Abstract
Lithium has recently been suggested to have neuroprotective effects in several models of neurodegenerative disease including Parkinson’s disease (PD). Levodopa (L-Dopa) replacement therapy remains the most common and effective treatment for PD, although it induces the complication of L-Dopa induced dyskinesia after years of use. Here we examined the potential use of lithium in combination with L-Dopa/Carbidopa for both reducing MPTP-induced abnormal involuntary movements (AIMs) as well as protecting against cell death in MPTP-lesioned mice. Chronic lithium administration (0.127% LiCl in the feed) in the presence of daily L-Dopa/Carbidopa injection for a period of 2 months was sufficient to effectively reduce MPTP-induced AIMs in mice. Mechanistically, lithium was found to suppress MPTP-induced calpain activities in vivo coinciding with down-regulation of calpain-1 but not calpain-2 expression in both the striatum (ST) and the brain stem (BS). Calpain inhibition has previously been associated with increased levels of the rate-limiting enzyme in dopamine synthesis, tyrosine hydroxylase (TH), which is probably mediated by the up-regulation of the transcription factors MEF-2A and 2D. Lithium was found to induce up-regulation of TH expression in the ST and the BS, as well as in N27 rat dopaminergic cells. Further, histone acetyltransferase (HAT) expression was substantially up-regulated by lithium treatment in vitro. These results suggest the potential use of lithium in combination with L-Dopa/Carbidopa not only as a neuroprotectant, but also for reducing AIMs and possibly alleviating potential side-effects associated with the current treatment for PD.
Keywords: combination therapy, MPTP, neurodegenerative disease, calpain 1, AIMs
1. Introduction
Parkinson’s disease (PD) is a progressive movement disorder with a prevalence of 1.8% in individuals of 65 years or older (de Rijk et al., 2000; Bogaerts et al., 2008); its etiology is largely unknown in most cases. Dopamine replacement therapy using levodopa (L-Dopa) has remained the most effective treatment to reduce motor symptoms in PD. Currently most patients taking L-Dopa also receive the DOPA decarboxylase inhibitor, Carbidopa, for higher efficacy and lower side-effects (Celesia and Wanamaker, 1976; Wajsbort et al., 1978). However, chronic L-Dopa treatment results in abnormal involuntary movements (AIMs) including dyskinesia, in approximately 30% patients after 4–6 years of treatment; almost 90% of patients suffer from this complication after 9 years of chronic use (Ahlskog and Muenter, 2001).
Lithium has been the most commonly-used mood-stabilizing drug for the treatment of bipolar disorder. However, its recently discovered neuroprotective and neurotrophic properties have suggested it as a potential repurposed therapeutic for several neurodegenerative conditions (Li et al., 2002; Pies, 2002). Chronic lithium treatment was found to significantly attenuate neurodegeneration in the trigeminal, facial, ambiguus, and hypoglossal nuclei associated with an animal model of amyotrophic lateral sclerosis (ALS) (Ferrucci et al., 2010). In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication PD mouse model, lithium was reported to prevent MPTP-induced neurotoxicity via regulation of Bcl-2 and Bax (Youdim and Arraf, 2004). Currently, lithium is in widespread “off label” usage for PD. It has been suggested that its co-administration could possibly provide some level of symptomatic relief against L-Dopa-induced dyskinesia (LID) and akinesia associated with “on-off” phenomenon complications following long-term L-Dopa treatment (Smith, 1976; Coffey et al., 1982). Lithium appears to be relatively safe at lower therapeutic dosages, readily penetrates the blood brain barrier, and low cost generic versions are available, making lithium an appealing drug for potential use in PD. Recently we demonstrated that chronic oral lithium administration was sufficient to prevent age-related, pesticide-induced alpha-synuclein aggregation and associated neuronal loss in multiple brain regions in a pan-neuronal human alpha-synuclein A53T mutant over-expressing transgenic line (Kim et al., 2011a).
The potential mechanisms by which lithium may modulate dopamine levels and reduce AIMs are largely unknown. Recent post-mortem studies, however, demonstrated that this could be via inhibition of a pathway involving calpain activation. Calpains have been shown to be elevated in human PD brains and calpain inhibition has been demonstrated to prevent reduction in the number of tyrosine hydroxylase (TH) positive cells in both MPTP and 6-hydroxydopamine (6-OHDA) treated rodent models of PD (Mouatt-Prigent et al., 1996; Crocker et al., 2003; Alvira et al., 2008). The calpain inhibitor MDL28170 was also shown to augment TH levels in the striatum of 6-OHDA lesioned rats, resulting in reductions in AIM frequencies (Chagniel et al., 2012). Here, we demonstrate that low-dose lithium with L-Dopa/Carbidopa reduces MPTP-induced AIMs in a mouse model coinciding with both calpain-1 inhibition and up-regulation of TH expression within both the striatum and the Substantia Nigra pars compacta (SNpc). These results suggest the potential use of lithium in combination with L-Dopa/Carbidopa (Sinemet®), not only as a neuroprotectant, but also for reducing AIMs and alleviating potential side-effects associated with currently available treatments for PD.
