Abstract
Wnt signaling pathways act at multiple locations and developmental stages to specify cell fate and polarity in vertebrate embryos. A long-standing question is how the same molecular machinery can be reused to produce different outcomes. The canonical Wnt/β-catenin branch modulates target gene transcription to specify cell fates along the dorsoventral and anteroposterior embryonic axes. By contrast, the Wnt/planar cell polarity (PCP) branch is responsible for cell polarization along main body axes, which coordinates morphogenetic cell behaviors during gastrulation and neurulation. Whereas both cell fate and cell polarity are modulated by spatially- and temporally-restricted Wnt activity, the downstream signaling mechanisms are very diverse. This review highlights recent progress in the understanding of Wnt-dependent molecular events leading to the establishment of PCP and linking it to early morphogenetic processes.
Keywords: Wnt, planar cell polarity, morphogenesis, neural tube, Xenopus
1. Introduction
Ever since Wnt proteins were discovered more than three decades ago, their roles in animal development have been expanding. Multiple branches of the Wnt pathway have been implicated in processes as diverse as body axis and cell fate specification, cell cycle regulation, planar and apical-basal cell polarity and protein stability (Acebron et al., 2014; Clevers and Nusse, 2012; Hikasa and Sokol, 2013; Semenov et al., 2007). During early embryonic development, canonical Wnt/β-catenin signaling functions to pattern the anteroposterior body axis throughout the animal kingdom (Petersen and Reddien, 2009). In lower vertebrates, the same pathway contributes to dorsoventral axis specification soon after fertilization (Sokol, 1999). The dorsoventral and the anteroposterior body axes are regulated by different sets of early and late gene targets, respectively (Hikasa and Sokol, 2013). Besides the transcriptional control of target genes, Wnt proteins also modulate the planar cell polarity (PCP) pathway, which is defined here as signaling leading to coordinated polarization of neighboring cells in the plane of the tissue. The Wnt/PCP pathway has been proposed to participate in numerous morphogenetic processes by regulating actomyosin dynamics, but the specific signaling mechanisms are poorly understood. In summary, the two major Wnt signaling branches play essential non-redundant roles in the control of fate specification and morphogenetic behavior, respectively, in the vertebrate embryo.
Despite significant effort, molecular connections between Wnt ligands and downstream targets remain unclear. Wnt signaling leads to different outcomes, depending on the signaling machinery that is available in particular cells. Due to this context dependence, the conclusions obtained from pathway characterization in one model cannot be easily transferred to another. An important question is to determine how the same signaling machinery generates diverse outcomes when reutilized at multiple times and locations in the embryo. This review summarizes the current understanding of molecular interactions leading to the establishment of Wnt/β-catenin and Wnt/PCP signaling in diverse models and attempts to connect it to morphogenetic cell behaviors during early development.
2. Distinct molecular composition of diverse Wnt pathways
While different signaling branches of the Wnt pathway are clearly distinguishable by the processes they affect, one of the main questions in the field is how the signaling becomes channeled towards diverse cellular targets. Pathway specificity is commonly attributed to differences between individual Wnt ligands and receptors (Hikasa et al., 2002; Mikels and Nusse, 2006; Niehrs, 2012). This review only considers the Wnt/β-catenin and the Wnt/PCP pathway, two major signaling branches (Fig. 1). The Frizzled family of seven-transmembrane-domain receptors and the cytoplasmic adaptor Dishevelled are shared by both branches. The canonical pathway involves the LRP5/6 coreceptors, which stabilize β-catenin by negatively affecting the degradation complex, including GSK3, APC and Axin (Clevers and Nusse, 2012; MacDonald et al., 2009). Recently, the same complex was shown to contain the Hippo pathway components YAP and TAZ that are regulated by a Wnt signal (Azzolin et al., 2014). The primary pathway endpoint is the activation of target gene promoters triggered by the association of β-catenin and TCF transcription factors. For the detailed description of this pathway, the readers are referred to more comprehensive reviews (Clevers and Nusse, 2012; MacDonald et al., 2009).
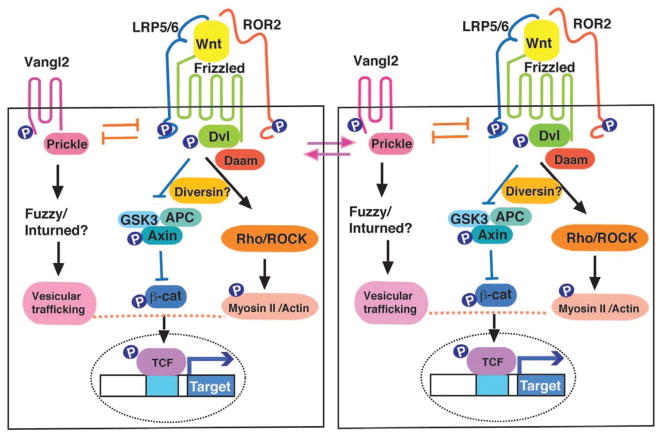
Figure 1. Two major Wnt pathway branches.
The canonical Wnt/β-catenin pathway antagonizes the β-catenin destruction complex, consisting of Axin, GSK3 and APC to promote target gene transcription. By contrast, the Wnt/PCP pathway is thought to involve Frizzled/Dishevelled signaling to locally activate RhoA, ROCK and Myosin II and, at the same time, precludes the recruitment of Vangl2/Prickle to the same membrane subdomain. Roles of the vertebrate PCP proteins Fuzzy, Inturned and Diversin in PCP are unclear. Besides the mutually antagonistic interactions inside the cell, PCP is coordinated by the positive feedback between the Frizzled/Dishevelled complex and the Vangl2/Prickle complex across the cell junction. Two neighboring epithelial cells are shown.
The composition of the Wnt/PCP pathway is different (Adler, 2012; Simons and Mlodzik, 2008). Besides Frizzled and Dishevelled, the pathway includes four other core PCP components: the transmembrane proteins Vang/Vangl2 (Taylor et al., 1998; Wolff and Rubin, 1998) and Flamingo/Celsr (Chae et al., 1999; Usui et al., 1999), the PET/LIM-domain protein Prickle (Gubb et al., 1999) and ankyrin-domain-containing Diego/Diversin (Feiguin et al., 2001). In Drosophila epithelia, the Frizzled/Dishevelled and the Vang/Prickle protein complexes segregate to nonoverlapping cellular domains. Core PCP proteins influence the localization and activity of specialized PCP effectors, such as Fuzzy (Collier and Gubb, 1997) and Inturned (Park et al., 1996). Other more generally acting PCP effectors, including Rho-associated protein kinase (ROCK) and the formin-related proteins Daam1 and Multiple wing hair (Mwh) (Habas et al., 2001; Strutt and Warrington, 2008; Winter et al., 2001; Yan et al., 2008), function by modulating actomyosin dynamics that is critical for cell shape regulation (Heisenberg and Bellaiche, 2013). Although it is clear that the cellular targets of PCP signaling are distinct from β-catenin, the molecular interactions between the core PCP proteins and their effectors are only beginning to be analyzed.
