Abstract
The members of the large family of claudin proteins regulate ion and water flux across the tight junction. Many claudins, e.g. claudins 2 and 15, accomplish this by forming size- and charge-selective paracellular channels. Claudins also appear to be essential for genesis of tight junction strands and recruitment of other proteins to these sites. What is less clear is whether claudins also form the paracellular seal. While this seal is defective when claudins are disrupted, some results, including ultrastructural and biochemical data, suggest that lipid structures are an important component of tight junction strands and may be responsible for the paracellular seal. This review highlights the current knowledge of claudins to barrier function and tight junction structure and suggests a model by which claudins and other tight junction proteins can drive assembly and stabilization of a lipid-based strand structure.
Introduction
Since their discovery in 1998 [1], claudin proteins have become a central focus of the tight junction research. It has become clear that expression of members of this large family of tetra-membrane spanning proteins modulates paracellular, i.e. tight junction, permeability to ions, water, in a size- and charge-selective manner [2–8]. Increases in paracellular conductance induced by specific claudins can be defined are either anion- or cation-selective [6, 9–13]. The conductance pathways that are enhanced by increased expression of pore-forming claudins are size-selective, and appear to only admit solutes and solvents with radii up to ~3.5Å [7, 8, 14–16]. These claudins are often referred to as “pore-forming” claudins. Other claudins have been described as “sealing” claudins [17, 18]. There is some evidence to support the idea that these claudins form paracellular seals, including the severe epidermal barrier defects in claudin-1-deficient mice [19] and the observation that expression of specific claudins reduces transepithelial ion conductance in cultured monolayers [20, 21]. However, while this is a convenient model, it may well be an oversimplification of a far more complex biology. In this review, we will explore the mechanisms by which claudins, other proteins, and lipids form and regulate the tight junction barrier, both at steady-state and in response to exogenous stimuli.
Claudins: Tight junction components, organizers, or both?
The initial report that identified claudins showed that claudin-1 and claudin-2 co-localized with occludin by fluorescence microscopy and were present within tight junction strands seen by freeze-fracture electron microscopy [1]. This was rapidly followed by the observation that, when expressed in fibroblasts, which lack tight junctions, claudin proteins concentrated at cell contact sites and induced formation of tight junction like strands [22]. This, along with the beaded appearance of tight junction strands was taken as evidence that the strands are composed primarily of claudins. However, it is important not to forget previous work concluding that tight junction strands are lipid-based [23–26] as well as more recent studies showing that tight junctions can be defined as low density, cholesterol- and glycolipid-rich, detergent-resistant membrane domains [27–31]. It may, therefore, be more accurate to think of claudins as essential organizers of tight junction strands. This view is supported by the observation that occludin and other members of the tight junction associated MARVEL protein (TAMP) family are recruited to strands by claudins [22, 32, 33].
Claudins as paracellular ion channels
Abundant data are available to support the conclusion that claudins form paracellular ion channels. Initial work demonstrated, for example, that the differences between MDCK cell lines characterized by high (MDCK I) and low (MDCK II) transepithelial electrical resistance (TER) were almost entirely explained by expression of claudin-2 in the latter, but not the former [16]. Specifically, claudin-2 expression in high resistance MDCK monolayers resulted in increased paracellular Na+ and K+ conductance without any effect on Cl− conductance or paracellular flux of larger solutes, including mannitol, lactulose, and 4kD dextran [2, 16]. This high capacity, size- and charge selective conductance route has been termed the pore pathway (Figure 1). Further study showed that treatment of cultured monolayers with the TH2 cytokine IL-13 induced claudin-2 expression as well as similar size- and charge-selective increases in paracellular permeability that could largely be prevented by inhibition of claudin-2 upregulation [8, 34]. Thus, while claudin-2 expression can regulate tight junction permeability to cations, it cannot explain differences in paracellular flux of larger molecules [2].
Figure 1. Distinct routes and regulatory mechanisms are involved in trans-tight junction flux.
Paracellular flux across the tight junction epithelium can defined as two distinct pathways, pore and leak. The cytokine IL-13 induces claudin-2 expression that, in turn, enhances water and small solute, e.g. ion, flux across the high capacity, size- and charge-slective pore pathway. Conversely, TNF activates myosin light chain kinase (MLCK) that triggers caveolin-mediated occludin removal from the tight junction. This results in increased macromolecular flux across the low capacitry, relatively nonslective leak pathway.
The ability to form charge- and size-selective channels has been linked to residues within the first extracellular loop of claudin proteins [3–5]. However, it is important to recognize that many, if not all, work exploring these issues in living cells and tissues is complicated by expression of claudins other than those mutated within the same cells. Despite this technical obstacle, mutagenesis studies have identified essential residues that define aspects of the conductance pore and are necessary for size-selectivity of claudin-2-based channels [14, 35, 36]. These studies have also mapped the sequences that form the interior of and entry to the claudin-2 channel [14, 15, 35–37].
The recently solved crystal structure of claudin-15 models of claudin interactions have provided new insight and allowed the generation of hypotheses that may define the protein domains that form the channel [38–40]. These models support the observation that claudin-2-based channels are size-selective, with a maximal radius of ~3.5 Ǻ, and can accommodate some cations that are larger than Na+, including methylamine and ethylamine (methylamine is only slight less permeable than Na+ [15]). Notably, tight junction channels traverse intercellular junctions and are, therefore, oriented parallel to the plasma membrane, i.e. orthogonal to transmembrane ion channels. The precise manner in which claudin monomers interact to form these channels and, potentially, the paracellular seal, remain areas of active investigation.
