Abstract
Studies have shown that administration of the β-lactam antibiotic, ceftriaxone (CEF) attenuates ethanol consumption and cocaine seeking behavior as well as preventing ethanol-induced downregulation of glutamate transporter 1 (GLT-1) expression in central reward brain regions. However, it is not known if these effects are compound-specific. Therefore, the present study examined the effects of two other β-lactam antibiotics, amoxicillin (AMOX) and amoxicillin/clavulanate (Augmentin, AUG), on ethanol drinking, as well as GLT-1 and phosphorylated-AKT (pAKT) levels in the nucleus accumbens (Acb) and medial prefrontal cortex (mPFC) of alcohol-preferring (P) rats. P rats were exposed to free-choice of ethanol (15% and 30%) for five weeks and were given five consecutive daily i.p. injections of saline vehicle, 100 mg/kg AMOX or 100 mg/kg AUG. Both compounds significantly decreased ethanol intake and significantly increased GLT-1 expression in the Acb. AUG also increased GLT-1 expression in the mPFC. Results for changes in pAKT levels matched those for GLT-1, indicating that β-lactam antibiotic-induced reductions in ethanol intake are negatively associated with increases in GLT-1 and pAKT levels within two critical brains regions mediating drug reward and reinforcement. These findings add to a growing literature that pharmacological increases in GLT-1 expression are associated with decreases in ethanol intake and suggest that one mechanism mediating this effect may be increased phosphorylation of AKT. Thus, GLT-1 and pAKT may serve as molecular targets for the treatment of alcohol and drug abuse/dependence.
Keywords: GLT-1, AKT, Amoxicillin, Augmentin, Clavulanate
Introduction
Glutamatergic neurotransmission in key mesocorticolimbic brain regions, such as the medial prefrontal cortex (mPFC) (Goldstein and Volkow, 2002) and the nucleus accumbens (Acb) (Childress et al., 1999; Obara et al., 2009), plays a key role in the development of ethanol and drug dependence. For example, clinical neuroimaging studies performed during periods of craving for drugs of abuse such as ethanol, heroin, methamphetamine, cocaine and nicotine revealed the critical role of glutamatergic projections from the mPFC to the Acb and the ventral-tegmental area (VTA) (Childress et al., 1999; Goldstein and Volkow, 2002). Furthermore, preclinical studies have demonstrated that ethanol consumption is associated with increased extracellular glutamate concentrations in several mesocorticolimbic brain regions (Dahchour et al., 2000; Ding et al., 2012a; Melendez et al., 2005; Moghaddam and Bolinao, 1994; Powell et al., 2013; Szumlinski et al., 2007). Extracellular glutamate concentrations are regulated by several glutamate transporters (Anderson and Swanson, 2000; Danbolt, 2001; Gegelashvili and Schousboe, 1997; Seal and Amara, 1999). Glutamate transporter 1 (GLT-1, a sodium dependent astro-glial protein) is responsible for 90% of glutamate reuptake from the synapse (Danbolt, 2001; Holmseth et al., 2012; Rothstein et al., 1996). It is noteworthy that a constituent GLT-1 gene knockout reduced glutamate reuptake by approximately 90% (Kiryk et al., 2008; Otis and Kavanaugh, 2000; Tanaka et al., 1997). Impaired glutamate uptake, often associated with downregulation of GLT-1 expression, has been implicated in the development of drug abuse using animal models (Knackstedt et al., 2009; Knackstedt et al., 2010; Nakagawa and Satoh, 2004; Rao and Sari, 2012; Sari et al., 2009; Sari et al., 2011; Sari and Sreemantula, 2012; Sari et al., 2013a; Sari et al., 2013b; Xu et al., 2003).
Pharmacological upregulation of GLT-1 expression in mesocorticolimbic brain regions can be induced by ceftriaxone (CEF, a β-lactam antibiotic). For instance, CEF increased mesocorticolimbic GLT-1 gene and/or protein expression and concomitantly attenuated (a) reinstatement to cocaine-seeking behavior (Fischer et al., 2013; Knackstedt et al., 2010; Sari et al., 2009), (b) relapse-like ethanol intake (Alhaddad et al., 2014) as well as (c) ethanol drinking in alcohol-preferring (P) rats (Sari et al., 2011; Sari et al., 2013b). Additionally, treatment with the neuroimmunophilin compound GPI-1046 (3-(3-pyridyl)-1-propyl (2S)-1-(3,3-dimethyl-1,2-dioxopentyl)-2-pyrrolidinecarboxylate) increased GLT-1 expression in the brain (Ganel et al., 2006; Sari and Sreemantula, 2012), and decreased ethanol consumption in male P rats (Sari and Sreemantula, 2012). These findings suggest that drugs upregulating central GLT-1 expression may serve as alternative treatments for drug abuse and dependence.
Interestingly, like CEF, amoxicillin was one of the compounds found to be proficient in upregulating GLT-1 expression (Rothstein et al., 2005). Therefore, in this study, we have investigated the effect of amoxicillin (AMOX) and amoxicillin/clavulanate (Augmentin, AUG) on ethanol intake in male P rats as illustrated in Figure 1A. The effect of AUG on sucrose intake as an appetitive control for drinking-motivated behavior was also examined (Figure 1B). Furthermore, we have examined the effects of these drugs on GLT-1 expression in the mPFC and Acb. The effects of AMOX and AUG treatments on pAKT expression, a signaling pathway involved in regulating GLT-1 expression (Wu et al., 2010), was also evaluated. Additionally, to confirm that AMOX or AUG do not exhibit disulfiram-like actions, liver mitochondrial aldehyde dehydrogenase 2 (ALDH2) enzyme activity was analyzed after treatment with these drugs.
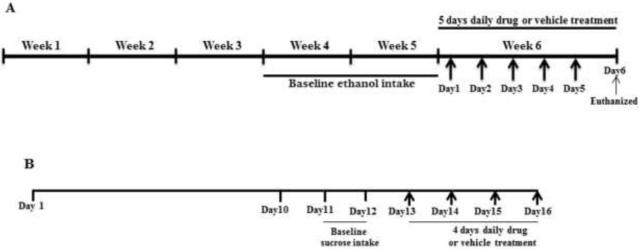
Figure 1.
