Abstract
Antibodies (inhibitors) developed by hemophilia B patients against coagulation factor IX (FIX) are challenging to eliminate because of anaphylaxis or nephrotic syndrome after continued infusion. To address this urgent unmet medical need, FIX fused with a transmucosal carrier (CTB) was produced in a commercial lettuce (Simpson Elite) cultivar using species specific chloroplast vectors regulated by endogenous psbA sequences. CTB-FIX (~1mg/g) in lyophilized cells was stable with proper folding, disulfide bonds and pentamer assembly when stored ~2 years at ambient temperature. Feeding lettuce cells to hemophilia B mice delivered CTB-FIX efficiently to the gut immune system, induced LAP+ regulatory T cells and suppressed inhibitor/IgE formation and anaphylaxis against FIX. Lyophilized cells enabled 10-fold dose escalation studies and successful induction of oral tolerance was observed in all tested doses. Induction of tolerance in such a broad dose range should enable oral delivery to patients of different age groups and diverse genetic background. Using Fraunhofer cGMP hydroponic system, ~870 kg fresh or 43.5 kg dry weight can be harvested per 1000 ft2 per annum yielding 24,000–36,000 doses for 20-kg pediatric patients, enabling first commercial development of an oral drug, addressing prohibitively expensive purification, cold storage/transportation and short shelf life of current protein drugs.
Keywords: cGMP plant production, chloroplast, hemophilia, lettuce, molecular pharming, oral tolerance
1. Introduction
Biopharmaceuticals produced in current systems are prohibitively expensive and are not affordable for large majority of the global population. The cost of protein drugs ($140 billion in 2013) exceeds GDP of >75% of countries around the globe, making them unaffordable in these countries [1]. One third of global population earning <$2 per day can’t afford any protein drug. Although recombinant insulin is sold commercially for five decades, it is still not affordable for a large majority of global population. This is because of their production in prohibitively expensive fermenters, purification, cold storage/ transportation, short shelf life and sterile delivery methods.
Production of biopharmaceutical proteins in plants provides a cost-effective solution. Oral delivery of protein drugs bioencapsulated in plant cells significantly reduces downstream processing costs by eliminating expensive purification, cold storage/transportation, and short shelf life [2–4]. Plant cells expressing therapeutic proteins can be inexpensively processed by lyophilization and stored at room temperature for several months or years without impacting their therapeutic efficacy [4,5]. Plants can also be grown in the field with appropriate biological containment of foreign genes, such as maternal inheritance or male sterility and/or harvest of vegetative tissues before flowering [6].
Oral delivery of protein drugs has been elusive for decades because of their degradation in the digestive system, inability to cross the gut epithelium and delivery to target cells. However, several recent studies have unequivocally shown that plant cell wall protects expressed protein drugs from acids and enzymes in the stomach via bio-encapsulation [2–4]. Human digestive enzymes are incapable of breaking down glycosidic bonds in carbohydrates that make up plant cell wall. However, when intact plant cells containing protein drugs reach the gut, commensal microbes digest plant cell wall and release protein drugs in the gut lumen. Bacteria inhabiting the human gut have evolved to utilize complex carbohydrates in plant cell wall and are capable of utilizing almost all plant glycans [7,8]. Fusion of the (nontoxic) Cholera toxin B subunit (CTB) to a reporter protein (GFP) expressed in chloroplasts and bioencapsulated in plant cells was orally delivered across the gut epithelium through GM1 receptors and furin cleavage site engineered between CTB-GFP released GFP into circulation or different tissues [9]. Fusion of CTB to therapeutic proteins facilitate their effective oral delivery for induction of oral tolerance [10–13] or deliver functional proteins to sera [5,14,15] or across blood brain or retinal barriers [15,16].
Hemophilia is an X-linked bleeding disorder caused by mutations in coagulation factor VIII (FVIII, hemophilia A) or factor IX (FIX, hemophilia B). Hemophilia patients are currently treated by replacement therapy with recombinant or plasma-derived factor concentrate. However, 20–30% of patients with severe hemophilia A (as defined by <1% coagulation activity) and ~5% of severe hemophilia B patients develop inhibitory antibodies (or “inhibitors”), which seriously complicate treatment and increase morbidity and mortality of the disease [17–19]. Therefore, immune tolerance induction to eliminate these inhibitors is required to restore effectiveness of factor replacement therapy. Current clinical protocols for reversal of the antibody response via immune tolerance induction (ITI) require frequent high-dose factor administration for prolonged periods (from months to more than 1 year), and the costs are extremely high (often exceeding one million dollars) [18]. Among hemophilia B patients, those with F9 gene deletions are at an elevated risk for inhibitor formation. Often ITI protocols for FIX inhibitors cannot be completed because of anaphylactic reactions or development of nephrotic syndrome [20]. There are currently no prophylactic ITI protocols to prevent inhibitor formation. Such a protocol should ideally avoid immune suppressive drugs because inhibitors tend to form at a very young age (during the first 50 days of exposure) [18]. To address this unmet medical need, we are developing an oral tolerance protocol using clotting factors expressed in plant cells [11–13,21].
In this study, we created FIX-transplastomic plants in an edible system ideal for oral delivery. Production of protein drugs in chloroplasts offers several unique advantages including high-level expression (up to 70% total leaf protein) [22], transgene containment from pollen transmission via maternal inheritance of transgenes and lack of gene silencing/position effect due to site specific integration of the transgenes [23]. Although we suppressed inhibitor formation and anaphylaxis against human FIX in hemophilia B mice using tobacco cells [11, 13] further clinical development was not feasible. We report here the first successful development and large scale production of CTB-FIX in a cGMP facility in an edible crop plant (lettuce) and evaluation of therapeutic efficacy in a wide dose range and product stability.
2. Materials and methods
2.1. Construction of lettuce chloroplast transformation vector
To construct the lettuce chloroplast CTB-FIX expression vector, PCR was first performed to amplify the CTB-FIX fusion gene with the primer set NdeI-CTB-Fw (5′ TTCATATGACACCTCAAAATATTACTGATT 3′, the underlined nucleotides represent the start codon of CTB fusion tag) and XbaI-FIX-Rv (5′ GATCTAGATTAAGTGAGCTTTG TTTTTTCCT 3′, the underlined nucleotides indicate the stop codon of FIX) from a template plasmid pLD CTB-FFIX [11]. The CTB-FIX PCR products were cloned into pCR-Blunt II-TOPO Vector (Life Technologies Co., Carlsbad, CA). After verification of nucleotide sequence, the NdeI-CTB-FIX-XbaI fragment was subcloned into an NdeI-XbaI digested intermediate vector pDVI-1 harboring a lettuce psbA promoter-5′ UTR and lettuce psbA 3′ UTR. The CTB-FIX expression cassette including the lettuce psbA promoter-5′ UTR /CTB-FIX /lettuce psbA 3′ UTR was obtained by SalI-NotI digestion and then cloned into SalI-NotI digested pLS-LF vector [10] to create the CTB-FIX lettuce chloroplast expression vector pLS-CTB-FIX (Fig. 1A).