3. Role of Wnt signaling in embryonic axis specification
3.1 Cortical rotation and the dorso-ventral axis specification
Whereas the mature Xenopus oocyte has clear animal-vegetal polarity, the dorsal-ventral body axis is specified in the embryo only after fertilization. This event requires a microtubule-dependent relocalization of some cortical components to the future dorsal side of the egg, a process known as cortico-cytoplasmic rotation (Gerhart et al., 1989; Sokol, 1999). The Wnt/β-catenin pathway is essential for this process, as depletion of β-catenin by different means inhibits the development of dorsal and anterior structures (Heasman et al., 1994). Conversely, injection of exogenous Wnt1 or Wnt8 RNA triggers ectopic dorsal axial development (Smith and Harland, 1991; Sokol et al., 1991). This activity can be mimicked by Dishevelled and β-catenin (Funayama et al., 1995; Sokol et al., 1995) and both proteins accumulate at the dorsal side of early embryos soon after fertilization (Larabell et al., 1997; Schneider et al., 1996). Dishevelled associates with vegetally localized microtubules that are required for dorsal-ventral axis specification (Miller et al., 1999). These findings support the hypothesis that the Wnt/β-catenin pathway functions to regulate dorsal-ventral axis specification.
Other observations indicate that Wnt signaling plays more complex roles in body axis specification. Blocking Dishevelled function with dominant interfering constructs did not inhibit dorsal structures arguing against the canonical role of this protein in axis specification (Sokol, 1996). Furthermore, Wnt11b, a noncanonical Wnt ligand, was shown to be required for dorsal development in Xenopus (Tao et al., 2005), implicating PCP signaling in this process. Although dorsal accumulation of β-catenin is commonly explained by its stabilization in response to canonical pathway activity, β-catenin may be also recruited to the dorsal side of the zygote by Wnt/PCP signaling. Indeed, both cortical and nuclear enrichment of β-catenin is known to take place in worm embryonic cells in response to different Wnt signaling branches (Sawa, 2012; Schneider and Bowerman, 2007; Sugioka et al., 2011). Consistent with the above hypothesis, Vangl2 has been proposed to play a role in Xenopus oocyte polarity (Cha et al., 2011). Additional experiments are warranted to investigate these new functions of PCP proteins in animal-vegetal and dorsal-ventral axis specification.
3.2 Anteroposterior patterning during gastrulation
Just a few hours after fertilization, the maternal β-catenin pathway activates many dorsal-specific target genes in the region, known as the dorsal signaling center (Spemann’s organizer in amphibians or the node in mammals). The organizer is characterized by many early Wnt/β-catenin targets, including goosecoid, nodal-related genes and genes encoding Wnt antagonists such as Frzb and Dkk-1 (Harland and Gerhart, 1997; Hikasa and Sokol, 2013). With the onset of gastrulation, Wnt signaling undergoes a time-dependent switch. The antagonists secreted by the early organizer (‘head’) gradually inhibit subsequent Wnt signaling in the future anterior end of the embryo. By contrast, late (‘tail’) organizer cells start to express a number of Wnt ligands that are responsible for posterior patterning (Hikasa and Sokol, 2013; Zhang et al., 2011). Additionally, some mediators of posterior Wnt signals are only active at gastrula stages. One such factor is homeodomain-interacting protein kinase 2, which mediates TCF3-dependent transcriptional repression in response to late Wnt signaling (Hikasa et al., 2010). As a result, a gradient of Wnt signaling activity is established and maintained along the anteroposterior axis during gastrulation and later development (Itoh and Sokol, 1997; Itoh et al., 1995; Kiecker and Niehrs, 2001). For a detailed discussion of the role of the Wnt/β-catenin pathway in anteroposterior axis specification, see recent reviews, e. g. (Hikasa and Sokol, 2013; Petersen and Reddien, 2009).
4. Establishing planar cell polarity axes
4.1 Tissues manifesting PCP
Whereas Wnt/β-catenin signaling activity is marked by a specific target gene signature, PCP signaling is manifested by cell polarization in the plane of epithelial and nonepithelial tissues as evidenced by morphological and molecular markers. Classical examples of PCP are cuticular hairs of the Drosophila wing or ommatidia of the eye (Adler, 2012; Simons and Mlodzik, 2008). More recently, the spectrum of known tissues with PCP has been expanded to include mammalian cochlea (Montcouquiol et al., 2003), limb mesenchyme (Gao et al., 2011), ascidian notochord (Jiang et al., 2005), vertebrate skin (Devenport and Fuchs, 2008), axial mesoderm and neuroectoderm (Devenport, 2014; Nishimura et al., 2012; Ossipova, 2015a; Tissir and Goffinet, 2013; Wang and Nathans, 2007).
Vertebrate epidermis and neuroectoderm are among the earliest examples of vertebrate PCP. Lack of Fz3 and Fz6 activity in the mouse skin leads to hair swirls reminiscent of wing hair patterns in Drosophila (Wang and Nathans, 2007). The Xenopus skin contains numerous multiciliated cells with hundreds of basal bodies that are coordinately polarized along the anteroposterior axis in a PCP-dependent manner (Mitchell et al., 2007; Mitchell et al., 2009). Whereas these studies documented the existence of PCP in the Xenopus early embryo, it has been challenging to visualize it with molecular markers. However, exogenous Drosophila Prickle was distributed to the anterior domain of cells in zebrafish neuroectoderm and axial mesoderm, while Dishevelled localization was biased to the posterior cortex (Ciruna et al., 2006; Yin et al., 2008). In the mouse skin, core PCP proteins similarly accumulate at anteroposterior cell boundaries (Devenport and Fuchs, 2008; Devenport et al., 2011). Consistent with these findings, Celsr1, Daam1, ROCK and Dishevelled were found enriched at anteroposterior cell faces in the chick and mouse neuroectoderm (McGreevy et al., 2015; Nishimura et al., 2012). In Xenopus, both endogenous Vangl2 and exogenous Vangl2/Prickle complexes accumulate at the most anterior edge of cells at the neural midline (Ossipova, 2015a). Mammalian axial mesoderm tissues, i. e. mouse notochord and the posterior node, are also polarized along the anteroposterior axis (Antic et al., 2010; Borovina et al., 2010; Hashimoto et al., 2010; Song et al., 2010). These studies reveal the early establishment of the anteroposterior PCP (AP-PCP, Fig. 2).
