Abstract
Antagonists and inhibitory molecules responsible for maintaining tissue homeostasis can present a significant barrier to healing when tissue engineering/regenerative medicine strategies are employed. One example of this situation is the up-regulation of antagonists such as noggin in response to increasing concentrations of bone morphogenetic protein 2 (BMP-2) present from endogenous bone repair processes or delivered exogenously from biomaterials (synthetic bone grafts). While recombinant human (rh)BMP-2 delivered from synthetic bone grafts has been shown to be an effective alternative to autografts and allografts, the supraphysiological doses of rhBMP-2 have led to clinically-adverse side effects. The high rhBMP-2 dosage may be required, in part, to overcome the presence of antagonists such as noggin. Small interfering RNA (siRNA) is an appealing approach to overcome this problem because it can knock-down antagonists or inhibitory molecules in a temporary manner. Here, we conducted fundamental studies on the delivery of siRNA from material surfaces as a means to knock-down antagonists like noggin. Non-viral cationic lipid (Lipofectamine)-siRNA complexes were delivered from a fibrin hydrogel surface to MC3T3-E1 preosteoblasts that were treated with a supraphysiological dose of rhBMP-2 to achieve noggin mRNA expression levels higher than cells naïve to rhBMP-2. Confocal microscopy and flow cytometry showed intracellular uptake of siRNA in over 98% of MC3T3-E1 cells after 48 hours. Doses of 0.5μg and 1μg noggin siRNA were able to significantly reduce noggin mRNA to levels equivalent to those in MC3T3-E1 cells not exposed to rhBMP-2 with no effects on cell viability.
Keywords: tissue engineering, natural polymer, transfection, regenerative medicine, mRNA
Graphical Abstract
1. Introduction
Two million bone graft procedures are performed worldwide each year to repair bone defects sustained from trauma, tumor resections, and orthopedic surgery [1]. The therapeutic gold standard for non-unions is the use of autologous bone grafts, and allogeneic bone grafts such as demineralized bone matrix are also widely used. However, due to limited donor tissue and donor site morbidity (autografts) and concerns about immunological rejection (allografts) a number of alternative bone grafts have been developed [1]. These devices aim to provide a material component (e.g., collagen, ceramic, or synthetic polymer) that acts as an osteoconductive matrix for cell attachment and migration. They also provide an osteoinductive mitogenic agent or growth factor to promote bone regeneration [2].
One growth factor of particular interest for bone regeneration, due to its osteoinductive capability, is bone morphogenetic protein-2 (BMP-2) [3]. In native bone regeneration, BMP-2 is uniquely required for initiation of bone healing [4] as well as for long-term duration of the healing process by its continuous expression [5]. BMP-2 acts as a chemoattractant of mesenchymal progenitor cells and stimulates their differentiation into osteoblasts [6]. Administration of exogenous BMP-2 alone can promote the bone healing process [7], but its short half-life [8], the presence of receptor-binding antagonists [9], and its potent morphogenetic potential require that it be incorporated into or onto a material to confine the effects of BMP-2 to the local implant site.
In several FDA-approved products, recombinant human BMP-2 (rhBMP-2) is adsorbed to collagen-based osteoconductive matrices [10]. Unfortunately, the supra-physiological dose of rhBMP-2 [11] delivered into the local environment from these products has led to complications including heterotrophic ossification, seroma, and edema [12–14]. The high doses of rhBMP-2 required to achieve successful bone regeneration may stem from endogenous, self-limiting regulation of BMP-2 expression through extracellular inhibitors and antagonists [9, 15]. Signaling regulators such as noggin are produced by local osteoblasts and have a high affinity to bind BMP-2, thus preventing it from binding to its receptor [16]. Consequently, BMP-2 efficacy is greatly decreased due to a temporal and spatial increase of noggin expression as a result of increases in BMP-2 levels [17]. This negative feedback mechanism provides a way to self-regulate endogenous BMP-2 levels under healing conditions and in clinical examples when rhBMP-2 is delivered exogenously as part of a synthetic bone graft. Considerable effort has been focused on the exploration of controlling the amount of rhBMP-2 delivered at the implant site either through alternative carrier systems [18–20] or through the use of other delivery strategies [21–23].
However, reducing the amount of rhBMP-2 alone does not address the underlying role that antagonists, such as noggin, play in mitigating effects of BMP-2. One approach to address the role of BMP-2 antagonists is through the delivery of small interfering RNA (siRNA) to cells involved in production of the antagonists. Since its discovery, siRNA has garnered excitement for its potential in therapeutic applications ranging from anti-cancer therapeutics [24] to treatment of chronic neurological conditions [25] by targeting specific mRNAs that code for proteins of interest [26, 27]. A key limitation to successful application of siRNA as a therapeutic strategy has been delivery to the site of action. Systemic delivery is challenging because siRNA is unstable in blood, which necessitates use of complexing agents (e.g., polycations) to protect the siRNA and achieve long circulation times. Other challenges include achieving localization to the target tissue, localization to the target cells, and achieving cellular uptake of the siRNA [28, 29]. However, for tissue engineering/regenerative medicine applications these barriers are less problematic as the siRNA can be directly implanted at the site of action (i.e., tissue in need of repair). Recent progress has led to successful incorporation of siRNA into synthetic [30–33], natural [34, 35], and composite [36, 37] biomaterials for local delivery of sequence-specific mRNA targets. Here, we have investigated the ability to achieve high levels of target (noggin) knockdown by a simple approach in which the siRNA is complexed with a cationic lipid and adsorbed to the material surface.
In terms of bone regeneration, siRNA targeting guanine nucleotide-binding protein alpha-stimulating activity polypeptide 1 (GNAS1) and prolyl hydroxylase domain-containing protein 2 (PHD2) have been administered directly to mesenchymal stem cells to promote their differentiation towards an osteogenic phenotype [38]. siRNA has also been used in directing transcription factor and osteogenic pathway regulation to accelerate bone fracture healing [38, 39]. Noggin has been investigated as a siRNA target due to its well-known antagonistic role against BMP-2. Several groups have used transfection methods including non-viral carriers [40], electroporation [41], or viral gene delivery strategies [42]. Others have developed novel carrier systems for siRNA to be delivered in conjunction with rhBMP-2 [43]. Not only has knockdown of noggin inhibition been shown to increase osteogenesis but it also lowers the effective rhBMP-2 dose required to elicit a favorable response [41, 43].
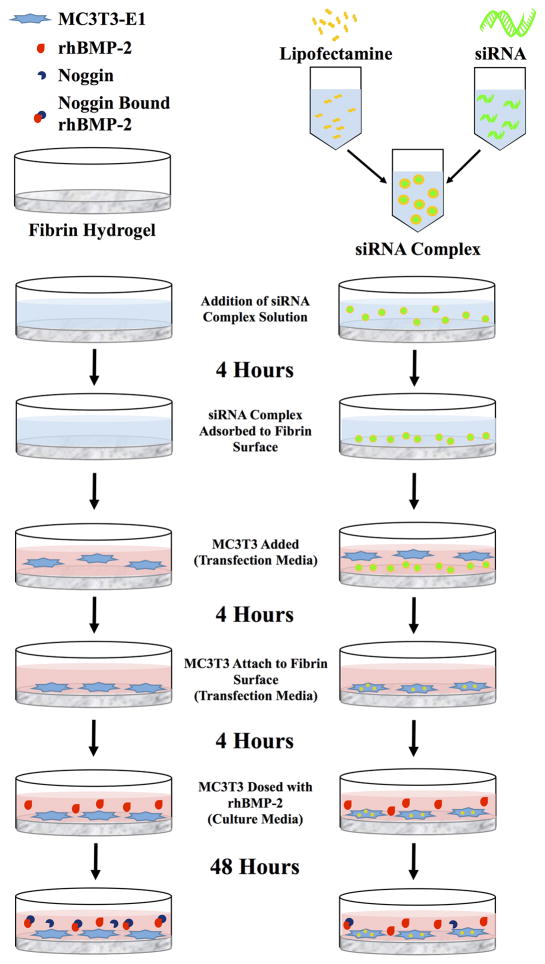
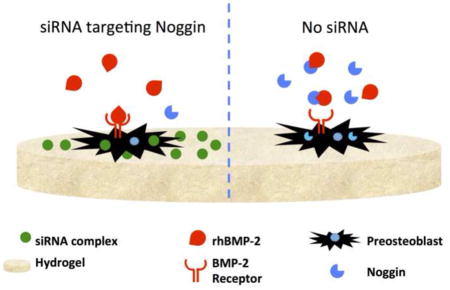
In the study reported here, we sought to use an approach in which siRNA could be delivered to cells from the surface of an osteoconductive natural polymeric biomaterial carrier that would ultimately be compatible with a local (implantable) delivery strategy. A number of natural polymers have been used for bone regeneration applications due to their inherent ability to promote cell attachment, biodegradation, and minimal toxicity. In these studies, we have used fibrin as the material due to its role in native bone healing [44], previous use as a BMP-2 carrier for bone regeneration [45, 46], and its suitability for local delivery of gene therapy agents including nucleic acids [47, 48]. Our approach was to complex the siRNA with a commercially available complexing agent (Lipofectamine, a cationic liposome) adsorbed to the surface of a biomaterial. We conducted a series of fundamental studies to determine: (1) the ability to adsorb siRNA complexes with a polycationic transfection vehicle to a fibrin hydrogel surface, (2) the uptake of these complexes into pre-osteoblast cells seeded onto the surface of the fibrin hydrogel, and (3) the effects of siRNA uptake on cell viability and knockdown of the target noggin mRNA in the preosteoblasts in conjunction with a supraphysiological dose of rhBMP-2 (study outline summarized in Figure 1). The studies demonstrate the utility of such a surface-mediated approach to achieve high levels of cell uptake and knockdown of the target mRNA, providing an approach to regulate noggin expression. The studies are general enough in nature to suggest that this approach would be more broadly applicable for the knockdown of other molecules inhibitory to regenerative processes.
