Highlights
-
•
A single dose of Advax-adjuvanted influenza vaccine in 7-day-old pups protected against lethal influenza infection.
-
•
Advax adjuvant enhanced both B-cell and T-cell memory in neonates.
-
•
Influenza protection in Advax-immunized neonates was dependent on memory B-cells.
-
•
Advax adjuvant confirmed to be safe and well tolerated in neonates.
Keywords: Vaccine, Neonate, Adjuvant, Influenza, Immunity
Abstract
Neonates are at high risk for influenza morbidity and mortality due to immune immaturity and lack of priming by prior influenza virus exposure. Inactivated influenza vaccines are ineffective in infants under six months and to provide protection in older children generally require two doses given a month apart. This leaves few options for rapid protection of infants, e.g. during an influenza pandemic. We investigated whether Advax™, a novel polysaccharide adjuvant based on delta inulin microparticles could help overcome neonatal immune hypo-responsiveness. We first tested whether it was possible to use Advax to obtain single-dose vaccine protection of neonatal pups against lethal influenza infection. Inactivated influenza A/H1N1 vaccine (iH1N1) combined with Advax™ adjuvant administered as a single subcutaneous immunization to 7-day-old mouse pups significantly enhanced serum influenza-specific IgM, IgG1, IgG2a and IgG2b levels and was associated with a 3–4 fold increase in the frequency of splenic influenza-specific IgM and IgG antibody secreting cells. Pups immunized with Advax had significantly higher splenocyte influenza-stimulated IFN-γ, IL-2, IL-4, and IL-10 production by CBA and a 3–10 fold higher frequency of IFN-γ, IL-2, IL-4 or IL-17 secreting T cells by ELISPOT. Immunization with iH1N1 + Advax induced robust protection of pups against virus challenge 3 weeks later, whereas pups immunized with iH1N1 antigen alone had no protection. Protection by Advax-adjuvanted iH1N1 was dependent on memory B cells rather than memory T cells, with no protection in neonatal μMT mice that are B-cell deficient. Hence, Advax adjuvant overcame neonatal immune hypo-responsiveness and enabled single-dose protection of pups against otherwise lethal influenza infection, thereby supporting ongoing development of Advax™ as a neonatal vaccine adjuvant.
1. Introduction
Young children, after the elderly, have the next highest influenza disease burden with pediatric cases representing ∼1–5% of annual influenza deaths [1]. Indeed, influenza-related hospitalization rates in children under 4 years are comparable to hospitalization rates in those over 85 years old [2]. Increased disease burden in young children reflects immune system immaturity, lack of previous influenza exposure, poor hygiene, and high communal contact in schools. Highlighting the high influenza transmission risk in children serological data from Hong Kong during the H1N1/2009 pandemic showed that 43% of children aged 5–14 years were infected early in the pandemic compared to ∼5% of those aged 30–59 years [3]. The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention and the American Academy of Pediatrics recommend that annual influenza vaccination be extended to all children over 6 months of age [4]. Because they are mostly immunologically naive to influenza young children generally require two doses a month apart to achieve seroprotection [4]. A recent Cochrane review estimated the efficacy of trivalent inactivated influenza virus (TIV) vaccine in preventing influenza at 59% in children over two years old [5]. Neither TIV or live attenuated influenza virus (LAIV) vaccines are indicated for children under 6 months due to poor neonatal immune responses to inactivated vaccines and increased respiratory side effects from LAIV vaccines [6]. This leaves infants under 6 months of age highly vulnerable to influenza infection, making it imperative to identify strategies to better protect this population.
Safety considerations must always be paramount when developing vaccines for young children given their sensitivity to pyrogens and febrile convulsions [7]. For this reason whole virus vaccines are relatively contraindicated in young children and highly purified split or subunit antigens are preferred [8]. This reduces vaccine pyrogenicity but at the expense of reduced immunogenicity. Adjuvants could be used to enhance the immunogenicity of influenza vaccines, however only a very limited number of adjuvants have been tested in young children and there is no guarantee that an adjuvant effective in adults will be similarly effective in neonates. Highlighting the potential difficulties in developing safe and effective influenza vaccines for young children, a split TIV vaccine (Fluvax®, CSL, Australia) was withdrawn from pediatric use after causing increased febrile convulsions [9], with an estimated three excess hospitalizations with febrile convulsions for every influenza hospitalization prevented [10]. Furthermore, Pandemrix®, a monovalent inactivated vaccine adjuvanted with AS03 (an emulsion of squalene oil, dl-α-tocopherol and polysorbate 80) was found during the 2009 influenza pandemic to be associated with an increased rate of narcolepsy among European children aged 4–19 years [11]. An MF59-adjuvanted influenza vaccine was recently shown after two immunizations a month apart to induce higher antibody titers than unadjuvanted vaccine in children aged 6–36 months [12]. However, no influenza vaccine has yet been shown to be effective and safe for infants under 6 months of age making it imperative to develop an effective influenza vaccine for these very young infants.
Advax™ adjuvant is a novel polysaccharide adjuvant derived from microparticles of polyfructofuranosyl-d-glucose (delta inulin) [13] that when formulated with inactivated or recombinant vaccine antigens has proved effective in enhancing humoral and cellular immunity in adult animals against a broad range of pathogens including Japanese encephalitis virus, West Nile virus, anthrax, African horse sickness, HIV, listeria and hepatitis B, amongst others [14], [15], [16], [17], [18], [19], [20], [21]. Advax enhanced humoral and cellular immunity in mice to inactivated influenza antigen, translating into improved protection against influenza challenge [22]. This vaccine was safe even when administered to pregnant mice, overcoming pregnancy-associated immune suppression and enhancing vaccine immunogenicity in pregnant dams and with a secondary benefit of enhanced protection of pups via breast milk transfer of influenza-specific IgG [23]. Advax similarly enhanced vaccine protection of adult ferrets against high pathogenicity avian H5N1 influenza [24]. In a clinical trial of a pandemic influenza vaccine it was confirmed in adults to enhance seroprotection with minimal side effects [25]. To date, Advax adjuvant has not been tested in infants. Given the problem of vaccine hyporesponsiveness in young children, the current study was undertaken to test the ability of Advax adjuvant to improve influenza vaccine immunogenicity and protection in neonatal mice. A particular objective was to test whether Advax adjuvant could be used to achieve single-dose influenza protection of neonates, thereby helping avoid the need for two immunizations as currently recommended for influenza protection of young children over 6 months of age [4].