2. Results
2.1. In the hindlimb clasping test, the combination of lithium and L-Dopa/Carbidopa reduces MPTP-induced Abnormal Involuntary Movements (AIMs) in MPTP-lesioned mice
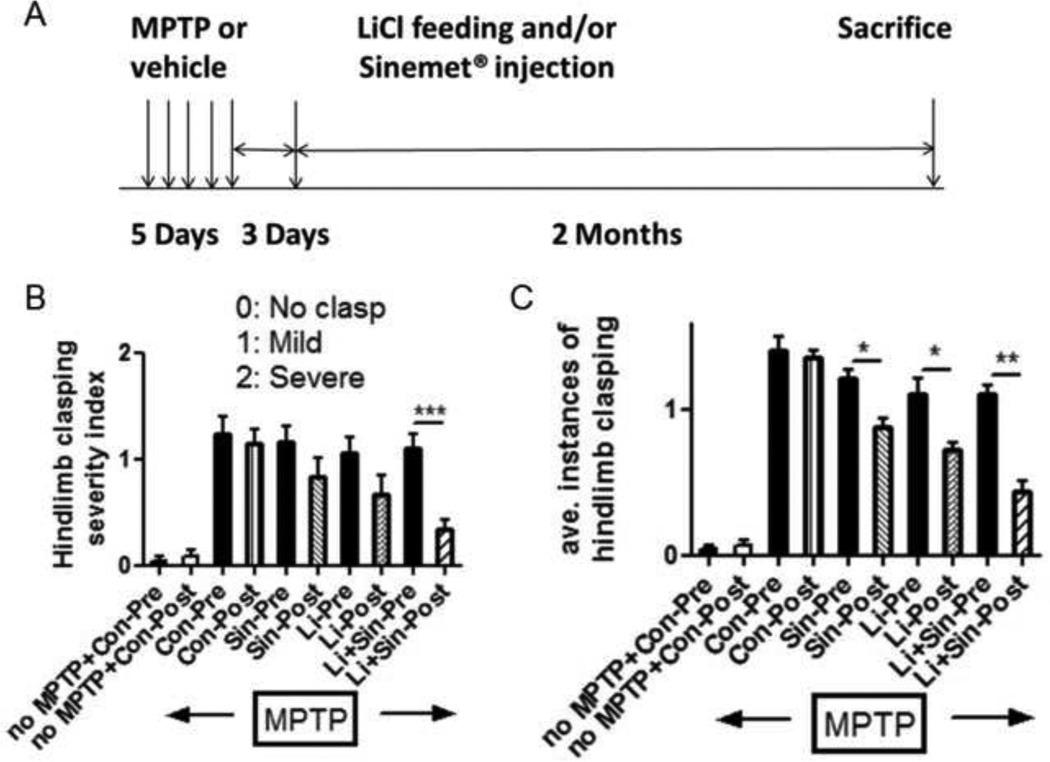
Using the hindlimb clasping test, we assessed AIMs after chronic MPTP treatment via analysis of hindlimb clasping severity and average instances of hindlimb clasping in the various experimental paradigms. In the comparison of pre-treatment (black filled bars) and post-treatment conditions (lined bars), we found that only the combination of lithium and L-Dopa/Carbidopa significantly reduced the severity of hindlimb clasping behavior (Fig. 1B, p<0.001), while we detected significant reductions in the average instances of hindlimb clasping in both lithium- and L-Dopa/Carbidopa-treated mice (p<0.05) as well as in the combination therapy (p<0.01, Fig. 1C. Supplementary videos are available on-line). The hindlimb severity index appeared to have higher variation in each group than counting the average instances of clasping, thus we found no significance in both lithium- and L-Dopa/Carbidopa-treated groups (Fig. 1B). However, we consistently found significant behavioral improvement from the combination therapy (Fig. 1B, C).
Figure 1.