Figure 1.
Schematic of surface-mediated transfection experimental design.
2. Materials and Methods
2.1 Complexation of siRNA with Polycationic Transfection Vehicles
Several types of siRNA were used for the experiments described below. Accell eGFP siRNA (Thermo Fisher Scientific, Waltham, MA) was used for complexing assays and particle size analysis. Accell Control siRNA #1 Non Targeting (Thermo Fisher Scientific, Waltham, MA) and All Stars Negative Control - Alexa 488 siRNA (Qiagen, Venlo, Netherlands) were used for non-target controls. Accell SmartPool Noggin siRNA (Thermo Fisher Scientific, Waltham, MA) was used as the sequence to target noggin mRNA. The same techniques were used to form complexes with Lipofectamine 2000 (Life Technologies, Grand Island, NY) for each type of siRNA used. Briefly, 20mM siRNA and Lipofectamine 2000 transfection agent were diluted into working concentrations (0.1μg/mL, 0.25μg/mL, 0.5μg/mL, 1μg/mL) with sterile RNase-free water (Thermo Fisher Scientific, Waltham, MA) under aseptic conditions. siRNA complexes were formed by using manufacturer’s directions for Lipofectamine 2000. For delivery of 1μg siRNA (n=3), 1.47μL Lipofectamine 2000 was added to RNase-free water for a total volume of 25μL in a sterile 1.5mL conical tube. In a different sterile 1.5mL conical tube, 3.76μL of 20μM siRNA stock solution was added for a total volume of 25μL of RNase-free water. The equal-volume Lipofectamine and siRNA solutions were then mixed together, allowed to complex at room temperature for 20 minutes, and then immediately used for subsequent experiments. For lower siRNA doses, Lipofectamine and siRNA volumes were adjusted accordingly. The Lipofectamine-siRNA complexes were measured for particle diameter by dynamic light scattering (DLS) on a Zetasizer Nano (Malvern, Worcestershire, UK) at an siRNA concentration of 1 μg/mL.
2.2 Fibrin Hydrogel Preparation
Fibrinogen powder from bovine plasma (Sigma-Aldrich, St. Louis, MO) was dissolved with sterile RNase-free water under aseptic conditions in a 37°C water bath to achieve a 200mg/mL fibrinogen solution. Fibrinogen solution was used to coat the bottom of glass slides (50 μL), 48-well plates (100 μL), 24-well plates (50 μL), or 6-well plates (240 μL) depending on the experiment. One part fibrinogen solution was mixed with three parts thrombin working solution, which consists of 13 parts PBS to one part 5.5mg/mL CaCl2 (Thermo Fisher Scientific, Waltham, MA) and one part 250U/mL bovine plasma thrombin (Sigma-Aldrich, St. Louis, MO). The resulting 5% (w/v) fibrin hydrogel was allowed to polymerize overnight at 37°C in a cell culture incubator (37oC, humidified atmosphere, 5% CO2).
2.3 Scanning Electron Microscopy
Scanning electron microscopy was used to assess siRNA complex adsorption and particle diameter after adsorption to fibrin hydrogels. Fibrin hydrogels were fabricated as described above in a 24-well plate. A 5mm biopsy punch was taken and placed into a 1.5mL conical tube. Sterile RNase-free water containing Lipofectamine-siRNA complexes prepared as described above were added to the top of the fibrin hydrogels and allow to adsorb for 4 hours. RNase-free water and siRNA alone (not complexed with Lipofectamine 2000) served as controls. Hydrogels were then taken through alcohol dehydration steps, critical point dried, and gold sputter coated. Adsorbed siRNA complexes were imaged with Zeiss Supra 35 VP FE-SEM (Jena, Germany) at 15,000 x magnification. Five images were taken of separate areas of the fibrin surface Lipofectamine-siRNA complexes in the image were later measured for particle diameter in Image J software.
2.4 siRNA Complex Desorption Kinetics
To determine the stability of siRNA complex adsorption to the fibrin hydrogels, we conducted a desorption (release) study under serum conditions at 37°C in a cell culture incubator. Four-hundred μL of fibrin hydrogels were fabricated as described above in a 48-well plate. Lipofectamine 2000 complexed with 0.5μg Alexa 488 siRNA in sterile RNAase-free water was allowed to adsorb to the fibrin gel surface for 4 hours. The hydrogel was then washed one time with 500 μL of PBS. The non-adsorbed material (from removed initial incubation and the wash) was collected for subsequent quantification. Two hundred μL of fresh culture media (alpha Minimal Essential Media with 10% fetal bovine serum and 1% antibiotic/antimycotic) the same as used below for cell culture was added to each well. At pre-assigned time points (1.5, 3, 6, 12, 24, 48, 72 hours), the media was removed from each well and replaced with 200uL of fresh media. All collected samples were frozen at −20°C until thawed for analysis. A final concentration of 1% TritonX-100 (Sigma Aldrich, St. Louis, MO) and 2% SDS (Sigma Aldrich, St. Louis, MO) was added to each sample in order to ensure release of the Alexa 488 siRNA from the Lipofectamine (decomplexation) to prevent possible fluorescence signal quenching. Samples were added to a 96-well black plate and fluorescence readings were made on a BioTek platereader at 485nm/525 nm (Ex/Em).
2.5 MC3T3-E1 Cell Culture
Mouse preosteoblast MC3T3-E1 Subclone 4 cells (ATCC® CRL-2593™; Manassas, VA) were cultured as recommend in complete growth media of Alpha Minimum Essential Medium (α-MEM) supplemented with 10% fetal bovine serum (FBS), and 1% antibiotic-antimycotic (A-A; Gibco, Life Technologies Grand Island, NY) at 37°C in a cell culture incubator. Cells were cultured on 150 mm cell culture plates (Thermo Fisher Scientific, Waltham, MA) until 60–70% confluent. At this point media was removed, cells were washed with 10 mL PBS, and detached with 0.25% Trypsin-EDTA (Gibco, Life Technologies Grand Island, NY) for 5 minutes in the cell culture incubator. Complete growth medium (5 mL) was added and cells were centrifuged at 1500 rpm for 5 minutes. Cells were re-suspended and plated 1:8 with complete growth media. In the following experiments, “transfection media” refers to α-MEM supplemented with 10% FBS alone without the addition of antibiotic-antimycotic.
2.6 Flow Cytometry
Flow cytometry was used to assess the uptake of siRNA complexes (or uncomplexed siRNA control) in MC3T3-E1 cells. For these studies siRNA labeled with Alexa 488 was used to allow detection by the fluorescence detectors. Fibrin hydrogels were coated on the bottom of a 6-well plate as described earlier and allowed to polymerize overnight. After one wash in PBS, Alexa 488 siRNA complexes were adsorbed to the hydrogel surface as before. No siRNA (water only) or uncomplexed siRNA (no Lipofectamine 2000) served as controls. 300uL containing 0.5ug Alexa 488 siRNA (in Lipofectamine 2000 or uncomplexed) was allowed to adsorb to the fibrin hydrogel surface for 4 hours. At this time, siRNA solutions were removed and the hydrogel surface was washed once with PBS. Immediately afterwards, 40,000 MC3T3-E1 cells were seeded onto the hydrogel surface with 300 μL of transfection media (αMEM with 10%FBS) for 4 hours. The transfection media was removed and the hydrogel surface was washed once with PBS. Then 1mL of culture media (αMEM; 10%FBS; 1%A-A) was added to each well and cells were placed into culture for 48 hours. After this 48-hour culture, media was removed and cells were washed once with PBS and detached from the hydrogel surface by two 5 minute washes of 0.25% trypsin (Gibco, Life Technologies Grand Island, NY). Wells were washed once with culture media to ensure collection of cells. Cells were collected into 15mL conical tubes and centrifuged for 5 minutes at 1500 rpm, the supernatant was removed, cells were washed in 1mL of PBS and centrifuged again, the PBS supernatant was removed, and the cell pellet was suspended in 500 μL of sterile BD FACsFlow sheath fluid (BD, Franklin Lakes, NJ). Each 500 μL cell suspension was then pipetted through a 0.4 μm cell strainer (BD, Franklin Lakes, NJ) into a polystyrene flow cytometer tube (BD, Franklin Lakes, NJ) and placed on ice. Cell-associated Alexa 488 siRNA uptake was then measured with a BD FACScan (BD, Franklin Lakes, NJ). Untreated cells (cells only control with no Alexa 488 siRNA) served as negative controls to determine acquisition parameters and establish gating regions. BD Cell Quest Pro software (BD, Franklin Lakes, NJ) was used for data acquisition and analysis.
2.7 Confocal Microscopy
Confocal microscopy was used to assess cellular uptake and distribution of siRNA in MC3T3-E1 cells. Confocal microscopy was used in order to allow determination of whether siRNA was located intracellularly or extracellularly (e.g., on the fibrin surface). A microscope slide was thinly coated with fibrin hydrogels as described above and allowed to polymerize for four hours. 0.5ug Alexa 488 siRNA was complexed with Lipofectamine 2000 as described above. Fibrin hydrogels washed with sterile RNase-free Sterile RNase-free water (no siRNA) or uncomplexed Alexa 488 siRNA (no Lipofectamine 2000) suspended in sterile RNase-free served as controls. 150 μL of solution (water, siRNA alone, or siRNA complexed with Lipofectamine) was added drop-wise to the top of the fibrin-coated coverslip and allowed to adsorb for 4 hours. After 4 hours, the coverslips were washed with 200μL sterile PBS. MC3T3 were passaged in transfection media (αMEM; 10% FBS) and 20,000 cells (in 150uL transfection media) were seeded onto each fibrin gel. Some gels were imaged without cells to visualize siRNA-Lipofectamine complexes on the surface of the gels. The cells were allowed to attach for 4 hours after which the transfection media was removed and replaced with normal culture media. After 48 hours the gels were washed with PBS and fixed in 10% neutral-buffered formalin (methanol free) for 10 minutes. Cells were counter-stained for F-actin with an Alexa 555 phalloidin dye (Invitrogen, Carlsbad, CA) and for nuclei with 300nM DAPI (Invitrogen). The slides and gels were then coverslipped with MM83 mounting media (Leica, Wetzlar, Germany) and allowed to dry overnight in the dark at 4°C. Confocal imaging (Zeiss 710 Laser Scanning Confocal System, Jena, Germany) of experimental groups was performed with a 20x objective. The Zeiss LSM Image Browser was used for image processing.