To date, most measures of tight junction conductance have relied on measurements across relatively large multicellular sheets that include many paracellular channels [41]. Higher resolution approaches, including measurements using scanning electrodes [42, 43], have been unable to detect single channel events. As a result, many have concluded that tight junction channels are static, i.e. are either open or closed, but do not regularly transition between these states. Recent work demonstrating that protein interactions at the tight junction are highly dynamic and are modulated during barrier regulation [33, 41, 44–49] has caused some to question this view. One recent report describes a modified patch-clamp approach that allowed development of a gigaOhm seal over the intercellular junction. This made it possible to detect of single channel opening and closing events [50, 51]. The frequency of these events correlated with claudin-2 expression, although the amplitude was similar in monolayers with low or high claudin-2 expression. These data suggest that paracellular channels formed by claudin-2 are actively gated. Although it has not been reported, the same is likely true for many other claudins, e.g. claudins 15 and 16 [3, 52, 53]. Differences in gating behavior could explain why, for example, mouse intestinal epithelia coordinately downregulate claudin-2 and upregulate claudin-15, both of which form paracellular Na+ channels, in the first month of life [54]. What is less clear, however, is the role claudins play in regulating macromolecular paracellular flux and, even more fundamentally, the manner in which they contribute to genesis of the tight junction barrier.
Functions of ‘sealing’ claudins
As noted above, expression of some claudins, e.g. claudin-4, can increase transepithelial electrical resistance, i.e. reduce paracellular ion conductance [20]. Thus, claudin-4 expression can enhance paracellular barrier function. It is not clear if this is due to a primary sealing property of claudin-4 or reflects the ability of claudin-4 to disrupt pores formed by other claudins. For example, claudin-4 expression in low resistance MDCK II monolayers, which express claudin-2, reduces paracellular Na+ flux but does not affect paracellular mannitol flux [20]. This is inconsistent with a general barrier-enhancing function of claudin 4 and, alternatively, suggests that claudin-4 may act by antagonizing claudin-2 function [4]. Such a conclusion is supported by the observation that claudin-4 reduces charge selectivity in claudin-2-expressing MDCK II, but not claudin-2-deficient LLCPK1, monolayers, despite reducing overall paracellular ion conductance in both [5]. One potential explanation for these observations is direct competitive inhibition of pore-forming claudins, e.g. claudin-2, by claudin-4. Alternatively, claudin-4 may regulate other tight junction components to inhibit claudin pore function [46, 55]. Either model is consistent with data indicating that claudin-4 can complex with claudin-8 to form a paracellular anion channel [10]. While these data cannot exclude a model in which claudin-4 directly seals the paracellular space, they do emphasize the importance of considering the cellular context, including which other claudin proteins are expressed, when assessing function of any individual claudin.
Regulation of paracellular macromolecular flux
In molecular terms, enhanced tight junction permeability to macromolecules, i.e. the leak pathway, has been most closely associated with occludin or ZO-1 knockdown in cultured monolayers in vitro [45, 48, 56–58]. Stimulus-induced occludin endocytosis causes similar increases in macromolecular paracellular flux, both in vitro [59, 60] and in vivo [61]. These changes are often linked to actomyosin cytoskeletal regulation and can, for example, be triggered by pro-inflammatory cytokines that activate myosin light chain kinase, e.g. tumor necrosis factor-α (TNF), LIGHT, IL-1β, [60, 62–66], as well as direct latrunculin A-induced actin depolymerization [62]. Consistent with the critical role of occludin in this process, blockade of occludin endocytosis prevents both cytokine and latrunculin A-induced macromolecular barrier loss in vitro [45, 59, 60] as well as TNF-induced barrier loss in vivo [61] (Figure 1). Moreover, in vivo occludin overexpression attenuates TNF-induced barrier loss [61], and the barrier defects observed in occludin-deficient monolayers are qualitatively and quantitatively similar to those observed after stimulus-induced occludin endocytosis [45, 56]. Consistent with this being the primary mechanism of TNF-induced barrier loss, TNF is unable to elicit further barrier loss in occludin-deficient monolayers [45, 58]. Thus, caveolar endocytosis and occludin removal from the tight junction are critical regulators of TNF-induced barrier loss. Although occasional reports have linked endocytosis of tight junction proteins, including occludin, to micropinocytosis and clathrin-coated pit-mediated endocytosis, these have only been observed in highly artificial systems, such as calcium chelation [67] or supraphysiologic interferon-γ exposure [68], while interferon-γ does not induce barrier loss in vivo [69]. In contrast, both pharmacologic inhibitors of caveolar endocytosis and caveolin-1 knockout prevent TNF-induced occludin internalization, barrier loss, and diarrhea in vitro and in vivo [60, 61].
Potential contributions of claudins to paracellular macromolecular flux
TNF has been variably reported to decrease or increase claudin-2 expression in vitro [55, 70, 71]. It is however notable that these studies treated monolayers with TNF or cocktails of IFNγ and TNF for 24 to 72 hrs, times which are more than sufficient to allow for compensatory changes in other tight junction proteins. In some cases, high TNF concentrations that also induced apoptosis were used, which further complicates analysis. Conversely, no claudin-2 upregulation was detectable when cultured monolayers were treated with TNF for only 4 hours, which was sufficient to induce barrier loss without apoptosis [8, 45, 63, 64, 66] and is similar to the kinetics of in vivo TNF-induced barrier loss, which also depends on MLCK-mediated occludin internalization and is claudin-2-independent [61, 62, 69, 72–75]. Moreover, unlike TNF-induced macromolecular, i.e. leak pathway, barrier loss, claudin-2-dependent increases in pore pathway flux are not affected by MLCK inhibition [8].
Despite the above, claudin-2 expression is not entirely unrelated to cytoskeletally-regulated paracellular macromolecular flux. One study, for example, found that transgenic, intestinal epithelial-specific expression of constitutively-active MLCK increased both macromolecular and charge-selective (cation) flux in mouse colon [8, 76]. This was associated with increases in mucosal IL-13 and epithelial claudin-2 expression, suggesting that a primary MLCK-induced defect in the paracellular barrier to macromolecular flux can elicit increases in claudin-2 expression in vivo [8]. Conversely, colonic epithelial claudin-2 upregulation was attenuated during chronic, immune-mediated colitis in mice lacking epithelial long MLCK, but restored upon epithelial-specific expression of constitutively-active MLCK [77]. Thus, while acute TNF-induced barrier loss is primarily, if not exclusively, due to MLCK-dependent occludin internalization, changes in claudin-2 and possibly claudin-4 expression can occur secondarily. Nevertheless, while claudins do not appear to contribute significantly to initial phases of TNF-induced increases in leak pathay flux, claudin expression may be critical to development of the paracellular macromolecular barrier. For example, claudin-1-deficient mice have a severely impaired epidermal barrier that allows markedly increased flux of a ~600 Da probe that likely crosses, at least in part, via the leak pathway [19].