Time scales for lithium feeding and/or L-dopa/Carbidopa injection followed by chronic MPTP injection. Lithium was fed in food (0.127% LiCl) and L-dopa/Carbidopa was injected daily for 2 months (A). Abnormal involuntary movements (AIMs) were assessed via the hindlimb clasping test. The hindlimb clasping severity index (B) or average instances of clasping (C) were quantified. Pre-drug treatment (black bars) was compared with post-drug treatment (lined bars) and displayed as mean ± SEM: *: p<0.05, **: p<0.01 as assessed by unpaired t-test (n=6–9 in each group). Only significant comparison was displayed and not significant (n.s) was not labeled. Hindlimb clasping was counted for a 10 second period on 3 consecutive days and data combined for statistical analysis. Abb.: Pre: pre-drug treatment, Post: Post-drug treatment, Con: vehicle treated control, Sin: Sinemet (L-dopa/Carbidopa), Li: lithium feed.
2.2. Lithium up-regulates TH expression in the ST and the SN as assessed by immunohistochemistry and Western blot analysis
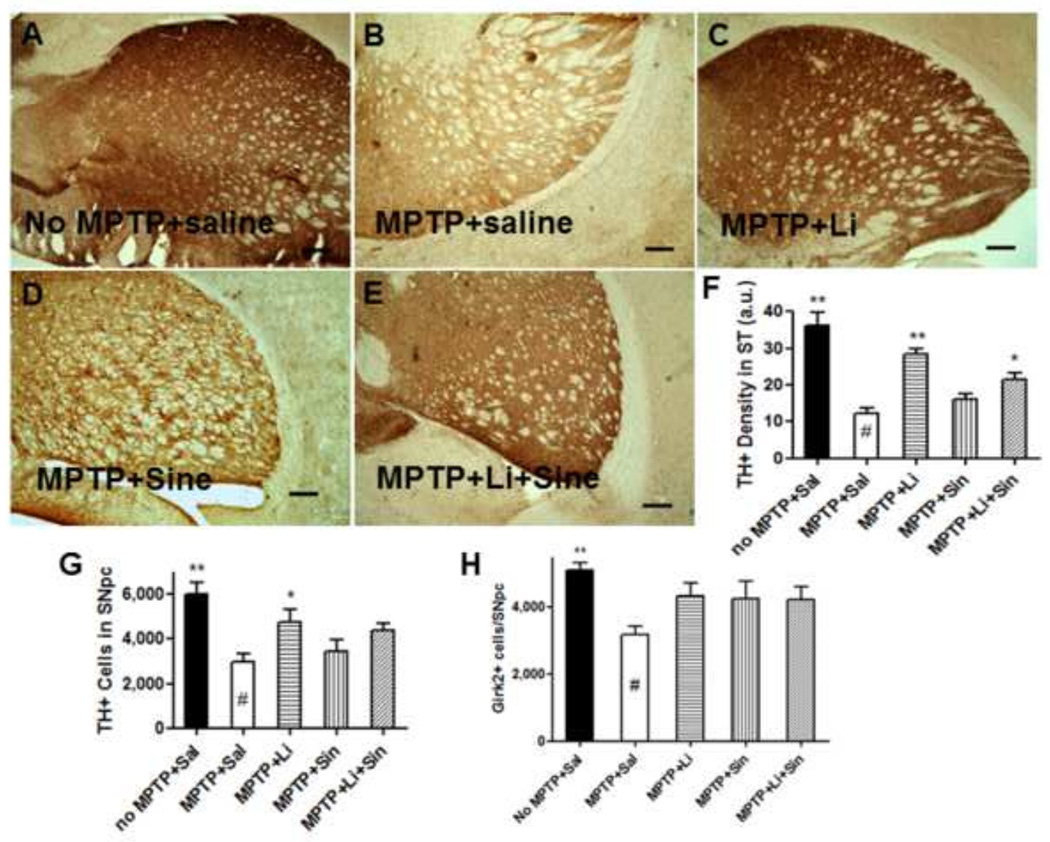
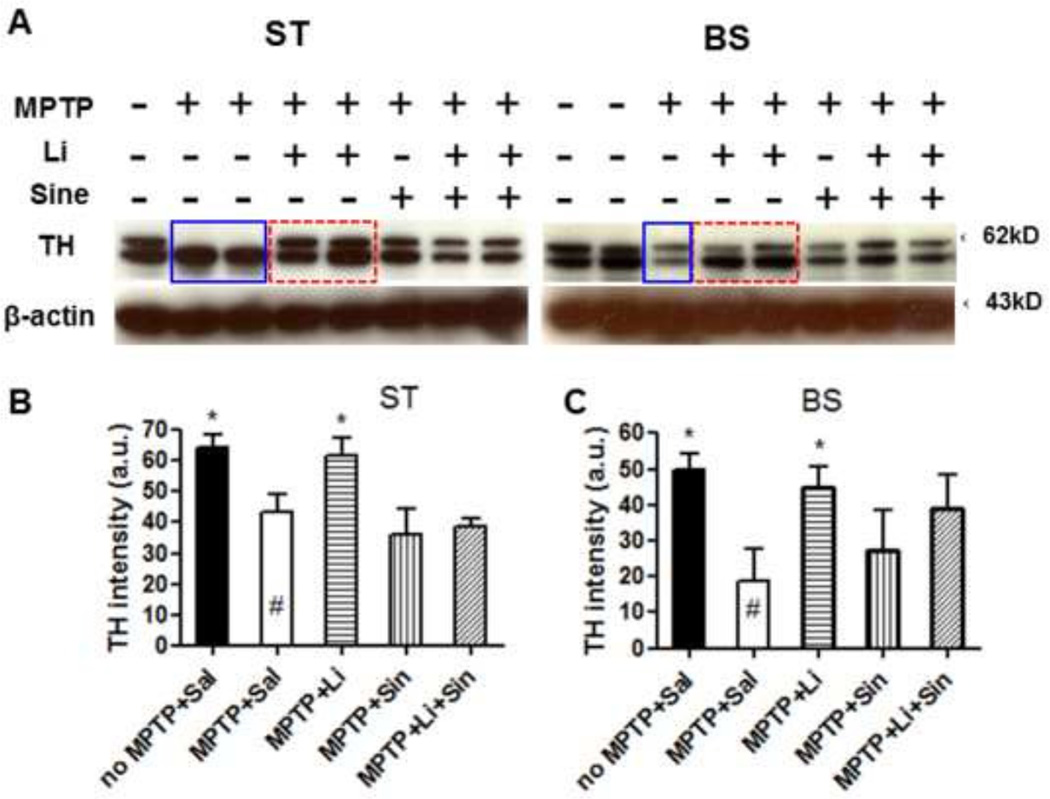
Following 5 days of chronic MPTP injection, lithium feeding, L-Dopa/Carbidopa daily injections or both were administered for a 2 month period. Lithium administration was found to significantly up-regulate TH protein levels in the ST in the presence or absence of L-Dopa/Carbidopa, compared with MPTP-saline controls (Fig. 2B, C, and E). In the ST, L-Dopa/Carbidopa alone did not significantly impact TH levels compared with MPTP-saline control (Fig. 2B vs. E, see Fig. 2F). In the Substantia Nigra pars compacta (SNpc), we also found higher numbers of TH+ cells in