Abstract
During development, vertebrate embryos produce serially repeated elements, the somites, on each side of the midline. These generate the vertebral column, skeletal musculature and dermis. They form sequentially, one pair at a time, from mesenchymal tissue near the tail. Somite development is a complex process. The embryo must control the number, size, and timing of somite formation, their subdivision into functional regions along three axes, regional identity such that somites develop in a region-specific way, and interactions with neighbouring tissues that coordinate them with nearby structures. Here we discuss many timing-related mechanisms that contribute to set up the spatial pattern.
Keywords: somite, segmentation, time-space conversion, pattern formation, segmentation clock
Introduction
During development of vertebrate embryos, a series of repeated elements, called somites, is laid down on either side of the main axis of the body (neural tube and notochord) (for reviews see [1–4]. In amniotes (reptiles, birds, mammals) each somite is initially an epithelial sphere, composed of about 1,000–2,0000 cells, arranged around a central lumen (which contains some mesenchymal cells and extracellular matrix) and surrounded by a basal lamina of fibronectin and other extracellular matrix materials. Some hours after its formation, the cells in the ventromedial part of each somite (adjacent to the notochord and ventral neural tube) becomes mesenchymal again – this is the sclerotome, whose cells will contribute to the vertebral column. The dorsolateral part (adjacent to the surface ectoderm) remains epithelial for longer – this is the dermomyotome, which eventually splits further into the dermatome most dorsally (still epithelial), which will contribute to the dermis of the trunk, and the myotome which will generate the epaxial muscles that extend and flex the vertebral column. The hypaxial musculature arises from cells at the ventrolateral edge of the dermomyotome, which migrate to invade the limbs and flank body wall. The sclerotomal contribution to the vertebral column generates the vertebral centra, the neural arches and associated spinous and transverse processes (including the proximal part of the ribs), as well as the annulus fibrosus of the intervertebral discs (which arises mainly from the mesenchymal cells that once resided in the somitic lumen [5, 6]). The nucleus pulposus (centre) of the inter-vertebral discs derives mainly from the notochord. Somite cells also generate inter-vertebral blood vessels [7–9].
The complexity of the pattern of somites is much greater than is apparent at first sight. Each somite is further subdivided into molecularly distinct rostral and caudal halves and this property is crucial both for dictating the segmental pattern of components of the peripheral nervous system (the motor nerves and neural crest derivatives including the autonomic ganglia, dorsal root ganglia and their central and peripheral projections) which are all confined to the rostral half [10–12], and for maintaining the segmental pattern by preventing mixing of cells between adjacent somites [13]. These properties later allow the process of “resegmentation” by which each vertebral centrum is formed from the caudal half of one sclerotome and the rostral half of the next sclerotome [14]. Moreover, somites are not identical along the length of the body axis – they have characteristic shapes and other properties in different regions (occipital, cervical, thoracic, lumbar, etc.). These properties are encoded by expression of combinations of Hox genes (see XXXX in this issue). Somites are also subdivided into a medial and a lateral half – these arise from distinct regions of the anterior primitive streak during gastrulation [15, 16] – after their formation, each somite rotates by about 45° so that the original North pole is relocated more laterally, and such that the original lateral half contributes mainly to the hypaxial musculature whereas the medial half generates the myotome and epaxial muscles (see above) [17]. The medial-lateral subdivision is further reinforced by medial signals from the notochord (Sonic hedgehog and probably Noggin) and dorsolateral signals from the surface ectoderm (BMP) [18, 19].
Although we now know a considerable amount about the signals and responses that set up the gross subdivisions of the somites and establish the various cell fates as well as regional properties, it is perhaps surprising that we know comparatively little about the mechanisms responsible for controlling the gross pattern including the total number of somites, the size of each individual somite (which varies somewhat along the axis), or what regulates the timing of formation of each somite so that it is so precise and synchronous on both sides of the embryo, or how somites acquire their regional addresses (Hox code) along the length of the body axis. It is clear that all of these aspects involve the conversion of temporal information into spatial patterns, and therefore that timing must be involved in several different ways. Several models have been put forward to explain different aspects of somite formation, including the clock-and-wavefront model [20] and several newer versions of it [4, 21–27], the cell cycle model [28–30], the delayed coupling model (multiple interacting oscillators) [31], clock-and-induction [32] and clock-and-trail models [33], and a traction-based model [34]. However to date, none of these fully explains all aspects of somite patterning. This review briefly draws attention to several aspects of somite formation that involve timing issues that appear to have been overlooked by much of the current literature. We will focus mainly on the chick embryo but make some reference to other model systems.