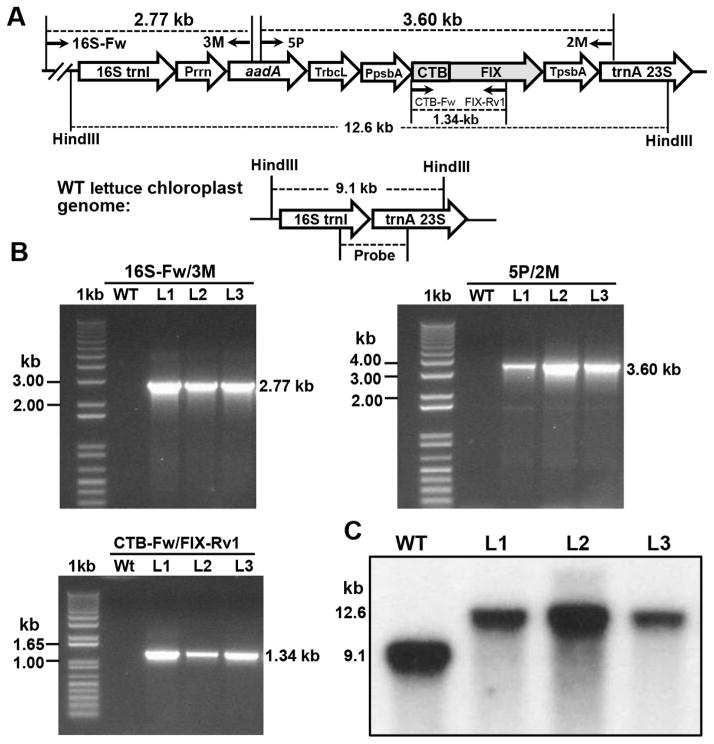
Figure 1. CTB-FIX lettuce chloroplast expression vector and evaluation of site-specific integration the lettuce chloroplast genome.
(A) Schematic diagram of CTB-FIX lettuce expression vector pLS-CTB-FIX. Homologous chloroplast genome flanking sequences include 16S (16S rRNA), isoleucine tRNA (trnI), alanine tRNA (trnA) genes. A glycine-proline-glycine-proline hinge and furin cleavage site (RRKR) are inserted between CTB and the FIX sequence. The CTB-FIX expression is regulated by the lettuce psbA promoter-5′ UTR (PpsbA) and 3′ UTR (TpsbA). The selection marker gene aadA (encoding aminoglycoside 3′-adenylyltransferase gene to confer spectinomycin resistance) is driven by a ribosomal RNA operon promoter (Prrn) with GGAG ribosome binding site. (B) PCR analysis of the CTB-FIX transplastomic lines. The primer annealing sites (16S-Fw/3M, 5P/2M and CTB-Fw/FIX-Rv1) for PCR analysis of control and transplastomic lines are shown in Figure 1a. L1, L2 and L3 represent three independent transplastomic lines. (C) Evaluation of homoplasmy in CTB-FIX-transplastomic lines. Total lettuce DNA was digested with HindIII and probed with 32P-labeled 1.12 kb trnI/trnA flanking region fragment. Untransformed line generates a 9.1 kb hybridizing fragment while transplastomic lines generate 12.6 kb fragment due to site specific integration of the transgene cassette.
2.2. Transformation and characterization of lettuce transplastomic lines
Lettuce (Lactuca sativa) cv. Simpson Elite leaves were bombarded with the CTB-FIX expression vector and the chloroplast transformants were selected on spectinomycin as previously described [24]. In order to identify the site specific integration of the CTB-FIX transgene into the chloroplast genome and to verify the homoplasmic plants, PCR analysis and Southern blot assays were performed according to previous reports from our laboratory [24,25].
2.3. Lyophilization of the CTB-FIX transplastomic leaves
Frozen lettuce leaves expressing CTB-FIX fusion proteins were freeze-dried in a lyophilizer (Genesis 35×L, SP Scientific, Stone Ridge, NY) at −40 °C, −30 °C, −20 °C, −15 °C, −10°C, −5°C and 25 °C for a total of 72 h under vacuum 400 mTorr. The lyophilized leaves were ground in a coffee grinder (Hamilton Beach, Southern Pines, NC) at maximum speed for 3 times (each time, pulse on 6 s and off 90 s). The fine powder or lyophilized leaves were stored in moisture free containers at room temperature with silica gels up to 2 years.
2.4. Analysis of CTB-FIX expression in transplastomic lettuce leaves
Immunoblot analysis and quantitation of the CTB-FIX fusion protein were carried out by previously reported protocols [5,24]. In order to analyze the pentameric structure of CTB-FIX fusion protein expressed in the transformed lettuce chloroplasts, GM1-ganglioside receptor binding ELISA and non-reducing gel-western blot assays were performed as reported earlier [5].
2.5. Hemophilia B mouse experiments
Hemophilia B mice with F9 gene deletion on C3H/HeJ background (C3H/HeJ F9−/−) were bred as previously published [11,26,27]. Male mice approximately 2 months of age were used at the onset of experiments and housed under special pathogen-free conditions at the University of Florida under institutionally approved protocols. Lyophylized plant material was rehydrated in sterile PBS to a final volume of in 200 μl per gavage dose (containing 1.5–15 μg of CTB-FIX antigen) and briefly homogenized on ice for <30 sec with an OMNI International (GLH-2596) probe. Oral delivery was performed twice per week for 8 weeks by gavage using a 20-G bulb-tipped gastric gavage needle. For FIX replacement therapy, mice were administrated 1 IU hFIX (Benefix, Pfizer, New York, NY) into the tail vein once per week for 8 weeks. Blood was collected by tail bleed into citrate buffer. To prevent fatal anaphylaxis in control animals (that did not receive oral tolerance), anti-histamine and anti-platelet activating factor (anti-PAF) were administered starting at the 4th injection [11,13]. Antibody formation against FIX in murine plasma was measured by Bethesda assay (using a fibrometer from Stago, Pasippany, NJ) and by immunoglobulin-specific ELISA as published [11,13]. Frozen tissue sections from small intestine were prepared for immunohistochemistry and stained for FIX and CD11c antigens as previously documented [13].