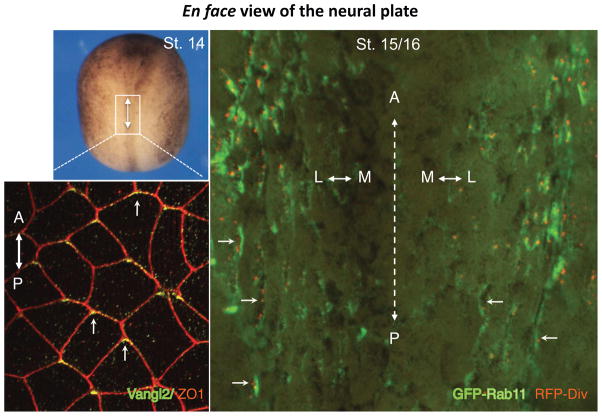
Figure 2. Anteroposterior and mediolateral PCP in Xenopus neural plate.
En face views of the Xenopus neural plate shown for stage 14–16 embryos. Top left, whole embryo view. White box corresponds to the area with the magnified image shown below. Bottom left, immunostaining for Vangl2 and ZO1 reveals anterior accumulation of Vangl2 in neural midline cells (arrows). On the right, the middle part of the neural plate exhibits mediolateral planar polarity of the cells mosaically expressing GFP-Rab11 (green) and RFP-Diversin (red puncta) that are enriched at medial domains of cells in the lateral neural plate. Arrows mark cell polarization towards the midline (dotted line). Anteroposterior (A–P) and mediolateral (M–L) axes are indicated.
It is currently unknown when AP-PCP first develops in vertebrate embryos. The Xenopus skin has been proposed to acquire PCP after or during gastrulation (Mitchell et al., 2009), but the molecular markers for its detection are still not available. In the Xenopus neural plate, AP-PCP is detectable from the beginning of neurulation, and it might be established even earlier. It is possible that AP-PCP derives from the original animal-vegetal polarity of the egg, but this hypothesis has not been tested experimentally. Alternatively, AP-PCP may reflect various cell behaviors in the neural plate, as suggested for the Drosophila wing (Aigouy et al., 2010). Consistent with this model, an early study showed that the majority of neural midline cells are displaced towards the anterior of the embryo (Burnside and Jacobson, 1968).
Besides AP-PCP, mediolaterally-directed PCP (ML-PCP) pattern has been reported in Xenopus neuroectoderm. In the lateral neural plate, the recycling endosome marker Rab11, the associated exocyst component Sec15 and the core PCP protein Diversin are polarized towards the midline in the mirror-symmetric manner (Ossipova et al., 2014), and this localization tightly correlated with centrosome position (Fig. 2, unpublished data). This Rab11 polarity was dependent on Vangl2 and Diversin signaling and was consistent with apical constriction behavior of cells at the dorsolateral hinges of the neural plate (Ossipova et al., 2014). These findings are remarkably similar to the observations in zebrafish neural progenitors showing midline-directed polarization of Rab11 and the centrosome, which were proposed to drive apical lumen formation in the neural tube (Buckley et al., 2013). Curiously, the behavior of these neural progenitors is also known to be under PCP control (Ciruna et al., 2006). This ML-PCP might relate to medially oriented monopolar protrusions of Xenopus deep neuroectoderm cells, which were described by earlier work and suggested to drive mediolateral intercalations (Elul and Keller, 2000). The above observations define two axes of PCP in the neural plate: AP-PCP along the anteroposterior axis and ML-PCP along the mediolateral axis. At present, the functional significance of the two PCP axes is unknown, however, it might reflect differential behaviors of cells at the neural midline and in the hinges undergoing apical constriction (see section 5).
4.2 Mechanisms for establishing PCP
4.2.1 Interactions between core PCP proteins
Several mechanisms have been put forward to account for core PCP protein polarization. A common model is based on feedback amplification mechanisms, such as the preferential association of PCP proteins between neighboring cells and their mutual exclusion within the same cell. The Frizzled/Dishevelled complex can promote Vangl2/Prickle enrichment across the cell junction between neighboring cells, either via a direct Frizzled-Vang interaction (Wu and Mlodzik, 2008) or with the help of Flamingo/Celsr, a cadherin-like protein (Chen et al., 2008). Intracellularly, Prickle, a PET-LIM domain-containing adaptor protein, can negatively affect the recruitment of Dishevelled to the cell membrane (Tree et al., 2002), and the association of Prickle to Dishevelled is competitively inhibited in vitro by Diego, a homologue of vertebrate Diversin (Jenny et al., 2005). These antagonistic protein interactions lead to the amplification of initially small asymmetries and the completion of PCP protein segregation to non-overlapping membrane domains (Li et al., 2013; Strutt et al., 2011).
It is still unknown which cytoskeletal and membrane components are needed to organize and maintain these domains. Among possible mechanisms, both polarized microtubule-based transport (Matis et al., 2014; Olofsson et al., 2014; Sepich et al., 2011; Shimada et al., 2006) and directed vesicular sorting (Guo et al., 2013; Ossipova et al., 2014) were proposed to regulate PCP protein distribution. Consistent with the important role of microtubules, Prickle might function by regulating polarized microtubule growth, although direct molecular mediators are not known (Matis et al., 2014; Olofsson et al., 2014). Prickle directly associates with Vangl2 (Bastock et al., 2003; Jenny et al., 2003), but its function in PCP is poorly understood. In Xenopus, overexpressed Vangl2 is not visibly polarized in the neural plate, but its polarity becomes readily apparent in the presence of exogenous Prickle, indicating that Prickle activity is limiting (Ossipova, 2015a). Moreover, polarized Prickle/Vangl2 complex formation appears to require the physical interaction of the two proteins, as suggested by loss of PCP for Vangl2 protein lacking Prickle-binding domain (unpublished observations).
An alternative model for PCP protein segregation is based on selective ubiquitin-dependent degradation. In Drosophila, Dishevelled is regulated by Cullin-3 (Strutt et al., 2013), whereas Prickle stability is under control of Vang and SkpA, a subunit of the SCF E3 ligase complex (Strutt et al., 2013). In vertebrates, Prickle1 and Prickle2 can be degraded by Smurf E3 ligases after their binding to Dvl in response to Wnt5a (Liu et al., 2014; Narimatsu et al., 2009). Notably, proteasome-dependent degradation is one of the major cellular responses to Wnt5a signaling (Carvallo et al., 2010; Iioka et al., 2007). To what degree protein degradation contributes to setting up PCP remains an open question.