A conveyor-belt of cells: age order within the presomitic mesoderm
An important feature of somitogenesis is that it proceeds in head to tail order, separating cohorts of cells into somites in a sequential, orderly manner. In the chick, one somite forms every 90 minutes or so; this varies somewhat among different vertebrates: faster in teleost and anuran amphibian embryos and slower in most mammals. Each somite condenses into an epithelial ball from the bilateral strips of paraxial presomitic mesoderm (PSM) that lie caudal to the forming somites, where most of the cells have mesenchymal morphology. In turn, cells enter into the PSM from its caudal end, coming from the tip of the primitive streak including part of Hensen’s node. In chick embryos, from the time a new cell enters the PSM at its caudal end to the time it becomes part of a formed somite corresponds approximately to 18–20 hours, during which interval 12–13 somites will have formed from cells that were located at a more advanced (cranial) position when the cell entered the PSM [28, 29]. A further 8–10 hours (about 6 somites worth) then elapse before the newly-formed epithelial somite separates into dermomyotome dorsally and sclerotome ventromedially. Figure 1 shows a Scanning Electron Micrograph of a stage 12 chick embryo fractured through the middle of the PSM, aligned with diagrams of the stages of somite development.
Figure 1.
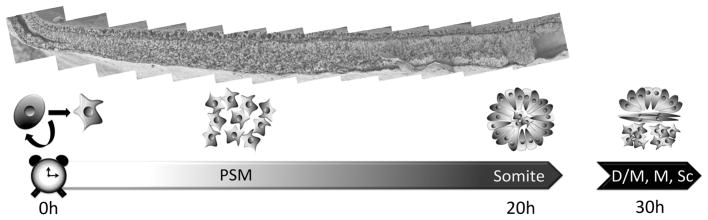
The upper part of the figure shows a Scanning Electron Micrograph (SEM) of an embryo at the 15 somite stage (stage HH12), fractured parallel to the head-tail axis, along the length of the PSM. The section covers the PSM from the tail (left) to the most recently formed somite. Below this, the diagrams schematise the various cell states and morphologies that cells pass through as they progress through the process of somite development. Cells (at least some of them derived from self-renewing stem cells at the primitive streak) enter the caudal PSM (time “0h” in the time scale below), where they have a mesenchymal morphology. Their sojourn in the PSM lasts about 20 hours, and they exit as a newly-formed somite with epithelial structure (surrounding a central core of cells that remains mesenchymal). Later (30 hours, equivalent to about 7 somites) the somite subdivides into the dorsally located epithelial dermomyotome (D/M), the ventrally located mesenchymal sclerotome (Sc), and a myotome (M) made up of an elongated stack of cells. The dermomyotome will give rise to dermis and hypaxial muscle, the myotome will generate epaxial muscles of the trunk and the sclerotome will generate the vertebrae and contribute to intersegmental blood vessels.
The above observations highlight the fact that at any one time, there is a “conveyor-belt” of cells that enter the PSM caudally and transit cranially as more cells enter behind them, at about the same rate as cells leave the tip of the PSM as they condense to form somites. Thus the length of the PSM remains approximately constant until the tail bud starts to form, when ingression seems to slow down or stop and the PSM shortens. The most caudal (tail) somites also tend to be much smaller than those in the trunk.
Resident stem cells and transiting cells
Lineage analysis of single identified somite progenitor cells (prospective single cell lineage analysis) has to date only been performed in the chick [15, 29]. This revealed that progenitors of the medial half of the somite are located in the lateral quadrant of Hensen’s node, whereas the lateral half is derived from cells located in the primitive streak just behind the node [15]. Interestingly, when a single cell is labelled by injection of a fluorescent tracer (lysine-fluorescein-dextran) in the former region, its descendants are found in small clusters at regular intervals along the PSM and eventually in the medial part of the somites, interspersed with unlabelled PSM cells. The space between successive clusters is approximately 6–8 somites – each cluster spans only 1–2 somites. At the caudal end, a single fluorescent cell can still be seen in the node, the original site of labelling. These observations can be explained most easily by suggesting that cells in the lateral quadrant of the chick Hensen’s node are self-renewing stem cells [35–37]: at each cell division one daughter remains in place whereas the other daughter leaves to enter the PSM. Cells in the node are not synchronous, so cells enter as a continuous stream into the PSM but at the next cell cycle, the same progenitors are responsible for the next part of the stream. There is therefore a covert (hidden) organisation within the PSM.
Results from the mouse are consistent with this view. Using ingenious genetic tools, Nicolas and colleagues have shown that the pattern of clonal distribution of descendants of a single labelled progenitor colonise most of the length of the somite-derived skeletal muscles along the body of the mouse, down to the tail [38, 39]. This genetic analysis did not reveal the location or time of labelling of the clonal founders. However an impressive later study, using a series of grafting experiments, demonstrated clearly that the chordoneural hinge (a structure that includes at least part of the late node) colonises the somite mesoderm in a manner consistent with it containing resident stem cells [40].
Progenitors of the lateral PSM located in the chick primitive streak behind the node do not seem to behave as resident stem cells. Rather, they appear to transit through the streak as in the more “classical” view of gastrulation: they seem to enter and leave the region at about the same rate [15, 16, 35, 41].