How do claudins contribute to tight junction barrier function?
The clearest example of the relationship between claudins and tight junction barrier function is provided by a landmark study of a mouse epithelial line (Eph4) engineered to be deficient in both ZO-1 and ZO-2 [78]. These cells failed to develop tight junctions by several measures. First, they did not traffic claudin-3, and presumably other claudins, as well as occludin and JAM to the tight junction. In addition, these monolayers displayed negligible transepithelial electrical resistance (TER) and markedly increased paracellular flux of 40kD dextran, a leak pathway probe [78]. Further, monolayers of these ZO-1- and ZO-2-deficient cells did not develop the strands that characterize freeze-fracture electron microscopic views of the tight junction. Nevertheless, transmission electron micrographs demonstrated closely-apposed plasma membranes, consistent with tight junctions, at the expected location. Moreover, representative apical and basolateral membrane proteins were correctly distributed, suggesting that “fence” function remained intact [78], suggesting that the tight junction is not required for maintenance of plasma membrane domains [79, 80].
The data above could be taken as evidence that ZO-1- and ZO-2-mediated recruitment of claudins is necessary for tight junction assembly and paracellular barrier function. Consistent with this, re-expression of ZO-1 or ZO-2 restored transepithelial resistance, freeze fracture strand formation, and claudin-3 recruitment to tight junctions in Eph4 cells [78]. Finally, an elegant approach that drove the amino terminal half of ZO-1 to lateral membrane resulted in focal claudin-3 recruitment as well as formation of complex freeze-fracture strands at these sites. However, it is not known if this retargeting of ZO-1 to lateral membranes was sufficient to recruit other tight junction proteins, e.g. occludin and JAM, or restore paracellular barrier function. While these would be important questions to address, the story is complicated by observations that combined suppression of ZO-1 and ZO-2 expression does not block tight junction assembly in MDCK cells [81–85], and that the PDZ1 domain of ZO-1 is not required for rescue of ZO-1 function in MDCK cells [85]. This distinction between MDCK and Eph4 cells has been ascribed to ZO-3 expression in the former, but not the latter. However, that has not been formally demonstrated by ZO-3 knockdown in ZO-1-and ZO-2-deficient MDCK cells. Nevertheless, these elegant studies do demonstrate that, in Eph4 cells, ZO-1 and ZO-2 are critical for recruitment of claudin-3, occludin, and JAM to the tight junction and in turn necessary for strand formation. Together with other studies showing that claudins 1 and 2 recruit occludin, tricellulin, and marvelD3 to tight junction strands [22, 33] and that this results in altered tight junction strand architecture [33], these data indicate that the claudin, zonula occludens, and TAMP families play cooperative roles in the development of tight junctions.
Proteins, lipids, or both?
The data discussed above as well as other studies definitively demonstrate that claudins and other tight junction proteins, including the tight junction-associated marvel proteins (TAMPs) occludin, tricellulin, and marvelD3, as well scaffolding proteins, e.g. ZO-1, ZO-2, and cingulin, are important for correct tight junction assembly and function. It is also clear that claudin proteins form channels that mediate flux across the pore pathway, and that occludin, tricellulin, ZO-1, and ZO-2 contribute to development and regulation of the barrier to macromolecular flux. However, the nature of the barrier remains to be defined.
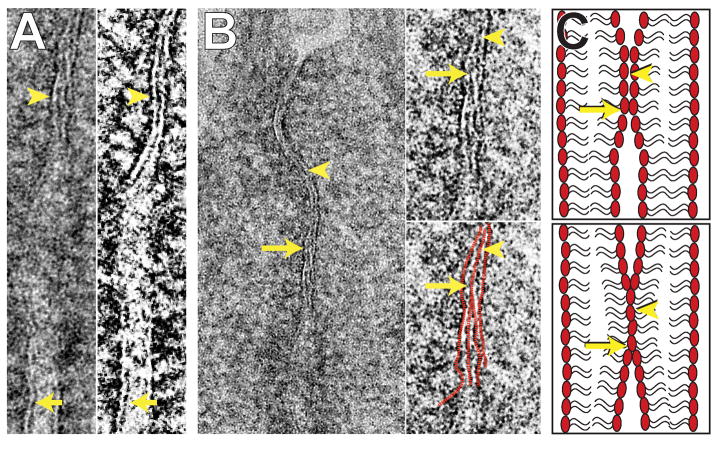
Many have proposed that the barrier is formed by cis interactions between claudins within an individual cell and trans- interactions between claudins in adjacent cells, i.e. across the paracellular space [33, 38, 86–90]. However, several points argue against a model where the barrier is composed solely of tight junction proteins. First, fluorescence microscopy, including super resolution microscopy, shows beaded distributions of individual proteins at the tight junction [91]. In the case of claudins, these may correspond to particles within tight junction strands, as seen by freeze-fracture immunoelectron microscopy [1]. Thus, morphological analyses of individual protein species, including claudins, as well as the beaded distribution of particles within strands suggest that the protein distribution at the tight junction is discontinuous. This makes it difficult to understand how the discontinuities do not allow for a shunt pathway across the tight junction at these sites. In contrast, both freeze-fracture and transmission electron micrographs demonstrate the continuity of strands and tri-laminar structures (Figure 2A), respectively, at the tight junction.
Figure 2. Ultrastructure suggests that membrane fusion may occur at the tight junciton.
A. Transmission electron microscopy (left) and tomogropahy (right) of intestinal epithelia porcessed using high pressure freezing/freeze substitution (HPF/FS) to avoid artifacts induced by chemical fixatives show trilaminar structure at the tight junction (arrowhead) that is distinct from the dual bilaminar memrbanes seen in other regions of cell apposition. B. Left. Transmission electron microscopy of mouse jejunal enterocytes processed as in A show the trilaminar tight junction structure (arrowhead) and a subjacent Y-shaped structure (arrow) where the central layer bifurcates into two outer plasma membrane leaflets as the tight junction transitions toward the adherens junction. Right. High magnification inset of the site of central layer (arrowhead) bifurcation (arrow). The layers are traced in red in the lower panel. C. Two potential models to explain the ultrastructure observed are shown. Membranes may merely be so closely apposed that the outer leaflets of the lipid bilayers are indistiguishable, but still separate (upper panel). Alternatively, the outer plasma membrane leaflets may actually fuse (lower panel). The sites of the tri-laminar structure (arrowheads) and subjacent bifurcation (arrows) are indicated.