4.2.2. Upstream regulators and downstream effectors of PCP
Whereas the interactions between core PCP proteins have been actively studied, less is known about upstream regulators of PCP. In Drosophila wing epithelium, the protocadherins Fat and Dachsous have been argued to provide a long-range cue for core PCP proteins (Ma et al., 2003). Alternatively, these proteins may function in parallel to core PCP components as a separate polarity-generating module (Donoughe and DiNardo, 2011; Lawrence et al., 2007; Matakatsu and Blair, 2012). For a detailed discussion of the Fat/Dachous system, see recent reviews (Goodrich and Strutt, 2011; Matis and Axelrod, 2013).
There has been a long-standing debate on the involvement of Wnt ligands in PCP. Several studies demonstrate Wnt protein requirement for PCP, both in vertebrates (Gao et al., 2011; Ossipova, 2015a; Qian et al., 2007). and in Drosophila (Wu et al., 2013). This role is reminiscent of cell polarization in response to Wnt proteins in other models (Habib et al., 2013; Lin et al., 2010; Witze et al., 2008). For example, Wnt signals modulate mitotic spindle assembly and developmental fates of early C. elegans blastomeres (Goldstein et al., 2006; Schlesinger et al., 1999; Sugioka et al., 2011; Walston et al., 2004). Similarly, application of Wnt-containing beads to human ES cells can direct their asymmetric division (Habib et al., 2013). Supporting an instructive role for Wnt signals in PCP, Wg- and Dwnt4-expressing clones can produce long range PCP in the Drosophila wing (Wu et al., 2013). Mouse and Xenopus studies are also consistent with PCP instructed by Wnt activity gradients (Andre et al., 2015; Gao et al., 2011; Ossipova, 2015a). Specifically, in the Xenopus early neural plate, the anterior intracellular accumulation of Vangl2 is largely detectable at the posterior end of the embryo (Ossipova, 2015a) in agreement with posterior expression of several Wnt genes (Hikasa and Sokol, 2013; Li et al., 2008; Wolda et al., 1993). These observations indicate that the graded instructive activity of Wnt ligands is responsible for both canonical and non-canonical (PCP) signaling in the early embryo. On the other hand, ubiquitously expressed Wnt11 can rescue abnormal morphogenesis of wnt11/silberblick zebrafish mutant embryos, suggesting a permissive role for Wnts (Heisenberg et al., 2000). Thus, additional experiments are warranted to determine whether Wnt ligands play an instructive or permissive role in PCP.
Wnt effects on PCP are likely mediated by Frizzled in combination with ROR and Ryk coreceptors (Andre et al., 2012; Grumolato et al., 2010; Macheda et al., 2012; Nishita et al., 2006; Yamamoto et al., 2007), rather than LRP5/6, which is specific for the canonical pathway. Interestingly, in Drosophila, Wg/Dwnt4 effects on PCP were proposed to be due to the negative regulation of Frizzled signaling (Wu et al., 2013). Wnt signals establish PCP through the phosphorylation of Dishevelled, Frizzled and Vangl2 (Gao et al., 2011; Gonzalez-Sancho et al., 2013; Shafer et al., 2011; Yanagawa et al., 1995) or through the modulation of PCP protein stability (Andre et al., 2012; Liu et al., 2014; Narimatsu et al., 2009; Strutt et al., 2013). Some of these mechanisms are likely to be context-dependent or may operate redundantly in any particular system.
PCP is established through the effects of core proteins on their cellular targets, which ultimately lead to changes in actomyosin dynamics and vesicular trafficking. Downstream molecular effectors can be subdivided into a PCP-specific class that includes Fuzzy and Inturned (Collier and Gubb, 1997; Gubb and Garcia-Bellido, 1982; Park et al., 1996) and molecules with more general roles, such as Rho-associated kinase (ROCK), Myosin II (Winter et al., 2001) and the formin-like proteins Daam1 and Mwh (Habas et al., 2001; Yan et al., 2008). The localization of both Fuzzy and Inturned is regulated by core PCP proteins (Adler, 2012; Strutt and Warrington, 2008). Both Fuzzy and Inturned are needed for ciliogenesis (Park et al., 2006; Zilber et al., 2013), but the importance of cilia in PCP remains questionable, given that the majority of Drosophila cells lack cilia (Wallingford and Mitchell, 2011). Inturned has been shown to bind Mwh, thereby connecting PCP signaling to actomyosin regulation (Lu et al., 2010; Strutt and Warrington, 2008). Similarly, the complex of Dishevelled and Daam1 can upregulate RhoA, ROCK and Myosin II activity (Habas et al., 2001; Shindo and Wallingford, 2014). By contrast, Fuzzy has been implicated in vesicular trafficking (Gray et al., 2009; Ooi et al., 2013). The connection between PCP and endocytosis is further strengthened by the interdependence of core PCP proteins and Rab5- or Rab11-mediated trafficking (Classen et al., 2005; Mottola et al., 2010; Ossipova et al., 2014). Notably, several PCP effectors mediate feedback control of core PCP protein localization. Interference with the activity of ROCK, Myosin II and Cofilin, an actin-remodelling factor, causes the disruption of PCP localization patterns in fly, ascidian, frog and mouse embryos (Mahaffey et al., 2013; Newman-Smith et al., 2015; Ossipova, 2015a; Yan et al., 2009). Although the proposed feedback may be indirect, it is consistent with the notion that PCP is sensitive to mechanical forces as proposed for Drosophila wing epithelium (Aigouy et al., 2010).
5. Linking cell polarity to morphogenetic movements
A major challenge in the PCP field is to explain how tissue polarization is related to specific cell behaviors. For example, mouse embryos with deficient PCP signaling develop pronounced neural tube defects, but it is unclear how lack of planar polarity results in the failure of the neural tube to close. Given a large number of developmental processes that involve PCP proteins, multiple context-dependent mechanisms are likely to be involved.
In general, many PCP-dependent morphogenetic events can be viewed as changes in cell shape that are coordinated between neighboring cells in a tissue (Fig. 3). One such process is convergent extension movement of the mesoderm and neuroectoderm, which is mediated by mediolateral cell intercalations (Elul and Keller, 2000; Keller, 2002; Wallingford et al., 2002). The conclusion that this cell behavior requires PCP came from the original observation that a dominant interfering form of Dishevelled blocked activin-dependent elongation of Xenopus animal pole explants (Sokol, 1996). The involvement of PCP in convergent extension has been confirmed in several experimental models and has been extensively reviewed (Gray et al., 2011).