Timing of ingression of cells through the primitive streak and regional identity
The above observations reveal that there is an age structure that parallels the location of cells along the head-tail axis: “older” descendants of the founder cells are located in more cranial somites, whereas “younger” descendants of at least some of the same founder cells are located more caudally. This age structure was noticed long ago, even before lineage analysis suggested the existence of stem cells, and was the basis of the original proposal that cells might translate this age structure into sequential activation of genes in the Hox clusters such that their identity might be fixed as a function of the time spent in the primitive streak, or following incorporation into the PSM [42–44]. Almost the opposite view was proposed more recently by Limura and colleagues, who suggested that the time of cell ingression through the primitive streak is determined by the Hox genes expressed by the cells prior to their ingression [45, 46]. It is difficult to reconclie the latter with the concept of resident stem cells since descendants of the same founder cells must express different Hox genes as they come to occupy different somites. However it is possible that this does apply to the precursors of the lateral PSM in the primitive streak caudal to the node, which do not seem to spend substantial periods of residence in the streak.
Relationships between the cell cycle and somitogenesis: the cell cycle model
To investigate the time at which cells in the PSM make the decision to come together and form a single somite, Primmett and colleagues applied a single, short heat shock to chick embryos and allowed them to develop further. In amphibian embryos this had been shown to result in a single, discrete anomaly a few somites in length, situated several somites behind the last formed one at the time of the shock [47–49]. In the chick, however, a similar treatment generates not one, but several discrete anomalies along the axis, separated by about 6–8 somites [50]. This suggests that the shock affects precursor cells, and that the effect of this becomes visible whenever the descendants of these cells generate somites – the first anomaly observed corresponds to when cells were about half-way along the PSM (6–7 prospective somites, or about 10 hours before segmenting). This raised the possibility that the 6–8 somite (10 hour) interval corresponds to the length of the cell cycle in these cells. Direct measurements of this using 3H-thymidine pulse-and-chase suggested that the cell cycle is indeed 10 hours, and the effects of heat shock can be phenocopied by short treatment with drugs that affect the cell cycle either in M- or S-phase [28]. Heat shock also causes similar repeated anomalies in zebrafish [51].
These results led to the “cell cycle model” of somite formation [28–30], which proposes that the number of cells that will segment together from the PSM is determined by a “gate” within the cell cycle, which groups together similarly aged cells by turning on gene(s) encoding cell adhesion or similar molecules that will be expressed one cell cycle later, causing the cells to come together to form a somite. The model implies that the timing of somite formation is carried by cells in a relatively cell-autonomous way, so that even if cells mix within the PSM, the gating mechanism can sort those cells of a similar stage of maturity and segregate them from differently aged neighbours. This also implies that cells “know” when they will segment from the time they enter the PSM.
Cell mixing and cell sorting
Labelling of a small group of cells in the prospective lateral somite territory of the primitive streak followed by incubation until labelled cells are incorporated into somites reveals a high amount of mixing caudally but gradual confinement of the labelled cells to the lateral halves of the somites. Similar results are obtained for medial precursors in Hensen’s node [15]. This is consistent with the proposals outlined above, in that cells appear to have a cell-intrinsic knowledge of their ultimate position and can find this position by sorting, despite a large amount of intermixing in the posterior PSM.
Although using a different terminology and different interpretation, a recent study [52] made similar findings by tracking individual cells as they progress along the PSM. Considerable cell mixing was seen caudally, decreasing in a cranial direction – this was called a “gradient of random cell motility” but is entirely consistent with the idea that cells can sort as they move along the PSM and approach the time of segmentation.
Clocks and wavefronts
Experiments largely done by Jonathan Cooke in the 1970s suggested that Xenopus embryos are able to regulate the size of somites according to overall body mass, such that larger or smaller embryos would make the correct number of somites but containing different numbers of cells. This suggested that at some point the embryo must be able to measure its mass which led to a model proposed by Cooke and Zeeman in 1976, based on the interaction between a cell-intrinsic clock and a wave travelling caudally along the axis, gating the clock into cohorts of cells that will segment together [20]. The speed of the wave would be adjusted in embryos of different sizes, allowing for size regulation. A modification of the model by Jonathan Slack suggested that the speed of the wavefront is in determined by the slope of a head-tail gradient [53].
The Clock
The clock-and-wavefront was almost forgotten until 1997, when Palmeirim and colleagues observed that the pattern of expression of the Hairy1 gene showed 3 different patterns in embryos of apparently the same age (same number of somites). Beautifully designed experiments where two halves of the same embryo were examined for Hairy1 expression after different periods of incubation then revealed an unexpectedly dynamic pattern, where a caudal-to-rostral wave of expression sweeps the PSM before ending in the formation of a strip in the future caudal half of the next somite to form, and this repeats itself every 90 minutes [54]. Thus, cells in the PSM experience about 12 of these waves before forming a somite [55]. Later other genes of the Notch pathway (as well as Wnt and FGF) have been shown to undergo similar but not identical patterns, suggesting a very complex array of oscillators accompanying the transit of cells along the PSM. For obvious reasons, this has generally been assumed to represent “the clock” predicted by the clock-and-wavefront model.