2. Materials and methods
2.1. Mice and immunization procedure
BALB/c, C57BL/6 (BL6) and μMT (BL6 background) mice were supplied by the central animal facility of Flinders University of South Australia. Each litter of neonatal mice (4–8 mice/group) were immunized at 7 days of age with a single subcutaneous (s.c.) injection in both hind thighs with 25 μl each of 1 to 15 μg β-propiolactone (BPL)-inactivated influenza A/Puerto Rico/8/34 (iH1N1) (Advanced Biotechnologies Inc., Columbia, MD, USA) alone or together with 1 mg Advax delta inulin adjuvant (Vaxine Pty Ltd., Adelaide, Australia). Control groups were injected with saline alone. All procedures were performed in accordance with the Animal Experimentation Guidelines of the National Health and Medical Research Council of Australia and approved by the Flinders Animal Welfare Committee. Three weeks post immunization, blood samples were collected by cheek vein bleeding or by retro-orbital plexus bleeding under anesthesia by intraperitoneal (i.p.) injection of 75 mg/kg ketamine and 1 mg/kg medetomidine. All immunization and challenge experiments were repeated at least 3 times to confirm reproducibility of results.
2.2. Influenza challenges
The virus used for challenge experiments was a mouse-adapted Influenza A/Puerto Rico/8/34 (H1N1) virus propagated in allantoic fluid of 10-day old embryonated hen's eggs and purified by sucrose density gradient ultracentrifugation and stored at −80 °C until used. The 50% mouse lethal dose (LD50) was estimated in adult BALB/c mice by the Reed-Muench method. One adult LD50 corresponded to 1250 TCID50 on MDCK cells. Unless otherwise indicated the virus challenge dose used was 6250 TCID50 (5 × LD50). This dose gave 100% lethality in unimmunized neonatal mice. For challenge studies, unimmunized control groups were routinely included to ensure that the challenge dose was completely lethal. In all cases, 100% lethality was always observed in control unimmunized infant mice. Mice were infected by intranasal administration of 30 μl of A/H1N1 virus. A sickness scoring system based on coat condition, posture and activity was used to assess the extent of clinical disease. Mice were evaluated daily and scored for individual symptoms. Ruffled fur (absent = 0; slightly present = 1; present = 2), hunched back (absent = 0; slightly present = 1; present = 2) and activity (normal = 0; reduced = 1; severely reduced = 2) were evaluated. The final score was the addition of each individual symptom score (e.g. an animal showing slightly ruffled fur (1), slightly hunched back (1) and reduced activity (1) was scored as 3. Mice were euthanized if they became moribund or developed a clinical score of 6. Although it was not possible to blind the challenges, bodyweight and clinical scores were independently audited by the Flinders University animal welfare committee.
2.3. ELISA assays
Influenza virus specific antibodies were determined by ELISA, as previously described [22]. Briefly, inactivated A/Puerto Rico/8/34 (PR8) antigen (Charles River, CT, USA) was used to coat 96-well ELISA plates. After blocking, 100 μl diluted serum samples were added followed by biotinylated anti-mouse IgG, IgG1, IgG2a/c, IgG2b, IgG3 and IgM (Abcam) with HRP-conjugated Streptavidin (BD Biosciences) was added for 1 hr. After washing, TMB substrate (KPL, Gaithersburg, MD, USA) was added for 10 min before the reaction was stopped with 100 μl 1 M Phosphoric Acid. The optical density was measured at 450 nm (OD450 nm) using a VersaMax plate reader and analyzed using SoftMax Pro Software. Average OD450 nm values obtained from negative control wells were subtracted.
2.4. Surface plasmon resonance (SPR)
SPR experiments were performed with a BIAcore X100 (GE Healthcare) and CM5 sensor chip at 25 °C using running buffer HBS–EP + (10 mM HEPES, 0.15 M NaCl, 3 mM EDTA, 0.005% v/v surfactant P20, pH 7.4). PR8 antigen was covalently immobilized on fc2 using wizard amine coupling method. The CM5 chip was activated with EDC/NHS for 7 min, followed by an injection of 30 μg/ml PR8 in10 mM sodium acetate, pH 4.5. The excess activated carboxyl groups were blocked with ethanolamine for 7 min to reach the final immobilization level of 4951 RU on fc2. Pooled mouse antisera for each vaccine group was 40-fold diluted in HBS-EP+ buffer and injected onto the surface fc2 and reference surface fc1 at 5 μl/min for 10 min. Bound anti-PR8 antibodies were further characterized by a sequential injection of 20 μl anti-mouse subclass specific antibodies (20 μg/ml) with the order of anti-IgG3, anti-IgG2b, anti-IgG2a, anti-IgG1 and anti-IgM for 180 s at 5 μl/min. The chip was regenerated by 10 mM Glycine pH 1.7 for 30 s and 0.05% SDS for 30 s. Sensorgrams were corrected by subtracting the signal from the reference flow channel fc1.
2.5. Preparation of single-cell suspensions from bone marrow and spleen
Mice were killed by cervical dislocation then bones and spleens were aseptically collected. Bone marrow was isolated from femur by flushing 3% FBS/PBS. Spleens were homogenized by grinding with the plunger from a 5 ml syringe on a 70 μm cell strainer (BD Biosciences) and were treated with red blood cell (RBC) lysis buffer. Cells were stained with trypan blue and live cells were counted by a hemocytometer.
2.6. Cytokine assays
Splenocytes (2 × 105 cells/well) were re-stimulated for 3 days in U–bottom 96-well plates (Greiner Bio-one) with 0.5 μg/ml PR8 antigen, the supernatants harvested and cytokines measured by mouse Th1/Th2/Th17 cytokine CBA kit (BD Biosciences) and analyzed by FCAP Array Software (Soft Flow Hungary Ltd.).