Timeline of experiments
Results
Effect of chronic ethanol on GLT-1 expression in Acb and mPFC
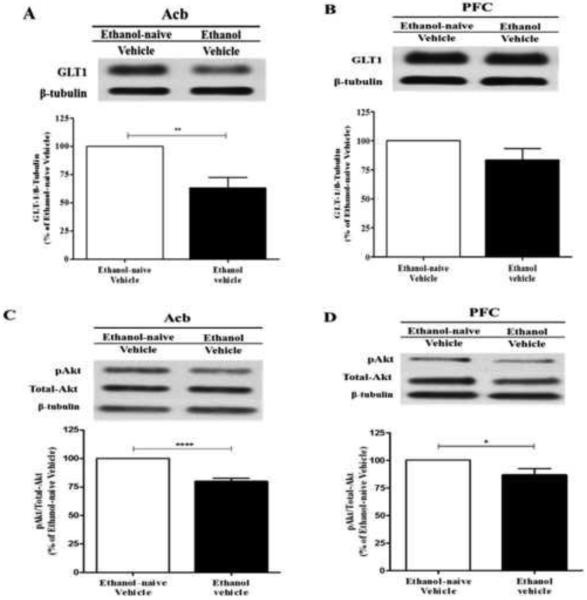
We analyzed the effect of chronic ethanol consumption on GLT-1 expression in Acb and mPFC. The effect of chronic ethanol consumption on GLT-1 expression is illustrated in Figure 2A (Acb) and Figure 2B (mPFC) as compared to the ethanol-naïve vehicle group. The Western blots analyzed using independent t-test demonstrated significant downregulation of GLT-1 in ethanol vehicle group as compared to ethanol-naïve vehicle group in Acb (p<0.01; Figure 2A). However, there was no significant change in the GLT-1 expression in ethanol vehicle group as compared to ethanol-naïve vehicle group in mPFC (Figure 2B).
Figure 2.
Effects of chronic ethanol on GLT-1 and pAKT expression in the Acb and mPFC (n=6/treatment group: ethanol vs. water). (A) Western Blot analysis revealed significant downregulation of GLT-1 expression in the Acb following chronic ethanol consumption by male P rats as compared to the ethanol naïve group (100%). (B) Western Blot analysis revealed no significant change of GLT-1 expression in the mPFC following chronic ethanol consumption as compared to the ethanol naïve group (100%). (C) Western Blot analysis revealed significant downregulation of pAKT levels in the Acb following chronic ethanol consumption as compared to the ethanol naïve group (100%). (D) Western Blot analysis revealed significant downregulation of pAKT levels in the mPFC following chronic ethanol consumption as compared to the ethanol naïve group (100%). *, p<0.05; **, p<0.01; ****, p<0.0001.
Effect of chronic ethanol on pAKT expression in Acb and mPFC
Furthermore, we investigated the effect of chronic ethanol consumption on phosphorylation of AKT in Acb and mPFC. Figure 2C and Figure 2CD illustrates the effect of chronic ethanol consumption on pAKT expression in Acb and mPFC, respectively, as compared to the ethanol-naïve vehicle group. An independent t-test revealed significant downregulation of pAKT in the ethanol vehicle group as compared to ethanol-naïve group in Acb (p<0.0001; Figure 2C) and mPFC (p<0.05; Figure 2D).
Concentration of amoxicillin in plasma and CSF after amoxicillin and Augmentin injection
The average plasma (n=3) and CSF (n=5) concentration of AMOX after its administration (i.p., 100mg/kg) was found to be 29.82±4.1 µg/mL and 352.87±20.58 ng/mL, respectively. However, the average concentration of AMOX in plasma (n=3) and CSF (n=2) after AUG administration (i.p., 100mg/kg) in P rats was found to be 7.3±0.79 µg/mL and 270.99±3.75 ng/mL, respectively.
Effects of amoxicillin and Augmentin on ethanol intake in male P rats
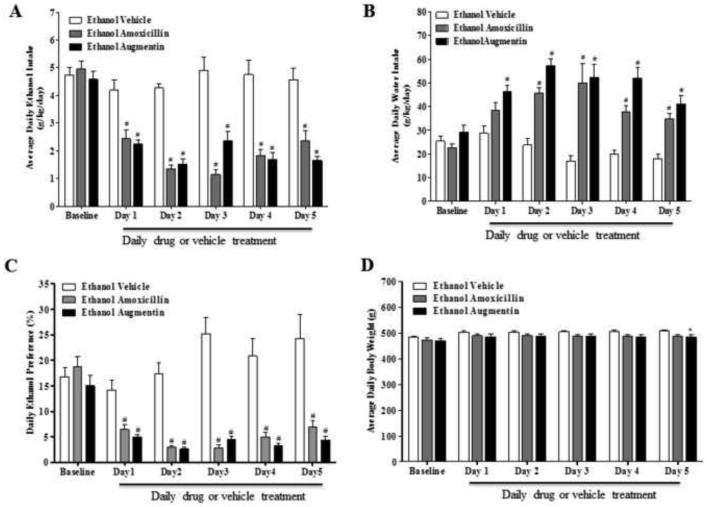
Figure 3A illustrates the effects of AMOX and AUG treatment on daily average ethanol consumption (grams/kg body weight/day) in male P rats. The timeline of the present chronic ethanol drinking paradigm with its dosing regimen has been illustrated in Figure 1A. Statistical analysis using GLM Repeated Measures revealed significant main effects of Day [F(1,5)=31.537, p<0.001], and Treatment [F(2,33)=46.108, p<0.001] as well as a significant Treatment × Day interaction [F(2,10)=8.698, p<0.001]. One-way ANOVA followed by two-sided Dunnett’s t-tests demonstrated a significant reduction in ethanol intake starting 24 hours after the first injection in all animals treated with AMOX (p<0.001) or AUG (p<0.001) compared to ethanol vehicle animals.
Figure 3.
(A) Effects of amoxicillin (AMOX) or Augmentin (AUG) on average daily ethanol intake (g/kg/day) in male P rats (n=12/β-lactam treatment group). A mixed ANOVA followed by Dunnett's t-tests revealed a significant reduction in ethanol intake by the AMOX and AUG treated groups starting on Test Day 1 and lasting through the end of the study as compared to the ethanol vehicle group. (B) Effects of AMOX and AUG on average daily water intake (g/kg/day) in male P rats. A mixed ANOVA followed by Dunnett's t-tests revealed a significant increase in water intake in AUG treated animals starting on Day 1 and lasting through the end of the study as compared to ethanol vehicle group; whereas the AMOX treated group had a significant increase in water intake, as compared to the ethanol vehicle group, starting on Day 2 and lasting though the end of the study. (C) Effects of AMOX and AUG on ethanol preference (%) in male P rats. A mixed ANOVA followed by Dunnett's t-tests revealed a significant decrease in ethanol preference in the AMOX and AUG treated groups starting on Day 1 and lasting through the end of the study as compared to the ethanol vehicle group. (D) Effects of AMOX and AUG on body weight in male P rats. A one-way ANOVA followed by Dunnett’s t-test revealed no significant difference with AMOX treatment as compared to the ethanol vehicle group. However, Dunnett’s t-test revealed there was a very modest, but significant, difference in body weight of the AUG treated group as compared to the ethanol vehicle group on the 5th day of treatment. All data are expressed as mean ± SEM. #, p<0.001; *, p<0.05.