2.6. Scaled-up hydroponic cGMP production of CTB-FIX biomass
A hydroponic system amenable to development into a cGMP pilot-scale production system was assembled. The hydroponic system consisted of two wire shelving units. In each rack there were four growing areas that were illuminated with three sealed lighting fixtures containing 2-T8 fluorescent bulbs. Two growth trays containing rockwool horticultural substrate (Grodan, The Netherlands) were placed on each shelf. The nutrient comprised a 20-10-20 ratio of nitrogen, phosphorus and potassium.
CTB-FIX seeds were placed on the rockwool surface at 2 inch × 2 inch spacing. Plants received an average of 70 – 90 μmol m−2 s−1 of light on an 18 hour photoperiod. The temperature and humidity in the growth room ranged from 23–26°C and 20–60%, respectively. This hydroponic plant growth system is a small-scale arrangement that can be scaled to cGMP pilot scale production [28]. For a comparison of germination and biomass accumulation rates, CTB-FIX plants were grown alongside the commercial cultivar Simpson Elite.
3. Results
3.1. Lettuce CTB-FIX chloroplast expression vector
Our goal is to create an edible leafy crop plant (lettuce) capable of producing adequate levels of FIX antigen in chloroplasts to facilitate clinical translational studies. In order to enhance transformation efficiency, lettuce species specific chloroplast vector was constructed using endogenous full length (~2 kb /flank) flanking sequences. Likewise, we used lettuce psbA promoter, 5′UTR and 3′UTR to enhance transgene expression. These concepts formed the basis for design and construction of pLS-CTB-FIX expression vector (Fig. 1A). For expression of FIX antigen, an N-termial truncated version (47th – 461st amino acids) of human FIX cDNA (FR846240.1) excluding the signal peptide (1st – 28th) and propeptide (29th – 46th) was used to generate the CTB-FIX expression vector. The vector contained the spectinomycin selection marker gene aadA which could be removed by direct repeats (see discussion section). In addition, a glycine-proline-glycine-proline (GPGP) hinge to prevent steric hindrance was created between CTB and FIX fusion elements. A furin cleavage site (RRKR) was also included in this CTB-FIX expression vector [11]. While this construct lacks the FIX propeptide (which is required for γ-carboxylation) and thus cannot produce functional FIX, the entire amino acid sequence of mature FIX is included, covering all potential CD4+ T cell epitopes for tolerance induction.
3.2. Characterization of CTB-FIX transplastomic lettuce lines
CTB-FIX transplastomic lettuce lines were created by biolistic particle bombardment of lettuce leaves from a commercial cultivar (Simpson Elite) with the expression vector pLS-CTB-FIX. PCR results showed the correct sizes of amplicons, 2.77 kb with primer set 16S-Fw/3M, 3.60 kb with 5P/2M and 1.34 kb with CTB-Fw/FIX-Rv1 (Fig. 1A and 1B). The site-specific integration of the CTB-FIX gene into the chloroplast genome was further confirmed by Southern blot analysis probed with the lettuce trnI and trnA flanking sequence. All three independent transplastomic lines showed distinct hybridizing fragments in Southern blots with the expected size of 12.6 kb but not the 9.1 kb fragment from untransformed wild type plants (Fig. 1A, 1C), confirming that all three CTB-FIX transplastomic lines were homoplasmic (in which all chloroplast genome have been transformed with insertion of the transgene cassette).
3.3. Folding, stability and CTB-FIX pentamer assembly in lyophilized lettuce leaves
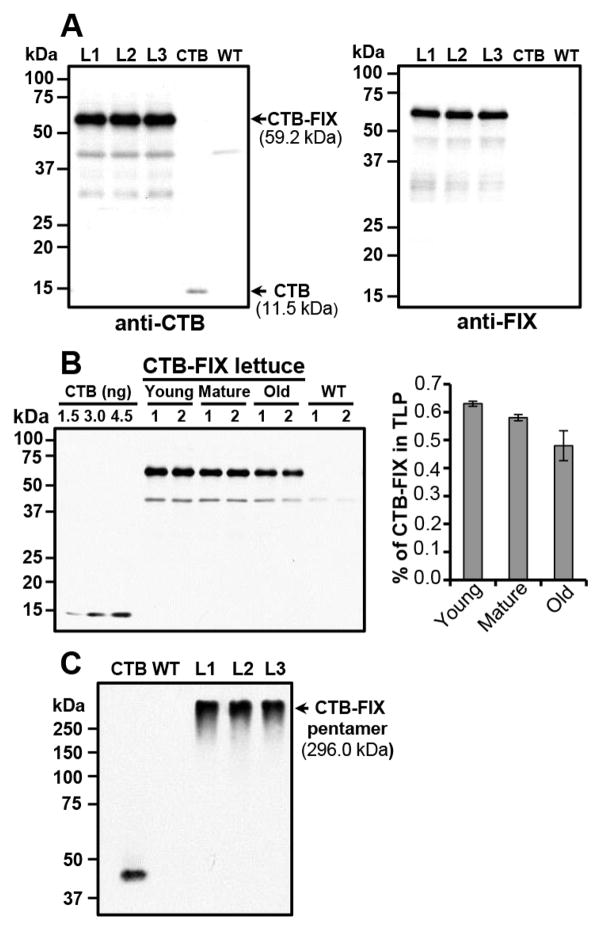
Total leaf proteins were extracted with a fully denatured and reducing buffer to analyze CTB-FIX protein. As shown in Figure 2A, the monomer CTB-FIX fusion protein with the correct molecular mass of 59.2 kDa was detected with anti-CTB or FIX antibody. All transplastomic lettuce lines expressing CTB-FIX were fertile and set seeds. In T1 plants, CTB-FIX expression levels of the young, mature and old leaves were 0.63%, 0.58% and 0.48% of total leaf protein (TLP) respectively (Fig. 2B). The CTB-FIX concentration of the frozen mature leaves was up to 58.38 μg per g of fresh leaves. CTB-FIX pentamer (296.0 kDa) properly assembled in the transgenic lettuce chloroplasts (Fig. 2C). Absence of any cleaved products affirms stability of assembled pentamers in plant extracts.
Figure 2. Evaluation of CTB-FIX expression in the lettuce transplastomic lines.