Figure 3. Regulation of morphogenetic behaviors by Wnt/PCP signaling.
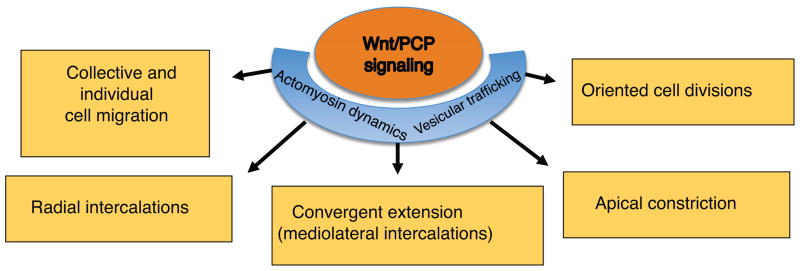
The Wnt/PCP pathway modulates actomyosin dynamics and vesicular trafficking to control mediolateral and radial cell intercalations, apical constriction, collective and individual cell migration and oriented cell division. Together, these cell behaviors ensure proper vertebrate neural tube closure.
The second type of cell migratory behavior that requires PCP signaling is radial intercalation. Radial cell intercalations, known in early embryos as epiboly movements, occur along the apical-basal embryo axis and result in tissue thinning and spreading (Keller, 1980; Walck-Shannon and Hardin, 2014). Accumulating evidence points to the involvement of PCP in radial intercalations. Zebrafish embryos with disrupted function of Flamingo/Celsr exhibit epiboly defects (Carreira-Barbosa et al., 2009). Consistent with defective radial intercalation, mutations in the Wnt5a gene and its receptor Ror2 inhibit gut elongation in mouse embryos (Cervantes et al., 2009; Yamada et al., 2010). Significantly, interference with the functions of Vangl2, Prickle3 and Dvl in Xenopus embryos revealed defective epiboly movements, causing thickening of both neural and epidermal ectoderm at both gastrula and neurula stages (Ossipova, 2015b). This thickening is also observed in embryos depleted of GEF-H1, a guanine nucleotide exchange factor that binds Dishevelled and might, therefore, be involved in PCP signaling (Itoh et al., 2014).
Another example of radial intercalation is the behavior of multiciliated cells (MCCs) in the Xenopus epidermis. These cells form in the inner layer of nonneural ectoderm but later integrate into the superficial layer (Deblandre et al., 1999; Drysdale and Elinson, 1992). Knockdown of different core PCP components blocks radial intercalation, causing MCCs to ectopically differentiate in the inner layer of the skin. The kinesin motor protein KIF-13 was shown to interact with Dishevelled and was proposed to play a key role in this process (Ossipova, 2015b). Besides mediolateral and radial cell intercalations, PCP signaling is involved in other cell behaviors, such as those occurring during angiogenesis (Cirone et al., 2008), neuronal migration (FBMN) in zebrafish and mouse embryos (Glasco et al., 2012; Jessen et al., 2002), during oriented cell divisions (Gong et al., 2004; Lake and Sokol, 2009; Segalen et al., 2010), the migration of lateral line primordia in zebrafish (Mirkovic et al., 2012) and branching morphogenesis of the lung and the kidney (Miller et al., 2011; Tissir and Goffinet, 2013; Yates et al., 2010a; Yates et al., 2010b) (Fig. 3). Notably, cell intercalations are essential for germ band extension in Drosophila, however, this process does not seem to require core PCP components (Zallen, 2007; Zallen and Wieschaus, 2004).
Neural tube defects in PCP mutants are commonly explained by the inhibition of convergent extension movements. According to one mechanism, anteroposterior enrichment of PCP proteins at specific cell junctions in chick neural plate is needed to shorten these junctions, stimulating cell neighbor exchange during convergent extension (Nishimura et al., 2012). The PCP proteins Dishevelled and Daam1 recruit PDZ-RhoGEF to these junctions, leading to localized actomyosin contractions, convergent extension and neural plate folding in the direction perpendicular to the anteroposterior axis. Of note, the same molecular interactions can be also interpreted as driving apical constriction, another cell behavior critical for neural tube closure (Martin and Goldstein, 2014; Sawyer et al., 2010; Schroeder, 1970). According to this interpretation, the actomyosin contractility at anteroposterior cell junctions promotes the constriction of neuroepithelial cell surfaces along the perpendicular (i. e. mediolateral) axis. Indeed, core PCP proteins were recently reported to be involved in apical constriction, although it remains unclear whether actomyosin contractility is active at one or both edges of the constricting cell (Ossipova et al., 2015; Ossipova et al., 2014). The link between PCP and apical constriction machinery is further supported by a study showing that Shroom, a key modulator of apical constriction, is planar polarized in the mouse neural plate and interacts with Dishevelled (McGreevy et al., 2015).
Besides actomyosin dynamics, neural tube closure may be promoted by the removal of the cell membrane via endocytosis (Lee and Harland, 2010; Lofberg, 1974). Strikingly, Rab11 recycling endosomes and active Myosin II accumulate at medial apical edges of cells in the superficial layer of the neural plate (Ossipova et al., 2014). Besides Rab11, the component of the exocyst Sec15 and the core PCP protein Diversin exhibit similar mediolateral polarization in the neural plate (Ossipova et al., 2014). This mediolateral polarity depends on PCP signaling, and, conversely, PCP protein trafficking requires Rab11 function. According to this model, apical constriction is anisotropic and requires ML-PCP (Ossipova et al., 2014). The mechanism by which PCP proteins polarize Rab11 endosomes is currently unclear, but it is likely to involve the activity of Fuzzy, a PCP effector implicated in vesicular trafficking (Gray et al., 2009; Ooi et al., 2013). Taken together, the above observations suggest several molecular mechanisms that may link PCP to morphogenetic cell behaviors (Fig. 3). To distinguish between different models, it would be important to characterize actomyosin dynamics in cells located in the different areas of the neural plate. Future studies are necessary to conclusively determine which of these mechanisms operate during vertebrate neurulation.