the MPTP+Lithium group compared with the MPTP+Saline control (Fig. 2G, p<0.05). However, this finding was not confirmed by another dopaminergic cell counting method using Girk2 staining, although the trend was clearly observed in the lithium treated group (Fig. 2H). In addition to the immunohistochemistry and stereological analysis of TH levels, we also performed Western blot analysis in order to quantify TH protein expression levels in both the ST and the brain stem (BS). These data show that lithium up-regulates TH expression, while L-Dopa/Carbidopa may either not affect TH expression or provide partial negative feedback to the lithium-induced TH up-regulation (Fig. 3A) (Blunt et al., 1991; Riverol et al., 2014; Lieu et al., 2014). Therefore, we found statistically significant increases only in lithium-fed mice compared with saline controls following MPTP exposure (Fig. 3B, C).
Figure 2.
Lithium up-regulates TH protein expression levels in the striatum (ST) and the brain stem (BS) in vivo. No MPTP+Saline (A), MPTP+Saline (B), MPTP+Lithium (C), MPTP+Sinemet® (D), and, MPTP+Lithium+Sinemet® (E). Background corrected density of TH immunostaining in the ST was quantitated (F). Numbers of TH+ cells (G) and Girk2+ cells (H) in the SNpc were stereological counted and quantitated via one-way ANOVA, Dunnett’s post hoc test (n=6–8 each): *: p<0.05, **: p<0.01, Bar: 250 µm.
Figure 3.
TH protein levels are up-regulated by lithium in both the ST and the BS as assessed by Western blot analysis (A). Relative TH intensity was measured in the ST (B) and the BS (C) and normalized for β-actin. One-way ANOVA, Dunnett’s post hoc test (n=6–8 each): *, p<0.05, **, p<0.01.