(A) Western blot analysis of CTB-FIX in three independent lines and WT controls. Plant total leaf proteins (5 μg/lane) were loaded from WT and CTB-FIX plants. CTB, 3 ng per lane. L1, L2, L3: 3 independent lettuce transplastomic lines. WT, untransformed wild type plant. Antibody titers: rabbit anti-CTB polyclonal antibody 1:10,000; goat anti-FIX polyclonal antibody 1:2,000. (B) Quantitation of CTB-FIX expression at different developmental stages. Total leaf protein (1.5 μg per lane) was loaded from young, mature and old leaves (2.5-months-old plants grown in the greenhouse). Probe: anti-CTB rabbit polyclonal antibody (titer 1:10,000). Right bar graph shows CTB-FIX concentrations of young, mature and old leaves. Data shown are means ± SD of two replicates from leaves collected from two independent lines. (C) Non-reducing gel western blot analysis. Probe, anti-CTB rabbit polyclonal antibody (titer 1:10,000). Protein concentration loaded: CTB, 3 ng per lane; CTB-FIX (L1, L2 and L3) and WT leaf extracts, 5 μg (TLP) per lane.
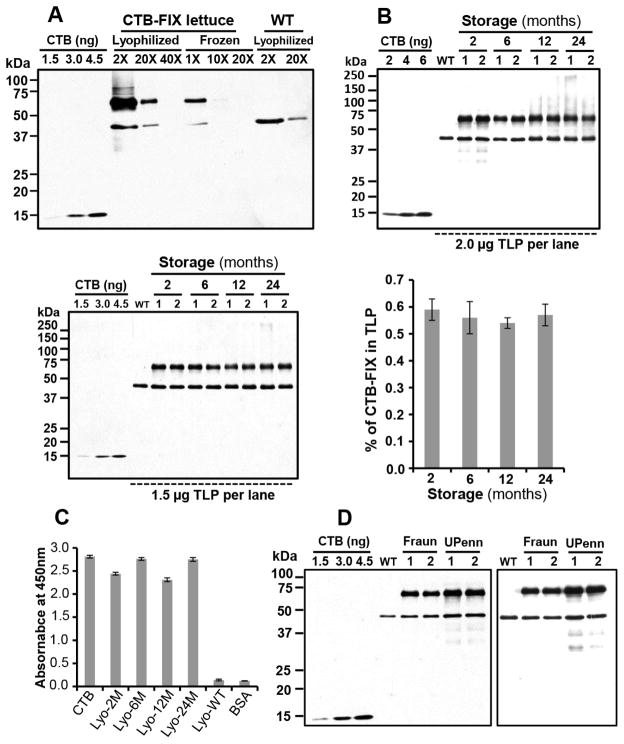
Frozen CTB-FIX lettuce mature leaves were freeze-dried using a lyophilizer as described in the methods section. Figure 3A shows a direct comparison of protein concentrations in lyophilized and frozen leaf samples by weight (5–200 μg, 1–40 X dilution) and an increase of 18–21 fold CTB-FIX protein concentration was observed after lyophilization. The intact monomer band of CTB-FIX fusion protein was observed without any detectable degradation of CTB-FIX in all tested lyophilized samples after storage for 2, 6, 12 and 24 months, at ambient temperature (Fig. 3B), confirming that CTB-FIX protein is stable in the freeze-dried lettuce leaves up to 2 years. The stability of the CTB-FIX fusion protein concentrations was similar in all tested samples when normalized for total protein concentration. CTB-FIX concentrations were 0.59% TLP (equivalent to1478.54 μg/gDW) after 2 month storage, 0.56% TLP (equivalent to 1155.60 μg/gDW) after 6 month storage, 0.54% TLP (equivalent to 846.43 μg/gDW) after 12 month storage and 0.57% TLP (equivalent to 927.02 μg/gDW) after 24 month storage. The results of Paired student’s t-test indicated absence of any significant difference in % TLP of CTB-FIX among lyophilized samples stored for different durations at ambient temperature.
Figure 3. Evaluation of CTB-FIX concentration and stability after long term storage of lyophilized lettuce leaves.
(A) Comparison of CTB-FIX protein concentration of lyophilized CTB-FIX lettuce leaves with the frozen leaf samples. 1× 40× indicate fold of dilution. One fold (1×) equals 200 μg (in3 weight) of leaf ground powder. (B) Evaluation of CTB-FIX protein stability in lyophilized lettuce leaves during long term storage at ambient temperature. Two independent blots by loading either 2.0 μg or 1.5 μg of TLP per lane are shown. Probe: anti-CTB rabbit polyclonal antibody (titer, 1:10,000). Right bar graph shows the CTB-FIX concentrations of the 4 batches of lyophilized leaves after different periods of storage (2, 6, 12 and 24 months). Data shown are means ± SD of three independent experiments. (C) GM1 binding assay. CTB: CTB standard protein (5 ng). Lyo-2M, Lyo-6M, Lyo-12M, Lyo-24M: Total soluble protein (TSP, 100 μg) from the lyophilized CTB-FIX expressing leaves after storage for 2, 6, 12 and 24 months at room temperature. Lyo-WT, protein extract (100 μg, TSP) from lyophilized and untransformed wild type leaves. BSA, bovine serum albumin (100 μg). Data shown are means ± SD of two independent experiments. (D) Comparative analysis of CTB-FIX protein concentrations in lyophilized lettuce leaves from Fraunhofer (Fraun, hydroponic system) with leaf samples collected from the Daniell lab greenhouse at University of Pennsylvania (UPenn, potted soils). 1.5 μg TLP per lane was loaded. Probe: anti-CTB rabbit polyclonal antibody (titer, 1:10,000). Left panel represents results of the 1st leaf harvest (plant ages: Fraunhofer, 32-days-old; UPenn, 66-days-old) and the right panel shows results of the 2nd leaf harvest (plant ages: Fraunhofer, 51-days-old; UPenn, 86-days-old).
A plasma membrane receptor (GM1-ganglioside) binds CTB in vivo, and pentamer formation of CTB is required for binding to GM1 receptor [29,30]. To further test if the pentameric structure with disulfide bonds was maintained in lyophilized lettuce leaves, GM1-ganglioside receptor binding ELISA was carried out using the lyophilized leaves after long term storage at ambient temperature. CTB-FIX fusion protein extracts from all tested samples showed strong binding affinity to GM1 (Fig. 3C), demonstrating the presence and functional stability of the CTB-FIX pentamers in lyophilized leaves even after long term storage (~2 years).