2.7. In vivo CD8 T-cell depletion
For CD8+ T-cell depletion, 250 μg anti-mouse CD8a (Clone 53-6.72) (Bio X Cell, West Lebanon, U.S.A.) was injected i.p. at days −3, −1, +3 and +7 of virus challenge. Depletion was verified by flow cytometry using peripheral blood cells treated with RBC lysis buffer and rat anti-mouse CD16/CD32 (Clone 2.4G2), APC-anti-mouse CD4 (Clone RM4-5) and PE-anti-mouse CD8a (Clone 53-6.72) (BD Biosciences) and confirmed more than 95% reduction in CD8+ T cells. Rat IgG enriched from naive rat serum by 30% ammonium sulfate precipitation was used as a negative control.
2.8. ELISPOT
Ninety-six-well multiScreen filter plates (Millipore, USA) were pre-wetted with 35% ethanol then washed twice with PBS before coating. Plates were coated at 4 °C overnight with 10 μg/ml PR8 for B-cell ELISPOTs or 5 μg/ml anti-mouse IL-2, IL-4, or IFN-γ (all from BD Biosciences) or IL-17 mAb (BioLegend, San Diego) for T-cell ELISPOTs. The plates were washed with PBS, then blocked with RPMI 1640 + 10% FCS. A total of 4 × 105 splenocytes and 5 μg/ml PR8 were added in triplicates before the plates were incubated at 37 °C for 24 h (B-cell ELISPOT) or 48 h (T-cell ELISPOT). The plates were washed 3 times with PBS-Tween 20 and 2 μg/ml detection antibodies in PBS + 10% FCS were added and incubate for overnight at 4 °C. After discarding detection antibodies, plates were washed 3 times with PBS/Tween 20 and incubated with 1:200 dilution of HRP-Streptavidin (BD Biosciences) for 1 h at RT. After washing spots were visualized using AEC substrate (BD Biosciences). The plates were washed twice with water, dried, and analyzed by Immunospot ELISPOT system (Cellular Technology, Shaker Heights, OH, USA).
2.9. In vivo CTL assay
Splenocytes were harvested from naive BL6 mouse spleens and half of the splenocytes were pulsed with 5 μM NP366 peptide (sequence ASNENMETM) for 2 h at 37 °C. The remaining half was incubated without peptide. After washing with PBS, cells were labeled by incubation for 7 min at RT with either 5 μM CFSE (Life Technologies) (peptide-pulsed cells, CFSEhi) or 0.5 μM CFSE (un-pulsed cells, CFSElow). CFSE-labeled cells were washed twice with PBS + 10% FCS. A mixture of 2 × 106 CFSEhi and 2 × 106 CFSElow cells was then injected intravenously through tail vein. After 18 hr, animals were sacrificed, splenocytes collected and analyzed by flow cytometry.
2.10. Statistical analysis
Mann-Whitney or Kruskal–Wallis tests were performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego). Survival curves were created using the Kaplan-Meier method and statistical analyses of survival curves used a log-rank (Mantel–Cox) test. For all comparisons, p < 0.05 was considered to represent a significant difference. All data shown as mean ± SEM. In figures * p < 0.05; ** p < 0.01; and *** p < 0.001.
3. Results
3.1. A single dose of Advax-adjuvanted influenza vaccine provides protection of neonatal mice
We first asked whether a single immunization with iH1N1 with or without adjuvant could protect neonatal mice. Although an intramuscular immunization route is often used in adult immunization studies, it is not possible to use this route to administer vaccine to very small pups and hence the subcutaneous (s.c.) route was used. Seven-day-old BALB/c pups were immunized s.c. in the hind limb with 1, 5 or 15 μg of iH1N1 with or without Advax adjuvant. When they reached 4 weeks of age immunized pups were challenged intranasally with homologous influenza virus. Immunization with iH1N1 alone provided no protection of pups, with rapid weight loss and death by 10 days post-challenge, even at the highest 15 μg iH1N1 dose (Fig. 1 ). By contrast, complete protection was seen in pups immunized with 15 μg iH1N1 + Advax adjuvant (100% survival) with the pups continuing to gain weight throughout the challenge period consistent with a lack of clinical disease. Partial protection was also seen in pups immunized with 5 μg iH1N1 + Advax (60% survival), although the survivors lost ∼20% body weight before recovering. Immunization with 1 μg iH1N1 + Advax was not protective (0% survival). This indicated that 7-day-old neonatal mice could be protected with a single high dose of iH1N1 providing it was formulated with Advax adjuvant.
Fig. 1.
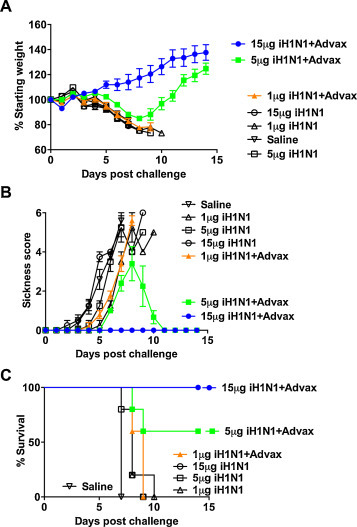
Single-dose vaccine protection of neonates. Seven-day-old pups (n = 5/gp) were immunized once s.c. with indicated dose of iH1N1 alone or with Advax adjuvant then challenged 3 weeks later with H1N1 virus. Shown are body weights (A), sickness scores (B) and survival rates (C). All data in figures are shown as mean ± SEM. Experiment was repeated twice with similar results.
3.2. Advax adjuvant overcomes neonatal B-cell hyporesponsiveness
To better understand the mechanism whereby Advax adjuvant provided enhanced single-dose vaccine protection of neonatal pups, the challenge study was repeated using the 15 μg iH1N1 + Advax dose that provided complete single-dose protection in the first study. Immunized pups were bled at 3 weeks of age for measurement of serum anti-influenza antibodies and then challenged at 4 weeks of age with homologous virus. Pups immunized with iH1N1 + Advax had significantly increased serum influenza-specific IgM, IgG1, IgG2a and IgG2b levels 3 weeks post-immunization, whereas pups immunized with iH1N1 alone had low to undetectable levels of all antibody isotypes (Fig. 2A). To compare relative amounts of each antibody isotype in immunized pups, surface plasmon resonance (SPR) was performed using pooled pre-challenge sera from each vaccine group. This revealed that pups immunized with iH1N1 + Advax produce predominantly IgM and to a lesser extent IgG1, but not IgG2a, 2b or 3 (Fig. 2B). This contrasted with the result of previously published studies in adult mice where immunization with iH1N1 + Advax induced IgG2a and 2b as well as IgG1 [22], [23]. This suggests that the 7-day-old pups had a marked Th2 isotype bias to influenza immunization, consistent with data reported elsewhere [26]. A subset of immunized pups from each group were sacrificed 2 weeks post-immunization and the frequency of influenza-specific IgM and IgG antibody secreting cells (ASC) measured by ELISPOT. Consistent with serum antibody, a significantly increased (∼3–4 fold) frequency of influenza-specific IgM and IgG ASC was seen in the spleens of pups immunized with iH1N1 + Advax versus those immunized with iH1N1 alone (Fig. 2C). When challenged at 3 weeks post-immunization, pups immunized with iH1N1 + Advax gained weight, had minimal clinical disease and had high levels of survival (survival 8/9, 89%, p < 0.0001). By contrast, pups immunized with iH1N1 alone, lost weight, had high clinical scores and all succumbed to infection (Fig. 2D).