Gradients and Wavefronts
To establish the position of the predicted wavefront, Pourquié and colleagues inverted small pieces of PSM at different locations and found that rostral rotations resulted in inversion of the pattern, whereas rotations performed more caudally than about half-way along the PSM length had no such effect. This was interpreted to mean that this point corresponds to the “determination front” predicted by the clock-and-wavefront model [56]. It was then found that FGF8 transcription is graded along the PSM, highest caudally, and a “threshold” was proposed to exist around the middle of the PSM [56, 57]. This “determination front” and associated FGF gradient (which incorporates elements of both a wave and a gradient) has been widely assumed to correspond to the wave of the clock-and-wavefront model, and also to support Slack’s interpretation (see above).
In contrast with the cell cycle model, the clock-and-wavefront model assumes that there is no pre-set pattern within the PSM, and assigns this pattern to a population of cells that is generally thought to be naïve.
Some problems with the clock & wavefront model
Despite the apparent correspondence of many experimental results with aspects of the clock-and-wavefront model, several aspects are difficult to explain without making more assumptions, or are inconsistent with the model as proposed. The questions and problems include:
Can the final number of somites be regulated as a function of body length? This was the original premise that led to the clock-and-wavefront model, but is based on observations in anuran amphibians, where the entire presomitic material is present in the embryo at one time and it is therefore possible for the embryo to subdivide this material according to its total length. In amniotes the process of somitogenesis continues for several days; at the time somitogenesis starts, the PSM only contains cells for the first 10–15 somites, so it is difficult to envisage a mechanism to scale somite size according to the overall size of the embryo;
Notch pathway mutants seem to start to engage in somite formation with approximately the correct pattern, but somites seem to fall apart a little later, perhaps coinciding with when rostral-caudal differences are established [58–61];
The period of the clock does not correspond exactly to that of somite formation. It was shown recently that the speed of the waves of “segmentation clock” gene expression changes along the zebrafish PSM and is influenced by the rate of tissue shortening as the PSM material runs out. A “Doppler shift” was proposed to be involved in the mechanisms setting up somite size [62].
Is there a “determination front”, and if so, where does it lie? The “determination front” and its position in the middle of the PSM was predicted by rotation of small pieces of PSM. However, the observations of cell sorting in the PSM and the “gradient in random cell motility” (see above), decreasing in a caudal-to-rostral direction, could provide a different interpretation: rotations performed near the tip of the PSM effectively rotate the pattern, whereas rotations performed behind the middle of the PSM have no effect simply because the appropriate cells have not yet sorted out from neighbours that will not segment at the same time. FGF clearly does play an important role in controlling maturation of cells in the PSM, but not necessarily by providing a discrete “determination front”;
The extensive cell mixing in the caudal PSM seems inconsistent with a key instructive role for either clocks or oscillators. If cells destined to contribute to different somites are mixed up for around half of the passing waves of gene expression (half of their sojourn in the PSM), how can these waves be instructive in terms of which cells will segment together? This is also incompatible with cell lineage results, which suggest that the age of entry into the PSM is followed quite strictly as they and their descendants become incorporated into somites;
Occipital somites form in a different order and without a clock. The first 5 somites do not give rise to vertebrae, but rather contribute to the occipital bone – they also support the development of the XII cranial nerve and Froriep’s ganglion instead of dorsal root ganglia as in the trunk [63]. These first 5 somites do not form sequentially as in the trunk but almost simultaneously (see [64]): the second somite is the first to form, followed by the first and third almost at once. There is considerable variability between embryos and in some embryos all 5 form almost synchronously. Moreover, “segmentation clock” genes do not appear to be expressed cyclically in the PSM that gives rise to these somites [65]. These findings are not easily reconciled with the clock-and-wavefront model; some other mechanism must be responsible for apportioning cells to each somite in the occipital region;
Somites can be induced to form in the absence of a clock. When the lateral plate [66, 67] or its precursor, the posterior primitive streak [68] are exposed to Noggin to block BMP signalling, the fate of the cells can be converted from lateral plate to somite. When Noggin is administered very evenly to the cells of a primitive streak explant, this can be induced to form somites. These are within the normal size range for somites, they express somite markers including markers of dermomyotome and sclerotome as well as Hox genes revealing a trunk identity. However many somites form simultaneously from such explants, and their formation is not predicted by the expression patterns of “segmentation clock” genes [64]. The somites that form from the explants are arranged in three-dimensions (as a “bunch-of-grapes”) rather than linearly. Therefore in this experimental paradigm, somite formation can be dissociated from both the molecular clock and wavefronts (as the 3-d arrangement is incompatible with this).