3.4. Scale up of biomass and CTB-FIX dose evaluation in capsules
CTB-FIX lettuce plants were transplanted into potted soils in the greenhouse to set seeds. For production of CTB-FIX in a scalable hydroponic system, CTB-FIX seeds were germinated on rockwool or in Petri dishes on filter paper with the nutrient solution. The germination rate of CTB-FIX lettuce seeds on rockwool was 93% when compared to 100% in Petri dishes on filter paper with the nutrient solution. After 32 days of growth, the WT Simpson Elite plants produced more leaf biomass (236.72 g per flat) than CTB-FIX plants (144.89 g per flat). This difference in biomass accumulation was anticipated based on the additional burden of constitutively synthesizing a foreign human protein (Fig. 4A). The CTB-FIX protein concentration in the lyophilized lettuce leaves collected from the hydroponic system contained 0.38% TLP (equivalent to 1,007.55 μg/gDW) which is lower than 0.56% TLP (equivalent to 1,459.59 μg/gDW) in lyophilized CTB-FIX lettuce leaves harvested from the Daniell lab greenhouse at the University of Pennsylvania (Fig. 3D). Two batches of leaves from different harvests at Fraunhofer (32, 51 days old) and UPenn greenhouse (66, 86 days old) samples were analyzed. Based on the current yield of FIX-lettuce production in the hydroponic system, ~870 kg of fresh FIX-lettuce leaves (~43.5 kg dry weight) can be harvested per thousand ft2 of growth room annually. Up to 43.5 g of FIX can be produced from 43.5 kg of dry FIX-lettuce leaves, yielding 24,000–36,000 doses for 20-kg pediatric patients (based on the lowest or highest effective dose for induction of oral tolerance). After lyophilization, the freeze-dried FIX lettuce leaves were ground into fine powder and used to prepare FIX-lettuce capsules (Fig. 4B).
Figure 4. Industrial production of FIX bioencapsulated in lettuce cells.
(A) Biomass production of CTB-FIX lettuce. CTB-FIX lettuce plants were grown in potted soils in the Daniell lab greenhouse at the University of Pennsylvania or in Fraunhofer cGMP hydroponic system (Upper 2 panels). The lower 2 panels show the growth status of CTB-FIX lettuce (cv. Simpson Elite, 24 days old) and wild type (WT) lettuce (cv. Simpson Elite, 24 days old). (B) Capsule preparation. A large scale production of FIX-expressing lettuce was established in Fraunhofer USA (Newark, DE). After harvesting and lyophilization (Lyophilizer: Genesis 35×L, SP Scientific, Stone Ridge, NY) of the fresh leaves, the freeze-dried FIX leaves were powdered and prepared as capsules.
3.5. Oral delivery of CTB-FIX lettuce cells suppressed inhibitor formation against FIX in hemophilia B mice
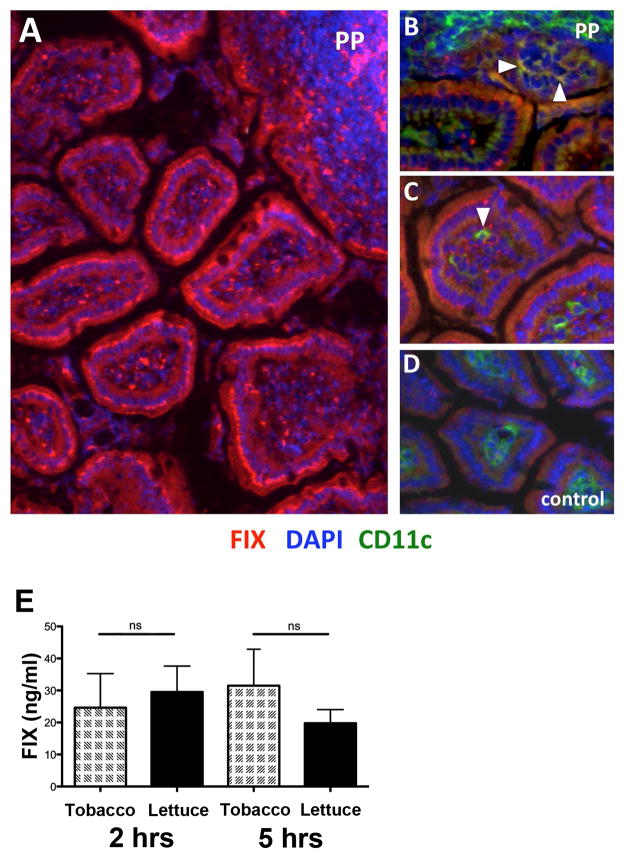
First, we wanted to confirm that oral administration of CTB-FIX bioencapsulated in lettuce cells results in delivery of the FIX antigen to the gut immune system. Lyophilized lettuce (after storage for eight months at ambient temperature) plant cells (containing 10 μg CTB-FIX) were resuspended in sterile PBS and delivered to hemophilia B mice by oral gavage. Five hours later, the small intestine was removed. Immunohistochemical staining of cryosections confirmed delivery of FIX antigen to the gut epithelium and to Peyer’s patches (Fig. 5A), with efficiency similar to our previous observations on FVIII and FIX delivery of tobacco cells [11–13]. Uptake of FIX antigen to dendritic cells (DC) was demonstrated by co-localization with CD11c+ cells (DCs, Fig. 5B–D). Systemic delivery of FIX antigen was observed 2 and 5 hrs after gavage of lettuce cells at levels similar to those achieved from feeding of identical antigen doses contained in tobacco cells (Fig. 5E).
Figure 5. FIX antigen delivery to the small intestine of hemophilia B mice.
(A) FIX (red) delivered to epithelium of intestinal villi and to Peyer’s patches (PP). DAPI was used to stain nuclei blue. (B) Examples of co-localization (arrows) of FIX antigen and CD11c+ cells, indicating antigen delivery to DC in PP. (C) Examples of co-localization (arrows) of FIX antigen and CD11c+ cells, indicating antigen delivery to DC in villi. Mice had received oral gavages of CTB-FIX expressing lettuce (but no i.v. FIX). (D) No FIX stain was observed in mice that received oral gavage of WT lettuce (shown), or in intestinal sections of CTB-FIX fed mice when the primary antibody against FIX was omitted during staining (not shown). Original magnification for all panels: 200×. (E) Systemic human FIX levels as measured by ELISA 2 and 5 hrs after oral gavage of 10 μg CTB-FIX bioencapsulated in lettuce or tobacco cells. Data shown are average ± SD (n=3/group). Differences between lettuce and tobacco were not significant (“ns”).