2.3. Lithium suppresses calpain activity in vivo and in vitro
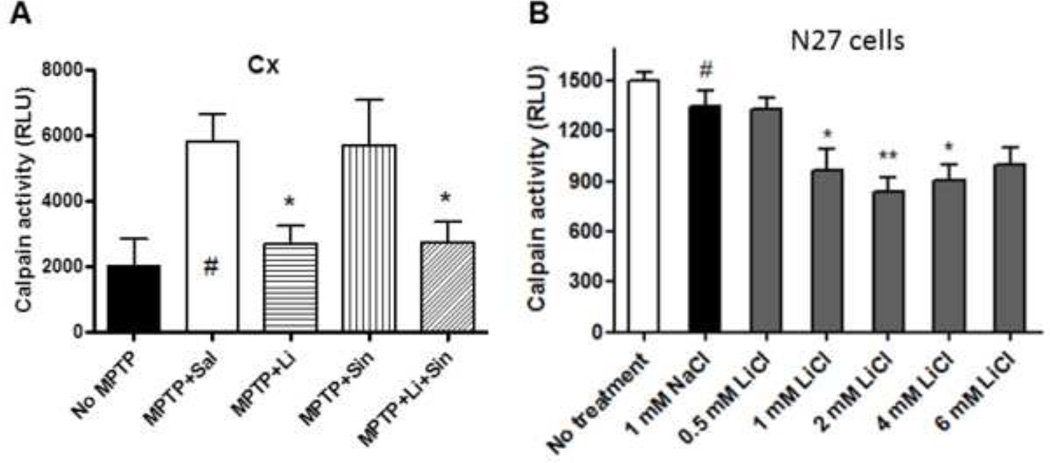
To better understand the potential mechanisms by which lithium may be eliciting TH up-regulation and reducing AIMs, we assessed calpain activities with different treatments in in vivo samples following exposure to MPTP and in in vitro N27 cells with different concentrations of lithium. In samples of the whole brain tissue, we found that lithium significantly suppresses MPTP-induced calpain activity regardless of the presence of L-Dopa/Carbidopa (p<0.05, Fig. 4A). We used the whole brain tissue without cerebellum for the calpain activity assay without treating protease inhibitors since we had limited amounts of total proteins from the ST and the BS. We also found that calpain activity was significantly inhibited in 1–4 mM LiCl-treated N27 cells. However, concentrations higher than 4 mM LiCl were less effective in suppressing calpain activity, as previously reported (*: p<0.05, **: p<0.01, Fig. 4B) (Kim et al., 2011a; Nciri et al., 2012).
Figure 4.
Lithium suppresses calpain activity in the whole brain tissues (A) in vivo and in N27 cells in vitro (B). Mean density ± SEM after background correction (a.u.: arbitrary unit), One-way ANOVA, Dunnett’s test: *: p<0.05, compared with MPTP + Saline (#) or 1 mM NaCl control (#), n=4–8 in each group.
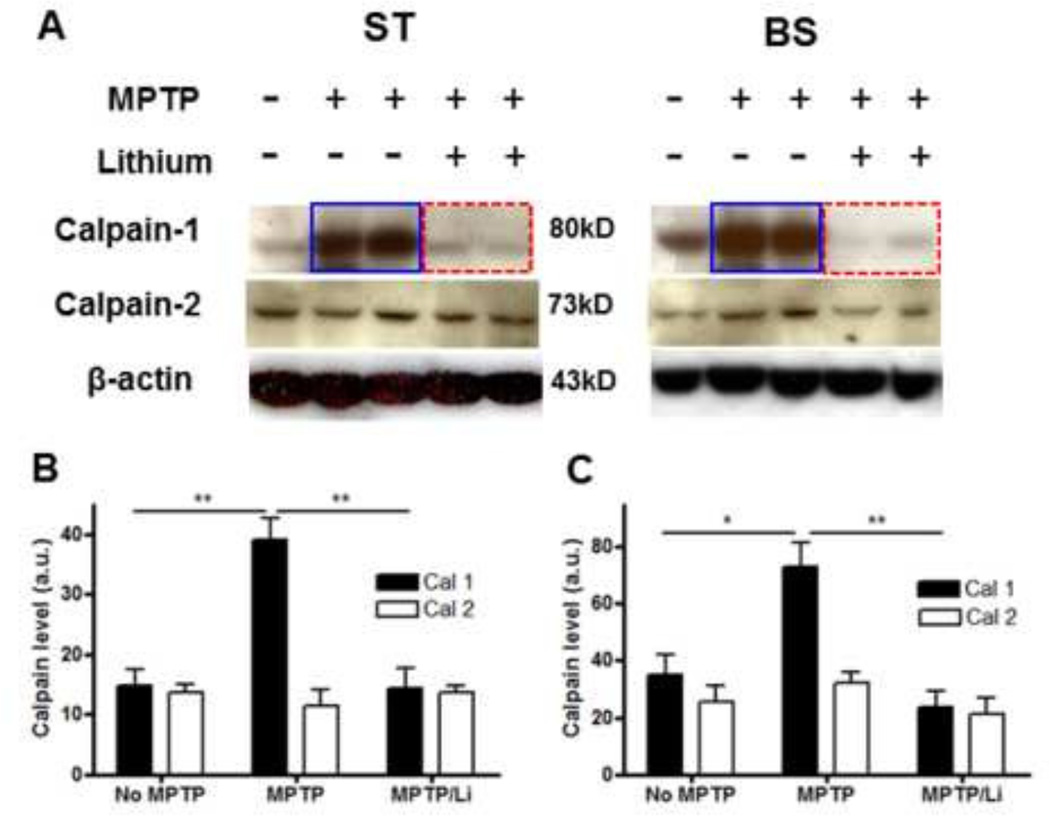
2.4. Lithium suppresses MPTP-induced calpain-1 but not calpain-2 expression in the ST and the BS