6. Conclusions
Studies using a number of in vivo models revealed essential and fundamental roles of Wnt signaling in cell fate and cell polarity specification in vertebrate embryos. The canonical Wnt/β-catenin pathway modulates target gene transcription to specify cell fates along the dorsoventral and anteroposterior embryonic axes. Strikingly, both Wnt/β-catenin and Wnt/PCP signaling branches appear to operate along the anteroposterior embryonic axis. Recent biochemical and cell biological experiments provide insights into PCP protein interactions, leading to the establishment of tissue polarity along different embryonic axes. Although the initial cues causing early PCP are unknown, an attractive hypothesis is that anteroposterior PCP derives from the original animal-vegetal polarity of the oocyte. Alternatively, PCP may result from local cell-cell interactions, which sense and interpret a long-range gradient of Wnt activity secreted from the posterior end of the vertebrate embryo. While it is becoming clear that PCP proteins coordinate diverse morphogenetic processes by modulating actomyosin dynamics and vesicular trafficking, specific molecular mechanisms remain to be demonstrated.
Acknowledgments
The author thanks Daniel Kessler and members of the Sokol laboratory for comments on the manuscript and numerous discussions. I apologize to those whose work could not be cited here due to space constraints and acknowledge the grant support from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acebron SP, Karaulanov E, Berger BS, Huang YL, Niehrs C. Mitotic wnt signaling promotes protein stabilization and regulates cell size. Mol Cell. 2014;54:663–674. doi: 10.1016/j.molcel.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Adler PN. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol. 2012;101:1–31. doi: 10.1016/B978-0-12-394592-1.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Andre P, Song H, Kim W, Kispert A, Yang Y. Wnt5a and Wnt11 regulate mammalian anterior-posterior axis elongation. Development. 2015;142:1516–1527. doi: 10.1242/dev.119065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre P, Wang Q, Wang N, Gao B, Schilit A, Halford MM, Stacker SA, Zhang X, Yang Y. The Wnt coreceptor Ryk regulates Wnt/planar cell polarity by modulating the degradation of the core planar cell polarity component Vangl2. J Biol Chem. 2012;287:44518–44525. doi: 10.1074/jbc.M112.414441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–412. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- Buckley CE, Ren X, Ward LC, Girdler GC, Araya C, Green MJ, Clark BS, Link BA, Clarke JD. Mirror-symmetric microtubule assembly and cell interactions drive lumen formation in the zebrafish neural rod. EMBO J. 2013;32:30–44. doi: 10.1038/emboj.2012.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside MB, Jacobson AG. Analysis of morphogenetic movements in the neural plate of the newt Taricha torosa. Dev Biol. 1968;18:537–552. doi: 10.1016/0012-1606(68)90025-0. [DOI] [PubMed] [Google Scholar]
- Carreira-Barbosa F, Kajita M, Morel V, Wada H, Okamoto H, Martinez Arias A, Fujita Y, Wilson SW, Tada M. Flamingo regulates epiboly and convergence/extension movements through cell cohesive and signalling functions during zebrafish gastrulation. Development. 2009;136:383–392. doi: 10.1242/dev.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvallo L, Munoz R, Bustos F, Escobedo N, Carrasco H, Olivares G, Larrain J. Non-canonical Wnt signaling induces ubiquitination and degradation of Syndecan4. J Biol Chem. 2010;285:29546–29555. doi: 10.1074/jbc.M110.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes S, Yamaguchi TP, Hebrok M. Wnt5a is essential for intestinal elongation in mice. Dev Biol. 2009;326:285–294. doi: 10.1016/j.ydbio.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha SW, Tadjuidje E, Wylie C, Heasman J. The roles of maternal Vangl2 and aPKC in Xenopus oocyte and embryo patterning. Development. 2011;138:3989–4000. doi: 10.1242/dev.068866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae J, Kim MJ, Goo JH, Collier S, Gubb D, Charlton J, Adler PN, Park WJ. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development. 1999;126:5421–5429. doi: 10.1242/dev.126.23.5421. [DOI] [PubMed] [Google Scholar]
- Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, Nusse R, Axelrod JD. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–1105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirone P, Lin S, Griesbach HL, Zhang Y, Slusarski DC, Crews CM. A role for planar cell polarity signaling in angiogenesis. Angiogenesis. 2008;11:347–360. doi: 10.1007/s10456-008-9116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Collier S, Gubb D. Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development. 1997;124:4029–4037. doi: 10.1242/dev.124.20.4029. [DOI] [PubMed] [Google Scholar]
- Deblandre GA, Wettstein DA, Koyano-Nakagawa N, Kintner C. A two-step mechanism generates the spacing pattern of the ciliated cells in the skin of Xenopus embryos. Development. 1999;126:4715–4728. doi: 10.1242/dev.126.21.4715. [DOI] [PubMed] [Google Scholar]
- Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014;207:171–179. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Oristian D, Heller E, Fuchs E. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat Cell Biol. 2011;13:893–902. doi: 10.1038/ncb2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoughe S, DiNardo S. dachsous and frizzled contribute separately to planar polarity in the Drosophila ventral epidermis. Development. 2011;138:2751–2759. doi: 10.1242/dev.063024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale TA, Elinson RP. Cell Migration and Induction in the Development of the Surface Ectodermal Pattern of the Xenopus laevis Tadpole. Development, Growth & Differentiation. 1992;34:51–59. doi: 10.1111/j.1440-169X.1992.00051.x. [DOI] [PubMed] [Google Scholar]
- Elul T, Keller R. Monopolar protrusive activity: a new morphogenic cell behavior in the neural plate dependent on vertical interactions with the mesoderm in Xenopus. Dev Biol. 2000;224:3–19. doi: 10.1006/dbio.2000.9746. [DOI] [PubMed] [Google Scholar]
- Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Danilchik M, Doniach T, Roberts S, Rowning B, Stewart R. Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development. 1989;107(Suppl):37–51. doi: 10.1242/dev.107.Supplement.37. [DOI] [PubMed] [Google Scholar]