Prior to the discovery of tight junction proteins, a series of studies concluded that tight junction strands are, at least in part, composed of inverted micelles, i.e. lipids arranged in hexagonal HII arrays [23–26, 92–96]. This is consistent with early interpretations of transmission electron micrographs that described tight junctions as “a region in which the membranes of adjoining cells come together and fuse [97].” This comment was accompanied by a footnote explaining that “By membrane fusion we mean the mergence of the outer leaflets of the apposed membranes into a single leaflet of about the same thickness (20 to 30 Å) as each of the contributory layers.” This description was accompanied by images of Y-shaped profiles at the tops and bottoms of tight junctions where it became impossible to resolve separate outer leaflets of adjacent cells and these were replaced by a “fusion line” [97]. The inner leaflets remained intact at these sites (Figure 2B).
It is also notable that, even in glutaraldehyde-fixed specimens, tight junction strands seen by freeze-fracture electron microscopy are converted into discontinuous arrays of intramembranous particles by impregnation with 100% glycerol [92]. Because proteins are heavily crosslinked by glutaraldehyde fixation, this result suggests that non-protein components are important to strand structure. Further analyses from at least two different groups [23, 24] showed that, particularly where ice crystal artifacts were limited, “Two cylinders, one belonging to each of the pair of membranes participating in the tight junction, are seen” [23]. While the identification of protein components shifted focus away from lipids, the observations above that suggest a critical lipid component have never been explained by a protein-limited model of tight junction structure.
In addition to morphological analyses, such as those above, it is well-recognized that the tight junction is a raft-like, cholesterol-rich lipid domain [28, 98, 99]. Indeed, the recognition that tight and adherens junction structures and proteins were enriched in detergent-insoluble, low-density membrane fractions was a breakthrough that allowed generation of the antigen pools that led to identification of occludin and the claudins [1, 100–103]. Further supporting the importance of lipids in tight junction structure, tight junction-enriched, hepatocyte canalicular fractions isolated without the use of detergents showed that the characteristic tri-laminar structure of the tight junction could be disrupted by deoxycholate, even though perijunctional microfilaments survived this treatment [104]. Finally, MLCK activation, which as discussed above enhances paracellular permeability, alters the density, and presumably lipid-protein composition of detergent-insoluble, low density tight junction fractions [31]. MLCK activation also alters the dynamics of ZO-1 exchange at the tight junction in a manner that depends on interactions mediated by the ZO-1 actin binding region [48]. These observations may link protein interactions to tight junction lipid composition.
A role for proteins in organizing lipids at the tight junction is further suggested by the observation that strands form at sites of membrane apposition in fibroblasts transected to express claudins [105]. It is however important to recognize that these strands are not tight junctions, as they are induced by claudins tagged with EGFP at the carboxyl terminus, which blocks the PDZ-mediated interactions with ZO-1 and ZO-2 that are necessary for claudin trafficking in epithelial cells, and do not form barriers. Similarly, ZO-1/ZO-2 deficient epithelia, strands formed in lateral membranes after trafficking of ZO-1 PDZ domain dimers to those sites, although barrier function was not assessed [78].
A role for proteins in organizing lipids at the tight junction is suggested by the observation that strands form at sites of membrane apposition in fibroblasts transected to express claudins [105]. It is however important to recognize that these strands are not tight junctions, as they are induced by claudins tagged with EGFP at the carboxyl terminus, which blocks the PDZ-mediated interactions with ZO-1 and ZO-2 that are necessary for claudin trafficking in epithelial cells, and do not form barriers. Nevertheless, the complex movements of these tight junction-like strands is suggestive of lipid movement and raises the possibility that similar behaviors may also characterize bona fide tight junction strands within epithelia. The idea of continuous tight junction remodeling at steady-state is supported by fluorescence recovery after photobleaching studies that show rapid exchange of many proteins within the epithelial tight junction [47, 49, 89]. It is therefore possible that some component of fluorescence recovery is related to the dynamic behavior of tight junction strands. It cannot however be that simple, as the limited exchange of claudins 1 and 2 within both epithelial tight junctions [46, 49] and tight junction-like strands, even when the latter are actively moving [33, 105], indicates that the mobility of strands and exchange of proteins within the strands are physiochemically distinct.
A cooperative model that integrates tight junction structure and function
We propose a model wherein the interaction between tight junction proteins in combination with membrane lipids organized as inverted micelles results in the formation of a unique lipid-protein structure that allows for both paracellular permeability barrier-forming properties of the tight junction. Abundant data, including those presented above, are consistent with a cooperative model in which ZO-1 and ZO-2 recruit claudins to the nascent tight junction and, in turn, claudins begin to organize the specialized lipid domains within the tight junction. Recent functional and structural data indicating that transmembrane domains are critical for integration of claudins into the tight junction [86, 88, 106] as well as studies demonstrating that palmitoylation is essential for claudin incorporation into and stabilization at the tight junction [107, 108] are consistent with this model. One could posit that these specialized claudin-lipid domains, i.e. strands, then recruit additional proteins, including occludin and other TAMPs, which is supported by the observation that TAMPs are recruited into claudin-based fibroblast strands [22, 32, 33] (Figure 3). Such a model would be consistent with the observed mobility of strands in fibroblasts despite limited exchange of claudin-1 within these strands.
Figure 3. A lipid-protein model of tight junction structure that may unify ultrastructural, biochemical, and functional data.