2.8 rhBMP-2 Dose Effect on MC3T3-E1 Noggin Expression
It is known that noggin expression is up-regulated in response to increased BMP-2 concentration. We assessed expression of noggin mRNA in response to increasing doses of BMP-2 by reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) in order to ensure appropriate cell response to BMP-2 (i.e., up-regulation of noggin mRNA). Fibrin hydrogels were coated on 24-well plates as previously described and allowed to polymerize overnight at 37°C followed by a RNase-free water wash. MC3T3-E1 cells passaged with transfection media were seeded at 20,000 cells/well and allowed to attach for 4 hours. The transfection media was then removed and replaced with 200uL of culture media supplemented with varying doses of rhBMP-2: 0, 0.001, 0.01, 0.1, 1, and 10 μg/mL rhBMP-2 (n=3 for each). After 24 hours the cells were harvested with 0.25% trypsin by two 5 minute incubations at 37°C and then washed with culture media. Trypsin and media washes were collected into 15mL conical tubes and centrifuged for 5 minutes at 1500 rpm. Supernatant was removed and cells were then resuspended in 500μL PBS and centrifuged for 5 minutes at 1500rpm. Supernatant was then removed and the cell pellet was frozen at −20°C until RT-qPCR was performed (see section 2.11).
2.9 Cell Activity (MTS Assay)
An MTS assay was used to determine effects of the siRNA and siRNA complexes on cell viability. These viability assays were conducted in the presence of rhBMP-2 to achieve up-regulation of noggin mRNA expression (as in Sections 2.8 and 2.10). Fibrin hydrogels were allowed to form overnight at 37°C in a 48-well plate as described above. Lipofectamine 2000 in sterile RNAase-free water was complexed with either 0.25 μg Noggin (NOG) siRNA or Non-Targeting (NT) siRNA as previously described and allowed to adsorb to the fibrin gel surface for 4 hours (n=3). Uncomplexed (0.25 μg NOG or NT siRNA) and sterile RNase-free water served as negative controls (n=3). After 4 hours, wells were washed with sterile PBS and 10,000 MC3T3-E1 were seeded onto each well with transfection media. After another 4 hours, transfection media was removed and replaced with 100μL culture media alone or containing 10 μg/mL rhBMP-2. Cells were incubated for 48 hours at which point the culture media was removed and replaced with 200μL of CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI) for 1 hour in a cell culture incubator according to the manufacturer’s directions. The reaction was stopped by lysis of cells with a 10% SDS solution after 1 hour, and 200μL of the cell lysate was transferred to a 96-well plate. Absorbance readings were then taken immediately at 490 nm and compared with a standard curve of known MC3T3-E1 cell number absorbance values. Cell numbers were normalized to MC3T3-E1 treated with no siRNA and 10μg/mL rhBMP-2 (± SEM).
2.10 siRNA Dose Effect on MC3T3 Noggin Expression
Fibrin hydrogels were coated on 24-well plates as previously described and allowed to polymerize overnight at 37°C followed by a RNase-free water wash. Lipofectamine was complexed as previously described with either 0.5 μg NT (non-target control), 0.5μg Alexa 488 (non-target control), or NOG siRNA at various doses per well: 0μg, 0.1μg, 0.25μg, 0.5μg, 1μg. Water, 2.2 μL Lipofectamine alone (vehicle-only concentration which would complex 0.5μg siRNA), or uncomplexed NOG siRNA served as controls. Each treatment (or control) was allowed to adsorb to the fibrin surface for 4 hours in RNAase-free water. After 4 hours, wells were washed with sterile PBS and 20,000 MC3T3-E1 were seeded onto each well with transfection media. After another 4 hours, transfection media was removed and replaced with 200 μL culture media alone or containing 10 μg/mL rhBMP-2. Cells were then incubated for 48 hours at which time they were harvested with 0.25% trypsin by two 5 minute incubations at 37°C and washed with culture media. Trypsin and media washes were collected into 15 mL conical tubes and centrifuged for 5 minutes at 1500 rpm. Supernatant was removed, cells were re-suspended in 500μL PBS, and centrifuged for 5 minutes at 1500 rpm. Supernatant was removed and the cell pellet was frozen at −20°C until RT-qPCR was performed.
2.11 RT-qPCR
RNA extraction was performed by using a Maxwell 16 LEV simplyRNA Tissue Kit (Promega, Madison, WI) followed by RNA quantification with a NanoDrop 2000 (Thermo Scientific, Wilmington, DE). 50 ng of sample RNA was immediately reverse transcribed into cDNA with an Omniscript RT kit according to the manufacturer’s instructions (Qiagen, Venlo, Netherlands) and frozen at -20° C until analyzed by qPCR. cDNA samples were mixed with QuantiTech SYBR Green PCR kit (Qiagen, Venlo, Netherlands) and appropriate primers (QuantiTech Primer Assay, Qiagen, Venlo, Netherlands) for mouse noggin (QT00256585), beta-Actin (QT00095242), or GAPDH (QT01658692). Quantative PCR was performed on a Bio-Rad iCycler (Bio Rad, Hercules, CA) for 40 cycles. Data were analyzed with Biogazelle QBase+ software (Zwijnaarde, Belgium). For the dose response of noggin expression to increasing rhBMP-2 dose (section 2.8) noggin expression was normalized to MC3T3-E1 cells that had not received rhBMP-2 (0 μg/mL). For experiments to determine levels of knockdown after treatment with siRNA in cells that had been exposed to 10 μg/mL of rhBMP-2, noggin expression was normalized to MC3T3-E1 cells that were exposed to 10 μg/mL of rhBMP-2 but were not treated with siRNA.
2.12 Statistical Analysis
All statistical analysis was performed by using Minitab 16 (Minitab, State College, PA). Normalized values (± SEM) were compared by using One-Way ANOVA with a Tukey’s post hoc test.
3. Results
3.1 siRNA Complex Adsorption and Size Analysis
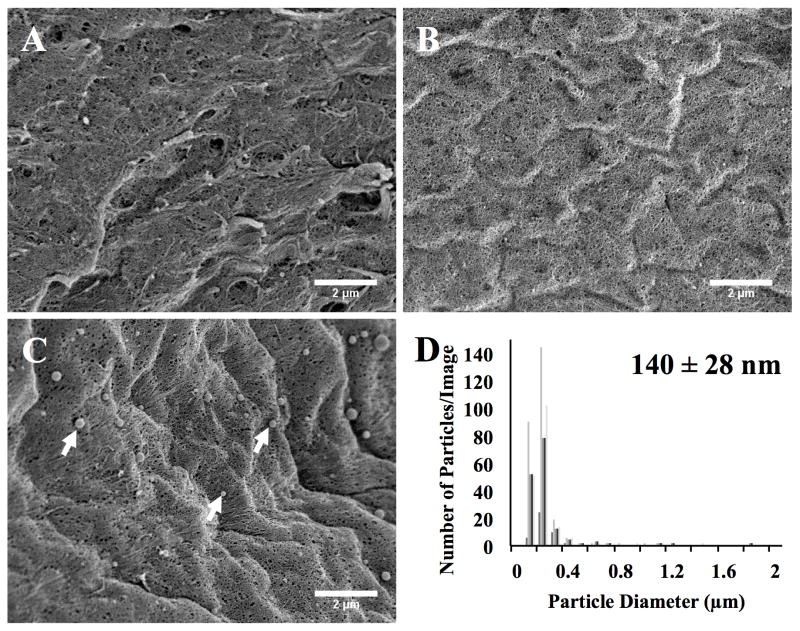
Cellular uptake of Lipofectamine complexes (and other non-viral gene delivery vehicles) is based partly on the size of the complex. To ensure that the adsorption process did not lead to aggregation of the siRNA complexes, we assessed particle diameter by DLS before adsorption and by SEM image analysis after adsorption. DLS of the Lipofectamine complexes showed particles of 128 nm ± 30 nm (mean diameter ± standard deviation). SEM images of the surface of the fibrin hydrogel (at 5000 x magnification) in the presence of RNase-free water (no siRNA; Figure 2A), uncomplexed siRNA (Figure 2B), or Lipofectamine-siRNA complexes (Figure 2C) are shown after 4 hours of adsorption. As expected, no complexes were observed when water only or when uncomplexed siRNA were used (Figure 2A and 2B). Lipofectamine-siRNA complexes were observed to be adsorbed to the fibrin surface (Figure 2C) where several of the complexes are indicated by the white arrows. The particle diameter distribution was determined by ImageJ analysis of 5 different regions of the gels, and the adsorbed complexes were found to have a diameter of 140 nm ± 28 nm (mean diameter ± STDEV) (Figure 2D). Minimal aggregation of the complexes was observed. It therefore appears that adsorption of Lipofectamine-siRNA complexes to the fibrin hydrogel did not affect the diameter size as determined by comparison to the DLS measurements of complexes in solution and the SEM measurements of the complexes after adsorption.
Figure 2.