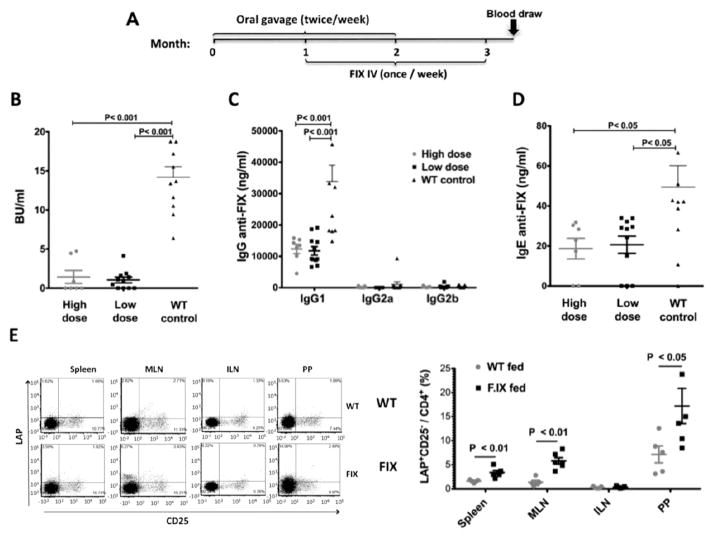
Next, hemophilia B mice received oral gavages of the lettuce material twice per week for 2 months (Fig. 6A). A ten-fold dose escalation (1.5 μg or 15 μg) of CTB-FIX (in 1.9 or 19 mg lyophilized lettuce cells) was investigated. Control mice received WT untransformed lettuce. During the second month of this regimen, all mice (n=11 for control and low dose, n=7 for high dose) were additionally i.v. injected with recombinant FIX (1 IU) once per week. This replacement therapy was continued for 1 month after oral gavages had been stopped (Fig. 6A). Blood samples were collected 1 week after the last FIX injection. FIX inhibitor titer was robustly suppressed by oral delivery of CTB-FIX expressing lettuce cells for both doses (Fig. 6B). Average titers were 15–fold lower, and 10 of 11 mice in the low-dose and 5 of 7 mice in the high-dose group had very low (<2 BU) to undetectable inhibitor titers. In contrast, control mice formed high-titer inhibitors (11/11 >5BU, with 9/11 >10 BU). IgG1 was the dominant subclass of IgG produced against FIX. Average IgG1 titers were suppressed by 3–fold, and – in contrast to control mice – no titers >20,000 ng/ml were measured (Fig. 6C). Because repeated i.v. delivery of FIX causes not only inhibitor formation but also fatal anaphylaxis in the C3H/HeJ F9−/− strain, control mice were additionally treated with drugs that prevent anaphylactic reactions. Mice that had received oral tolerance using high- or low-dose CTB-FIX in lettuce, despite not being treated with these drugs, did not develop anaphylaxis, consistent with suppression of IgE formation (Fig. 6D). At the end of these experiments, different organs were analyzed for induction of LAP+ Treg by flow cytometry. A significant increase in the frequency of LAP+CD25−CD4+ Treg was found in the spleen, mesenteric lymph nodes, and Peyer’s patches (but not in control lymph node) of FIX fed mice (Fig. 6E).
Figure 6. Suppression of inhibitor and IgE formation against FIX in hemophilia B mice.
(A) Mice were given two gavages weekly (of different CTB-FIX doses) of lettuce cells and were challenged with i.v. injections of FIX. (B) Anti-FIX titers 1 week after the last i.v. injection of FIX are graphed as inhibitor titers in BU/ml. (C) IgG1, 2a, and 2b anti-FIX titers. (D) IgE anti-FIX titers. (E) Frequencies of LAP+CD25−CD4+ Treg in the spleen, mesenteric lymph nodes, Peyer’s patches, and control (inguinal) lymph nodes of FIX fed and control mice. Flow plots on the left show individual representative examples. Graph to the right shows results for individual mice (n=5/group) as well as averages ± SD.
4. Discussion
Even though one protein drug made in carrot cells has been approved by FDA [1], this did not eliminate prohibitively expensive fermentation, purification, cold storage or transportation costs. Therefore, this reports the first clinical advancement of a protein drug made in edible plant cells for oral drug delivery. Successful generation of transplastomic lettuce plants at an industrial scale is a major step towards development of a clinical protocol for inducing oral tolerance in hemophilia patients.
In addition, fusion to CTB ensures highly efficient targeting of the gut immune system for oral tolerance induction [10–13]. CTB assembles into a stable pentameric ring consisting of five identical 11.5-kDa polypeptide monomers. CTB pentamers bind to GM1 ganglioside receptors on the membranes of gut epithelial cells and on DCs [10–13, 31, 32]. Role of CTB in induction of oral tolerance has been reported by several investigators [10–13, 33, 34]. Clinical data to support the safety of CTB administration to humans [35] has already been approved for human use a decade ago and is used by hundreds of millions of people around the globe [36]. The successful induction of oral tolerance by CTB-autoantigen conjugates in experimental systems has been also proven to be safe in clinical trials [37]. Extensive pre-clinical evaluation leading to these clinical studies have shown that recombinant CTB or CTB fusions do not cause histopathology in the small intestine, nor do they increase vascular permeability [38].
CTB may enhance tolerance induction also by induction of indoleamine 2, 3-dioxygenase (which is known to aid in Treg induction) in DC [39]. Upon uptake by epithelial cells, FIX is cleaved off at the engineered Furin cleavage site and in part systemically delivered [11]. Furthermore, FIX antigen is delivered to several subsets of antigen presenting cells (APCs) in the GALT. These include F4/80+ cells in the duodenum and CD11c+ DC (including tolerogenic CD103+ DC) in the lamina propria and in Peyer’s patches (PP) throughout the small intestine [13]. Direct uptake by DC that sample the gut lumen and transport to DC in PP by M cells likely also contribute to antigen uptake resulting in an increase in several subsets of DCs and CD4+ T cells. IL-10 dependent and antigen-specific immune regulatory response ultimately suppresses systemic antibody formation via induction of CD4+CD25+FoxP3+ Treg and CD4+CD25−FoxP3−LAP+ Treg [13]. LAP+ Treg overexpress TGF-β (resulting in detectable latency associated peptide (LAP) on the cell surface, suppress via a TGF-β dependent mechanism, and may be more immune suppressive than FoxP3+ Treg [40,41]. Upon systemic challenge with FVIII or FIX, our oral tolerance protocol increases the overall frequency of LAP+ Treg, and these induced Treg up-regulate IL-10 and TGF-β expression in response to antigen [12, 13].
Use of freeze-dried plant cells facilitates pharmaceutical production and formulation. In this study, we observed that CTB-FIX fusion protein in lyophilized lettuce can be stored at room temperature up to 2 years without any detectable degradation of this protein. We used material that had been stored for 8 months to tolerize the hemophilic mice over a wide dose range. The long-shelf-life of freeze-dried CTB-FIX lettuce leaves eliminates the need for expensive protein purification, cold storage, cold transportation and addresses a major challenge in production and delivery of current protein drugs. Therefore, lettuce-made FIX would be ideal for large scale and cost effective production of this biopharmaceutical for translational studies of hemophilia B.