Effects of amoxicillin and Augmentin on alcohol preference in male P rats
Figure 3C demonstrates the effects of AMOX and AUG on alcohol preference in male P rats. Daily percent alcohol preference was calculated (total ethanol consumption/total fluid consumption × 100) from daily alcohol and water consumption of male P rats as described previously (Lee et al., 2013). GLM Repeated Measures revealed significant main effects of Day [F(1,5)=12.053, p<0.001] and Treatment [F(2,33)=36.740, p<0.001] as well as a significant Treatment × Day interaction [F(2,10)=7.963, p<0.001]. One-way ANOVA demonstrated significant differences in percent ethanol preference in AMOX and AUG treated animal groups as compared to the ethanol vehicle group. Dunnett’s t-tests revealed a significant reduction in percent ethanol preference in AMOX (p<0.001) and AUG (p<0.001) treated animal groups as compared to the ethanol vehicle group starting on day 1 of treatment which continued throughout the treatment period.
Effects of amoxicillin and Augmentin on water intake in male P rats
The effects of AMOX and AUG on water intake are illustrated in Figure 3B. GLM Repeated Measures revealed significant main effects of Day [F(1,5)=12.290, p<0.001] and Treatment [F(2,33)=33.147, p<0.001] as well as a significant Treatment × Day interaction [F(2,10)=5.791, p<0.001]. Dunnett’s t-tests following significant one-way ANOVAs revealed a significant increase in water intake in all animals treated with AMOX (p<0.001) or AUG (p<0.001) compared to ethanol vehicle animals. The increase in water intake was evident throughout the study in AUG treated group (as compared to ethanol vehicle group); however, a significant increase in water intake was only observed from day 2 to day 5 of the treatment period in AMOX treated group as compared to the ethanol vehicle group.
Effect of Augmentin on sucrose intake in male P rats
Effect of AUG on sucrose (10%) intake was examined. A GLM repeated measures analysis revealed significant main effects of Day [F(1,7)=2.906, p:S0.01] and Treatment [F(1,10)=7.865, p<0.05] as well as a significant Day × Treatment interaction [F(1,7)=4.172, p:S0.001]. However, unpaired t-tests did not reveal any significant change in sucrose intake after AUG treatment on Test Day 4 nor across the post-treatment interval (compared to the ethanol vehicle group). The values (ml/kg/day; mean ± SEM) were Baseline: 36±1.2 and 37±1.9 (p>0.05); Test Day 1: 41±1.4 and 31±1.5 (p<0.05); Test Day 2: 43±0.7 and 28±3.1 (p<0.05); Test Day 3: 42±1.1 and 25±2.5 (p<0.05); Test Day 4: 38±4.4 and 34±5.5 (p>0.05); Post-Test: 37±2.8 and 32±3.1 (p>0.05) for the saline and AUG groups, respectively.
Effects of amoxicillin and Augmentin on body weight of male P rats
Figure 3D illustrates the effects of AMOX and AUG on body weight of male P rats. Body weights of animals were monitored throughout the study. A GLM repeated measures analysis revealed a significant main effect of Day [F(1,5)=92.985, p<0.001] but not Treatment (p>0.10) and a significant Day × Treatment interaction [F(2,10)=2.179, p<0.05]. One-way ANOVA did not show any significant effect of AMOX treatment on body weight compared to the ethanol vehicle group (p>0.05). However, one-way ANOVA revealed a significant effect on the body weight of animals treated with AUG as compared to ethanol vehicle group on Day 5 (p<0.05). Nevertheless, there was no significant effect on body weight from Day 1 through Day 4 of AUG treated group as compared to the ethanol vehicle group.
Effects of amoxicillin and Augmentin on GLT-1 expression in Acb and mPFC
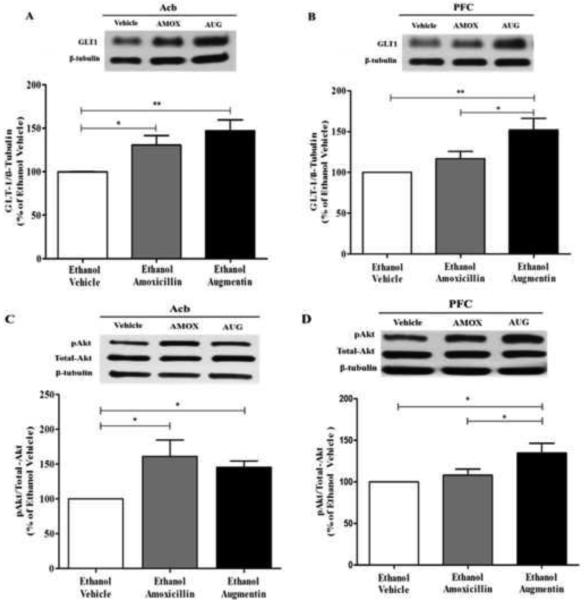
Figure 4A illustrates the effects of AMOX and AUG on GLT-1 expression in Acb. One-way ANOVA analysis of the blots revealed a significant main effect of Treatment in Acb [F(2, 15) = 6.364; p<0.05]. Furthermore, a Newman-Keuls multiple-comparison post-hoc test, revealed a significant increase in GLT-1 expression in both AMOX (p<0.05) and AUG (p<0.01) treated groups compared to ethanol vehicle group in Acb (Figure 4A).
Figure 4.
Effects of amoxicillin (AMOX) and Augmentin (AUG) on GLT-1 and pAKT expression in Acb and mPFC of ethanol-experienced P rats (n=6/β-lactam treatment group). (A) There was a significant increase in Acb GLT-1 expression, of chronic ethanol consuming P rats, induced by AMOX and AUG, as compared to the ethanol naïve group (100%). (B) There was no significant upregulation of mPFC GLT-1 expression, of chronic ethanol consuming P rats, following AMOX treatment as compared to the ethanol vehicle group (100%). However there was a significant upregulation of mPFC GLT-1 expression following AUG treatment as compared to both the AMOX treated group and the ethanol vehicle group (100%). (C) As with Acb GLT-1 expression, both AMOX and AUG significantly upregulated Acb AKT phosphorylation in chronic ethanol consuming P rats. (D) As with mPFC GLT-1 expression, there was no significant upregulation of mPFC AKT phosphorylation in chronic ethanol consuming P rats following AMOX treatment as compared to the ethanol vehicle group (100%). However there was a significant upregulation of mPFC AKT phosphorylation in chronic ethanol consuming P rats following AUG treatment as compared to both the AMOX treated group and the ethanol vehicle group (100%). Data are expressed as mean ± SEM. *, p<0.05; **, p<0.01.
Figure 4B illustrates the effects of AMOX and AUG on GLT-1 expression in mPFC. Interestingly, one-way ANOVA revealed a significant difference between the treatment and the ethanol vehicle group [F(2,12) = 7.507; p<0.01]. A Newman-Keuls multiple-comparison post-hoc test revealed a significant upregulation of GLT-1 expression in AUG treated group (p<0.01) but not in AMOX treated group as compared to ethanol vehicle animals in mPFC. Additionally, there was a significant upregulation in GLT-1 expression in AUG treated group as compared to AMOX treated group (p<0.05) (Figure 4B).