FE-SEM images of the fibrin hydrogel surface after being treated for 4 hours with: (A) RNase-free water, (B) uncomplexed siRNA, or (C) Lipofectamine siRNA-GFP complexes (arrows). (D) ImageJ particle size analysis of adsorbed Lipofectamine siRNA-GFP complexes on the fibrin surface correlates with DLS analysis of solution suspension of Lipofectamine siRNA nanoparticles (numeric insert). ImageJ measurement of the diameter size distribution of the adsorbed siRNA complexes showed complex diameters centered near 200nm (15k x magnification; scale bar denotes 2μm).
3.2 Release Kinetics
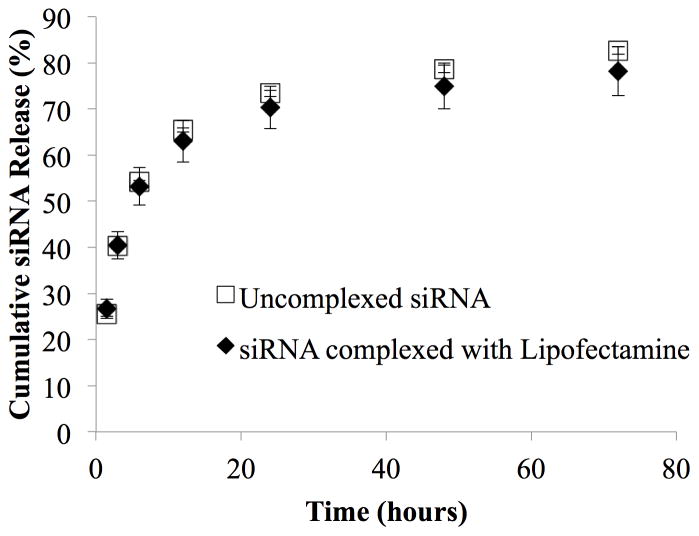
The goal of the approach described in this manuscript is to achieve delivery of siRNA to cells via surface-mediated delivery to cells in contact with the biomaterial. To assess release of the siRNA or siRNA complexes from the fibrin surface, we performed an experiment with fluorescently-labeled siRNA to measure the amount of siRNA eluting from the surface after adsorption. The amount of siRNA not eluted therefore indicates the amount remaining associated with the biomaterial surface. The rate of siRNA (alone or as a complex) release was measured over the course of 72 hours under serum conditions, which exceeds the length of time used for the cell studies reported below. After an initial wash, 58.9 ± 0.9% of free siRNA and 39.9 ± 4.0% of siRNA complexed with Lipofectamine remained adsorbed to the surface. Figure 3 shows the release profiles of siRNA that had remained adsorbed to the fibrin surface after the initial PBS wash. After three days ~ 80% of the both the free and complexed siRNA was released (where the 80% refers to the amount of release of the originally adsorbed material). Thus, approximately 20% of the initially adsorbed siRNA remained bound to the surface after 72 hours in the presence of serum.
Figure 3.
Release kinetics of uncomplexed siRNA and siRNA-Alexa 488 complexed with Lipofectamine from fibrin hydrogel surface (n=3, error bars denote ± standard deviation). Approximately 80% of the adsorbed siRNA was released over the course of 72 hours, indicating that ~ 20% remained adsorbed to the fibrin surface.
3.3 Flow Cytometry
In order to successfully knock down noggin, we reasoned that a large percentage of the cells would require uptake of the Lipofectamine-siRNA complexes. To quantify the number of cells with associated siRNA, we performed flow cytometry of cells after culture on the fibrin surfaces in the presence of fluorescently-labeled siRNA. We used cells cultured on fibrin without siRNA to gate (Figure 4A) and compared cell-association with uncomplexed siRNA (Figure 4B) and Lipofectamine-siRNA complexes (Figure 4C). The number of cells falling into the gated region was measured after 48 hours of culture with the fibrin surfaces containing siRNA and the number of cells (“events”) was determined by using the appropriate fluorescence channel for Alexa 488. Results show that after 48 hours in culture with surfaces coated with uncomplexed siRNA, only 7.18 ± 0.62% of cells had taken up the siRNA and the fluorescence intensity in these cells was low. In contrast, 98.45±0.06% of cells were positive for Alexa 488 siRNA that had been complexed with Lipofectamine. These results demonstrate the importance of complexation for uptake of the siRNA by MC3T3-E1 cells.
Figure 4.
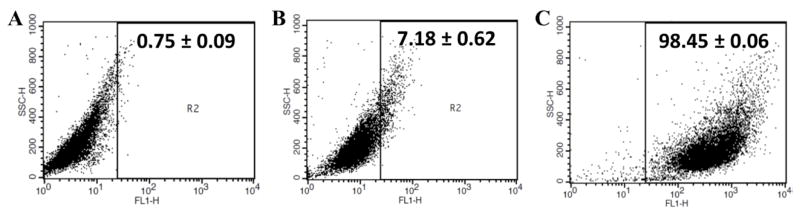
Transfection efficiency was quantified by using flow cytometry 48 hours after seeding on fibrin hydrogels. (A) Normal MC3T3-E1 were seeded on fibrin only to serve as a negative control for gating purposes. (B) Uncomplexed siRNA-Alexa 488 or (C) Lipofectamine siRNA-Alexa 488 complexes were then measured to record the percentage of cells which expressed Alexa 488 (n = 3; mean ± SEM). Numerical inserts indicate the percentage of events (cells) within the gating region that were therefore positive for siRNA-Alexa 488.
3.4 Confocal Microscopy
We used confocal microscopy to image cells seeded onto thin fibrin gels in order to allow assessment of the distribution of the siRNA with the cells that were quantified above by flow cytometry. Confocal (rather than epifluorescence) microscopy was used in order to take sections through cells so that it could be determined whether siRNA was located intracellularly or extracellularly (either remaining on the fibrin surface or at the cell membrane). Fluorescently-labeled siRNA (Alexa 488 siRNA) and MC3T3-E1 cells were used in the same manner as in the flow cytometry study. Phalloidin was used to label actin filaments to demark the cellular cytoplasm and thus intracellular siRNA. Cells were also counterstained with DAPI for nuclear staining to allow determination of whether the siRNA was cytoplasmic or nuclear. Figures 5A and 5B show MC3T3-E1 cells in the absence of siRNA or in the presence of uncomplexed siRNA after 48 hours in culture. In each case, no fluorescence signal for the siRNA is detectable, and in the case of uncomplexed siRNA this indicates a lack of detectable internalization of the siRNA. In contrast, Figures 5C-5D (siRNA complexed with Lipofectamine) shows obvious intracellular siRNA (several areas of green fluorescence indicative of Alexa 488 siRNA are shown by green arrows) after 48 hours in culture, but there is no noticeable change in cell morphology or actin filament arrangement. Qualitatively, the results are consistent with the flow cytometry data where nearly all cells had some level of siRNA uptake when delivered with Lipofectamine, and these cells clearly had greater intracellular fluorescence signal than when siRNA was uncomplexed. We note that the confocal microscopy results are not as quantitative as the flow cytometry data as the images were optimized to minimize background autofluorescence from the fibrin. Thus, it is possible that there is additional siRNA within the cells or on the cell surface that was below the detection limit for the imaging parameters used, making the more punctate areas (possibly endosomal siRNA) easier to visualize. In Figure 5C, siRNA-Lipofectamine complexes are not observed on the surface of the fibrin hydrogel because the image section was taken outside the plane of the fibrin surface. Figure 5D was taken in a region in which intracellular siRNA (green arrow) and extracellular siRNA likely bound to the fibrin surface (white arrow head) can be observed. To further verify that siRNA was intracellular, we constructed a z-stack image series based on Figure 5D. Figure 5E shows an orthogonal view constructed from the z-stack of Figure 5D and further confirms that siRNA complexes co-localized with cells (as indicated by actin staining) were intracellular. We did not observe co-localization of siRNA with the nucleus.
Figure 5.
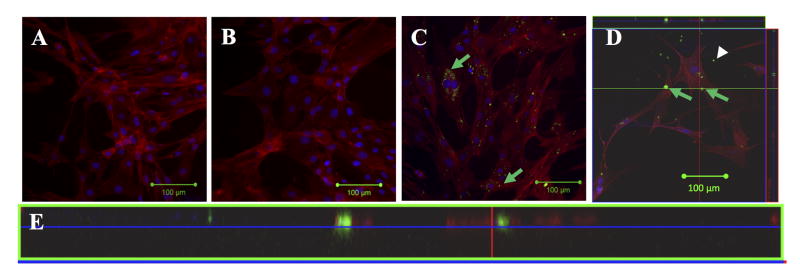
Confocal images of MC3T3-E1 cells at 48 hours after seeding on fibrin hydrogels. (A) Cells alone; (B) Uncomplexed Alexa 488 siRNA; (C) Intracellular (green arrows) siRNA complexed with Lipofectamine and delivered from fibrin surface; (D) Field of view in which both intracellular (green arrows) and extracellular (fibrin-adsorbed; white arrowhead) siRNA complexed with Lipofectamine can be observed; (E) Z-stack orthogonal view through plane indicated by green line in (D) to show that the siRNA is localized between actin filaments, indicating that siRNA is intracellular and not below the cell surface on the fibrin hydrogel. Red indicates phalloidin, blue in DAPI, and green (only visible in C-E) is Alexa 488 siRNA. Green arrows indicate intracellular siRNA and white arrowhead indicates extracellular siRNA in (C-D). Images were taken with a 20X objective and scale bars indicate 100 μm.