In the present study, no significant difference for oral tolerance induction was observed between low-dose-(1.5 μg of CTB-FIX) and high-dose-treated (15 μg of CTB-FIX) hemophilia B mice, which may be due to highly efficient CTB-mediated delivery of FIX antigen. This will be a major advantage in further clinical advancement to overcome potential differences among patients in efficiency of oral delivery of protein drugs bioencapsulated in plant cells. In the unlikely event of requiring lyophilized materials with higher dose (which could be simply achieved by taking more capsules), codon optimized genes could be used instead of native human genes. An additional improvement needed may be the removal of selectable marker gene from transformed chloroplast genomes. Several methods are readily available for removal of the selection marker genes [42, 43]. Precise excision of the selectable marker gene (aadA) was also accomplished recently from the integration site (trnA/trnI) used in this study with Bxb1 recombinase and attP/attB recognition sites [44].
The demonstration of growing CTB-FIX and WT lettuce (cv. Simpson Elite ) plants in a controlled environment hydroponic system illustrates that transformed plants performed well using scalable production methods that are translatable to cGMP (current Good Manufacturing Practices). There was no need to germinate seeds in the presence of antibiotic. The indoor hydroponic system does not require use of pesticides and herbicides. This system can also avoid soil borne diseases. The fast growth rate is another unique advantage of the hydroponic system and one-month-old FIX-lettuce leaves were ready for the first harvest. This opens a path towards human clinical evaluation of CTB-FIX, as well as other similarly expressed proteins. For example, existing plant-based biopharmaceutical production facilities operated under the FDA’s GMP guidelines can potentially be modified to accommodate lettuce biomass production [28]. Under current growth condition, the CTB-FIX concentration in the lettuce leaves harvested from the hydroponic system was not as high as that from UPenn Daniell lab greenhouse (1mg/g vs 1.5 mg CTB-FIX/g DW). This difference may be mainly due to different light source and 3-fold lower intensity (sunlight ~280 μmol m−2 s−1 vs hydroponic system ~90 μmol m−2 s−1 ) because of the light-regulated psbA promoter-5′ UTR determining expression level of CTB-FIX protein.
Mechanistic studies to understand molecular basis of tolerance conferred by different blood clotting factors expressed in plant cells and efficacy of this system to not only induce tolerance but also accomplish reversal [11–13] augurs well for further clinical advances. In this study for oral tolerance induction, the propeptide sequence of FIX was not included in the CTB-FIX fusion protein expressed in lettuce chloroplasts. It has been demonstrated that this propeptide is required for gamma-carboxylation of the Gla domain of the mature protein to produce functional FIX [45]. Therefore, the lettuce chloroplast-expressed FIX from this study does not have blood clotting activity. Future studies will explore delivery of functional clotting factors. Most importantly, elimination of prohibitively expensive fermentation, purification, cold storage/transportation, short shelf life of current protein drugs makes this novel approach highly efficient, cost-effective and environmentally friendly for large scale production of therapeutic proteins in plants, a major milestone in advancing this field.
Acknowledgments
This research was supported by Novo Nordisk, NIH grants R01 HL107904 and R01 HL109442 to H.D. and R.W.H. L.Z. was supported by grant no. 81371748 by The National Nature Science Foundation of China. The support of Rebecca Snow was also invaluable to this project.
Footnotes
Author contributions
HD and RWH conceived and supervised the study and designed experiments. JS, RWH and HD wrote the manuscript. JS contributed data to figures 1–3; Data and related text for figure 4 was contributed by JS, SL, AK, JHN and SJS; LZ, AS, XW contributed data in figures 5, 6. All authors participated in data analysis.
Competing financial interests
Henry Daniell is an inventor in several US and international patents on chloroplast transformation technology to produce biopharmaceuticals, in particular induction of oral tolerance. Henry Daniell and Roland Herzog are co-inventors in hemophilia patents. Novo Nordisk is currently funding the hemophilia project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walsh G. Biopharmaceutical benchmarks. Nat Biotechnol. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]
- 2.Jin SX, Daniell H. Engineered chloroplast genome just got smarter. Trends Plant Sci. 2015 doi: 10.1016/j.tplants.2015.07.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan HT, Daniell H. Plant-made vaccines against human diseases–are we there yet? Plant Biotechnol J. 2015 doi: 10.1111/pbi.12471. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon KC, Verma D, Singh ND, Herzog R, Daniell H. Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev. 2013;65:782–99. doi: 10.1016/j.addr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon KC, Nityanandam R, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol J. 2013;11:77–86. doi: 10.1111/pbi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arlen PA, Falconer R, Cherukumilli S, Cole A, Cole AM, Oishi KK, et al. Field production and functional evaluation of chloroplast-derived interferon-α2b. Plant Biotechnol J. 2007;5:511–25. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–31. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 9.Limaye A, Koya V, Samsam M, Daniell H. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J. 2006;20:959–61. doi: 10.1096/fj.05-5134fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B–proinsulin fusion protein in lettuce and tobacco chloroplasts – oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J. 2007;5:495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, et al. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci USA. 2010;107:7101–6. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman A, Su J, Shina L, Wang X, Herzog RW, Daniell H. Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood. 2014;124:1659–68. doi: 10.1182/blood-2013-10-528737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Su J, Sherman A, Rogers GL, Liao G, Hoffman BE, et al. Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood. 2015;125:2418–27. doi: 10.1182/blood-2014-08-597070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shenoy V, Kwon KC, Rathinasabapathy A, Lin S, Jin G, Song C, et al. Oral delivery of Angiotensin-Converting Enzyme2 and Angiotensin-(1–7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension. 2014;64:1248–59. doi: 10.1161/HYPERTENSIONAHA.114.03871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shil PK, Kwon KC, Zhu P, Verma A, Daniell H, Li Q. Oral Delivery of ACE2/Ang-(1–7) bioencapsulated in plant cells protects against experimental uveitis and autoimmune uveoretinitis. Mol Ther. 2014;22:2069–82. doi: 10.1038/mt.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohli N, Westerveld DR, Ayache AC, Verma A, Shil P, Prasad T, et al. Oral delivery of bioencapsulated proteins across blood–brain and blood–retinal barriers. Mol Ther. 2014;22:535–46. doi: 10.1038/mt.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berntorp E, Shapiro AD. Modern haemophilia care. Lancet. 2012;379:1447–56. doi: 10.1016/S0140-6736(11)61139-2. [DOI] [PubMed] [Google Scholar]