The “segmentation clock” and rostral-caudal subdivision of somites
The above issues raise the question of what is the main function of the “segmentation clock”, if it is not absolutely required to control somite size, number and other properties such as their Hox regional address. The experiments with streak explants treated with Noggin [64] suggest that the main property that is defective in these ectopic somites is their subdivision into rostral and caudal halves. This observation raises the possibility that the main function of the “segmentation clock” is to coordinate somite formation with their subdivision into rostral and caudal halves in precise positions. However we do not yet understand the precise mechanism responsible.
Is the medial PSM a pacemaker?
When cells from the lateral part of Hensen’s node (prospective medial PSM) are grafted into the presomitic mesoderm of a host embryo, the graft gives rise to somites that form independently from those of the surrounding host cells, as if they can dictate their own segmental pattern [16]. However, a similar experiment performed with the region of primitive streak that contains lateral PSM precursors does not generate separate somites, but the grafted cells incorporate into those formed by the host PSM ([16] and unpublished observations). This suggested that the medial PSM cells might act as a pacemaker, dictating the pattern of segmentation to surrounding cells. A similar conclusion was reached by Palmeirim and colleagues [69] from a different type of experiment: when the lateral and medial PSM are separated by a sagittal slit, the medial PSM forms small somites whereas the lateral PSM does not. While both experiments appear to indicate that the medial PSM acts as a pacemaker whereas the lateral PSM passively follows the instructions of its more medial neighbour, a more parsimonious explanation might be that separation of the lateral PSM from the midline causes an increase in BMP exposure by insulating it from the effects of Noggin and Sonic hedgehog from the midline (see [64, 66–68]).
Differences between species?
The above discussion has focused mainly on results from the chick embryo, a model amniote. Anamniotes (fish and amphibians) differ in several important respects. Among the main differences, anamniote somites are mainly composed of myotome (future axial muscle) and the sclerotome is almost absent [70]. The vertebral column, especially in Teleost fish, may derive much more prominently from the notochord, which may even have an intrinsic segmental pattern [71, 72]. In anuran amphibians, somites are not true epithelial spheres at the time of their formation; instead, cohorts of paraxial mesoderm cells align and then turn by 90° as they elongate to form the myotome. This then becomes chevron-shaped, with its mid-portion aligned with the notochord so that the dorsal part becomes epaxial and the ventral part hypaxial. In fish, an intermediate situation may exist, where the initial somites are somewhat epithelial although the overall pattern is more similar to that in amphibians than to the amniote pattern. The most important difference in terms of somite formation, however is that anamniote embryos do not undergo significant growth (increase in mass or volume) before hatching: the mass and volume of the fertilised egg is the same as that of the hatching free-swimming tadpole that arises about a day later. As a result, segmentation of the paraxial mesoderm into somites is more a matter of subdivision than in amniotes. In the latter, the PSM is very long and renews itself continuously for a long time. In zebrafish, the PSM appears to contain only about 5 presumptive somites worth of material at any one time and somites form very rapidly. This probably corrsponds to a single cell cycle’s worth of material (rather than the two cell cycles worth in chick). The shorter Teleost PSM appears to behave more like the anterior half of the chick PSM. Observations of cell movement together with fluorescent reporters of segmentation clock genes in the PSM suggest that there is relatively little cell movement within the PSM [73, 74] and it seems possible that a mechanism relying on repeated waves of gene expression may play a much more prominent role than in anamniotes.
Conclusion
This brief summary emphasizes that the development of somites is a much more complex process than it appears from its simple organization of repeated elements along the axis. Many properties of somitic cells need to be controlled in a consistent way. While mechanisms similar to the clock-and-wavefront model of Cooke and Zeeman [20] may well play an important role, it is now becoming clear that this cannot control all aspects of somite formation and that there are many other mechanisms that contribute to determine the number, size, regional identity, timing of formation, various subdivisions, interactions within and between tissues and other features. Whatever the mechanisms, somite formation remains a very clear example of time-space conversion, where temporal information of different types is converted to spatial patterns. The challenge for the future is to understand how all these properties are controlled and how they are integrated with each other.