This model assumes that the outer leaflets of adjacent plasma membranes fuse at the tight junction. Within these areas, lipids may organize in HII phase to form inverted micelles and even tubes. These bulges within the fusion site may represent the strands that characterize freeze-fracture images of the tight junction. As discussed in the text, we propose that the membrane fusion and HII phase transition depend on the unique lipid compoisiton of the tight junction as well as proteins, e.g. claudins, that organize these lipids to establish a barrier while also forming a trans-tight junction ion channel. The model suggests that the claudins actually form the pore that mediates pore pathway flux. Larger, micelles may form a conduit for the leak pathway. Occludin may serve to limit the stability of these micelles and macromolecular flux. This may explain enhanced leak pathway flux upon occludin depletion.
This model suggests that lipids are essential to tight junction structure and function. In such a model, cholesterol within these domains likely enhances membrane fluidity. When taken in this context, studies showing that cholesterol depletion by inhibition of cholesterol synthesis alters tight junction protein extractability [107] and enhances tight junction barrier function [28, 29] can be interpreted to mean that paracellular permeability requires such membrane fluidity. This is supported by studies showing reduced in paracellular conductance and occludin diffusion within the tight junction at temperatures between 14°C and 22°C [49, 109, 110], temperatures at which a phase transition is expected to occur. Interestingly, acute cholesterol removal using methyl-β-cyclodextrin increases paracellular conductance, i.e. reduces TER [49]. This observation confirms that the lipid composition of tight junction microdomains is critical and suggests that acute perturbation of these domains interferes with maintenance of the structures responsible for barrier function. While proteins are certainly affected, for example methyl-β-cyclodextrin markedly reduced occludin diffusion within the tight junction [49], the effect of cholesterol depletion on membrane structure is likely to be far greater. Thus, while indirect, these data also support speculation that lipids form the tight junction barrier.
Is it time to reconsider membrane fusion as a component of tight junction assembly?
On the basis of the data above and other work it is tempting to revisit the idea that lipid structures are of critical importance to tight junction structure and barrier function [111]. Could the earliest observations interpreted as membrane fusion [97] and lipid-based strands [23, 24, 92, 112] have been correct? If so, one might anticipate that lipids, and even some proteins, could diffuse between cells at these sites. However, several reports showing that a bulky glycolipid, Forssman antigen, suggest that such lipid diffusion does not occur and, therefore, that tight junctions are not sites of membrane fusion [113–115]. It must, however, be recognized by all that it is difficult to prove a negative conclusion. Further, subsequent studies documented diffusion of smaller lipids between cells [116–118]. As expected for diffusion within the tight junction, lipid transfer did not occur at temperatures below 10°C or when tight junctions were disrupted [116–118]. It therefore seems likely that the Forssman antigen failed to diffuse because of its size rather than the absence of intercellular lipid diffusion at the tight junction. Sufficient evidence is therefore available to re-consider the possibility that plasma membrane outer leaflets of adjacent cells do fuse at the tight junction.
Even if membrane fusion does occur at tight junctions, this does not demonstrate that the strands that have been the focus of so much attention are composed of lipids. Nevertheless, the observation that remarkable tight junction-like strands, complete with particles resembling those within actual tight junction strands, can form in artificial membranes created by mixing defined lipids, cholesterol, and, in some cases, Ca2+ suggests that this may be possible [24, 93]. A model of strands as lipidic structures organized by tight junction proteins is also consistent with reports that modified expression of either claudins or TAMPs [33, 119] or changes in membrane lipid content [120] can dramatically alter the appearance and organization of strand networks.
The idea that lipid structures form the barrier is attractive, as lipids are already widely used in biological systems to insulate distinct compartments from one another, e.g. in the nervous system. Further, it would explain how claudins contribute to assembly of tight junctions and development of barrier function without the complexities of a model in which claudins both create pores and form a barrier.
A potential explanation for paracellular macromolecular flux
If claudins define flux across the pore pathway, what then defines leak pathway flux, and how can this be interpreted in the context of a lipid strand model of the tight junction? One idea, is that breaks within and fusions between fibroblast strands result in transient, focal barrier loss that allows step-by-step flux of solutes through the anastomosing network [105]. This is a reasonable hypothesis that could also be consistent with occasional transfer of claudins between cells [121]. However, this model would predict that the leak pathway is nonselective, while data show that the leak pathway is size selective, but allows flux of molecules too large to fit within claudin pores. For example, occludin knockdown results in increased flux across a paracellular pathway with a radius of ~60Å [45]. While much larger than the ~3.5Å radius of claudin pores, this remains a highly selective barrier.
An alternative explanation for the leak pathway can be developed based on the lipidic tight junction strand model proposed over 30 years ago [23, 111], before the first tight proteins were discovered. This model can also explain the logarithmic relationship between strand number and paracellular macromolecular flux [122, 123] as well as observed impact of intercellular junction geometry on barrier function [124].
As proposed previously [23–26, 93–96, 111, 117], a lipid-based model of tight junction structure can be based on the idea that strands are composed of inverted micelles organized into hexagonal arrays. The dynamic fusion and fission of these strands, as observed in fibroblasts expressing claudins [105], is consistent with this model and may also explain the leak pathway. The inner dimensions of these micelles could be consistent with the 60Å radius paracellular pathway that is partially dependent on occludin function [45]. Together, these points can also explain the limited conductance of the leak pathway, as these tubular structures are expected to be unstable and to exist in continuity for only brief intervals. Finally, if one considers that a compact lipid might stabilize these arrays, the model is consistent with marked increase in paracellular 4kD dextran flux, with no effect on ion conductance, that follows linoleic acid enrichment [120].
Concluding thoughts
The discussion above is speculative and based on studies spanning 40 years. However, the model presented is consistent with data from morphological, functional, and structural analyses of tight junctions and tight junction proteins. Although the general hypothesis of lipidic tight junction strands is not novel, some of the refinements proposed here are. Nevertheless, it remains an untested model. A formal analysis of these ideas will be difficult without technological advances, including new methods of isolating pure tight junction strands or claudin-dependent reconstitution of strands within model membranes. Regardless, these data should give one pause as the field continues to move towards a protein-centric view of the tight junction.