- 18.DiMichele DM. Immune tolerance in haemophilia: the long journey to the fork in the road. Br J Haematol. 2012;159:123–34. doi: 10.1111/bjh.12028. [DOI] [PubMed] [Google Scholar]
- 19.Scott DW, Pratt KP, Miao CH. Progress toward inducing immunologic tolerance to factor VIII. Blood. 2013;121:4449–56. doi: 10.1182/blood-2013-01-478669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. 2007;138:305–15. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 21.Dolgin E. Immunology: Oral solutions. Nature. 2014;515:S166–67. doi: 10.1038/515S166a. [DOI] [PubMed] [Google Scholar]
- 22.Ruhlman T, Verma D, Samson N, Daniell H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010;152:2088–104. doi: 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniell H. Transgene containment by maternal inheritance: effective or elusive? Proc Natl Acad Sci USA. 2007;104:6879–80. doi: 10.1073/pnas.0702219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Daniell H. Engineering the chloroplast genome for hyperexpression of human therapeutic proteins and vaccine antigens. Methods Mol Biol. 2004;267:365–83. doi: 10.1385/1-59259-774-2:365. [DOI] [PubMed] [Google Scholar]
- 25.Verma D, Samson NP, Koya V, Daniell H. A protocol for expression of foreign genes in chloroplasts. Nat Protoc. 2008;3:739–58. doi: 10.1038/nprot.2007.522. [DOI] [PubMed] [Google Scholar]
- 26.Markusic DM, Brad E, Hoffman BE, Perrin GQ, Nayak S, Wang X, et al. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med. 2013;5:1698–709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Moghimi B, Zolotukhin I, Morel LM, Cao O, Herzog RW. Immune tolerance induction to factor IX through B cell gene transfer: TLR9 signaling delineates between tolerogenic and immunogenic B cells. Mol Ther. 2014;22:1139–50. doi: 10.1038/mt.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirz H, Sauer-Budge AF, Briggs J, Sharpe A, Sudong S, Sharon A. Automated Production of Plant-Based Vaccines and Pharmaceuticals. J Laboratory Automation. 2012;17:449–57. doi: 10.1177/2211068212460037. [DOI] [PubMed] [Google Scholar]
- 29.Daniell H, Lee SB, Panchal T, Wiebe PO. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J Mol Biol. 2001;311:1001–9. doi: 10.1006/jmbi.2001.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuji T, Watanabe K, Miyama A. Monomer of the B subunit of heat-labile enterotoxin from enterotoxigenic Escherichia coli has little ability to bind to GM1 ganglioside compared to its coligenoid. Microbiol Immunol. 1995;39:817–19. doi: 10.1111/j.1348-0421.1995.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 31.Kraehenbuhl JP, Hopkins SA, Kernéis S, Pringault E. Antigen sampling by epithelial tissues: Implication for vaccine design. Behring Inst Mitt. 1997;98:24–32. [PubMed] [Google Scholar]
- 32.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–67. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 33.Dénes B, Fodor I, Langridge WHR. Persistent suppression of Type 1 Diabetes by a multicomponent vaccine containing a cholera toxin B subunit-autoantigen fusion protein and Complete Freund’s Adjuvant. Clinical and Developmental Immunology. 2013 doi: 10.1155/2013/578786.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol. 1998;16:934–938. doi: 10.1038/nbt1098-934. [DOI] [PubMed] [Google Scholar]
- 35.Stål P, Befrits R, Rönnblom A, Danielsson Å, Suhr O, Ståhlberg D, et al. Clinical trial: the safety and short-term efficacy of recombinant cholera toxin B subunit in the treatment of active Crohn’s disease. Aliment Pharmacol Ther. 2010;31:387–95. doi: 10.1111/j.1365-2036.2009.04185.x. [DOI] [PubMed] [Google Scholar]
- 36.Hill DR, Ford L, Lalloo DG. Oral cholera vaccines: use in clinical practice. Lancet Infect Dis. 2006;6:361–73. doi: 10.1016/S1473-3099(06)70494-7. [DOI] [PubMed] [Google Scholar]
- 37.Stanford M, Whittall T, Bergmeier LA, Lindblad M, Lundin S, Shinnick T, et al. Oral tolerization with peptide 336–351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behcet’s disease. Clin Exp Immunol. 2004;137:201–8. doi: 10.1111/j.1365-2249.2004.02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goto N, Maeyama J, Yasuda Y, Isaka M, Matano K, Kozuka S, et al. Safety evaluation of recombinant cholera toxin B subunit produced by Bacillus brevis as a mucosal adjuvant. Vaccine. 2000;18:2164–71. doi: 10.1016/s0264-410x(99)00337-0. [DOI] [PubMed] [Google Scholar]
- 39.Mbongue JC, Nicholas DA, Zhang K, Kim N-S, Hamilton BN, Larios M, et al. Induction of Indoleamine 2, 3-Dioxygenase in Human Dendritic Cells by a Cholera Toxin B Subunit–Proinsulin Vaccine. PLoS ONE. 2015;10:e0118562. doi: 10.1371/journal.pone.0118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+CD25–LAP+ T cells. Nat Med. 2006;12:627–35. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 41.Scurr M, Ladell K, Besneux M, Christian A, Hockey T, Smart K, et al. Highly prevalent colorectal cancer-infiltrating LAP + Foxp3 T cells exhibit more potent immunosuppressive activity than Foxp3 + regulatory T cells. Mucosal Immunol. 2014;7:428–39. doi: 10.1038/mi.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iamtham S, Day A. Removal of antibiotic resistance genes from transgenic tobacco plastids. Nat Biotechnol. 2000;18:1172–76. doi: 10.1038/81161. [DOI] [PubMed] [Google Scholar]
- 43.Day A, Goldschmidt-Clermont M. The chloroplast transformation toolbox: selectable markers and marker removal. Plant Biotechnol J. 2011;9:540–53. doi: 10.1111/j.1467-7652.2011.00604.x. [DOI] [PubMed] [Google Scholar]
- 44.Shao M, Kumar S, Thomson JG. Precise excision of plastid DNA by large serine recombinase Bxb1. Plant Biotechnol J. 2014;12:322–29. doi: 10.1111/pbi.12139. [DOI] [PubMed] [Google Scholar]
- 45.Larson PJ, Stanfield-Oakley SA, VanDusen WJ, Kasper CK, Smith KJ, Monroe DM, et al. Structural integrity of the γ-carboxyglutamic acid domain of human blood coagulation factor IXa is required for its binding to cofactor VIIIa. J Biol Chem. 1996;271:3869–76. doi: 10.1074/jbc.271.7.3869. [DOI] [PubMed] [Google Scholar]