Effects of amoxicillin and Augmentin on pAKT level in Acb and mPFC
We further explored the effect of AMOX and AUG on pAKT, which is known to regulate GLT-1 expression (Li et al., 2006), in the Acb (Figure 4C) and the mPFC (Figure 4D). One-way ANOVA demonstrated a significant main effect of treatment on pAKT level in Acb [F(2,15) = 4.638; p<0.05] and in mPFC [F(2,15) = 5.370; p<0.05]. Newman-Keuls multiple comparison post-hoc test revealed a significant increase in pAKT level (p<0.05) with AUG treatment compared to the ethanol vehicle group in both Acb (Figure 4C) and mPFC (Figure 4D). A Newman-Keuls multiple comparison post-hoc test revealed a significant increase in pAKT level (p<0.05) with AMOX treatment compared to ethanol vehicle group in the Acb (Figure 4C), but not in the mPFC (Figure 4D).
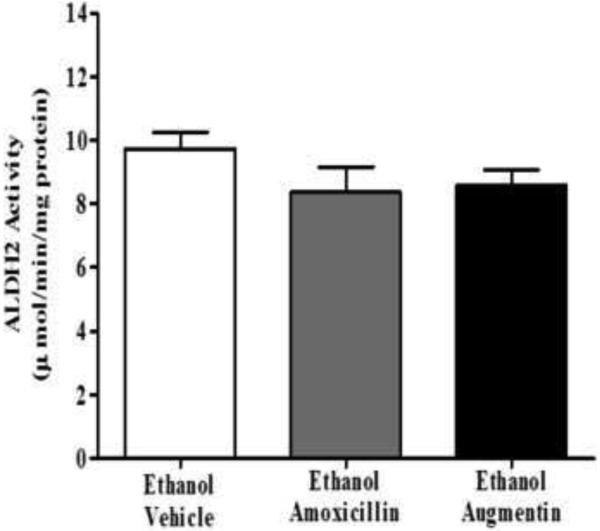
Effects of amoxicillin and Augmentin on hepatic ALDH2 activity
Additionally, we examined the effects of AMOX and AUG on ALDH2 activity compared to ethanol vehicle group (Figure 5). One-way ANOVA followed by Newman-Keuls multiple comparison post-hoc test revealed no significant effect of AMOX and AUG on hepatic ALDH2 activity as compared to ethanol vehicle group [F(2,15)=1.432].
Figure 5.
Effects of amoxicillin (AMOX, n=6) and Augmentin (AUG, n=6) on hepatic ALDH2 activity, as indicated by NADH production in the presence of acetaldehyde. P rats treated with AMOX or AUG did not display any significant changes in hepatic ALDH2 activity as compared to the ethanol saline-vehicle group (n=6). Enzyme activity (NADH production) is expressed as mean ± SEM
Discussion
The present study revealed a number of findings including (a) chronic ethanol-drinking significantly reduced GLT1 expression in the Acb but not mPFC of alcohol-preferring P rats; (b) chronic ethanol-drinking significantly reduced phosphorylation of AKT in both the Acb and mPFC of P rats; (c) administration of AMOX and AUG resulted in significant and appreciable levels of amoxicillin in the CSF and plasma of ethanol-drinking rats; (d) AMOX and AUG significantly decreased ethanol-drinking behavior of male P rats; (e) AMOX and AUG also significantly decreased ethanol preference in these rats; (f) AMOX and AUG increased water intake, possibly as a compensatory mechanism for the decreases in ethanol drinking; (g) AUG had only acute effects on the sucrose-drinking behavior of P rats, such that sucrose consumption returned to control levels on the fourth day of injections; (h) similarly, AMOX and AUG had very limited effects (no main effect of treatment but a significant treatment by day interaction) on body weight; (i) AMOX and AUG reversed the effects of chronic ethanol drinking on GLT1 expression in the Acb and mPFC of P rats; (j) AMOX reversed the effects of chronic ethanol drinking on pAKT levels in the Acb but not the mPFC; (k), however, unlike AMOX, AUG reversed the effects of chronic ethanol drinking on pAKT levels in both the Acb and mPFC; and (l) neither AMOX nor AUG altered hepatic ALDH2 activity in chronic ethanol-drinking P rats.
Elevated glutamatergic neurotransmission in the mPFC and Acb plays a critical role in ethanol dependence (c.f., Sari, 2013); with an increase in extracellular glutamate concentrations in regions of the mesocorticolimbic reward pathway, especially Acb, following ethanol consumption (Dahchour et al., 2000; Melendez et al., 2005; Moghaddam and Bolinao, 1994; Szumlinski et al., 2007). Extracellular glutamate concentrations are regulated by several glial glutamate transporters with GLT-1 eliminating more than 90% of the extracellular concentrations of glutamate. Downregulation of GLT-1 expression has been reported following self-administration of other drugs of abuse such as cocaine (Knackstedt et al., 2010) and nicotine (Knackstedt et al., 2009). In accordance with these findings, we also found a downregulation of GLT-1 expression in the Acb, but not in the mPFC, of the ethanol vehicle group as compared to the ethanol-naïve vehicle group following five weeks of chronic ethanol consumption. In addition, these findings are in agreement with other studies from our laboratory (Sari and Sreemantula, 2012; Sari et al., 2013b); such that CEF and GPI-1046 were effective in upregulating GLT-1 expression in both the Acb and mPFC, with a concomitant decrease in ethanol intake by adult male P rats given the same five week ethanol drinking paradigm employed in the present study.
Although AMOX is known to easily penetrate the blood-brain-barrier (BBB) in the presence of infections and inflammation, its ability to penetrate the BBB of ethanol-experienced healthy subjects remains debatable. Therefore, to further substantiate our hypothesis that AMOX and AUG were acting through a centrally-mediated mechanism of action, we performed an HPLC analysis of CSF samples collected from ethanol-consuming P rats to determine concentration levels of the drug. In support of our hypothesis, the present findings parallel previous studies demonstrating that AMOX is detected in the CSF after peripheral administration in the absence of any infection (Clumeck et al., 1978; Mingrino et al., 1981). However, further detailed analyses need to be performed to determine the dose-response relationship between the concentration of drug levels in the CSF and brain and changes in GLT-1 expression as well as attenuation of ethanol consumption. And, as noted below, regional and sub-regional tissue levels of these β-lactam compounds, following peripheral administration, need to be evaluated.