3.5 rhBMP-2 Dose Effect on MC3T3-E1 Noggin Expression
Noggin expression is up-regulated in response to increasing amounts of BMP-2. We performed RT-qPCR on noggin expression in response to increasing BMP-2 doses to confirm this up-regulation in our hands and also to determine the concentration of rhBMP-2 to use for the knockdown experiments (section 3.7). For this experiment, increasing concentrations of rhBMP-2 given in solution were used. RT-qPCR values were normalized to MC3T3-E1 that were not treated with rhBMP-2 (0 μg/mL). After 24 hours, concentrations above 0.01μg/mL rhBMP-2 resulted in significantly increased levels of noggin mRNA expression in MC3T3-E1 cells (Figure 6, *). In addition, each 10-fold increase above 0.001μg/mL rhBMP-2 resulted in a statistically significant difference in noggin mRNA compared to the next lower dose level (**). The greatest noggin mRNA expression was 20.82 ± 1.47 fold, which resulted from the highest rhBMP-2 concentration (10μg/mL). Therefore, we chose to use 10μg/mL rhBMP-2 for subsequent experiments on noggin knockdown with surface-mediated delivery of siRNA.
Figure 6.
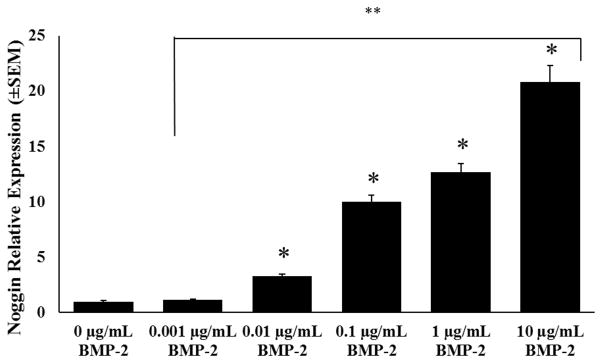
Noggin mRNA expression levels with increasing rhBMP-2 dose 24 hours after MC3T3-E1 were seeded on fibrin hydrogels. Values were normalized to MC3T3-E1 cells not treated with rhBMP-2 (0μg/mL rhBMP-2) noggin mRNA expression levels. Error bars denote standard error of the mean; *p < 0.05 vs. 0μg/mL BMP-2, **p < 0.05 between treatment group at next lower concentration.
3.6 Cell Viability in Response to Surface-Mediated siRNA Delivery
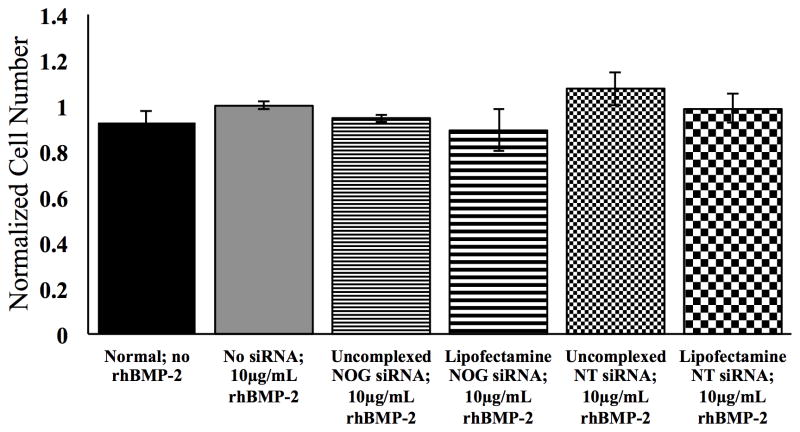
An MTS assay was performed 48 hours after MC3T3-E1 cells were seeded on fibrin hydrogels in order to determine effects on cell viability (Figure 7). These experiments were conducted after delivery of rhBMP-2 at 10 μg/mL. Cell viability values were normalized to MC3T3-E1 cells treated with 10μg/mL rhBMP-2 only (no siRNA). MC3T3-E1 cells without treatment (no siRNA and no rhBMP-2) did not show a difference in metabolic activity compared to the MC3T3 cells treated with 10μg/mL rhBMP-2. Uncomplexed NOG siRNA and NT siRNA as well as NOG siRNA and NT siRNA complexed with Lipofectamine coated onto the fibrin surfaces did not have a significant effect on the viability of MC3T3-E1 cells.
Figure 7.
Cell viability as determined by MTS assay preformed 48 hours after MC3T3-E1 cells were seeded on fibrin hydrogels with or without adsorbed 0.25μg siRNA alone or complexed with Lipofectamine 2000. Absorbance readings were correlated to average cell number and then normalized to MC3T3-E1 cells treated with no siRNA and 10μg/mL rhBMP-2. Error bars denote standard error the mean. No statistical differences were found between any of the groups, indicating minimal toxicity of the various siRNA treatments.
3.7 siRNA Dose Effect on MC3T3-E1 Noggin Expression
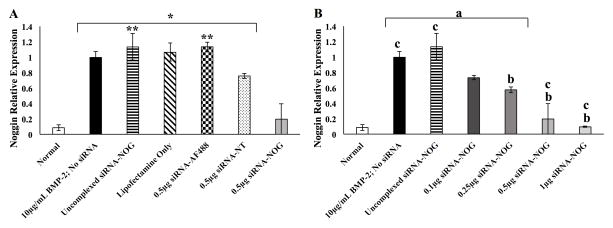
To determine the ability to achieve knockdown of noggin expression up-regulated by the presence of 10 μg/mL of rhBMP-2 (section 3.5), noggin expression in MC3T3-E1 cells was assessed by RT-qPCR 48 hours after delivery of 10 μg/mL of BMP-2 when the cells were cultured in the presence of adsorbed siRNA. siRNA for NOG was either uncomplexed or complexed with Lipofectamine. Several control siRNA groups were used. A non-target (NT) sequence and an Alexa 488 siRNA sequence were used in uncomplexed form or complexed with Lipofectamine to assess off-target effects of siRNA. Lipofectamine only (no siRNA; vehicle control) was also used as a negative control for siRNA knockdown of noggin. Basal noggin mRNA expression of MC3T3-E1 not supplemented with rhBMP-2 (0 μg/mL rhBMP-2) showed 91.5 ± 3.81% lower noggin mRNA expression than MC3T3-E1 cells treated with 10μg/mL rhBMP-2, consistent with the results shown in Figure 6 but in this case at a 48 hour (instead of 24 hour) time point. As shown in Figure 8A (where all siRNA doses were the same), 0.5μg of uncomplexed siRNA-NOG, Lipofectamine vehicle only (no siRNA), and 0.5 μg siRNA-Alexa 488 (siRNA-AF488) complexed with Lipofectamine were not significantly different in their noggin mRNA levels compared to cells treated with 10 μg/mL rhBMP-2 only, indicating virtually no knockdown of noggin. The NT siRNA complexed with Lipofectamine led to a 20% decrease in noggin expression, which we interpret to be off-target effects, though this was not statistically different than the MC3T3-E1 cells receiving 10 μg/mL rhBMP-2 with no siRNA treatment. In contrast the MC3T3-E1 cells culture in the presence of 0.5 μg NOG siRNA complexed with Lipofectamine led to a significant decrease in noggin expression compared to cells treated with only 10 μg/mL rhBMP-2. Thus, noggin knockdown was approximately 80.4% when MC3T3-E1 cells were cultured on fibrin surfaces with Lipofectamine-complexed siRNA against noggin.
Figure 8.
MC3T3-E1 cell noggin mRNA expression levels with indicated treatment by surface-mediated transfection of siRNA as quantified by qRT-PCR at 48 hours (n=3; mean relative quantity ± standard error of the mean). All groups received 10μg/mL rhBMP-2 except “Normal” cells that were not treated with rhBMP-2. Noggin mRNA levels were normalized to 10μg/mL BMP-2; no siRNA treatment. (A) siRNA groups treated at 0.5 μg per well including non-target (NT) control with Lipofectamine and siRNA-Alexa (siRNA-AF488) control with Lipofectamine as well as siRNA-NOG not complexed with Lipofectamine.*p<0.05 versus Normal (MC3T3-E1 cells not treated with rhBMP-2), **p<versus 0.5μg siRNA-NT (B) Lipofectamine siRNA-NOG complex dose response. ap<versus Normal, bp<versus 10μg/mL BMP-2; no siRNA, cp<0.05 versus 0.25μg siRNA-NOG.
We also conducted a dose-response experiment for a range of NOG siRNA concentrations (Figure 8B). There is a clear trend that with increasing amounts of surface-adsorbed Lipofectamine-siRNA-NOG complexes there is an increase in knockdown of noggin mRNA after 10 μg/mL rhBMP-2 treatment in MC3T3-E1 cells. Surface-mediated delivery of 0.1μg, 0.25μg, 0.5μg, and 1μg siRNA-NOG respectively resulted in 27.0 ± 2.7%, 42.6 ± 3.7%, 80.4 ± 19.6%, and 90.6 ± 0.6% knockdown of noggin mRNA when treated with 10μg/mL rhBMP-2 for 48 hours. MC3T3-E1 cells treated with 0.5μg or 1μg of siRNA-NOG and treated with 10 μg/mL rhBMP-2 had mRNA levels of noggin reduced to levels that were not significantly different (statistically) than noggin mRNA expression in cells not treated with rhBMP-2 (0μg/mL rhBMP-2). This demonstrates that, at the mRNA level, noggin expression can be reduced to near basal levels (i.e., levels of noggin in cells naïve to BMP-2 exposure) via siRNA delivery from the surface of fibrin hydrogels.
4. Discussion
The use of FDA-approved rhBMP-2 devices to enhance bone regeneration has increased over the past decade, bringing to light a number of drawbacks. First, given that there are roughly microgram quantities of BMP-2 that can be extracted from kilogram quantities of bone, the amount of rhBMP-2 delivered in a single treatment is several orders of magnitude greater than the amount of BMP-2 endogenously present in bone [49]. This supraphysiological rhBMP-2 dose directly leads to the larger secondary problems of adverse side effects such as edema and ectopic bone growth [50]. The regenerative potential of bone at a site of injury is often diminished by locally expressed inhibitory molecules such as the BMP-2 antagonist noggin. The current clinical strategy that uses supraphysiological doses of rhBMP-2 in the device helps to overcome the therapeutic threshold set by BMP-2 antagonists and inhibitors. Previous studies have focused on lowering loading concentrations by using alternative biomaterials to control the rate BMP-2 release from the carrier system [12, 18, 19, 51].