Next, we addressed the following questions: (1) what calpain isoforms are down-regulated by lithium following MPTP exposure and (2) whether the reduced activity is based on reductions in protein expression levels or not. In Western blot analysis, we found that calpain 1 but not calpain 2 expression was substantially suppressed by lithium in both the ST and the BS, as Kanagaraj et al. reported, 2014 (Fig. 5A). In conclusion, MPTP-induced calpain 1 up-regulation in the expression level was attributed to its higher activity and was significantly reduced in lithium-treated animals in both the ST (Fig. 5B) and the BS (Fig. 5C, *: p<0.05, **: p<0.01).
Figure 5.
Lithium suppresses MPTP-induced calpain 1 but not calpain 2 protein expression levels in both the ST and the BS. Examples of relative calpain 1 and 2 levels in Western blot analysis (A); density analysis was performed for the ST (B) and for the BS (C). Relative density is reported as mean ± SEM (n=4–6 in each group). *, p< 0.05, **, p < 0.01 versus controls as assessed by one-way ANOVA Tukey’s multiple comparison test.
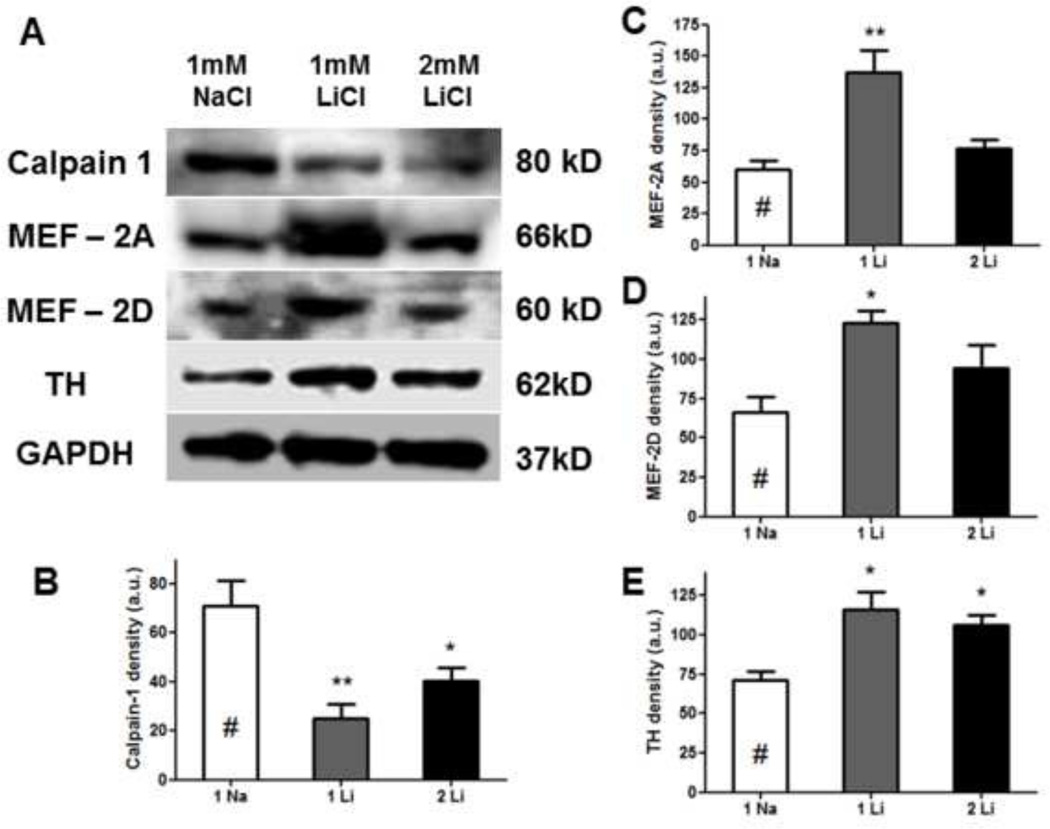
2.5. In Western blot analysis, Lithium suppresses calpain 1 expression while up-regulating MEF2 and TH in vitro
Western blot analysis of N27 cell cultures treated with 1mM LiCl or 2 mM LiCl for 24 hours showed down-regulation of calpain 1 in both concentrations, compared with 1mM NaCl control (*: p<0.05, **: p<0.01, Fig. 6B). Lithium also up-regulated both MEF-2A and MEF-2D, but only when the concentration of treatment was optimal (e.g. 1 mM LiCl, Fig. 6C, D). We consistently found that lithium induced a concomitant increase in TH expression (Fig. 6E) (Lieu et al., 2014).