CEF also has been shown to decrease ethanol preference with an associated upregulation of GLT-1 in mouse models (Lee et al., 2013). Based on the structural similarities between AMOX and CEF (i.e., the presence of a β-lactam ring) we hypothesized that the observed attenuation in ethanol intake and decreased ethanol preference induced by CEF would be mimicked by AMOX. In addition, we had hypothesized that there would be an associated increase in GLT-1 expression after AMOX and AUG treatment. In support of our hypothesis, we found a significant upregulation of GLT-1 expression in the Acb after both AMOX and AUG treatments. This upregulation of GLT-1 expression in the brain parallels the seminal study by Rothstein et al (2005) where he reported AMOX was found, amongst 1,040 compounds, to be one of the most potent stimulators of GLT-1 expression in an organotypic spinal cord culture.
In contrast with the Acb, which only receives glutamatergic projections from other brain regions, the PFC receives as well as sends glutamatergic projections to and from other brain reward regions, which are involved in addictive behavior (c.f., Rao et al., 2015b). In the present study, we found that ethanol-experience downregulated GLT-1 expression in the Acb, but not the mPFC; although, ethanol-experience did decrease phosphorylation of AKT in both brain regions. In addition, AMOX failed to significantly reverse the effects of ethanol-experience on both GLT-1 expression and the phosphorylation of AKT in the mPFC. One possibility for these differences in regional neuroadaptations, as indicated by significant effects of AUG in both brain regions while AMOX exerted significant effects only in the Acb, may be due to differences in the synaptic location and/or levels of GLT-1 expression between the mPFC and Acb. This hypothesis would require further investigation. Another possibility is there may be differences in regional or subregional tissue levels that are drug-dependent after systemic administration. The observation of brain-region-specific differences in tissue levels of systemically administered compounds has been reported previously (e.g., Ding et al., 2012b). To date, very little is known about the distribution of AMOX in the brain. Thus, further analyses need to be conducted to determine if there are β-lactam drug-dependent differences in tissue levels within the mesocorticolimbic system following peripheral administration.
The fact that AMOX failed to significantly reverse the effects of ethanol on GLT-1 expression may be due in part to the absence of an effect of ethanol on mPFC GLT-1 expression. Essentially, the trend for a significant effect of ethanol on mPFC GLT-1 expression may have allowed a sufficient dynamic range (magnitude of GLT-1 changes) for AUG to have a significant effect but the dynamic range was not sufficient for us to observe a significant effect by AMOX. However, this hypothesis does not appear to hold true for levels of AKT phosphorylation, such that ethanol significantly reduced AKT phosphorylation which was subsequently reversed by AUG, but not AMOX. An examination of the mPFC data (Figure 4) indicates that the magnitude of effect exerted by AUG was substantially greater than that of AMOX. We believe that this effect, in the mPFC, could be due to the presence of clavulanic acid in the AUG formulation. Ample evidence supports the role of clavulanic acid as a central nervous system modulator. Clavulanic acid also has a β-lactam structure with negligible intrinsic antibacterial activity and it crosses the BBB very easily, achieving a CSF/plasma ratio of ~0.25 (Nakagawa et al., 1994). It also appears to have anxiolytic effects in primates and rodents (Kim et al., 2009). In addition, clavulanic acid appears to have anti-seizure properties, at least in invertebrates (Rawls et al., 2010), although it is unclear whether this anti-seizure effect of clavulanic acid was due to its ability to enhance glutamate uptake or not. Importantly, a recent study revealed that clavulanic acid reduced reward, as measured by a morphine-induced conditioned place preference, in rats (Schroeder et al., 2014). Therefore, more studies are needed to establish the plausible role of clavulanic acid in upregulating GLT-1 expression and restoring glutamate homeostasis.
Despite the fact that there were no significant reductions in mPFC GLT-1 expression, between the ethanol vehicle and ethanol-naïve vehicle animals, we observed an increase in mPFC GLT-1 in ethanol-AUG treated animals. These findings are in agreement with previous reports that CEF, another β-lactam antibiotic, as well as GPI-1046 significantly upregulated GLT-1 expression in the mPFC (e.g., Sari and Sreemantula, 2012; Sari et al., 2013b). Importantly, the β-lactam attenuation of ethanol-intake by P rats was observed as early as the second day of injections in the present study. Although, we did not analyze GLT-1 expression in the brain at this time-point, we have shown previously that β-lactam-mediated GLT-1 upregulation can be anticipated as early as the second day of treatment (e.g., Rao et al., 2015a). While it has been previously reported that five days of β-lactam treatment are needed for a significant up-regulation of GLT-1 expression (e.g., Rothstein et al., 2005); the more rapid upregulation of GLT-1 expression, following β-lactam treatment, found by our laboratory (e.g., Rao et al., 2015a) may be due to methodological differences, including the model systems used to assess this effect. Nevertheless, further studies of GLT-1 expression manipulations are required to reveal the mechanism-of-action associated with this intriguing finding.
We tested AUG, rather than AMOX, because we were interested in the possible effects of clavulanic acid found in the AUG, but not AMOX, formulation given its putative behavioral effects, including disruption of a morphine-induced conditioned place preference (Schroeder et al., 2014). Whether AMOX affects sucrose intake still needs to be examined; but, given its limited effects on GLT-1 and pAKT modulation in the mPFC of ethanol-consuming P rats, AUG appears to hold greater promise for anti-addiction treatment via restoration of glutamate homeostasis in the mesocorticolimbic reward circuit.
Studies have reported that pAKT levels are downregulated following chronic alcohol treatment using in-vitro and/or in-vivo models (Antonelli et al., 2009; Cynkar et al., 2010). The present findings confirmed this effect, such that we found a significant downregulation in pAKT/total-AKT expression in the ethanol-vehicle group as compared to the ethanol-naïve group in the Acb and mPFC, ~25% and 15% respectively. In contrast, studies have also demonstrated that systemic administration and binge-like consumption of ethanol resulted in the activation of the AKT signaling pathway in the Acb (Cozzoli et al., 2009; Neasta et al., 2011). The disparity in findings can be explained by difference in the ethanol drinking paradigms used in these studies. The paradigm used in the present study involved uninterrupted access to ethanol for five-weeks, whereas a binge ethanol drinking paradigm was used where increased phosphorylation of AKT was observed, which involved limited access drinking with intermittent withdrawal periods (Cozzoli et al., 2009; Neasta et al., 2011).
Given ethanol’s modulation of AKT phosphorylation, we examined the effect of AMOX and AUG on pAKT (Ser473) levels in the Acb and mPFC of ethanol-experienced animals. Following treatment with AUG, there was an upregulation of pAKT levels in both the Acb and mPFC; contrarily, AMOX treatment upregulated pAKT expression only in the Acb and not in the PFC. Our interest in AKT phosphorylation also stemmed from findings of an important role for AKT signaling in upregulation of GLT-1 expression (Li et al., 2006; Wu et al., 2010). Of direct relevance to the present study, it has been reported that CEF upregulated GLT-1 expression in hippocampal astrocytes in-vitro and in-vivo through the PI3K/AKT/NF-KB pathway (Cigana et al., 2009). Therefore, we hypothesized that AMOX- and AUG-induced upregulation of GLT-1 expression is regulated by pAKT; and, while the present results provide some support for this hypothesis, further studies are needed.