Fig. 2.
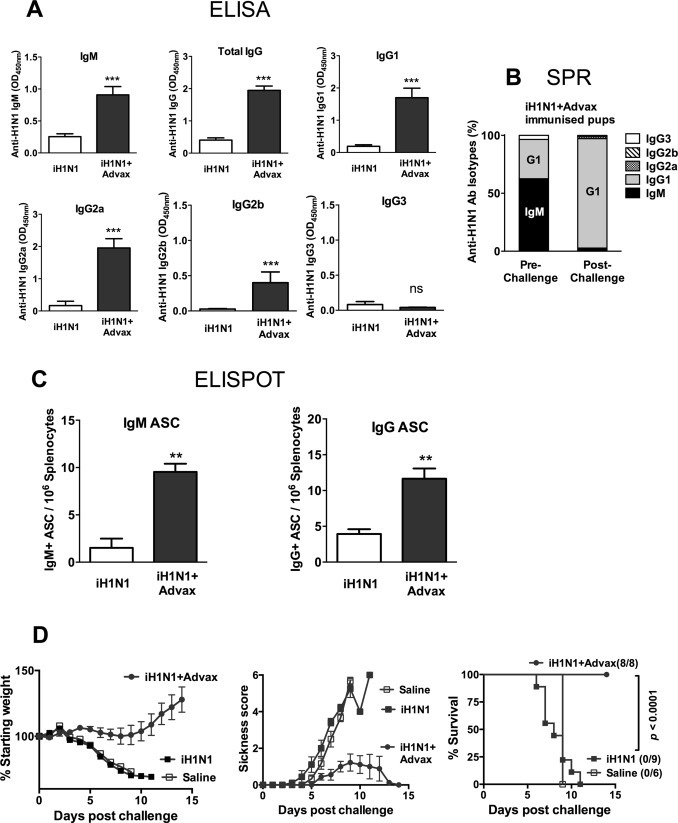
Advax adjuvant overcomes neonatal B-cell hyporesponsiveness. Seven-day-old pups (n = 9–10/gp) were immunized s.c. with 15 μg of iH1N1 alone or with Advax adjuvant. Serum was collected 3 weeks post-immunization for antibody determination by ELISA (A). Pooled sera from the iH1N1 + Advax immunised group pre- and post-challenge were captured on an iH1N1-immobilized Biacore chip for SPR analysis of the relative quantities of each antibody isotype as determined by sequential injection over the sensor surface and depicted as relative percentage of antibody isotypes (B). Pups from each group were sacrificed pre-challenge to determine the frequency of H1N1-specific ASC in their spleens by ELISPOT (C). Remaining immunized pups were challenged with H1N1 virus 3 weeks post-immunization and body weight, sickness score and survival rate monitored for 2 weeks (D).
Sera taken from iH1N1 + Advax survivors two weeks post-challenge showed a large rise in influenza-specific IgG1 from pre-challenge levels, with loss of the pre-challenge IgM component, (Fig. 2B), consistent with the vaccine-induced IgM-secreting B cells having undergone an isotype switch from IgM to IgG1 production in response to influenza virus exposure.
3.3. Advax adjuvant overcomes neonatal T-cell hyporesponsiveness
To further characterize the mechanism whereby Advax adjuvant enhances neonatal protection, memory T-cell responses were assessed in immunized pups. Splenocytes were isolated from pups 3 weeks post-immunization, stimulated with iH1N1 antigen and cytokines measured in the culture supernatants. Pups immunized with iH1N1 + Advax had significantly higher (∼2–3 fold) influenza-stimulated recall production of IFN-γ, IL-2, IL-4, and IL-10, but not IL-6 or TNF, when compared to pups immunized with iH1N1 alone (Fig. 3A). Thus although neonates typically have impaired Th1 vaccine responses [27], immunization with iH1N1 + Advax adjuvant was able to overcome this defect and enhance T-cell IFN-γ production. We next tested by cytokine ELISPOT whether higher cytokine recall responses in Advax-immunized pups reflected an increased frequency of influenza-specific memory T cells. Consistent with the bulk cytokine production data, pups immunized with iH1N1 + Advax had ∼3–10 fold higher frequencies of IFN-γ, IL-2, IL-4, and IL-17 secreting memory T cells when compared to pups immunized with iH1N1 alone (Fig. 3B). Thus, immunization of pups with iH1N1 + Advax adjuvant successfully overcame neonatal immune immaturity at both the B- and T-cell level, resulting in increased influenza-specific serum antibody levels, and increased frequency of anti-influenza memory B cells and Th1, Th2 and Th17 memory T cells.
Fig. 3.
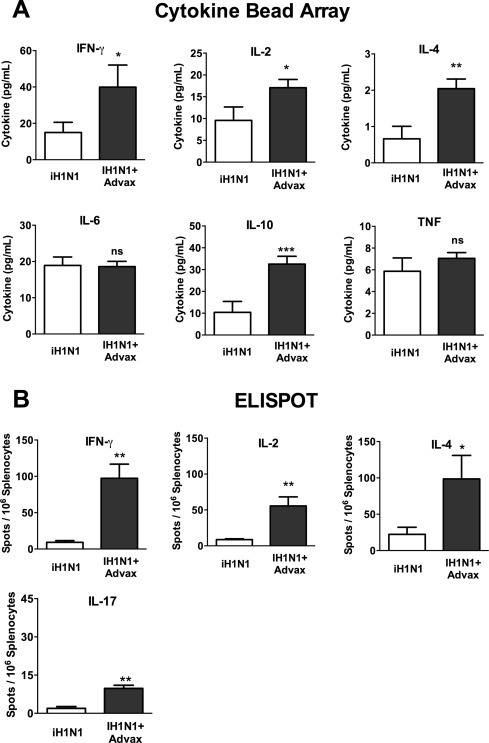
Advax adjuvant overcomes neonatal T-cell hyporesponsiveness. Splenocytes were harvested 2 weeks post-immunization of 7-day-old pups, stimulated with iH1N1 and cytokine levels in the culture supernatant determined by CBA (A). The frequency of H1N1-specific T cells in the spleen of the same mice was assessed by ELISPOT (B).
