.1016/j.memsci.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimmel JD, Arazawa DT, Ye SH, Shankarraman V, Wagner WR, Federspiel WJ. Carbonic anhydrase immobilized on hollow fiber membranes using glutaraldehyde activated chitosan for artificial lung applications. J Mater Sci: Mater Med. 2013 Nov;24(11):2611–2621. doi: 10.1007/s10856-013-5006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaar JL, Oh HI, Russell AJ, Federspiel WJ. Towards improved artificial lungs through biocatalysis. Biomaterials. 2007 Jul;28(20):3131–3139. doi: 10.1016/j.biomaterials.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snider MT, Chaudhari SN, Richard RB, Whitcomb DR, Russell GB. Augmentation of CO2 Transfer in Membrane Lungs by the Infusion of a Metabolizable Organic Acid. ASAIO Journal. 1987;33(3) [PubMed] [Google Scholar]
- 31.Zanella A, Patroniti N, Isgrò S, Albertini M, Costanzi M, Pirrone F, Scaravilli V, Vergnano B, Pesenti A. Blood acidification enhances carbon dioxide removal of membrane lung: an experimental study. Intensive Care Medicine. 2009 Jun;35:1484–1487. doi: 10.1007/s00134-009-1513-5. [DOI] [PubMed] [Google Scholar]
- 32.Zanella A, Giani M, Redaelli S, Mangili P, Scaravilli V, Ormas V, Costanzi M, Albertini M, Bellani G, Patroniti N, Pesenti A. Infusion of 2.5 meq/min of lactic acid minimally increases CO2 production compared to an isocaloric glucose infusion in healthy anesthetized, mechanically ventilated pigs. Critical Care. 2013 Nov;17(6):R268. doi: 10.1186/cc13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanella A, Mangili P, Redaelli S, Scaravilli V, Giani M, Ferlicca D, Scaccabarozzi D, Pirrone F, Albertini M, Patroniti N, Pesenti A. Regional Blood Acidification Enhances Extracorporeal Carbon Dioxide Removal: A 48-hour Animal Study. Anesthesiology. 2014 Feb;120(2):416–424. doi: 10.1097/ALN.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 34.Zanella A, Mangili P, Giani M, Redaelli S, Scaravilli V, Castagna L, Sosio S, Pirrone F, Albertini M, Patroniti N, Pesenti A. Extracorporeal carbon dioxide removal through ventilation of acidified dialysate: An experimental study. The Journal of Heart and Lung Transplantation. doi: 10.1016/j.healun.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Geers C, Gros G. Carbon Dioxide Transport and Carbonic Anhydrase in Blood and Muscle. Physiological Reviews. 2000 Jan;80(2):681–715. doi: 10.1152/physrev.2000.80.2.681. [DOI] [PubMed] [Google Scholar]
- 36.Gunnison AF. Sulphite toxicity: A critical review of in vitro and in vivo data. Food and Cosmetics Toxicology. 1981;19:667–682. doi: 10.1016/0015-6264(81)90519-8. [DOI] [PubMed] [Google Scholar]
- 37.Boone CD, Habibzadegan A, Tu C, Silverman DN, McKenna R. Structural and catalytic characterization of a thermally stable and acid-stable variant of human carbonic anhydrase II containing an engineered disulfide bond. Acta Crystallographica Section D Biological Crystallography. 2013 Aug;69(8):1414–1422. doi: 10.1107/S0907444913008743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook AD, Pajvani UB, Hrkach JS, Cannizzaro SM, Langer R. Colorimetric analysis of surface reactive amino groups on poly(lactic acid-co-lysine):poly(lactic acid) blends. Biomaterials. 1997 Nov;18(21):1417–1424. doi: 10.1016/s0142-9612(97)00075-6. [DOI] [PubMed] [Google Scholar]
- 39.Pocker Y, Beug MW. Kinetic studies of bovine carbonic anhydrase catalyzed hydrolyses of p-substituted phenyl esters. Biochemistry. 1972 Feb;11(5):698–707. doi: 10.1021/bi00755a005. [DOI] [PubMed] [Google Scholar]
- 40.MIZOCK BA, FALK JL. Lactic acidosis in critical illness. Critical Care Medicine. 1992;20(1) doi: 10.1097/00003246-199201000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock*. Critical Care Medicine. 2009 May;37(5):1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 42.Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care. 2009;13(3):R90. doi: 10.1186/cc7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutierrez G, Williams JD. The riddle of hyperlactatemia. Crit Care. 2009;13(4):176. doi: 10.1186/cc7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fall PJ, Szerlip HM. Lactic Acidosis: From Sour Milk to Septic Shock. J Intensive Care Med. 2005 Sep;20(5):255–271. doi: 10.1177/0885066605278644. [DOI] [PubMed] [Google Scholar]