Evidence suggests that the N-methylthiotetrazole (NMTT) moiety in β-lactam antibiotics is crucial in producing disulfiram-like effects on hepatic alcohol/aldehyde metabolizing enzymes, mainly aldehyde dehydrogenases (Brien et al., 1985; Matsubara et al., 1987). However, we hypothesized that the present observations were not due to secondary effects of AMOX or AUG, namely increased levels of acetaldehyde via disruption of hepatic ALDH2 (the primary effect of disulfiram) activity. Therefore, using the mitochondrial fraction from ethanol-experienced P rat liver tissue, we assessed ALDH2 activity via NADH production in the presence of acetaldehyde. Our finding that NADH production did not differ between the saline and two β-lactam groups supports our contention that the attenuating effects of AMOX and AUG on ethanol drinking were not due to a disulfiram-like aversive peripheral effect and were probably due to central effects.
Despite the fact that the present study provides compelling evidence that AMOX and AUG are efficacious in attenuating ethanol intake, the study has its limitations. One of the limitations was the selection of a single dose of each drug; which did not aid in establishing a clear dose-response relationship between these drugs and the observed effects. Further studies are required to establish this dose-response relationship. Also, recovery of ethanol intake following AMOX and AUG treatment was not assessed, thus it remains to be seen whether the observed effects of these drugs are long-term or if the ethanol intake is only reduced in the presence of the drugs. Finally, it would be important to study the effects of these drugs on sodium independent glutamate uptake since another β-lactam antibiotic, CEF, has also been shown to modulate the expression of the cysteine/glutamate exchanger (xCT) (Rao et al., 2015).
Conclusion
This study demonstrates, for the first time, the effectiveness of AMOX and AUG treatment in reducing ethanol intake in an animal model of alcoholism. This attenuation in ethanol intake was associated with an upregulation of GLT-1 in the Acb, but not in the PFC, following AMOX treatment; whereas AUG treatment upregulated GLT-1 expression in both the Acb and mPFC. The changes in GLT-1 expression were associated with a significant upregulation of phosphorylated AKT levels. These findings provide strong evidence for the efficacy of AMOX and AUG in attenuating ethanol intake, and possibly dependence, putatively by restoring glutamate homeostasis and implicate a role for pAKT in mediating this effect.
Experimental procedure
Drugs
AMOX and AUG (amoxicillin/clavulanate = 1g/200mg) were procured from GlaxoSmithKline (France). Both the drugs were dissolved in 0.9% NaCl solution vehicle and administered intraperitoneally (i.p.).
Animals
Male alcohol-preferring P rats were obtained from the breeding colonies at Indiana University School of Medicine, Indianapolis, IN at the age of 21-30 days and housed in the Department of Laboratory Animal Resources at the University of Toledo. All animals were housed in standard plastic tubs with corn-cob bedding and had ad lib access to food and water throughout the experimental procedures. Animal vivaria were maintained at a temperature of 21°C with 50% relative humidity on a 12-hour light/dark cycle (0600h/1800h). All of the experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Toledo in accordance with guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996).
At the age of three months, P rats were single housed in bedded plastic cages and randomly separated into four different groups: (a) Ethanol naïve vehicle group (water control) received i.p. injections of saline vehicle solution (n=6); (b) Ethanol vehicle group (ethanol control) received i.p. injections of saline vehicle solution (n=12); (c) Ethanol amoxicillin group (AMOX) which received 100 mg/kg, i.p. injections of AMOX (n=12); and (d) Ethanol Augmentin group (AUG) which received 100 mg/kg, i.p. injections of AUG (n=12). Ethanol control, ethanol AMOX and ethanol AUG groups had free access to water, 15% and 30% ethanol and food throughout the experimental procedures. We also tested the effect of AUG (100 mg/kg, i.p.) on sucrose intake as compared to a vehicle treated group (saline vehicle, i.p.).
Ethanol drinking procedures
At three months of age, male P rats were given free-choice access to ethanol (concurrently 15% and 30%, v/v), water and food ad lib for five weeks. Ethanol consumption for each animal was measured as grams of ethanol consumed per kilogram of body weight per day. Ethanol and water intakes as well as body weight were measured three times a week. The amount of ethanol and water consumed were determined to the nearest tenth of a gram by subtraction of the measured bottle weights from their initial weights. All animals were required to meet the criterion of 2:4 g/kg/day ethanol consumption for at least 2 weeks before saline vehicle or drug treatment to be included in the study, as adopted in previous studies (Sari et al., 2011; Sari and Sreemantula, 2012; Sari et al., 2013b). Figure 1A illustrates the timeline of the chronic ethanol drinking paradigm including the pharmacological treatment regimen. The average ethanol consumption for the two weeks prior to drug or vehicle treatment was taken as baseline drinking. During week 6, P rats were injected with AMOX (100 mg/kg, i.p.), AUG (100 mg/kg, i.p) or vehicle (0.9% saline, i.p.) daily for five consecutive days. All injection volumes were 2 ml/kg. During the sixth week, ethanol and water consumption were measured daily along with animal body weight.
Sucrose drinking procedures
With respect to the sucrose drinking procedure, it is important to note that P rats have significantly higher inclination towards lower concentrations of sucrose as compared to selectively bred alcohol-non-preferring (NP) rats (Stewart et al., 1994), although both strains of rats exhibit comparable drinking levels of higher concentration sucrose solutions (more than 8%), supporting the use of a 10% sucrose concentration in the present study. Thus, AUG’s effects on sucrose (10%) intake were examined, as an appetitive control for drinking-motivated behavior. Figure 1B illustrates the sucrose drinking procedure including the treatment regimen. Continuous free-choice access to the 10% sucrose solution, water and food was given to the male P rats throughout the study. Animals were divided into two groups: (a) Saline vehicle (control) group which was injected with saline, i.p. and (b) AUG group which was injected with 100 mg/kg of AUG, i.p. Starting on day 13, both groups received their respective treatments for four consecutive days. Baseline sucrose intake was taken as the average sucrose intake on days 11 and 12. Body weight of animals, sucrose and water intakes were measured daily starting 2 days prior to injections (i.e., day 11 through the end of the study).
Brain tissue harvesting
All animals were euthanized 24 hours after receiving their last injection by CO2 inhalation and rapidly decapitation with a guillotine. The brains were dissected and flash-frozen and stored at −70°C. Stereotaxic microdissections were carried out using a cryostat to isolate the medial PFC (mPFC) and Acb (core and shell) as described previously (Sari and Sreemantula, 2012). Stereotaxic coordinates for the identification of PFC and Acb in the rat brain were obtained from Paxinos and Watson’s Stereotaxic Atlas (Paxinos and Watson, 2007).