3.4. B cells are required for neonatal influenza protection by Advax-adjuvanted vaccine
Interestingly, although iH1N1 + Advax induced memory T-cell responses belonging to both Th1 and Th2 subsets as indicated by their patterns of cytokine production, the antibody production in these pups was solely Th2 in nature (IgM and IgG1) even after influenza virus challenge. We asked, therefore, whether influenza protection in iH1N1 + Advax immunized pups was dependent on memory B- or T-cells. μMT mice lack mature B cells and ability to make antibody [28]. Hence, to test the role of memory B cells in neonatal protection we immunized C57BL6 wildtype (WT) or μMT pups with iH1N1 + Advax and then challenged them with influenza virus 3 weeks post-immunization. As previously observed, C57BL6 WT pups immunized with iH1N1 alone lost weight and succumbed to infection, whereas those immunized with iH1N1 + Advax survived and gained weight throughout the challenge period (Fig. 4 ). By contrast, all neonatal μMT pups including those immunized with iH1N1 + Advax lost weight and died. This indicates that protection of pups with iH1N1 + Advax requires functional B cells and is not conferred by memory T cells alone. Consistent with this result, CD8 T-cell depletion of iH1N1 + Advax immunized WT pups at the time of challenge did not reduce survival, indicating that CD8 T cells were not required for protection of Advax-immunized WT pups (Fig. 4). We next asked whether the inability of T cells to provide protection in μMT pups reflected an inability of neonates to make cytotoxic T lymphocytes (CTL). To address this question we performed in vivo CTL assays post-challenge in WT pup survivors previously immunized with iH1N1 + Advax. Neonatal iH1N1 + Advax-immunized survivors all exhibited a high level of target cell killing indicating that they were able to generate anti-influenza CTL's in response to viral exposure (data not shown).
Fig. 4.
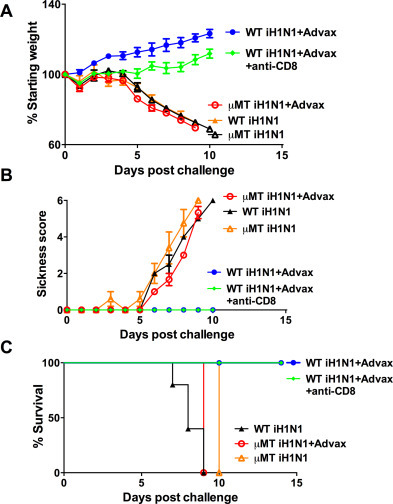
Vaccine protection of neonates is B-cell dependent. Seven-day-old wild type (WT) and μMT mice were immunized s.c. with 15 μg of iH1N1 alone or with Advax adjuvant. One group of WT mice also received anti-mouse CD8 antibody to deplete CD8+ cells during challenge. μMT pups were challenged with 1250 TCID50 of H1N1 virus. Shown are post-challenge body weights (A), sickness scores (B) and survival rates (C).
3.5. Absence of adverse effects in neonates receiving Advax-adjuvanted vaccine
Potential adverse effects of Advax adjuvant were assessed in immunized pups, including inspection for injection site reactions and monitoring of feeding behavior and growth. Pups receiving iH1N1 + Advax had no evidence of injection site lesions, and showed normal development and weight gain comparable to control pups (Fig. 5 ). This is consistent with the absence of adverse effects of Advax adjuvant previously seen in immunization studies of adult mice including pregnant dams [22], [23].
Fig. 5.
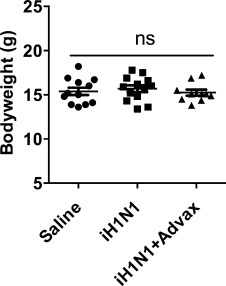
Advax adjuvant does not affect neonatal growth. Seven-day-old pups (n = 10–13/gp) were immunized once s.c. with saline or 15 μg iH1N1 with or without Advax adjuvant and the bodyweight of each pup recorded 3 weeks later. ns = no significant difference between groups.
4. Discussion
These results confirm that Advax adjuvant is able to successfully overcome neonatal hypo-responsiveness to inactivated influenza immunization, thereby enabling robust single-dose protection of 7-day-old pups against otherwise lethal influenza infection. Immunization of 7-day-old pups with a single dose of iH1N1 + Advax adjuvant induced high serum IgM and IgG1 levels and increased the frequency of splenic influenza-specific ASC. Advax adjuvant also enhanced cellular immunity, increasing frequencies of influenza-specific memory T cells and increasing recall cytokine production.
The effectiveness of Advax adjuvant in protection of neonates stands in contrast to other adjuvants that have been shown to be ineffective in inducing neonatal influenza protection. For example, an alum-adjuvanted influenza vaccine provided no protection in neonatal mice [29], despite use of a very similar influenza model to the one in the current study. This can be explained by the fact that alum adjuvant has reduced ability to induce IgG production, B-cell affinity maturation and germinal center formation in neonates [30]. The squalene oil emulsion adjuvant MF59 is currently the only adjuvant in an approved human seasonal influenza vaccine. It was recently reported that immunization of neonatal mice at 7 and 21 days of age with MF59-adjuvanted influenza vaccine failed to provide protection or to enhance antibody titers, consistent with a complete lack of MF59 effectiveness in neonates despite it enhancing protection in older infant and adult mice given the same two dose vaccine regimen [31]. This stands in marked contrast to the outcomes seen in the current study where Advax adjuvant induced high serum levels of IgM and IgG1 in 7-day-old mice after just a single vaccine dose and protected them against an otherwise highly lethal influenza challenge. Hence, Advax adjuvant appears unique in its ability to overcome neonatal immune hyporesponsiveness and induce vaccine protection in such young mice.