Acknowledgments
We thank members of our laboratory whose data and discussion have stimulated development of the hypothesis presented. Work in the authors’ laboratory is supported by the NIDDK (R01DK61931, R01DK68271), The University of Chicago Cancer Center (P30CA14599), and The University of Chicago Institute for Translational Medicine (UL1RR024999).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–50. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–76. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 3.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol - Cell Physiol. 2002;283:C142–7. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 4.Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol - Cell Physiol. 2003;284:C1346–54. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 5.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol - Renal Physiol. 2003;285:F1078–84. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 6.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol - Renal Physiol. 2006;291:F1288–99. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 7.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 8.Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–46. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, et al. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci. 2009;122:1507–17. doi: 10.1242/jcs.040113. [DOI] [PubMed] [Google Scholar]
- 10.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci USA. 2010;107:18010–5. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krug SM, Gunzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, et al. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell Mol Life Sci. 2012;69:2765–78. doi: 10.1007/s00018-012-0949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA. 2010;107:8011–6. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem. 2003;278:17350–9. doi: 10.1074/jbc.M213286200. [DOI] [PubMed] [Google Scholar]
- 14.Angelow S, Yu AS. Structure-function studies of claudin extracellular domains by cysteine-scanning mutagenesis. J Biol Chem. 2009;284:29205–17. doi: 10.1074/jbc.M109.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, et al. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–27. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–72. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milatz S, Krug SM, Rosenthal R, Gunzel D, Muller D, Schulzke JD, et al. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta. 2010;1798:2048–57. doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Gunzel D, Fromm M. Claudins and other tight junction proteins. Comprehensive Physiology. 2012;2:1819–52. doi: 10.1002/cphy.c110045. [DOI] [PubMed] [Google Scholar]
- 19.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–27. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Skare IB, et al. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J Cell Sci. 2000;113(Pt 19):3387–98. doi: 10.1242/jcs.113.19.3387. [DOI] [PubMed] [Google Scholar]
- 22.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kachar B, Reese TS. Evidence for the lipidic nature of tight junction strands. Nature. 1982;296:464–6. doi: 10.1038/296464a0. [DOI] [PubMed] [Google Scholar]
- 24.Meyer HW. Tight junction strands are lipidic cylinders. Naturwissenschaften. 1983;70:251–2. doi: 10.1007/BF00405446. [DOI] [PubMed] [Google Scholar]
- 25.Hein M, Madefessel C, Haag B, Teichmann K, Post A, Galla HJ. Implications of a non-lamellar lipid phase for the tight junction stability. Part II: Reversible modulation of transepithelial resistance in high and low resistance MDCK-cells by basic amino acids, Ca2+, protamine and protons. Chem Phys Lipids. 1992;63:223–33. doi: 10.1016/0009-3084(92)90038-q. [DOI] [PubMed] [Google Scholar]
- 26.Hein M, Post A, Galla HJ. Implications of a non-lamellar lipid phase for the tight junction stability. Part I: Influence of basic amino acids, pH and protamine on the bilayer-hexagonal II phase behaviour of PS-containing PE membranes. Chem Phys Lipids. 1992;63:213–21. doi: 10.1016/0009-3084(92)90037-p. [DOI] [PubMed] [Google Scholar]
- 27.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, et al. Tight junctions are membrane microdomains. J Cell Sci. 2000;113:1771–81. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- 28.Lynch RD, Tkachuk LJ, Ji X, Rabito CA, Schneeberger EE. Depleting cell cholesterol alters calcium-induced assembly of tight junctions by monolayers of MDCK cells. Eur J Cell Biol. 1993;60:21–30. [PubMed] [Google Scholar]
- 29.Stankewich MC, Francis SA, Vu QU, Schneeberger EE, Lynch RD. Alterations in cell cholesterol content modulate Ca(2+)-induced tight junction assembly by MDCK cells. Lipids. 1996;31:817–28. doi: 10.1007/BF02522977. [DOI] [PubMed] [Google Scholar]
- 30.Francis SA, Kelly JM, McCormack J, Rogers RA, Lai J, Schneeberger EE, et al. Rapid reduction of MDCK cell cholesterol by methyl-beta-cyclodextrin alters steady state transepithelial electrical resistance. Eur J Cell Biol. 1999;78:473–84. doi: 10.1016/s0171-9335(99)80074-0. [DOI] [PubMed] [Google Scholar]
- 31.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 32.Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, et al. Tight junction-associated MARVEL proteins marvelD3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200–13. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cording J, Berg J, Kading N, Bellmann C, Tscheik C, Westphal JK, et al. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci. 2013;126:554–64. doi: 10.1242/jcs.114306. [DOI] [PubMed] [Google Scholar]
- 34.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterol. 2005;129:550–64. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Angelow S, Linge A, Zhuo M, Yu AS. Claudin-2 pore function requires an intramolecular disulfide bond between two conserved extracellular cysteines. Am J Physiol - Cell Physiol. 2013;305:C190–6. doi: 10.1152/ajpcell.00074.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Zhuo M, Pei L, Rajagopal M, Yu AS. Comprehensive cysteine-scanning mutagenesis reveals Claudin-2 pore-lining residues with different intrapore locations. J Biol Chem. 2014;289:6475–84. doi: 10.1074/jbc.M113.536888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Zhuo M, Pei L, Yu AS. Conserved aromatic residue confers cation selectivity in claudin-2 and claudin-10b. J Biol Chem. 2013;288:22790–7. doi: 10.1074/jbc.M113.484238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki H, Tani K, Tamura A, Tsukita S, Fujiyoshi Y. Model for the Architecture of Claudin-Based Paracellular Ion Channels through Tight Junctions. J Mol Biol. 2015;427:291–7. doi: 10.1016/j.jmb.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Saitoh Y, Suzuki H, Tani K, Nishikawa K, Irie K, Ogura Y, et al. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science. 2015;347:775–8. doi: 10.1126/science.1261833. [DOI] [PubMed] [Google Scholar]