Western Blot protocol for detection of GLT-1, pAKT and total-AKT
Isolated mPFC and Acb brain regions from rats were analyzed for changes in GLT-1 and pAKT relative to total-AKT and β-tubulin using the Western Blot assay. In brief, extracted proteins were transferred on a PVDF membrane (Bio-Rad, Hercules, CA) electrophoretically. The membranes containing protein were blocked using 3% milk in TBST for 30 minutes at room temperature, and then incubated overnight at 4°C with: guinea pig-anti GLT-1 antibody (1:5000; Millipore), rabbit pAKT Ser473 (1:5000; Cell Signaling Technology), or mouse anti-AKT (1:5000; Cell Signaling Technology). Mouse anti β-tubulin antibody was used to assess equivalent protein loading (loading control). Membranes were then washed and incubated with horseradish peroxidase (HRP) donkey-anti-Guinea pig IgG (H+L) secondary antibody (1:5000), anti-mouse IgG, HRP-linked secondary antibody (1:5000), or anti-rabbit IgG, HRP-linked secondary antibody (1:5000). After incubation with an HRP Chemiluminescent kit (SuperSignal West Pico, Pierce Inc.), membranes were exposed to Kodak BioMax MR film (Fisher Inc.), and the films were developed on a SRX-101A machine. Digitized images of immunoreactive proteins were quantified using the MCID system (GE Healthcare Niagara Inc., US). The data are presented as percentage ratios of GLT-1/β-tubulin and pAKT/total-AKT, relative to ethanol vehicle control (100% control-value) levels.
Aldehyde dehydrogenase enzyme assay
β-lactam induced attenuation of ethanol consumption might also be attributed to a disulfiram-like aversive effect of the drug (Hauser et al., 2012), which prevents the conversion of acetaldehyde to acetate through inhibition of aldehyde dehydrogenase. Of the subtypes, hepatic type 2 aldehyde dehydrogenase (ALDH2) enzyme accounts for 60% of hepatic acetaldehyde metabolism (Weiner, 1987). In order to determine the effects of AMOX or AUG on ALDH2 activity in the presence of acetaldehyde, NADH production was determined after treating P rats for 5 consecutive days with AMOX (n=6) or AUG (n=6) compared to an ethanol vehicle (6) treated group. All animals were given free-choice access to ethanol (concurrently 15% and 30%, v/v), water and food ad lib for five weeks prior to treatment with either drug or vehicle. P rats were euthanized 24 hours after the last injection and their liver was dissected and stored at −80°C. Liver tissue (1.2 g-1.5 g) was homogenized in 3 volumes (w/v) of ice-cold 0.25 M sucrose solution using a mechanical homogenizer. The homogenate was then subjected to centrifugation for 10 min. The pellet was discarded and the supernatant was further centrifuged for 20 min. The pellet obtained after this step was washed with 1.5 ml of 0.25 M sucrose solution and subjected to centrifugation for 15 min. The final pellet obtained, mitochondrial fraction, was resuspended in 0.5 ml of sucrose solution containing 1% sodium deoxycholate and stored at −80 °C until testing. ALDH2 activity was analyzed using acetaldehyde as the substrate as described previously (Karamanakos et al., 2007). The assay mixture comprised of 50 µM acetaldehyde, 75 mM sodium pyrophosphate buffer (pH 8.0), 1 mM pyrazole, and 2 µM rotenone. NADH formation was determined spectrophotometrically at 340 nm over a 5 min time period. The ALDH2 activity was reported as µmol of NADH production/min/mg protein.
Analytical determination of AMOX and AUG in male P rats’ plasma and CSF
Approximately one hour after receiving a 100 mg/kg i.p. injection of AMOX or 100 mg/kg of AUG, P rats were euthanized, and blood and cerebrospinal fluid (CSF) were collected and immediately frozen at −80°C for further analysis. The blood was collected by cardiac puncture while CSF was collected by puncturing the cisterna magna using a needle attached to a syringe. A high-performance liquid chromatography system (HPLC) (Waters Alliance 2695 separation module, Milford, MA) equipped with a Kinetex C18 column (250 × 4.6 mm, Phenomenex) and UV/Visible detector was used for analyses. AMOX was analyzed with a mobile phase containing phosphate buffer, pH 5 and acetonitrile (98:2) pumped at a flow rate of 1 ml/min. The retention time of AMOX (Amax= 230 nm) was found to be 7.8 min. Different calibration standards of AMOX were prepared in the mobile phase. For the calibration curve, each standard was analyzed in triplicate and the average peak area was plotted against the concentration. The drug content was determined quantitatively by plotting a calibration curve. The assay method was found to be linear in the range of 0.78 −50 µg/ml with a correlation coefficient of 0.9994. The limit of detection and limit of quantification of AMOX was found to be 0.060 µg/ml and 0.20 µg/ml, respectively. The intra- and inter-day reliability (measured by %RSD) exceeded 98%.
Statistical analyses
General Linear Model (GLM) Repeated Measures statistical analyses were conducted to determine a significant main effect of Day or Treatment or Day × Treatment interaction on body weight, or ethanol, water and sucrose intake of the ethanol vehicle, ethanol AMOX and ethanol AUG groups. Day served as the repeated measure (within-subjects variable) and β-lactam treatment served as the between-subjects variable. A significant main effect or interaction was followed by one-way ANOVAs and Dunnett t-test planned comparisons to determine the effects of AMOX or AUG vs control treatment for each test day. Effects of chronic ethanol on protein expression were determined by performing independent t-tests on immunoblots comparing the ethanol-naïve vehicle group with the ethanol vehicle group. Effects of β-lactam treatments on protein expression were determined by one-way ANOVAs and Newman-Keuls multiple-comparisons on the immunoblots, with the ethanol vehicle group serving as the control. All statistical analyses were based on a p<0.05 level of significance.
Highlights.
>Augmentin and amoxicillin treatments reduced alcohol intake.
> These drugs upregulated GLT-1 and pAKT levels in the NAc and PFC.
> Augmentin and amoxicillin have been detected in CSF.