Neonatal mice did require a higher antigen dose than adult mice for complete influenza protection. Neonatal mice are known to have higher susceptibility to lethal influenza than adults [32], which likely reflects immaturity of both innate and adaptive immune systems and similarly increased neonatal susceptibility to influenza is also seen in human epidemiological data [1]. This high neonatal susceptibility to influenza makes the search for an effective neonatal vaccine strategy imperative. How the influenza antigen requirement required for complete protection of neonates in the current study might translate to doses needed to protect human neonates cannot be predicted in advance of human studies, but it must be noted that the vaccine regimen used in the current study was protective after just a single dose, whereas typically even older human infants require at least 2 doses for seroprotection. The World Health Organization consequently recommends that infants aged 6–35 mouths receive two 7.5 μg doses of split influenza vaccine a month apart. Hence even though a high dose of antigen was needed in the current study for single dose protection of neonates, there should be considerable scope to reduce the neonatal vaccine dose by using a more typical two-dose regimen of Advax-adjuvanted influenza vaccine. For example, in adult mice we have found protection with a two-dose regimen requires as little as one-hundredth the dose required for single-dose protection. In future studies we will confirm whether a two-dose regimen of Advax-adjuvanted influenza vaccine significantly reduces the antigen dose required for neonatal protection.
Neonatal protection with the Advax-adjuvanted influenza vaccine was dependent on functional B cells as protection was not seen in immunized neonatal μMT mice that are deficient in B cells and are unable to make immunoglobulin. Neonatal protection with Advax-adjuvanted influenza vaccine was not dependent on CD8 T-cell immunity as protection was observed even when CD8 T cells were depleted prior to challenge. The fact that T cells were unable to mediate protection in immunized neonates in the absence of antibody was surprising as the T-cell cytokine phenotype generated in immunized pups appeared very similar to the phenotype previously seen in adult mice in which T-cell protection could be demonstrated [22]. This suggested that T cells in immunized pups might have a defect in effector function, e.g. in cytotoxic T lymphocyte (CTL) differentiation. However when in vivo CTL assays were performed post-challenge in surviving iH1N1 + Advax immunized WT pups, high levels of killing of influenza peptide-labeled target cells was seen (data not shown), suggesting the neonatal T cells were indeed able to differentiate into CTLs. The lack of T-cell protection in neonatal μMT pups could instead reflect slower differentiation of neonatal CD8 T cells into lytic CTL effectors, with delayed CTL differentiation having been shown to be the reason for defective neonatal protection against herpes simplex virus [33]. Furthermore, in contrast to adults, neonatal T cells were shown to fail to proliferate and migrate into the lungs in response to influenza infection, consistent with qualitative differences in neonatal and adult T-cell function [32].
How did Advax adjuvant enhance protective B cell immunity in the immunized pups? Impaired antibody production and affinity maturation in immunized neonates have been correlated with impaired expansion of T follicular helper (TFH) lymphocytes, as shown in neonatal mice immunized with tetanus toxoid absorbed to alum adjuvant [34]. Poor neonatal germinal center formation post-immunization has been correlated with a delayed maturation of follicular dendritic cells (fDCs) [35]. Notably, MF59-adjuvanted influenza vaccine failed to induce germinal centers and antibody responses in 7-day-old mice associated with a failure of induction of functional TFH cells [31]. Thus most adjuvants with the exception of Advax are incapable of correcting neonatal immune defects in B-cell function. The central importance of TFH and fDC in optimal neonatal vaccine responses suggests that Advax through a yet to be determined mechanism improve activation and expansion of neonatal TFH cells and/or fDCs.
Interestingly, although iH1N1 + Advax immunization induced almost exclusively IgM and IgG1 isotypes in pups consistent with a Th2-dominated response, it induced memory T cells belonging to Th1 and Th17 as well as the Th2 subset. This is interesting as Th2 recall responses typically dominate in neonates [36]. Both murine [37] and human [38] newborns have been reported to have impaired IL-17 and IFN-γ responses, with the latter caused by low dendritic cell IL-12 production [27]. It was therefore surprising that Advax-adjuvanted vaccine induced such a robust IFN-γ (Th1) recall response in neonates. The mechanism by which Advax overcame the normal neonatal Th2 bias is not known but may potentially be very advantageous. Excess Th2 bias can cause eosinophilic lung immunopathology as has been seen after either SARS coronavirus (SARS)[39], or respiratory syncytial virus (RSV) [40] immunization. A recent study showed formulation of inactivated or recombinant SARS vaccine with Advax adjuvant not only enhanced protection against SARS but also prevented eosinophilic lung immunopathology [39]. Hence, the ability of Advax adjuvant to enhance neonatal Th1 responses and reduce their otherwise overwhelming Th2 bias could be of major benefit for neonatal vaccines particularly where there might be risk of Th2 bias otherwise inducing eosinophilic lung immunopathology.
A key consideration when developing vaccine adjuvants for potential use in neonates is safety and tolerability. Children are highly prone to febrile convulsions and at least one seasonal inactivated influenza vaccine has had to be withdrawn due to excess reactogenicity and pyrogenicity in young children [10]. Although in this study 7-day-old pups received exactly the same Advax dose (1 mg) previously used in adult mouse studies, no adjuvant-related adverse effects were observed. Future dose-response studies will test whether lower doses of Advax can be used for pups. However it was a testament to Advax's safety that pups which weighted just a few grams were able to tolerate the full adult dose. When administered to adult mice, even human sized doses of 10–20 mg of Advax did not induce pyrexia (unpublished data). While we had no means to measure temperature in the 7-day-old pups, there was no indication of pyrexia post-immunization, which in neonates would typically be associated with a loss of body weight. Similarly, no adverse effects on pregnant dams or their pups were observed when Advax was administered during pregnancy [23]. Advax has also already been shown to be safe and well tolerated in adult human trials, with no evidence of any potential to induce pyrexia [25], [41].