- 40.Tamura A, Tsukita S. Paracellular barrier and channel functions of TJ claudins in organizing biological systems: Advances in the field of barriology revealed in knockout mice. Semin Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 41.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fromm M, Krug SM, Zeissig S, Richter JF, Rosenthal R, Schulzke JD, et al. High-resolution analysis of barrier function. Ann N Y Acad Sci. 2009;1165:74–81. doi: 10.1111/j.1749-6632.2009.04047.x. [DOI] [PubMed] [Google Scholar]
- 43.Gitter AH, Bertog M, Schulzke J, Fromm M. Measurement of paracellular epithelial conductivity by conductance scanning. Pflugers Arch. 1997;434:830–40. doi: 10.1007/s004240050472. [DOI] [PubMed] [Google Scholar]
- 44.Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol. 2014;36C:204–12. doi: 10.1016/j.semcdb.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buschmann MM, Shen L, Rajapakse H, Raleigh DR, Wang Y, Wang Y, et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell. 2013;24:3056–68. doi: 10.1091/mbc.E12-09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193:565–82. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paschoud S, Yu D, Pulimeno P, Jond L, Turner JR, Citi S. Cingulin and paracingulin show similar dynamic behaviour, but are recruited independently to junctions. Mol Membr Biol. 2011;28:123–35. doi: 10.3109/09687688.2010.538937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, et al. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci USA. 2010;107:8237–41. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–95. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber CR. Dynamic properties of the tight junction barrier. Ann N Y Acad Sci. 2012;1257:77–84. doi: 10.1111/j.1749-6632.2012.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber CR, Shen L, Nelson D, Yu A, Wang Y, Wang Y, et al. Patch Clamp Recordings Reveal Paracellular (Tight Junction) Ion Channels. Gastroenterol. 2012;142:S-108–S-9. [Google Scholar]
- 52.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–6. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 53.Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterol. 2011;140:913–23. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6:581–8. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Capaldo CT, Farkas AE, Hilgarth RS, Krug SM, Wolf MF, Benedik JK, et al. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol Biol Cell. 2014;25:2710–9. doi: 10.1091/mbc.E14-02-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, et al. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol - Cell Physiol. 2005;288:C1231–41. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 57.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–40. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010:2844–52. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–36. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterol. 2007;132:2383–94. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–26. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–15. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterol. 2006;131:1153–63. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterol. 2002;123:163–72. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 65.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–9. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–19. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–88. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–33. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 69.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–94. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, et al. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci. 2010;123:4145–55. doi: 10.1242/jcs.070896. [DOI] [PubMed] [Google Scholar]
- 71.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–62. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 72.Nishida M, Yoshida M, Nishiumi S, Furuse M, Azuma T. Claudin-2 Regulates Colorectal Inflammation via Myosin Light Chain Kinase-Dependent Signaling. Dig Dis Sci. 2013 doi: 10.1007/s10620-012-2535-3. [DOI] [PubMed] [Google Scholar]
- 73.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-alpha modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol - Gastrointest Liver Physiol. 2005;288:G422–30. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 74.Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-alpha-induced regulation of myosin light chain kinase gene activity. J Cell Mol Med. 2008;12:1331–46. doi: 10.1111/j.1582-4934.2008.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graham WV, Wang F, Clayburgh DR, Cheng JX, Yoon B, Wang Y, et al. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205–15. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- 76.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterol. 2009;136:551–63. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterol. 2013;145:407–15. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–54. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 79.Hernandez S, Chavez Munguia B, Gonzalez-Mariscal L. ZO-2 silencing in epithelial cells perturbs the gate and fence function of tight junctions and leads to an atypical monolayer architecture. Exp Cell Res. 2007;313:1533–47. doi: 10.1016/j.yexcr.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 80.Mandel LJ, Bacallao R, Zampighi G. Uncoupling of the molecular ‘fence’ and paracellular ‘gate’ functions in epithelial tight junctions. Nature. 1993;361:552–5. doi: 10.1038/361552a0. [DOI] [PubMed] [Google Scholar]
- 81.Ikenouchi J, Umeda K, Tsukita S, Furuse M. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 2007;176:779–86. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci. 2009;1165:113–20. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fanning AS, Van Itallie CM, Anderson JM. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell. 2012;23:577–90. doi: 10.1091/mbc.E11-09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodgers LS, Beam MT, Anderson JM, Fanning AS. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J Cell Sci. 2013;126:1565–75. doi: 10.1242/jcs.113399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McNeil E, Capaldo CT, Macara IG. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2006;17:1922–32. doi: 10.1091/mbc.E05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suzuki H, Nishizawa T, Tani K, Yamazaki Y, Tamura A, Ishitani R, et al. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science. 2014;344:304–7. doi: 10.1126/science.1248571. [DOI] [PubMed] [Google Scholar]