Acknowledgments
This work was supported by Award Number R01AA019458 (Y.S.) and U24AA015512 as well as U01AA13522 (RLB) from the National Institutes on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
Abbreviations
- AMOX
amoxicillin
- Augmentin, AUG
amoxicillin/clavulanate
- GLT-1
glutamate transporter 1
- Acb
nucleus accumbens
- PFC
prefrontal cortex
- VTA
ventral-tegmental area
- CEF
ceftriaxone
- P
alcohol-preferring rats
- ALDH2
aldehyde dehydrogenase 2
- NMTT
N-methylthiotetrazole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate concentrations in P rats. Psychopharmacology (Berl) 2014;231:4049–57. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Antonelli A, et al. Second surgery for renal relapse after nephron sparing surgery: review of seven cases. Arch Ital Urol Androl. 2009;81:218–22. [PubMed] [Google Scholar]
- Brien JF, et al. A comparative study of the inhibition of hepatic aldehyde dehydrogenases in the rat by methyltetrazolethiol, calcium carbimide, and disulfiram. Can J Physiol Pharmacol. 1985;63:438–43. doi: 10.1139/y85-076. [DOI] [PubMed] [Google Scholar]
- Childress AR, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigana C, et al. Pseudomonas aeruginosa exploits lipid A and muropeptides modification as a strategy to lower innate immunity during cystic fibrosis lung infection. PLoS One. 2009;4:e8439. doi: 10.1371/journal.pone.0008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clumeck N, et al. Amoxicillin entry into human cerebrospinal fluid: comparison with ampicillin. Antimicrob Agents Chemother. 1978;14:531–2. doi: 10.1128/aac.14.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, et al. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–68. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynkar W, et al. Classification of Tempranillo wines according to geographic origin: combination of mass spectrometry based electronic nose and chemometrics. Anal Chim Acta. 2010;660:227–31. doi: 10.1016/j.aca.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Dahchour A, et al. Effects of ethanol on extracellular amino acid levels in high-and low-alcohol sensitive rats: a microdialysis study. Alcohol Alcohol. 2000;35:548–53. doi: 10.1093/alcalc/35.6.548. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Ding ZM, et al. Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcohol Clin Exp Res. 2012a;36:633–40. doi: 10.1111/j.1530-0277.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, et al. Neuroprotective Effects of Ischemic Preconditioning and Postconditioning on Global Brain Ischemia in Rats through the Same Effect on Inhibition of Apoptosis. Int J Mol Sci. 2012b;13:6089–101. doi: 10.3390/ijms13056089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV. Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J Neurosci. 2013;33:9319–27. doi: 10.1523/JNEUROSCI.3278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganel R, et al. Selective up-regulation of the glial Na+-dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiol Dis. 2006;21:556–67. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, et al. Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (p) rats. Alcohol Clin Exp Res. 2012;36:1963–72. doi: 10.1111/j.1530-0277.2012.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S, et al. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J Neurosci. 2012;32:6000–13. doi: 10.1523/JNEUROSCI.5347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanakos PN, et al. Pharmaceutical agents known to produce disulfiram-like reaction: effects on hepatic ethanol metabolism and brain monoamines. Int J Toxicol. 2007;26:423–32. doi: 10.1080/10915810701583010. [DOI] [PubMed] [Google Scholar]
- Kim DJ, et al. Clavulanic acid: a competitive inhibitor of β-lactamases with novel anxiolytic-like activity and minimal side effects. Pharmacol Biochem Behav. 2009;93:112–20. doi: 10.1016/j.pbb.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Kiryk A, et al. Behavioral characterization of GLT1 (+/−) mice as a model of mild glutamatergic hyperfunction. Neurotox Res. 2008;13:19–30. doi: 10.1007/BF03033364. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–5. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–4. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, et al. Striatal adenosine signaling regulates EAAT2 and astrocytic AQP4 expression and alcohol drinking in mice. Neuropsychopharmacology. 2013;38:437–45. doi: 10.1038/npp.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LB, et al. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem. 2006;97:759–71. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- Matsubara T, et al. Effects of β-lactam antibiotics and N-methyltetrazolethiol on the alcohol-metabolizing system in rats. Jpn J Pharmacol. 1987;45:303–15. doi: 10.1254/jjp.45.303. [DOI] [PubMed] [Google Scholar]
- Melendez RI, et al. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–33. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Mingrino S, Scanarini M, Magliulo E. [Penetration of amoxicillin into the cerebrospinal fluid] Arch Sci Med (Torino) 1981;138:33–5. [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci Lett. 1994;178:99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, et al. [Penetration of potassium clavulanate/ticarcillin sodium into cerebrospinal fluid in neurosurgical patients] Jpn J Antibiot. 1994;47:93–101. [PubMed] [Google Scholar]
- Nakagawa T, Satoh M. Involvement of glial glutamate transporters in morphine dependence. Ann N Y Acad Sci. 2004;1025:383–8. doi: 10.1196/annals.1307.047. [DOI] [PubMed] [Google Scholar]
- Neasta J, et al. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry. 2011;70:575–82. doi: 10.1016/j.biopsych.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, et al. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33:1924–34. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Kavanaugh MP. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J Neurosci. 2000;20:2749–57. doi: 10.1523/JNEUROSCI.20-08-02749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sixth Academic Press; New York: 2007. [Google Scholar]
- Powell DR, et al. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab. 2013;304:E117–30. doi: 10.1152/ajpendo.00439.2012. [DOI] [PubMed] [Google Scholar]
- Rao PS, Sari Y. Glutamate transporter 1: target for the treatment of alcohol dependence. Curr Med Chem. 2012;19:5148–56. doi: 10.2174/092986712803530511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, et al. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3868-3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PSS, Bell RL, Engleman EA, Sari Y. Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci Neuropharmacol. 2015;9:144. doi: 10.3389/fnins.2015.00144. http://dx.doi.org/10.3389/fnins.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, et al. beta-lactamase inhibitors display anti-seizure properties in an invertebrate assay. Neuroscience. 2010;169:1800–4. doi: 10.1016/j.neuroscience.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–86. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sari Y, et al. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–43. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, et al. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–46. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–35. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y. Potential therapeutic role of glutamate transporter 1 for the treatment of alcohol dependence. OA Alcohol. 2013;1:6. doi: 10.13172/2053-0285-1-1-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, et al. Effects of ceftriaxone on the acquisition and maintenance of ethanol drinking in peri-adolescent and adult female alcohol-preferring (P) rats. Neuroscience. 2013a;241:229–38. doi: 10.1016/j.neuroscience.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, et al. Ceftriaxone treatment affects the expression of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Mol Neurosci. 2013b;51:779–87. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JA, et al. Clavulanic acid reduces rewarding, hyperthermic and locomotor-sensitizing effects of morphine in rats: a new indication for an old drug? Drug Alcohol Depend. 2014;142:41–5. doi: 10.1016/j.drugalcdep.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol. 1999;39:431–56. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Russell RN, Lumeng L, Li T-K, Murphy JM. Consumption of sweet, salty, sour and bitter solutions by selectively bred alcohol-preferring P and -nonpreferring NP lines of rats. Alcohol Clin Exp Res. 1994;18:375–381. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, et al. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology (Berl) 2007;190:415–31. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Tanaka K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Weiner H. Subcellular localization of acetaldehyde oxidation in liver. Ann N Y Acad Sci. 1987;492:25–34. doi: 10.1111/j.1749-6632.1987.tb48650.x. [DOI] [PubMed] [Google Scholar]
- Wu X, et al. PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem Biophys Res Commun. 2010;393:514–8. doi: 10.1016/j.bbrc.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Xu NJ, et al. Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses. J Neurosci. 2003;23:4775–84. doi: 10.1523/JNEUROSCI.23-11-04775.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]