Acknowledgments
Our research on somite formation was funded by the Medical Research Council (G0700095) and by the National Institutes of Health (R01GM076692). We thank Mark Turmaine for expert help with scanning electron microscopy which led to the production of Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keynes RJ, Stern CD. Mechanisms of vertebrate segmentation. Development. 1988;103(3):413–29. doi: 10.1242/dev.103.3.413. [DOI] [PubMed] [Google Scholar]
- 2.Christ B, Ordahl CP. Early stages of chick somite development. Anatomy and embryology. 1995;191(5):381–96. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- 3.Pourquie O. The chick embryo: a leading model in somitogenesis studies. Mech Dev. 2004;121(9):1069–79. doi: 10.1016/j.mod.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Benazeraf B, Pourquie O. Formation and segmentation of the vertebrate body axis. 2013;29:1–26. doi: 10.1146/annurev-cellbio-101011-155703. Annual review of cell and developmental biology. [DOI] [PubMed] [Google Scholar]
- 5.Huang R, et al. Function of somite and somitocoele cells in the formation of the vertebral motion segment in avian embryos. Acta Anat (Basel) 1996;155(4):231–41. doi: 10.1159/000147811. [DOI] [PubMed] [Google Scholar]
- 6.Huang R, et al. The fate of somitocoele cells in avian embryos. Anatomy and embryology. 1994;190(3):243–50. doi: 10.1007/BF00234302. [DOI] [PubMed] [Google Scholar]
- 7.Beddington RS, Martin P. An in situ transgenic enzyme marker to monitor migration of cells in the mid-gestation mouse embryo. Somite contribution to the early forelimb bud. Molecular biology & medicine. 1989;6(4):263–74. [PubMed] [Google Scholar]
- 8.Wilting J, et al. Angiogenic potential of the avian somite. Developmental dynamics: an official publication of the American Association of Anatomists. 1995;202(2):165–71. doi: 10.1002/aja.1002020208. [DOI] [PubMed] [Google Scholar]
- 9.England MA. Aspects of somite formation in the early chick embryo. In: Bellairs R, Ede DA, Lash JW, editors. Somites in developing embryos. Plenum Publishing Corp; New York: 1986. pp. 47–60. [Google Scholar]
- 10.Keynes RJ, Stern CD. Segmentation in the vertebrate nervous system. Nature. 1984;310(5980):786–9. doi: 10.1038/310786a0. [DOI] [PubMed] [Google Scholar]
- 11.Rickmann M, Fawcett JW, Keynes RJ. The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. Journal of embryology and experimental morphology. 1985;90:437–55. [PubMed] [Google Scholar]
- 12.Bronner-Fraser M, Stern C. Effects of mesodermal tissues on avian neural crest cell migration. Developmental biology. 1991;143(2):213–7. doi: 10.1016/0012-1606(91)90071-a. [DOI] [PubMed] [Google Scholar]
- 13.Stern CD, Keynes RJ. Interactions between somite cells: the formation and maintenance of segment boundaries in the chick embryo. Development. 1987;99(2):261–72. doi: 10.1242/dev.99.2.261. [DOI] [PubMed] [Google Scholar]
- 14.Remak R. Untersuchungen über die Entwicklung der Wirbeltiere. Berlin: Reimer; 1855. [Google Scholar]
- 15.Selleck MA, Stern CD. Fate mapping and cell lineage analysis of Hensen’s node in the chick embryo. Development. 1991;112(2):615–26. doi: 10.1242/dev.112.2.615. [DOI] [PubMed] [Google Scholar]
- 16.Selleck MAJ, Stern CD. Commitment of mesoderm cells in Hensen’s node of the chick embryo to notochord and somites. Development. 1992;114(2):403–415. [Google Scholar]
- 17.Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. 1992;114(2):339–53. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- 18.Pourquie O, et al. Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proceedings of the National Academy of Sciences of the United States of America; 1993; pp. 5242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasiliauskas D, Hancock S, Stern CD. SWiP-1: novel SOCS box containing WD-protein regulated by signalling centres and by Shh during development. Mech Dev. 1999;82(1–2):79–94. doi: 10.1016/s0925-4773(99)00014-3. [DOI] [PubMed] [Google Scholar]
- 20.Cooke J, Zeeman EC. A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J Theor Biol. 1976;58(2):455–76. doi: 10.1016/s0022-5193(76)80131-2. [DOI] [PubMed] [Google Scholar]
- 21.Aulehla A, et al. A beta-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nature cell biology. 2008;10(2):186–93. doi: 10.1038/ncb1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray PJ, Maini PK, Baker RE. The clock and wavefront model revisited. Journal of theoretical biology. 2011;283(1):227–38. doi: 10.1016/j.jtbi.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Santillan M, Mackey MC. A proposed mechanism for the interaction of the segmentation clock and the determination front in somitogenesis. PloS one. 2008;3(2):e1561. doi: 10.1371/journal.pone.0001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroter C, Oates AC. Segment number and axial identity in a segmentation clock period mutant. Current biology: CB. 2010;20(14):1254–8. doi: 10.1016/j.cub.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 25.Hester SD, et al. A multi-cell, multi-scale model of vertebrate segmentation and somite formation. PLoS computational biology. 2011;7(10):e1002155. doi: 10.1371/journal.pcbi.1002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dequeant ML, et al. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science. 2006;314(5805):1595–8. doi: 10.1126/science.1133141. [DOI] [PubMed] [Google Scholar]