- Glasco DM, Sittaramane V, Bryant W, Fritzsch B, Sawant A, Paudyal A, Stewart M, Andre P, Cadete Vilhais-Neto G, Yang Y, et al. The mouse Wnt/PCP protein Vangl2 is necessary for migration of facial branchiomotor neurons, and functions independently of Dishevelled. Dev Biol. 2012;369:211–222. doi: 10.1016/j.ydbio.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Takeshita H, Mizumoto K, Sawa H. Wnt signals can function as positional cues in establishing cell polarity. Dev Cell. 2006;10:391–396. doi: 10.1016/j.devcel.2005.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho JM, Greer YE, Abrahams CL, Takigawa Y, Baljinnyam B, Lee KH, Lee KS, Rubin JS, Brown AM. Functional consequences of Wnt-induced dishevelled 2 phosphorylation in canonical and noncanonical Wnt signaling. J Biol Chem. 2013;288:9428–9437. doi: 10.1074/jbc.M112.448480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, Lee I, Weiss GS, Liu KJ, Marcotte EM, Wallingford JB, et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol. 2009;11:1225–1232. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- Gubb D, Green C, Huen D, Coulson D, Johnson G, Tree D, Collier S, Roote J. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 1999;13:2315–2327. doi: 10.1101/gad.13.17.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Zanetti G, Schekman R. A novel GTP-binding protein-adaptor protein complex responsible for export of Vangl2 from the trans Golgi network. Elife. 2013;2:e00160. doi: 10.7554/eLife.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E, Nusse R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12:170–176. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Bellaiche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell. 2010;19:521–532. doi: 10.1016/j.devcel.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H, Shibata M, Hiratani I, Taira M. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development. 2002;129:5227–5239. doi: 10.1242/dev.129.22.5227. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Sokol SY. Wnt signaling in vertebrate axis specification. Cold Spring Harb Perspect Biol. 2013;5:a007955. doi: 10.1101/cshperspect.a007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iioka H, Iemura S, Natsume T, Kinoshita N. Wnt signalling regulates paxillin ubiquitination essential for mesodermal cell motility. Nat Cell Biol. 2007;9:813–821. doi: 10.1038/ncb1607. [DOI] [PubMed] [Google Scholar]
- Itoh K, Ossipova O, Sokol SY. GEF-H1 functions in apical constriction and cell intercalations and is essential for vertebrate neural tube closure. J Cell Sci. 2014;127 doi: 10.1242/jcs.146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Sokol SY. Graded amounts of Xenopus dishevelled specify discrete anteroposterior cell fates in prospective ectoderm. Mech Dev. 1997;61:113–125. doi: 10.1016/s0925-4773(96)00627-2. [DOI] [PubMed] [Google Scholar]
- Itoh K, Tang TL, Neel BG, Sokol SY. Specific modulation of ectodermal cell fates in Xenopus embryos by glycogen synthase kinase. Development. 1995;121:3979–3988. doi: 10.1242/dev.121.12.3979. [DOI] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Munro EM, Smith WC. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Keller RE. The cellular basis of epiboly: an SEM study of deep-cell rearrangement during gastrulation in Xenopus laevis. J Embryol Exp Morphol. 1980;60:201–234. [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Lake BB, Sokol SY. Strabismus regulates asymmetric cell divisions and cell fate determination in the mouse brain. J Cell Biol. 2009;185:59–66. doi: 10.1083/jcb.200807073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J Cell Biol. 1997;136:1123–1136. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Harland RM. Endocytosis is required for efficient apical constriction during Xenopus gastrulation. Curr Biol. 2010;20:253–258. doi: 10.1016/j.cub.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Roszko I, Sepich DS, Ni M, Hamm HE, Marlow FL, Solnica-Krezel L. Gpr125 modulates Dishevelled distribution and planar cell polarity signaling. Development. 2013;140:3028–3039. doi: 10.1242/dev.094839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Baye LM, Westfall TA, Slusarski DC. Wnt5b-Ryk pathway provides directional signals to regulate gastrulation movement. J Cell Biol. 2010;190:263–278. doi: 10.1083/jcb.200912128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Lin C, Gao C, May-Simera H, Swaroop A, Li T. Null and hypomorph Prickle1 alleles in mice phenocopy human Robinow syndrome and disrupt signaling downstream of Wnt5a. Biol Open. 2014 doi: 10.1242/bio.20148375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofberg J. Apical surface topography of invaginating and noninvaginating cells. A scanning-transmission study of amphibian neurulae. Dev Biol. 1974;36:311–329. doi: 10.1016/0012-1606(74)90054-2. [DOI] [PubMed] [Google Scholar]
- Lu Q, Yan J, Adler PN. The Drosophila planar polarity proteins inturned and multiple wing hairs interact physically and function together. Genetics. 2010;185:549–558. doi: 10.1534/genetics.110.114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta–catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheda ML, Sun WW, Kugathasan K, Hogan BM, Bower NI, Halford MM, Zhang YF, Jacques BE, Lieschke GJ, Dabdoub A, et al. The Wnt receptor Ryk plays a role in mammalian planar cell polarity signaling. J Biol Chem. 2012;287:29312–29323. doi: 10.1074/jbc.M112.362681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey JP, Grego–Bessa J, Liem KF, Jr, Anderson KV. Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development. 2013;140:1262–1271. doi: 10.1242/dev.085316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Goldstein B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development. 2014;141:1987–1998. doi: 10.1242/dev.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Separating planar cell polarity and Hippo pathway activities of the protocadherins Fat and Dachsous. Development. 2012;139:1498–1508. doi: 10.1242/dev.070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis M, Axelrod JD. Regulation of PCP by the Fat signaling pathway. Genes Dev. 2013;27:2207–2220. doi: 10.1101/gad.228098.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis M, Russler-Germain DA, Hu Q, Tomlin CJ, Axelrod JD. Microtubules provide directional information for core PCP function. Elife. 2014;3:e02893. doi: 10.7554/eLife.02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreevy EM, Vijayraghavan D, Davidson LA, Hildebrand JD. Shroom3 functions downstream of planar cell polarity to regulate myosin II distribution and cellular organization during neural tube closure. Biol Open. 2015;4:186–196. doi: 10.1242/bio.20149589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol. 1999;146:427–437. doi: 10.1083/jcb.146.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Canny SG, Jang CW, Cho K, Ji H, Wagner DS, Jones EA, Habas R, McCrea PD. Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling. J Am Soc Nephrol. 2011;22:1654–1664. doi: 10.1681/ASN.2010101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovic I, Pylawka S, Hudspeth AJ. Rearrangements between differentiating hair cells coordinate planar polarity and the establishment of mirror symmetry in lateral-line neuromasts. Biol Open. 2012;1:498–505. doi: 10.1242/bio.2012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Mottola G, Classen AK, Gonzalez-Gaitan M, Eaton S, Zerial M. A novel function for the Rab5 effector Rabenosyn-5 in planar cell polarity. Development. 2010;137:2353–2364. doi: 10.1242/dev.048413. [DOI] [PubMed] [Google Scholar]
- Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Newman-Smith E, Kourakis MJ, Reeves W, Veeman M, Smith WC. Reciprocal and dynamic polarization of planar cell polarity core components and myosin. Elife. 2015;4 doi: 10.7554/eLife.05361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–1097. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, Takada S, Kikuchi A, Minami Y. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–562. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson J, Sharp KA, Matis M, Cho B, Axelrod JD. Prickle/spiny-legs isoforms control the polarity of the apical microtubule network in planar cell polarity. Development. 2014;141:2866–2874. doi: 10.1242/dev.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi YS, Stiles KM, Liu CY, Taylor GM, Kielian M. Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry. PLoS Pathog. 2013;9:e1003835. doi: 10.1371/journal.ppat.1003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Kim K, Sokol SY. Anteroposterior polarity of the neural plate is regulated by Wnt and Myosin signaling. Biology Open. 2015a doi: 10.1242/bio.201511676. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Chu C, Fillatre J, Brott BK, Itoh K, Sokol SY. The involvement of planar cell polarity in radial cell intercalations in Xenopus embryos. Dev Biol. 2015b doi: 10.1016/j.ydbio.2015.06.013. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Chuykin I, Chu CW, Sokol SY. Vangl2 cooperates with Rab11 and Myosin V to regulate apical constriction during vertebrate gastrulation. Development. 2015;142:99–107. doi: 10.1242/dev.111161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Kim K, Lake BB, Itoh K, Ioannou A, Sokol SY. Role of Rab11 in planar cell polarity and apical constriction during vertebrate neural tube closure. Nat Commun. 2014;5:3734. doi: 10.1038/ncomms4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Park WJ, Liu J, Sharp EJ, Adler PN. The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development. 1996;122:961–969. doi: 10.1242/dev.122.3.961. [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa H. Control of cell polarity and asymmetric division in C. elegans. Curr Top Dev Biol. 2012;101:55–76. doi: 10.1016/B978-0-12-394592-1.00003-X. [DOI] [PubMed] [Google Scholar]
- Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B. Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol. 2010;341:5–19. doi: 10.1016/j.ydbio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger A, Shelton CA, Maloof JN, Meneghini M, Bowerman B. Wnt pathway components orient a mitotic spindle in the early Caenorhabditis elegans embryo without requiring gene transcription in the responding cell. Genes Dev. 1999;13:2028–2038. doi: 10.1101/gad.13.15.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Schneider SQ, Bowerman B. beta-Catenin Asymmetries after All Animal/Vegetal- Oriented Cell Divisions in Platynereis dumerilii Embryos Mediate Binary Cell-Fate Specification. Dev Cell. 2007;13:73–86. doi: 10.1016/j.devcel.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Schroeder TE. Neurulation in Xenopus laevis. An analysis and model based upon light and electron microscopy. J Embryol Exp Morphol. 1970;23:427–462. [PubMed] [Google Scholar]
- Segalen M, Johnston CA, Martin CA, Dumortier JG, Prehoda KE, David NB, Doe CQ, Bellaiche Y. The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev Cell. 2010;19:740–752. doi: 10.1016/j.devcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131:1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Sepich DS, Usmani M, Pawlicki S, Solnica-Krezel L. Wnt/PCP signaling controls intracellular position of MTOCs during gastrulation convergence and extension movements. Development. 2011;138:543–552. doi: 10.1242/dev.053959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y. Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell. 2011;20:177–191. doi: 10.1016/j.devcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Shindo A, Wallingford JB. PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science. 2014;343:649–652. doi: 10.1126/science.1243126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Wnt signaling and dorso-ventral axis specification in vertebrates. Curr Opin Genet Dev. 1999;9:405–410. doi: 10.1016/S0959-437X(99)80061-6. [DOI] [PubMed] [Google Scholar]
- Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:3487. doi: 10.1242/dev.121.10.3487. [DOI] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D, Warrington SJ. Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development. 2008;135:3103–3111. doi: 10.1242/dev.025205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Searle E, Thomas-Macarthur V, Brookfield R, Strutt D. A Cul-3-BTB ubiquitylation pathway regulates junctional levels and asymmetry of core planar polarity proteins. Development. 2013;140:1693–1702. doi: 10.1242/dev.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Warrington SJ, Strutt D. Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Dev Cell. 2011;20:511–525. doi: 10.1016/j.devcel.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka K, Mizumoto K, Sawa H. Wnt regulates spindle asymmetry to generate asymmetric nuclear beta-catenin in C. elegans. Cell. 2011;146:942–954. doi: 10.1016/j.cell.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Shaping the nervous system: role of the core planar cell polarity genes. Nat Rev Neurosci. 2013;14:525–535. doi: 10.1038/nrn3525. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Walck-Shannon E, Hardin J. Cell intercalation from top to bottom. Nat Rev Mol Cell Biol. 2014;15:34–48. doi: 10.1038/nrm3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston T, Tuskey C, Edgar L, Hawkins N, Ellis G, Bowerman B, Wood W, Hardin J. Multiple Wnt signaling pathways converge to orient the mitotic spindle in early C. elegans embryos. Dev Cell. 2004;7:831–841. doi: 10.1016/j.devcel.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolda SL, Moody CJ, Moon RT. Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev Biol. 1993;155:46–57. doi: 10.1006/dbio.1993.1005. [DOI] [PubMed] [Google Scholar]
- Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–469. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol. 2013;15:1045–1055. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Udagawa J, Matsumoto A, Hashimoto R, Hatta T, Nishita M, Minami Y, Otani H. Ror2 is required for midgut elongation during mouse development. Dev Dyn. 2010;239:941–953. doi: 10.1002/dvdy.22212. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y. Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells. 2007;12:1215–1223. doi: 10.1111/j.1365-2443.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Huen D, Morely T, Johnson G, Gubb D, Roote J, Adler PN. The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics. 2008;180:219–228. doi: 10.1534/genetics.108.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Lu Q, Fang X, Adler PN. Rho1 has multiple functions in Drosophila wing planar polarity. Dev Biol. 2009;333:186–199. doi: 10.1016/j.ydbio.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- Yates LL, Papakrivopoulou J, Long DA, Goggolidou P, Connolly JO, Woolf AS, Dean CH. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet. 2010a;19:4663–4676. doi: 10.1093/hmg/ddq397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates LL, Schnatwinkel C, Murdoch JN, Bogani D, Formstone CJ, Townsend S, Greenfield A, Niswander LA, Dean CH. The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum Mol Genet. 2010b;19:2251–2267. doi: 10.1093/hmg/ddq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol. 2008;180:221–232. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Zhang B, Tran U, Wessely O. Expression of Wnt signaling components during Xenopus pronephros development. PLoS One. 2011;6:e26533. doi: 10.1371/journal.pone.0026533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilber Y, Babayeva S, Seo JH, Liu JJ, Mootin S, Torban E. The PCP effector Fuzzy controls cilial assembly and signaling by recruiting Rab8 and Dishevelled to the primary cilium. Mol Biol Cell. 2013;24:555–565. doi: 10.1091/mbc.E12-06-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]