- 45.Demers P, Elkouri S, Martineau R, Couturier A, Cartier R. Outcome with high blood lactate levels during cardiopulmonary bypass in adult cardiac operation. The Annals of Thoracic Surgery. 2000 Dec;70(6):2082–2086. doi: 10.1016/s0003-4975(00)02160-3. [DOI] [PubMed] [Google Scholar]
- 46.Sander R. Compilation of Henry’s law constants for inorganic and organic species of potential importance in environmental chemistry. Max-Planck Institute of Chemistry, Air Chemistry Department; 1999. [Google Scholar]
- 47.Langer T, Scotti E, Carlesso E, Protti A, Zani L, Chierichetti M, Caironi P, Gattinoni L. Electrolyte shifts across the artificial lung in patients on extracorporeal membrane oxygenation: Interdependence between partial pressure of carbon dioxide and strong ion difference. Journal of Critical Care. 2015 Feb;30(1):2–6. doi: 10.1016/j.jcrc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Fogh-Andersen N, Bjerrum PJ, Siggaard-Andersen O. Ionic binding, net charge, and Donnan effect of human serum albumin as a function of pH. Clinical Chemistry. 1993 Jan;39(1):48–52. [PubMed] [Google Scholar]
- 49.Vally H, Misso NLA, Madan V. Clinical effects of sulphite additives. Clinical & Experimental Allergy. 2009;39(11):1643–1651. doi: 10.1111/j.1365-2222.2009.03362.x. [DOI] [PubMed] [Google Scholar]
- 50.Schweigert BS, Chichester CO, Mrak EM. ADVANCES IN FOOD RESEARCH. Academic Press; 1986. [Google Scholar]
- 51.Lester MR. Sulfite sensitivity: significance in human health. J Am Coll Nutr. 1995 Jun;14(3):229–232. doi: 10.1080/07315724.1995.10718500. [DOI] [PubMed] [Google Scholar]
- 52.NB, Ar E. Final report on the safety assessment of sodium sulfite, potassium sulfite, ammonium sulfite, sodium bisulfite, ammonium bisulfite, sodium metabisulfite and potassium metabisulfite. Int J Toxicol. 2002 Dec;22(Suppl 2):63–88. doi: 10.1080/10915810305077x. [DOI] [PubMed] [Google Scholar]
- 53.Veldman A, Santamaria-Araujo JA, Sollazzo S, Pitt J, Gianello R, Yaplito-Lee J, Wong F, Ramsden CA, Reiss J, Cook I, Fairweather J, Schwarz G. Successful Treatment of Molybdenum Cofactor Deficiency Type A With cPMP. Pediatrics. 2010 May;125(5):e1249–e1254. doi: 10.1542/peds.2009-2192. [DOI] [PubMed] [Google Scholar]
- 54.Shapiro R. Genetic effects of bisulfite (sulfur dioxide) Mutation Research/Reviews in Genetic Toxicology. 1977;39(2):149–175. doi: 10.1016/0165-1110(77)90020-3. [DOI] [PubMed] [Google Scholar]
- 55.Gunnison AF, Farruggella TJ. Preferential S-sulfonate formation in lung and aorta. Chem Biol Interact. 1979 May;25(2–3):271–277. doi: 10.1016/0009-2797(79)90051-6. [DOI] [PubMed] [Google Scholar]
- 56.Gunnison AF, Palmes ED. Species variability in plasma S-sulfonate levels during and following sulfite administration. Chemico-Biological Interactions. 1978 Jun;21(2–3):315–329. doi: 10.1016/0009-2797(78)90029-7. [DOI] [PubMed] [Google Scholar]
- 57.Pundir CS, Rawal R. Determination of sulfite with emphasis on biosensing methods: a review. Anal Bioanal Chem. 2013 Apr;405(10):3049–3062. doi: 10.1007/s00216-013-6753-0. [DOI] [PubMed] [Google Scholar]
- 58.Sulfites as Food Additives. Nutrition Reviews. 1976;34(2):58–62. doi: 10.1111/j.1753-4887.1976.tb05697.x. [DOI] [PubMed] [Google Scholar]
- 59.Gunnison AF, Bresnahan CA, Palmes ED. Comparative sulfite metabolism in the rat, rabbit, and rhesus monkey. Toxicology and Applied Pharmacology. 1977 Oct;42(1):99–109. doi: 10.1016/0041-008x(77)90200-9. [DOI] [PubMed] [Google Scholar]
- 60.WJ, GJ, Jm W. Toxicity of intraperitoneal bisulfite. Clin Pharmacol Ther. 1967 Dec;9(3):328–332. doi: 10.1002/cpt196893328. [DOI] [PubMed] [Google Scholar]
- 61.Ji AJ, Savon SR, Jacobsen DW. Determination of total serum sulfite by HPLC with fluorescence detection. Clinical Chemistry. 1995 Jun;41(6):897–903. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.