Figure 6.
In Western blot analyses, lithium suppresses MPTP-induced calpain 1 expression, but up-regulates MEF 2A and 2D and tyrosine hydroxylase (TH) levels in rat dopaminergic N27 cells (A). As shown by quantitative density analyses, 1mM and/or 2mM LiCl inhibits calpain-1 expression (B), increases MEF-2A (C), MEF-2D (D), and TH (E) levels following normalization with the loading control, GAPDH. One-way ANOVA, Dunnett’s post-hoc test (n=3–4): *: p<0.05, **: p<0.01.
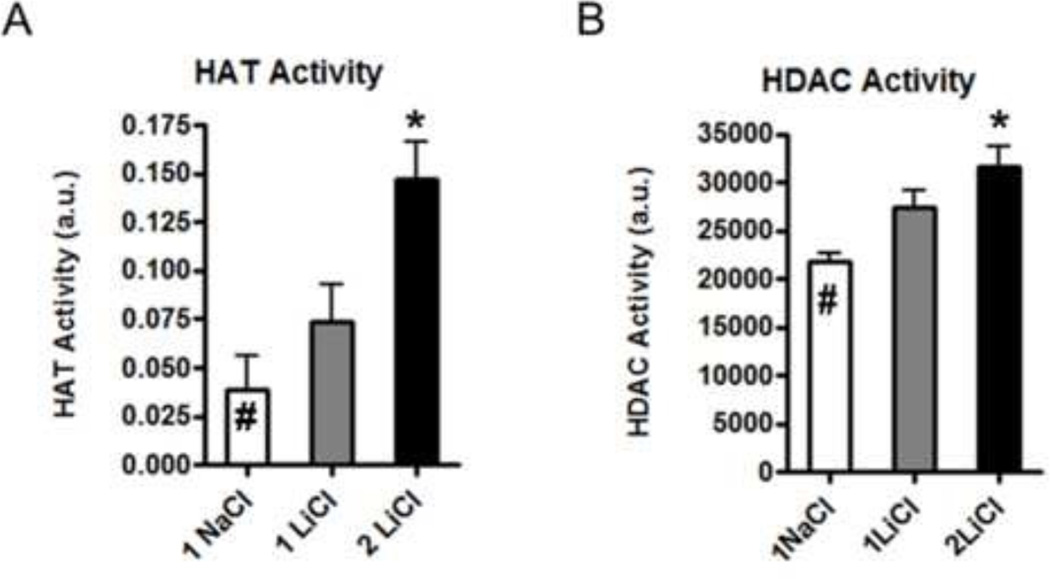
2.6. Lithium increases HAT activity relative to HDAC activity in vitro
After observing up-regulation of the transcription factors MEF-2A and -2D, we decided to assess whether this coincided with alterations in the activity of epigenetic regulation factors including HAT and HDAC. Based on enzymatic assays, we found that both were up-regulated by lithium in the dose-dependent manner, however, HAT activity was up-regulated by 400% while HDAC activity was increased by only 30% (Fig. 7).
Figure 7.
Lithium treatment (2mM LiCl) in the N27 cell cultures increased the HAT activity by 400% and HDAC activity by 30% compared to the sodium treated control (#). One-way ANOVA, Dunnett’s post hoc test (n=3 each): *, p<0.05, compared to control.