Indeed, this approach to deliver growth-promoting molecules such as BMP-2 is common in tissue engineering/regenerative medicine whereby these molecules are “pro-regeneration” by signaling cell growth, proliferation, and/or migration. The content of this manuscript reports the complimentary approach of reducing inhibitory molecules, which may ultimately allow efficacy or pro-regenerative molecules at lower doses. That is, this approach seeks to promote tissue regeneration by delivery of molecules such as siRNA that can knock down molecules typically inhibitory to regeneration. In this case, we investigated the ability to knock down the BMP-2 antagonist noggin through the use of siRNA. Because molecules that promote (bone) tissue formation such as rhBMP-2 can be adsorbed to biomaterials for delivery to cells, we were interested in investigating this approach for the delivery of siRNA.
We elected to use fibrin as the material “carrier” for the siRNA complexes in this study for several reasons. Fibrin is a native biopolymer in wound healing (including bone regeneration) and functions to provide a provincial matrix for cell infiltration prior to production of type 1 collagen, the main extracellular matrix component of bone. The capacity of fibrin to adsorb 6.7 times more serum proteins (including fibronectin and heparin) than collagen aids in fibrin’s role in adhesion, proliferation, differentiation, and maturation of osteoblasts [52, 53]. We have had previous success with fibrin for the delivery of plasmid DNA complexed with PEI with an overall positive zeta potential [47], which we attributed in part to the negative charge of fibrin at a neutral pH [54].
In order to achieve delivery of the siRNA complexes from the fibrin surface to cells, it is necessary that the siRNA complexes remain of small enough size to allow cellular internalization either directly from the fibrin surface or following desorption of the complexes from the surface and subsequent uptake from the surrounding media (in the case of in vitro experiments). Adsorption of the complexes to fibrin did not greatly alter their diameters compared to their solution-phase diameter (determined by DLS), remaining at ~100–200nm in diameter. While DLS accounts for hydrodynamic radius while SEM was performed on dry samples, it seems clear that the siRNA complexes remained of a sufficiently small diameter to allow for uptake by cells, which we explored in further detail (see below). Investigation into siRNA complex desorption from the fibrin hydrogel surface in the presence of serum (10% fetal bovine serum) showed that approximately 80% of uncomplexed siRNA an siRNA complexed with Lipofectamine (where siRNA was fluorescently labeled with Alexa 488) were released from the fibrin surface, indicating that approximately 20% of the complexes remained on the hydrogel surface after 3 days. Unlike a previous study that investigated plasmid DNA complexed with polyethylenimine (PEI) [47], the negative charge of fibrin does not appear to have as great an effect on surface retention of the Lipofectamine-siRNA complexes. Because some complexes are released from the surface while others remain associated with the fibrin surfaces, it is possible that uptake occurs through one of two mechanisms. Desorbed complexes may be subsequently taken up by the cells from the solution phase, or cells may take up the siRNA complexes directly from the surface upon contact [55, 56]. While we are unable to specify the specific mechanism of uptake, surface-based delivery of nucleic acids has shown the benefits of reduced mass transport and minimal transfection agent aggregation, leading to greater transfection efficacy [57] while using less material. In addition, this approach is akin to growth factor delivery from material surfaces and therefore of potential use in tissue engineering/regenerative medicine applications for local implantation of biomaterial-based constructs.
A number of strategies have been developed for surface-mediated delivery of plasmid DNA (pDNA) delivery [56, 58], but nuclear uptake of pDNA and subsequent transcription is only required for a relatively small population of cells to achieve transgene expression. siRNA does not require nuclear uptake since it acts in the cytoplasm on mRNA, but to achieve sufficient levels of knockdown of the target it would be necessary for nearly all cells to take up the siRNA complexes in order to achieve biologically-relevant knockdown. In other words, if a small fraction of the cells were to take up the complexes, the overall effect might be great on those cells, but minimal for the overall local cell population. Results of the flow cytometry study show that virtually all cells have siRNA associated with them (greater than 98%) when the siRNA was complexed with Lipofectamine. This is in contrast to uncomplexed siRNA, which showed only 7% of cells in the gated region with uptake and all at a relatively low signal for Alexa 488. This suggests that the coating of the surface with the complexes was effective for mediating delivery to cells, although the mechanism by which cells take up the complexed siRNA is unclear. While flow cytometry indicated a large fraction of cells with associated siRNA, we used confocal microscopy to determine if the siRNA was internalized by the cells and where it was distributed. Confocal images clearly demonstrated intracellular siRNA when delivered via Lipofectamine complexes as evidenced by their presence within the actin-stained region. The siRNA was not located in the nucleus, but we cannot rule out the possibility that they were located in the endosomes given the punctate staining patterns observed. If siRNA was located in the endosomes, it seems that sufficient levels of endosomal escape occurred to achieve the high levels of noggin knockdown observed. We again note that the imaging parameters (necessary to minimize autofluorescence from the fibrin) may lead to the inability to observe non-punctate siRNA within the cells. Thus, additional, non-endosomal siRNA is likely present in the cells at levels below the limits detectable by our imaging parameters, which leads to the high levels of noggin mRNA knockdown observed.
Effective nucleic acid delivery through transfection reagents is often sacrificed in order to minimize cellular toxicity [59], and our studies showed that cells had considerable uptake of siRNA and thus the Lipofectamine complexing agent. We therefore investigated whether the cellular uptake of the siRNA-complexes led to observable toxicity on cells. Even though 98.5 ± 0.1% of MC3T3-E1 were positive for siRNA uptake when complexed with Lipofectamine (flow cytometry results), this did not lead to observable or statistically significant reductions in cell viability as determined by the MTS assay. This may be because the amount of siRNA delivered to the cells from the fibrin surface, while efficient, may be lower than observed for solution-phase non-viral transfection. The time frame of uptake by delivery from the fibrin surface would also differ from solution-phase transfer. Internalization of siRNA would not only occur at the time of initial cell attachment to the hydrogel surface, but would essentially be “re-transfected” as the cells divide and/or migrate on the fibrin surface to areas previously unoccupied by cells but still containing siRNA complexes or as siRNA desorbs from the fibrin surface to achieve a more sustained transfection. Most commercially available transfection vehicles based on traditional solution-phase transfection are highly dependent on cell type and, on average, achieve ~70% transfection. In difficult to transfect cell lines and primary cells the transfection efficacy decreases to ~30%. Delivery from material surfaces has been recognized for its benefits in cell microarray technology [60] but has seen limited success being translated to tissue engineering/regenerative medicine applications. Delivery from the hydrogel surface and the related toxicity results indicate that this method can be easily applied to a number of tissue engineering applications beyond the noggin/bone regeneration focus of these experiments.
In our present study, the MC3T3-E1 pre-osteoblasts attached to a fibrin hydrogel showed up-regulation of noggin mRNA expression levels in response to increasing amounts of BMP-2 (Figure 6), as expected [17]. This may be akin to the mechanism by which the body responds to supraphysiological doses of rhBMP-2 in current technology in which the high BMP-2 dose ultimately leads to ectopic bone formation. After 48 hours, siRNA targeting noggin led to a dose-dependent reduction in noggin mRNA levels with the highest surface concentration of noggin siRNA causing the greatest amount of noggin mRNA knockdown (Figure 8B). Interestingly, the two highest noggin siRNA surface concentrations (0.5μg and 1μg/well) reached statistically equivalent noggin mRNA levels to MC3T3-E1 cells never exposed to BMP-2. Uncomplexed siRNA-NOG, Lipofectamine transfection reagent alone, and 0.5μg of siRNA-AF488 did not have an effect on noggin mRNA expression. Even though off-target effects of siRNA-NT were observed, the 0.5μg siRNA-NT dose did not achieve knockdown levels nearly as great as the equivalent siRNA-NOG dose (Figure 8A). Rather, the siRNA-NT was statistically similar to the 0.1μg siRNA-NOG concentration. This indicates that non-negligible levels of off-target knockdown are possible as indicated in this study with the knockdown from the NT siRNA. We note that no off-target effects were observed with the Alexa 488 siRNA control, so the off-target effects of the NT siRNA could be specific to that siRNA sequence.
One potential concern related to this study is the use of Lipofectamine, which is not generally recognized as suitable for systemic delivery of nucleic acids such as siRNA [61]. This is likely due to lack of salt stability and opsonization leading to reticulo-endothelial system uptake and hepatic clearance when delivered systemically [62]. In addition, there are concerns related to immunological response to cationic lipids such as Lipofectamine. While there are some reports of in vivo toxicity associated with cationic liposomes such as Lipofectamine, these generally do not exceed in vitro toxicity levels, suggesting that the low in vitro toxicity shown in our study may make this system suitable for in vivo use in terms of toxicity profiles. Toxicity, immune response, and delivery of siRNA are key challenges associated with its implementation and there are numerous other complexation strategies that may prove superior to the Lipofectamine used in this study. Examples include glycopolymers [63], dendrimers [64], chitosan [65], cell-penetrating peptides [66], and cationic cyclodextrins [67]. We do note that two other commercially available cationic materials (Dharmafect and 25 kDa linear polyethylenimine) were investigated but did not achieve the levels of knockdown of noggin mRNA expression observed with Lipofectamine as the complexing agent.