The ability of Advax adjuvant to enhance neonatal vaccine protection raises many questions, including the nature of the mechanisms whereby Advax adjuvant is able to correct for neonatal B- and T-cell immaturity. Notably, injection of Advax adjuvant alone without antigen did not provide any non-specific protection against influenza [22], [24]. This contrasts with the non-specific virus protection due to nonspecific innate immune activation seen with CpG oligonucleotides and other TLR agonists [42], [43]. This together with the fact that it doesn’t induce pyrexia even when administered at high doses suggests that Advax is not working through traditional TLR-mediated signaling pathways. However, the exact mechanism of action remains an area of active investigation.
In conclusion, this pilot study in 7-day-old mice showed Advax adjuvant was able to overcome neonatal immune immaturity with enhancement of influenza-specific antibody and T-cell responses and single-dose vaccine protection against otherwise lethal influenza infection. The use of Advax adjuvant was not associated with any adverse effects, supporting the strong safety data seen in other animal models and in adult human clinical trials. There is an ongoing need for an influenza vaccine effective in children under 6 months. The challenge now will be to see whether this promising murine data with Advax adjuvant translates into similar benefits in larger animal models and ultimately in human infants. These findings also raise the possibility that Advax adjuvant might similarly beneficially enhance the immunogenicity of other vaccines directed at neonates, for example RSV, TB or malaria vaccines.
Funding
This project has been funded with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract no. HHSN272200800039C and Collaborative Research Contact no. U01AI061142. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest statement
YHO, CHO and NP are associated with Vaxine Pty Ltd., (Adelaide Australia), which holds proprietary interests in Advax™ adjuvant. Animal challenge study results are overseen and audited by the Flinders University Animal Welfare Committee.
Acknowledgements
We thank Connie Li for technical assistance with the BIAcore studies and Samay Trec, Anna Lalusis-Derks, Marco Meier, Robb Muirhead and Annasaheb Kolpe for assistance with animal husbandry.
References
- 1.Wong K.K., Jain S., Blanton L., Dhara R., Brammer L., Fry A.M. Influenza-associated pediatric deaths in the United States, 2004–2012. Pediatrics. 2013;132(Nov (5)):796–804. doi: 10.1542/peds.2013-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brotherton J., McIntyre P., Puech M., Wang H., Gidding H., Hull B. Vaccine preventable diseases and vaccination coverage in Australia 2001 to 2002. Commun Dis Intell. 2004;28(Dec (Suppl 2)):vii-S116. [PubMed] [Google Scholar]
- 3.Wu J.T., Ma E.S., Lee C.K., Chu D.K., Ho P.L., Shen A.L. The infection attack rate and severity of 2009 pandemic H1N1 influenza in Hong Kong. Clin Infect Dis. 2010;51(Nov (10)):1184–1191. doi: 10.1086/656740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekker A., Chou C., Bernstein H.H. Update on universal annual influenza immunization recommendations for children. Curr Opin Pediatr. 2009;21(Feb (1)):122–126. doi: 10.1097/MOP.0b013e32832185af. [DOI] [PubMed] [Google Scholar]
- 5.Jefferson T., Rivetti A., Harnden A., Di Pietrantonj C., Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008;2:CD004879. doi: 10.1002/14651858.CD004879.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Vesikari T. Emerging data on the safety and efficacy of influenza vaccines in children. Pediatr Infect Dis J. 2008;27(11 Suppl):S159–S161. doi: 10.1097/INF.0b013e31818a545d. [DOI] [PubMed] [Google Scholar]
- 7.Kaczmarek M.C., Duong U.T., Ware R.S., Lambert S.B., Kelly H.A. The risk of fever following one dose of trivalent inactivated influenza vaccine in children aged ≥6 months to <36 months: a comparison of published and unpublished studies. Vaccine. 2013;31(Nov (46)):5359–5365. doi: 10.1016/j.vaccine.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Dowdle W.R., Millar J.D., Schonberger L.B., Ennis F.A., LaMontagne J.R. Influenza immunization policies and practices in Japan. J Infect Dis. 1980;141(2):258–264. doi: 10.1093/infdis/141.2.258. [DOI] [PubMed] [Google Scholar]
- 9.ACIP Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep. 2010;59(Aug (31)):989–992. [PubMed] [Google Scholar]
- 10.Kelly H., Carcione D., Dowse G., Effler P. Quantifying benefits and risks of vaccinating Australian children aged six months to four years with trivalent inactivated seasonal influenza vaccine in 2010. Euro Surveill. 2010;15(Sep (37)):1–9. [PubMed] [Google Scholar]
- 11.Partinen M., Saarenpaa-Heikkila O., Ilveskloski I. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS ONE. 2012;7(3):e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan T., Bravo L., Ceballos A., Mitha E., Gray G., Quiambao B. Enhanced and persistent antibody response against homologous and heterologous strains elicited by a MF59-adjuvanted influenza vaccine in infants and young children. Vaccine. 2014;32(Oct (46)):6146–6156. doi: 10.1016/j.vaccine.2014.08.068. [DOI] [PubMed] [Google Scholar]
- 13.Cooper P.D., Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising beta-d-[2 ≥ 1] poly(fructo-furanosyl) alpha-d-glucose polymers. Glycobiology. 2011;21(5):595–606. doi: 10.1093/glycob/cwq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cristillo A.D., Ferrari M.G., Hudacik L., Lewis B., Galmin L., Bowen B. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. J Gen Virol. 2011;92(Jan (Pt 1)):128–140. doi: 10.1099/vir.0.023242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckersley A.M., Petrovsky N., Kinne J., Wernery R., Wernery U. Improving the dromedary antibody response: the hunt for the ideal camel adjuvant. J Camel Pract Res. 2011;18(1):35–46. [Google Scholar]