- 27.Pourquie O. The segmentation clock: converting embryonic time into spatial pattern. Science. 2003;301(5631):328–30. doi: 10.1126/science.1085887. [DOI] [PubMed] [Google Scholar]
- 28.Primmett DR, et al. Periodic segmental anomalies induced by heat shock in the chick embryo are associated with the cell cycle. Development. 1989;105(1):119–30. doi: 10.1242/dev.105.1.119. [DOI] [PubMed] [Google Scholar]
- 29.Stern CD, et al. A cell lineage analysis of segmentation in the chick embryo. Development. 1988;104(Suppl):231–244. doi: 10.1242/dev.104.Supplement.231. [DOI] [PubMed] [Google Scholar]
- 30.Collier JR, et al. A cell cycle model for somitogenesis: mathematical formulation and numerical simulation. J Theor Biol. 2000;207(3):305–16. doi: 10.1006/jtbi.2000.2172. [DOI] [PubMed] [Google Scholar]
- 31.Morelli LG, et al. Delayed coupling theory of vertebrate segmentation. HFSP journal. 2009;3(1):55–66. doi: 10.2976/1.3027088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnell S, Maini PK. Clock and induction model for somitogenesis. Dev Dyn. 2000;217(4):415–20. doi: 10.1002/(SICI)1097-0177(200004)217:4<415::AID-DVDY8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Kerszberg M, Wolpert L. A clock and trail model for somite formation, specialization and polarization. J Theor Biol. 2000;205(3):505–10. doi: 10.1006/jtbi.2000.2085. [DOI] [PubMed] [Google Scholar]
- 34.Bard JB. Traction and the formation of mesenchymal condensations in vivo. Bioessays. 1990;12(8):389–95. doi: 10.1002/bies.950120809. [DOI] [PubMed] [Google Scholar]
- 35.Selleck MAJ, Stern CD. Evidence for stem cells in the mesoderm of Hensen’s node and their role in embryonic pattern formation. In: Bellairs R, Sanders EJ, Lash JW, editors. Formation and differentiation of early embryonic mesoderm. Plenum Press; New York: 1992. pp. 23–31. [Google Scholar]
- 36.Joubin K, Stern CD. Molecular interactions continuously define the organizer during the cell movements of gastrulation. Cell. 1999;98(5):559–71. doi: 10.1016/s0092-8674(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 37.Stern CD, et al. Head-tail patterning of the vertebrate embryo: one, two or many unresolved problems? Int J Dev Biol. 2006;50(1):3–15. doi: 10.1387/ijdb.052095cs. [DOI] [PubMed] [Google Scholar]
- 38.Nicolas JF, et al. Evidence in the mouse for self-renewing stem cells in the formation of a segmented longitudinal structure, the myotome. Development. 1996;122(9):2933–46. doi: 10.1242/dev.122.9.2933. [DOI] [PubMed] [Google Scholar]
- 39.Tzouanacou E, et al. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Developmental cell. 2009;17(3):365–76. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Cambray N, Wilson V. Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development. 2002;129(20):4855–66. doi: 10.1242/dev.129.20.4855. [DOI] [PubMed] [Google Scholar]
- 41.Psychoyos D, Stern CD. Fates and migratory routes of primitive streak cells in the chick embryo. Development. 1996;122(5):1523–34. doi: 10.1242/dev.122.5.1523. [DOI] [PubMed] [Google Scholar]
- 42.Gaunt SJ. Conservation in the Hox code during morphological evolution. The International journal of developmental biology. 1994;38(3):549–52. [PubMed] [Google Scholar]
- 43.Gaunt SJ, Strachan L. Forward spreading in the establishment of a vertebrate Hox expression boundary: the expression domain separates into anterior and posterior zones, and the spread occurs across implanted glass barriers. Dev Dyn. 1994;199(3):229–40. doi: 10.1002/aja.1001990307. [DOI] [PubMed] [Google Scholar]
- 44.Gaunt SJ, Strachan L. Temporal colinearity in expression of anterior Hox genes in developing chick embryos. Dev Dyn. 1996;207(3):270–80. doi: 10.1002/(SICI)1097-0177(199611)207:3<270::AID-AJA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 45.Iimura T, Denans N, Pourquie O. Establishment of Hox vertebral identities in the embryonic spine precursors. Current topics in developmental biology. 2009;88:201–34. doi: 10.1016/S0070-2153(09)88007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iimura T, Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 2006;442(7102):568–71. doi: 10.1038/nature04838. [DOI] [PubMed] [Google Scholar]
- 47.Cooke J, Elsdale T. Somitogenesis in amphibian embryos. III. Effects of ambient temperature and of developmental stage upon pattern abnormalities that follow short temperature shocks. Journal of embryology and experimental morphology. 1980;58:107–18. [PubMed] [Google Scholar]
- 48.Elsdale T, Pearson M, Whitehead M. Abnormalities in somite segmentation following heat shock to Xenopus embryos. Journal of embryology and experimental morphology. 1976;35(3):625–35. [PubMed] [Google Scholar]