Noggin regulation through siRNA delivery has been successful previously [40–43], but to our knowledge our work is the first to show a controlled dose-dependent regulation of noggin mRNA, which we attribute to the high transfection efficiency associated with delivery from the fibrin surface. This dose-dependent behavior is of particular interest due to the dualistic effects of both noggin and BMP-2 on osteogenic behavior of a number of cell types. Even though BMP-2 induces differentiation in MSCs, pre-osteoblasts, and immature osteoblasts [68] there are recent findings that show BMP-2 induces apoptosis in mature osteoblasts (but not MSCs) in a dose-dependent manner [69]. Along these lines, up-regulation of noggin inhibits apoptosis caused by BMP-2 [70]. On the other hand, complete knockdown of noggin decreases BMP-2 induced osteogenic differentiation of human MSCs [71]. Therefore, it is suggested that some noggin expression is necessary for suitable and controlled bone regeneration. Taken together, there must be a balance struck between noggin and BMP-2 regulation. This siRNA-based approach offers that possibility given that the siRNA knockdown is temporary. siRNA provides a short-term silencing of target genes for about one week, which correlates to the time when a supraphysiological burst release of rhBMP-2 is observed with current FDA-approved collagen devices. Surface-mediated delivery of siRNA would provide a finite duration of noggin silencing which would taper-off with time, thus allowing the native noggin-BMP-2 control mechanism to be slowly reset to normal physiological function. Once the siRNA-NOG is depleted, noggin expression would return to normal levels and thus control BMP-2 levels to prevent adverse effects like heterotopic ossification.
Clearly, inhibitory or antagonist molecules are not unique to bone regeneration. Chondroitin sulfate proteoglycans in nerve [72], inflammatory cytokines in skin [73], and molecules that inhibit endothelialization in the cardiovascular system [74] are potential targets of siRNA delivery from biomaterials used to promote healing/regeneration. Given use of fibrin as a coating [75, 76], this approach would also be compatible with recent efforts to modulate the response of macrophages and other inflammatory cells towards implanted materials [77, 78]. Given the low toxicities observed, it is also possible that this approach could be used to direct in vitro cultures of stem cells [79], though effects of the siRNA complexes would need to be examined for different cell types. Although we have not investigated the effects of mRNA knockdown on protein expression, this study demonstrates that efficient mRNA knockdown can be achieved in a non-toxic fashion by surface-mediated delivery of the siRNA.
Conclusion
The surface-mediated transfection method described here provides a system to locally deliver siRNA complexes adsorbed to a fibrin hydrogel surface. The system offers a simple, non-toxic method of surface-mediated delivery resulting in nearly 100% cellular uptake in MC3T3-E1 cells. Due to this high level of cellular uptake we were able to achieve controlled, dose-dependent noggin mRNA knockdown with increasing concentrations of siRNA-NOG. The levels of noggin mRNA in rhBMP-2 treated MC3T3-E1 cells with the two highest surface concentrations were reduced to levels similar to cells naïve to BMP-2 exposure. Ultimately, this approach may offer the potential to use reduced levels of rhBMP-2 for bone regeneration via knockdown of BMP-2 antagonists.
Acknowledgments
The authors acknowledge assistance from the Center for Nanotechnology and Molecular Materials at Wake Forest University (SEM imaging), Dr. Richard Edelmann and Matthew Duley at the Miami University Center for Advanced Microscopy and Imaging (confocal microscopy), and Dr. Andor Kiss at the Miami University Center for Bioinformatics and Functional Genomics (RT-qPCR). This work was partially supported by the National Institutes of Health (JMS; R01AR061391) and the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also partially supported by The Wake Forest University Health Sciences Translational Sciences Institute Pilot Grant and The Wake Forest Graduate School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36 (Suppl 3):S20–7. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Janicki P, Schmidmaier G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury. 2011;42 (Suppl 2):S77–81. doi: 10.1016/j.injury.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nature genetics. 2006;38:1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 5.De Marco AC, Jardini MA, Modolo F, Nunes FD, de Lima LA. Immunolocalization of bone morphogenetic protein 2 during the early healing events after guided bone regeneration. Oral surgery, oral medicine, oral pathology and oral radiology. 2012;113:533–41. doi: 10.1016/j.oooo.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Puleo DA. Dependence of mesenchymal cell responses on duration of exposure to bone morphogenetic protein-2 in vitro. J Cell Physiol. 1997;173:93–101. doi: 10.1002/(SICI)1097-4652(199710)173:1<93::AID-JCP11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Huang C, Xue M, Zhang X. Expression of endogenous BMP-2 in periosteal progenitor cells is essential for bone healing. Bone. 2011;48:524–32. doi: 10.1016/j.bone.2010.10.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto M, Takahashi Y, Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24:4375–83. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 9.Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Reviews in endocrine & metabolic disorders. 2006;7:51–65. doi: 10.1007/s11154-006-9000-6. [DOI] [PubMed] [Google Scholar]
- 10.Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnology letters. 2009;31:1817–24. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- 11.Vaibhav B, Nilesh P, Vikram S, Anshul C. Bone morphogenic protein and its application in trauma cases: a current concept update. Injury. 2007;38:1227–35. doi: 10.1016/j.injury.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Brown KV, Li B, Guda T, Perrien DS, Guelcher SA, Wenke JC. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release. Tissue engineering Part A. 2011;17:1735–46. doi: 10.1089/ten.TEA.2010.0446. [DOI] [PubMed] [Google Scholar]
- 13.Kugimiya F, Kawaguchi H, Kamekura S, Chikuda H, Ohba S, Yano F, et al. Involvement of endogenous bone morphogenetic protein (BMP) 2 and BMP6 in bone formation. J Biol Chem. 2005;280:35704–12. doi: 10.1074/jbc.M505166200. [DOI] [PubMed] [Google Scholar]
- 14.Garrett MP, Kakarla UK, Porter RW, Sonntag VK. Formation of painful seroma and edema after the use of recombinant human bone morphogenetic protein-2 in posterolateral lumbar spine fusions. Neurosurgery. 2010;66:1044–9. doi: 10.1227/01.NEU.0000369517.21018.F2. discussion 9. [DOI] [PubMed] [Google Scholar]
- 15.Ebara S, Nakayama K. Mechanism for the action of bone morphogenetic proteins and regulation of their activity. Spine (Phila Pa 1976) 2002;27:S10–5. doi: 10.1097/00007632-200208151-00004. [DOI] [PubMed] [Google Scholar]
- 16.Zhu W, Kim J, Cheng C, Rawlins BA, Boachie-Adjei O, Crystal RG, et al. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone. 2006;39:61–71. doi: 10.1016/j.bone.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura Y, Wakitani S, Nakayama J, Wakabayashi S, Horiuchi H, Takaoka K. Temporal and spatial expression profiles of BMP receptors and noggin during BMP-2-induced ectopic bone formation. J Bone Miner Res. 2003;18:1854–62. doi: 10.1359/jbmr.2003.18.10.1854. [DOI] [PubMed] [Google Scholar]
- 18.Kowalczewski CJ, Tombyln S, Wasnick DC, Hughes MR, Ellenburg MD, Callahan MF, et al. Reduction of ectopic bone growth in critically-sized rat mandible defects by delivery of rhBMP-2 from kerateine biomaterials. Biomaterials. 2014;35:3220–8. doi: 10.1016/j.biomaterials.2013.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisiel M, Klar AS, Ventura M, Buijs J, Mafina MK, Cool SM, et al. Complexation and sequestration of BMP-2 from an ECM mimetic hyaluronan gel for improved bone formation. PLoS One. 2013;8:e78551. doi: 10.1371/journal.pone.0078551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempen DH, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245–52. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413–8. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Migneco F, Lin CY, Hollister SJ. Chemically-conjugated bone morphogenetic protein-2 on three-dimensional polycaprolactone scaffolds stimulates osteogenic activity in bone marrow stromal cells. Tissue Eng Part A. 2010;16:3441–8. doi: 10.1089/ten.tea.2010.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wegman F, Geuze RE, van der Helm YJ, Cumhur Oner F, Dhert WJ, Alblas J. Gene delivery of bone morphogenetic protein-2 plasmid DNA promotes bone formation in a large animal model. J Tissue Eng Regen Med. 2014;8:763–70. doi: 10.1002/term.1571. [DOI] [PubMed] [Google Scholar]