- 16.Feinen B., Petrovsky N., Verma A., Merkel T.J. Advax-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clin Vaccine Immunol. 2014;21(4):580–586. doi: 10.1128/CVI.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larena M., Prow N.A., Hall R.A., Petrovsky N., Lobigs M. JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8+ T cells and pre-exposure neutralizing antibody. J Virol. 2013;87(Apr (8)):4395–4402. doi: 10.1128/JVI.03144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobigs M., Pavy M., Hall R.A., Lobigs P., Cooper P., Komiya T. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol. 2010;91(Jun (Pt 6)):1407–1417. doi: 10.1099/vir.0.019190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrovsky N., Larena M., Siddharthan V., Prow N.A., Hall R.A., Lobigs M. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. J Virol. 2013;87(Sep (18)):10324–10333. doi: 10.1128/JVI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Del Rio E., Marradi M., Calderon-Gonzalez R., Frande-Cabanes E., Penades S., Petrovsky N. A gold glyco-nanoparticle carrying a listeriolysin O peptide and formulated with Advax delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine. 2015;33(Mar (12)):1465–1473. doi: 10.1016/j.vaccine.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 21.Saade F., Honda-Okubo Y., Trec S., Petrovsky N. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31(Apr (15)):1999–2007. doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda-Okubo Y., Saade F., Petrovsky N. Advax a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012;30(Aug (36)):5373–5381. doi: 10.1016/j.vaccine.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honda-Okubo Y., Kolpe A., Li L., Petrovsky N. A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine. 2014;32(Aug (36)):4651–4659. doi: 10.1016/j.vaccine.2014.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Layton R.C., Petrovsky N., Gigliotti A.P., Pollock Z., Knight J., Donart N. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine. 2011;29(Aug (37)):6242–6251. doi: 10.1016/j.vaccine.2011.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon D.L., Sajkov D., Woodman R.J., Honda-Okubo Y., Cox M.M., Heinzel S. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine. 2012;30(Aug (36)):5407–5416. doi: 10.1016/j.vaccine.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrios C., Brandt C., Berney M., Lambert P.H., Siegrist C.A. Partial correction of the TH2/TH1 imbalance in neonatal murine responses to vaccine antigens through selective adjuvant effects. Eur J Immunol. 1996;26(11):2666–2670. doi: 10.1002/eji.1830261118. [DOI] [PubMed] [Google Scholar]
- 27.Debock I., Jaworski K., Chadlaoui H., Delbauve S., Passon N., Twyffels L. Neonatal follicular Th cell responses are impaired and modulated by IL-4. J Immunol. 2013;191(Aug (3)):1231–1239. doi: 10.4049/jimmunol.1203288. [DOI] [PubMed] [Google Scholar]
- 28.Bot A. Immunoglobulin deficient mice generated by gene targeting as models for studying the immune response. Int Rev Immunol. 1996;13(4):327–340. doi: 10.3109/08830189609061756. [DOI] [PubMed] [Google Scholar]
- 29.Khalil S.M., Tonkin D.R., Snead A.T., Parks G.D., Johnston R.E., White L.J. An alphavirus-based adjuvant enhances serum and mucosal antibodies, T cells, and protective immunity to influenza virus in neonatal mice. J Virol. 2014;88(Aug (16)):9182–9196. doi: 10.1128/JVI.00327-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pihlgren M., Tougne C., Bozzotti P., Fulurija A., Duchosal M.A., Lambert P.H. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J Immunol. 2003;170(Mar (6)):2824–2832. doi: 10.4049/jimmunol.170.6.2824. [DOI] [PubMed] [Google Scholar]
- 31.Mastelic Gavillet B., Eberhardt C.S., Auderset F., Castellino F., Seubert A., Tregoning J.S. MF59 mediates its B Cell adjuvanticity by promoting T follicular helper Cells and thus germinal center responses in adult and early life. J Immunol. 2015;194(May (10)):4836–4845. doi: 10.4049/jimmunol.1402071. [DOI] [PubMed] [Google Scholar]
- 32.Lines J.L., Hoskins S., Hollifield M., Cauley L.S., Garvy B.A. The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol. 2010;185(Sep (5)):2980–2988. doi: 10.4049/jimmunol.0903075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez M.A., Evans I.A., Hassan E.H., Carbone F.R., Jones C.A. Neonatal CD8+ T cells are slow to develop into lytic effectors after HSV infection in vivo. Eur J Immunol. 2008;38(1):102–113. doi: 10.1002/eji.200636945. [DOI] [PubMed] [Google Scholar]
- 34.Mastelic B., Kamath A.T., Fontannaz P., Tougne C., Rochat A.F., Belnoue E. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J Immunol. 2012;189(12):5764–5772. doi: 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- 35.Bjarnarson S.P., Adarna B.C., Benonisson H., Del Giudice G., Jonsdottir I. The adjuvant LT-K63 can restore delayed maturation of follicular dendritic cells and poor persistence of both protein- and polysaccharide-specific antibody-secreting cells in neonatal mice. J Immunol. 2012;189(Aug (3)):1265–1273. doi: 10.4049/jimmunol.1200761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adkins B., Du R.Q. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J Immunol. 1998;160(May (9)):4217–4224. [PubMed] [Google Scholar]
- 37.Lee H.H., Hoeman C.M., Hardaway J.C., Guloglu F.B., Ellis J.S., Jain R. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205(Sep (10)):2269–2280. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goriely S., Vincart B., Stordeur P., Vekemans J., Willems F., Goldman M. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166(Feb (3)):2141–2146. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 39.Honda-Okubo Y., Barnard D., Ong C.H., Peng B.H., Tseng C.T., Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015;89(Mar (6)):2995–3007. doi: 10.1128/JVI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You D., Marr N., Saravia J., Shrestha B., Lee G.I., Turvey S.E. IL-4Ralpha on CD4+ T cells plays a pathogenic role in respiratory syncytial virus reinfection in mice infected initially as neonates. J Leukoc Biol. 2013;93(Jun (6)):933–942. doi: 10.1189/jlb.1012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon D., Kelley P., Heinzel S., Cooper P., Petrovsky N. Immunogenicity and safety of Advax, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled phase 1 study. Vaccine. 2014;(Sep (27)) doi: 10.1016/j.vaccine.2014.09.034. (pii: S0264-410X(14)01293-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han Y., Bo Z.J., Xu M.Y., Sun N., Liu D.H. The protective role of TLR3 and TLR9 ligands in human pharyngeal epithelial cells infected with influenza A virus. Korean J Physiol Pharmacol. 2014;18(Jun (3)):225–231. doi: 10.4196/kjpp.2014.18.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parvizi P., Abdul-Careem M.F., Mallick A.I., Haq K., Haghighi H.R., Orouji S. The effects of administration of ligands for Toll-like receptor 4 and 21 against Marek's disease in chickens. Vaccine. 2014;32(Apr (17)):1932–1938. doi: 10.1016/j.vaccine.2014.01.082. [DOI] [PubMed] [Google Scholar]