- 49.Pearson M, Elsdale T. Somitogenesis in amphibian embryos. I. Experimental evidence for an interaction between two temporal factors in the specification of somite pattern. Journal of embryology and experimental morphology. 1979;51:27–50. [PubMed] [Google Scholar]
- 50.Primmett DR, Stern CD, Keynes RJ. Heat shock causes repeated segmental anomalies in the chick embryo. Development. 1988;104(2):331–9. doi: 10.1242/dev.104.2.331. [DOI] [PubMed] [Google Scholar]
- 51.Roy MN, V, Prince E, Ho RK. Heat shock produces periodic somitic disturbances in the zebrafish embryo. Mechanisms of development. 1999;85(1–2):27–34. doi: 10.1016/s0925-4773(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 52.Benazeraf B, et al. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466(7303):248–52. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slack J. From egg to embryo: Regional specification in early development. 2. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- 54.Palmeirim I, et al. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91(5):639–48. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- 55.Vasiliauskas D, Stern CD. Patterning the embryonic axis: FGF signaling and how vertebrate embryos measure time. Cell. 2001;106(2):133–6. doi: 10.1016/s0092-8674(01)00442-1. [DOI] [PubMed] [Google Scholar]
- 56.Dubrulle J, McGrew MJ, Pourquie O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106(2):219–32. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- 57.Dubrulle J, Pourquie O. fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature. 2004;427(6973):419–22. doi: 10.1038/nature02216. [DOI] [PubMed] [Google Scholar]
- 58.Barrantes IB, et al. Interaction between Notch signalling and Lunatic fringe during somite boundary formation in the mouse. Current biology: CB. 1999;9(9):470–80. doi: 10.1016/s0960-9822(99)80212-7. [DOI] [PubMed] [Google Scholar]
- 59.de la Pompa JL, et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124(6):1139–48. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- 60.Ferjentsik Z, et al. Notch is a critical component of the mouse somitogenesis oscillator and is essential for the formation of the somites. PLoS genetics. 2009;5(9):e1000662. doi: 10.1371/journal.pgen.1000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oka C, et al. Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development. 1995;121(10):3291–301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- 62.Soroldoni D, et al. Genetic oscillations. A Doppler effect in embryonic pattern formation. Science. 2014;345(6193):222–5. doi: 10.1126/science.1253089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim TM, et al. The differing effects of occipital and trunk somites on neural development in the chick embryo. Development. 1987;100(3):525–33. doi: 10.1242/dev.100.3.525. [DOI] [PubMed] [Google Scholar]
- 64.Dias AS, et al. Somites without a clock. Science. 2014;343(6172):791–5. doi: 10.1126/science.1247575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodrigues S, Santos J, Palmeirim I. Molecular characterization of the rostral-most somites in early somitic stages of the chick embryo. Gene expression patterns: GEP. 2006;6(7):673–7. doi: 10.1016/j.modgep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Tonegawa A, et al. Mesodermal subdivision along the mediolateral axis in chicken controlled by different concentrations of BMP-4. Development. 1997;124(10):1975–84. doi: 10.1242/dev.124.10.1975. [DOI] [PubMed] [Google Scholar]
- 67.Tonegawa A, Takahashi Y. Somitogenesis controlled by Noggin. Dev Biol. 1998;202(2):172–82. doi: 10.1006/dbio.1998.8895. [DOI] [PubMed] [Google Scholar]
- 68.Streit A, Stern CD. Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech Dev. 1999;85(1–2):85–96. doi: 10.1016/s0925-4773(99)00085-4. [DOI] [PubMed] [Google Scholar]
- 69.Freitas C, et al. Evidence for medial/lateral specification and positional information within the presomitic mesoderm. Development. 2001;128(24):5139–47. doi: 10.1242/dev.128.24.5139. [DOI] [PubMed] [Google Scholar]
- 70.Morin-Kensicki EM, Eisen JS. Sclerotome development and peripheral nervous system segmentation in embryonic zebrafish. Development. 1997;124(1):159–67. doi: 10.1242/dev.124.1.159. [DOI] [PubMed] [Google Scholar]
- 71.Fleming A, Keynes R, Tannahill D. A central role for the notochord in vertebral patterning. Development. 2004;131(4):873–80. doi: 10.1242/dev.00952. [DOI] [PubMed] [Google Scholar]
- 72.Morin-Kensicki EM, Melancon E, Eisen JS. Segmental relationship between somites and vertebral column in zebrafish. Development. 2002;129(16):3851–60. doi: 10.1242/dev.129.16.3851. [DOI] [PubMed] [Google Scholar]
- 73.Schroter C, et al. Topology and dynamics of the zebrafish segmentation clock core circuit. PLoS biology. 2012;10(7):e1001364. doi: 10.1371/journal.pbio.1001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soroldoni D, Oates AC. Live transgenic reporters of the vertebrate embryo’s Segmentation Clock. Current opinion in genetics & development. 2011;21(5):600–5. doi: 10.1016/j.gde.2011.09.006. [DOI] [PubMed] [Google Scholar]