- 24.Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release. 2014;194:238–56. doi: 10.1016/j.jconrel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Alegre P, Paulson HL. Technology insight: therapeutic RNA interference--how far from the neurology clinic? Nature clinical practice Neurology. 2007;3:394–404. doi: 10.1038/ncpneuro0551. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–2. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 27.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–38. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–77. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 30.Kim YM, Park MR, Song SC. Injectable polyplex hydrogel for localized and long-term delivery of siRNA. ACS Nano. 2012;6:5757–66. doi: 10.1021/nn300842a. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen K, Dang PN, Alsberg E. Functionalized, biodegradable hydrogels for control over sustained and localized siRNA delivery to incorporated and surrounding cells. Acta Biomater. 2013;9:4487–95. doi: 10.1016/j.actbio.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickerson EB, Blackburn WH, Smith MH, Kapa LB, Lyon LA, McDonald JF. Chemosensitization of cancer cells by siRNA using targeted nanogel delivery. BMC cancer. 2010;10:10. doi: 10.1186/1471-2407-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flessner RM, Jewell CM, Anderson DG, Lynn DM. Degradable polyelectrolyte multilayers that promote the release of siRNA. Langmuir. 2011;27:7868–76. doi: 10.1021/la200815t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khormaee S, Ali OA, Chodosh J, Mooney DJ. Optimizing siRNA efficacy through alteration in the target cell-adhesion substrate interaction. J Biomed Mater Res A. 2012;100:2637–43. doi: 10.1002/jbm.a.34202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartmann H, Hossfeld S, Schlosshauer B, Mittnacht U, Pego AP, Dauner M, et al. Hyaluronic acid/chitosan multilayer coatings on neuronal implants for localized delivery of siRNA nanoplexes. J Control Release. 2013;168:289–97. doi: 10.1016/j.jconrel.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Ma L, Liang J, Zhang B, Teng J, Gao C. RNAi functionalized collagen-chitosan/silicone membrane bilayer dermal equivalent for full-thickness skin regeneration with inhibited scarring. Biomaterials. 2013;34:2038–48. doi: 10.1016/j.biomaterials.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 37.Castleberry S, Wang M, Hammond PT. Nanolayered siRNA dressing for sustained localized knockdown. ACS Nano. 2013;7:5251–61. doi: 10.1021/nn401011n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rios CN, Skoracki RJ, Mathur AB. GNAS1 and PHD2 short-interfering RNA support bone regeneration in vitro and in an in vivo sheep model. Clin Orthop Relat Res. 2012;470:2541–53. doi: 10.1007/s11999-012-2475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tajima K, Takaishi H, Takito J, Tohmonda T, Yoda M, Ota N, et al. Inhibition of STAT1 accelerates bone fracture healing. J Orthop Res. 2010;28:937–41. doi: 10.1002/jor.21086. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen MK, Jeon O, Krebs MD, Schapira D, Alsberg E. Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation. Biomaterials. 2014;35:6278–86. doi: 10.1016/j.biomaterials.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayama K, Suzuki A, Manaka T, Taguchi S, Hashimoto Y, Imai Y, et al. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. Journal of bone and mineral metabolism. 2009;27:402–11. doi: 10.1007/s00774-009-0054-x. [DOI] [PubMed] [Google Scholar]
- 42.Wan DC, Pomerantz JH, Brunet LJ, Kim JB, Chou YF, Wu BM, et al. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem. 2007;282:26450–9. doi: 10.1074/jbc.M703282200. [DOI] [PubMed] [Google Scholar]
- 43.Manaka T, Suzuki A, Takayama K, Imai Y, Nakamura H, Takaoka K. Local delivery of siRNA using a biodegradable polymer application to enhance BMP-induced bone formation. Biomaterials. 2011;32:9642–8. doi: 10.1016/j.biomaterials.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Harwood PJ, Newman JB, Michael ALR. (ii) An update on fracture healing and non–union. Orthopaedics and Trauma. 2010;24:9–23. [Google Scholar]
- 45.Yang HS, La WG, Cho YM, Shin W, Yeo GD, Kim BS. Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Experimental & molecular medicine. 2012;44:350–5. doi: 10.3858/emm.2012.44.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osathanon T, Linnes ML, Rajachar RM, Ratner BD, Somerman MJ, Giachelli CM. Microporous nanofibrous fibrin-based scaffolds for bone tissue engineering. Biomaterials. 2008;29:4091–9. doi: 10.1016/j.biomaterials.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saul JM, Linnes MP, Ratner BD, Giachelli CM, Pun SH. Delivery of non-viral gene carriers from sphere-templated fibrin scaffolds for sustained transgene expression. Biomaterials. 2007;28:4705–16. doi: 10.1016/j.biomaterials.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 48.Kidd ME, Shin S, Shea LD. Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo. Journal of controlled release : official journal of the Controlled Release Society. 2012;157:80–5. doi: 10.1016/j.jconrel.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wozney JM. Overview of bone morphogenetic proteins. Spine (Phila Pa 1976) 2002;27:S2–8. doi: 10.1097/00007632-200208151-00002. [DOI] [PubMed] [Google Scholar]
- 50.Woo EJ. Adverse events reported after the use of recombinant human bone morphogenetic protein 2. J Oral Maxillofac Surg. 2012;70:765–7. doi: 10.1016/j.joms.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 51.de Guzman RC, Saul JM, Ellenburg MD, Merrill MR, Coan HB, Smith TL, et al. Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials. 2013;34:1644–56. doi: 10.1016/j.biomaterials.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Oh JH, Kim HJ, Kim TI, Woo KM. Comparative evaluation of the biological properties of fibrin for bone regeneration. BMB reports. 2014;47:110–4. doi: 10.5483/BMBRep.2014.47.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 54.Okay O. Hydrogel Sensors and Actuators. Springer; 2010. General properties of hydrogels; pp. 1–14. [Google Scholar]
- 55.Segura T, Shea LD. Surface-tethered DNA complexes for enhanced gene delivery. Bioconjug Chem. 2002;13:621–9. doi: 10.1021/bc015575f. [DOI] [PubMed] [Google Scholar]
- 56.Tokatlian T, Cam C, Segura T. Non-viral DNA delivery from porous hyaluronic acid hydrogels in mice. Biomaterials. 2014;35:825–35. doi: 10.1016/j.biomaterials.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jang JH, Bengali Z, Houchin TL, Shea LD. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. J Biomed Mater Res A. 2006;77:50–8. doi: 10.1002/jbm.a.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blocker KM, Kiick KL, Sullivan MO. Surface immobilization of plasmid DNA with a cell-responsive tether for substrate-mediated gene delivery. Langmuir. 2011;27:2739–46. doi: 10.1021/la104313z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breunig M, Lungwitz U, Liebl R, Goepferich A. Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc Natl Acad Sci U S A. 2007;104:14454–9. doi: 10.1073/pnas.0703882104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva JM, Mizuno H, Brady A, Lucito R, Hannon GJ. RNA interference microarrays: high-throughput loss-of-function genetics in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:6548–52. doi: 10.1073/pnas.0400165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R, et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release. 2010;142:245–50. doi: 10.1016/j.jconrel.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 62.Tseng YC, Mozumdar S, Huang L. Lipid-based systemic delivery of siRNA. Adv Drug Deliv Rev. 2009;61:721–31. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith AE, Sizovs A, Grandinetti G, Xue L, Reineke TM. Diblock glycopolymers promote colloidal stability of polyplexes and effective pDNA and siRNA delivery under physiological salt and serum conditions. Biomacromolecules. 2011;12:3015–22. doi: 10.1021/bm200643c. [DOI] [PubMed] [Google Scholar]
- 64.Nam JP, Nam K, Jung S, Nah JW, Kim SW. Evaluation of dendrimer type bio-reducible polymer as a siRNA delivery carrier for cancer therapy. J Control Release. 2015;209:179–85. doi: 10.1016/j.jconrel.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He C, Yin L, Song Y, Tang C, Yin C. Optimization of multifunctional chitosan-siRNA nanoparticles for oral delivery applications, targeting TNF-alpha silencing in rats. Acta Biomater. 2015;17:98–106. doi: 10.1016/j.actbio.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 66.Suhorutsenko J, Oskolkov N, Arukuusk P, Kurrikoff K, Eriste E, Copolovici DM, et al. Cell-penetrating peptides, PepFects, show no evidence of toxicity and immunogenicity in vitro and in vivo. Bioconjug Chem. 2011;22:2255–62. doi: 10.1021/bc200293d. [DOI] [PubMed] [Google Scholar]
- 67.O'Mahony AM, Ogier J, Darcy R, Cryan JF, O'Driscoll CM. Cationic and PEGylated Amphiphilic Cyclodextrins: Co-Formulation Opportunities for Neuronal Sirna Delivery. PLoS One. 2013;8:e66413. doi: 10.1371/journal.pone.0066413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366:51–7. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 69.Hyzy SL, Olivares-Navarrete R, Schwartz Z, Boyan BD. BMP2 induces osteoblast apoptosis in a maturation state and noggin-dependent manner. J Cell Biochem. 2012;113:3236–45. doi: 10.1002/jcb.24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merino R, Ganan Y, Macias D, Economides AN, Sampath KT, Hurle JM. Morphogenesis of digits in the avian limb is controlled by FGFs, TGFbetas, and noggin through BMP signaling. Dev Biol. 1998;200:35–45. doi: 10.1006/dbio.1998.8946. [DOI] [PubMed] [Google Scholar]
- 71.Chen C, Uludag H, Wang Z, Jiang H. Noggin suppression decreases BMP-2-induced osteogenesis of human bone marrow-derived mesenchymal stem cells in vitro. J Cell Biochem. 2012;113:3672–80. doi: 10.1002/jcb.24240. [DOI] [PubMed] [Google Scholar]
- 72.Siebert JR, Conta Steencken A, Osterhout DJ. Chondroitin sulfate proteoglycans in the nervous system: inhibitors to repair. BioMed research international. 2014;2014:845323. doi: 10.1155/2014/845323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 74.Daniel JM, Penzkofer D, Teske R, Dutzmann J, Koch A, Bielenberg W, et al. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res. 2014;103:564–72. doi: 10.1093/cvr/cvu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filova E, Brynda E, Riedel T, Bacakova L, Chlupac J, Lisa V, et al. Vascular endothelial cells on two-and three-dimensional fibrin assemblies for biomaterial coatings. J Biomed Mater Res A. 2009;90:55–69. doi: 10.1002/jbm.a.32065. [DOI] [PubMed] [Google Scholar]
- 76.Castagnola E, Ansaldo A, Maggiolini E, Angotzi GN, Skrap M, Ricci D, et al. Biologically compatible neural interface to safely couple nanocoated electrodes to the surface of the brain. ACS Nano. 2013;7:3887–95. doi: 10.1021/nn305164c. [DOI] [PubMed] [Google Scholar]
- 77.Zaveri TD, Lewis JS, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials. 2014;35:3504–15. doi: 10.1016/j.biomaterials.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andersen MO, Howard KA, Paludan SR, Besenbacher F, Kjems J. Delivery of siRNA from lyophilized polymeric surfaces. Biomaterials. 2008;29:506–12. doi: 10.1016/j.biomaterials.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 79.Solanki A, Shah S, Yin PT, Lee KB. Nanotopography-mediated reverse uptake for siRNA delivery into neural stem cells to enhance neuronal differentiation. Sci Rep. 2013;3:1553. doi: 10.1038/srep01553. [DOI] [PMC free article] [PubMed] [Google Scholar]