- 87.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 88.Rossa J, Ploeger C, Vorreiter F, Saleh T, Protze J, Gunzel D, et al. Claudin-3 and claudin-5 protein folding and assembly into the tight junction are controlled by non-conserved residues in the transmembrane 3 (TM3) and extracellular loop 2 (ECL2) segments. J Biol Chem. 2014;289:7641–53. doi: 10.1074/jbc.M113.531012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piontek J, Fritzsche S, Cording J, Richter S, Hartwig J, Walter M, et al. Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell Mol Life Sci. 2011;68:3903–18. doi: 10.1007/s00018-011-0680-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, et al. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22:146–58. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 91.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–49. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pinto da Silva P, Kachar B. On tight-junction structure. Cell. 1982;28:441–50. doi: 10.1016/0092-8674(82)90198-2. [DOI] [PubMed] [Google Scholar]
- 93.Van Venetie R, Verkleij AJ. Analysis of the hexagonal II phase and its relations to lipidic particles and the lamellar phase. A freeze-fracture study. Biochim Biophys Acta. 1981;645:262–9. doi: 10.1016/0005-2736(81)90197-8. [DOI] [PubMed] [Google Scholar]
- 94.Verkleij AJ, Momvers C, Leunissen-Bijvelt J, Ververgaert PH. Lipidic intramembranous particles. Nature. 1979;279:162–3. doi: 10.1038/279162a0. [DOI] [PubMed] [Google Scholar]
- 95.Kinnunen PK. Fusion of lipid bilayers: a model involving mechanistic connection to HII phase forming lipids. Chem Phys Lipids. 1992;63:251–8. doi: 10.1016/0009-3084(92)90041-m. [DOI] [PubMed] [Google Scholar]
- 96.Marrink SJ, Mark AE. Molecular view of hexagonal phase formation in phospholipid membranes. Biophys J. 2004;87:3894–900. doi: 10.1529/biophysj.104.048710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farquhar M, Palade G. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feltkamp CA, Van der Waerden AW. Junction formation between cultured normal rat hepatocytes. An ultrastructural study on the presence of cholesterol and the structure of developing tight-junction strands. J Cell Sci. 1983;63:271–86. doi: 10.1242/jcs.63.1.271. [DOI] [PubMed] [Google Scholar]
- 99.Kan FW. Cytochemical evidence for the presence of phospholipids in epithelial tight junction strands. J Histochem Cytochem. 1993;41:649–56. doi: 10.1177/41.5.8468446. [DOI] [PubMed] [Google Scholar]
- 100.Tsukita S, Tsukita S. Isolation of cell-to-cell adherens junctions from rat liver. J Cell Biol. 1989;108:31–41. doi: 10.1083/jcb.108.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Itoh M, Yonemura S, Nagafuchi A, Tsukita S, Tsukita S. A 220-Kd Undercoat-Constitutive Protein - Its Specific Localization at Cadherin-Based Cell Cell-Adhesion Sites. J Cell Biol. 1991;115:1449–62. doi: 10.1083/jcb.115.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- 104.Stevenson BR, Goodenough DA. Zonulae occludentes in junctional complex-enriched fractions from mouse liver: preliminary morphological and biochemical characterization. J Cell Biol. 1984;98:1209–21. doi: 10.1083/jcb.98.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc Natl Acad Sci USA. 2003;100:3971–6. doi: 10.1073/pnas.0630649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rossa J, Protze J, Kern C, Piontek A, Gunzel D, Krause G, et al. Molecular and structural transmembrane determinants critical for embedding claudin-5 into tight junctions reveal a distinct four-helix bundle arrangement. Biochem J. 2014;464:49–60. doi: 10.1042/BJ20140431. [DOI] [PubMed] [Google Scholar]
- 107.Lynch RD, Francis SA, McCarthy KM, Casas E, Thiele C, Schneeberger EE. Cholesterol depletion alters detergent-specific solubility profiles of selected tight junction proteins and the phosphorylation of occludin. Exp Cell Res. 2007;313:2597–610. doi: 10.1016/j.yexcr.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci. 2005;118:1427–36. doi: 10.1242/jcs.01735. [DOI] [PubMed] [Google Scholar]
- 109.Martinez-Palomo A, Meza I, Beaty G, Cereijido M. Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol. 1980;87:736–45. doi: 10.1083/jcb.87.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gonzalez-Mariscal L, Chavez de Ramirez B, Cereijido M. Effect of temperature on the occluding junctions of monolayers of epithelioid cells (MDCK) J Membr Biol. 1984;79:175–84. doi: 10.1007/BF01872121. [DOI] [PubMed] [Google Scholar]
- 111.Lee DB, Jamgotchian N, Allen SG, Abeles MB, Ward HJ. A lipid-protein hybrid model for tight junction. Am J Physiol - Renal Physiol. 2008;295:F1601–12. doi: 10.1152/ajprenal.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Staehelin LA, Mukherjee TM, Williams AW. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma. 1969;67:165–84. doi: 10.1007/BF01248737. [DOI] [PubMed] [Google Scholar]
- 113.Nichols GE, Borgman CA, Young WW., Jr On tight junction structure: Forssman glycolipid does not flow between MDCK cells in an intact epithelial monolayer. Biochem Biophys Res Commun. 1986;138:1163–9. doi: 10.1016/s0006-291x(86)80404-1. [DOI] [PubMed] [Google Scholar]
- 114.van Genderen IL, van Meer G, Slot JW, Geuze HJ, Voorhout WF. Subcellular localization of Forssman glycolipid in epithelial MDCK cells by immuno-electronmicroscopy after freeze-substitution. J Cell Biol. 1991;115:1009–19. doi: 10.1083/jcb.115.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Meer G, Gumbiner B, Simons K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature. 1986;322:639–41. doi: 10.1038/322639a0. [DOI] [PubMed] [Google Scholar]
- 116.Laffafian I, Hallett MB. Lipid-protein cargo transfer: a mode of direct cell-to-cell communication for lipids and their associated proteins. J Cell Physiol. 2007;210:336–42. doi: 10.1002/jcp.20851. [DOI] [PubMed] [Google Scholar]
- 117.Grebenkamper K, Galla HJ. Translational diffusion measurements of a fluorescent phospholipid between MDCK-I cells support the lipid model of the tight junctions. Chem Phys Lipids. 1994;71:133–43. doi: 10.1016/0009-3084(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 118.Turin L, Behe P, Plonsky I, Dunina-Barkovskaya A. Hydrophobic ion transfer between membranes of adjacent hepatocytes: a possible probe of tight junction structure. Proc Natl Acad Sci USA. 1991;88:9365–9. doi: 10.1073/pnas.88.20.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamazaki Y, Tokumasu R, Kimura H, Tsukita S. Role of claudin species-specific dynamics in reconstitution and remodeling of the zonula occludens. Mol Biol Cell. 2011;22:1495–504. doi: 10.1091/mbc.E10-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Calderon V, Lazaro A, Contreras RG, Shoshani L, Flores-Maldonado C, Gonzalez-Mariscal L, et al. Tight junctions and the experimental modifications of lipid content. J Membr Biol. 1998;164:59–69. doi: 10.1007/s002329900393. [DOI] [PubMed] [Google Scholar]
- 121.Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci. 2004;117:1247–57. doi: 10.1242/jcs.00972. [DOI] [PubMed] [Google Scholar]
- 122.Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Claude P. Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J Membr Biol. 1978;39:219–32. doi: 10.1007/BF01870332. [DOI] [PubMed] [Google Scholar]
- 124.Marcial MA, Carlson SL, Madara JL. Partitioning of paracellular conductance along the ileal crypt-villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J Membr Biol. 1984;80:59–70. doi: 10.1007/BF01868690. [DOI] [PubMed] [Google Scholar]