3. Discussion
In this study, we demonstrated that combined lithium (0.127% LiCl in food) and L-Dopa/Carbidopa (IP injection: 100 mg/kg levodopa and 25 mg/kg carbidopa) therapy significantly reduced MPTP-induced AIMs in mice as indicated by improvements in the hindlimb clasping test (Fig. 2, video data are supplemented). The combination therapy not only reduces MPTP-induced AIMs but also increases TH expression in both the ST and the brainstem (Farah et al., 2013; Lieu et al., 2014). We found that lithium treatment protects dopaminergic neurons in both the ST and the SNpc against MPTP-induced neurotoxicity, based on TH-labeled cell count. Lithium treatment, however, did not induce higher number of dopaminergic cells in the SNpc, based on Girk2 staining. These results suggest that higher number of TH+ cells induced by lithium within the SNpc is likely due to TH up-regulation in the region (n=6–9 in each group, Fig. 2G, H). Taken in total, our data suggest that relatively low-dose lithium (0.127 % LiCl in food) may not only induce dopamine synthesis, which can slow down the disease-associated neuropathologies, but also reduce MPTP-induced AIMs, perhaps by providing more uniform levels of dopamine than the fluctuating levels achieved with L-Dopa administration alone (Koh et al., 2008).
While a number of recent reports have indicated that lithium may be a potential therapeutic for various neurodegenerative diseases, in a few reported cases lithium treatment was suggested to be detrimental to PD patients and to cause side effects (Brandt-Christensen et al., 2006; Shen et al., 2007). However, lithium-induced side effects were reported to disappear after lowering the dosage or stopping the treatment. In order to reduce potential side effects of lithium, we lowered the concentration of LiCl to 0.127% in the feed from the previous study (0.255%) (Kim et al., 2011a). In the MPTP model, the combination therapy using the lower lithium dosage reduced MPTP-induced AIMs compared with lithium- and L-Dopa/Carbidopa only-treatment. Reported lithium dosages generally used for treatment of bipolar disorder (900–1,800 mg per day; 0.6–1.2 mM in the serum) are significantly higher than what we used in this current study, which was calculated to be in the range of 0.4–0.6 mM in serum (Riadh et al., 2011). We additionally observed none of the reported side effects of lithium therapy including diarrhea, copious urination, and other kidney or liver related symptoms from 0.127% lithium fed animals.
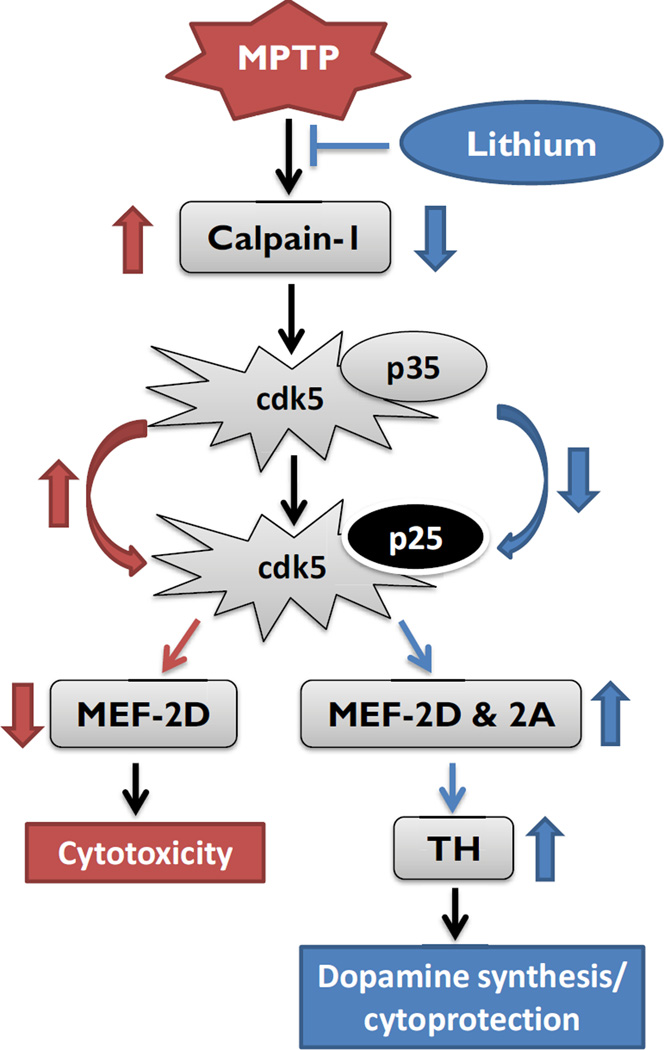
In this study, we further investigated the mechanism of lithium in inducing neuroprotective effects in PD models (Kim et al., 2011a; Lieu et al., 2014). Lithium-mediated increases in TH protein levels and reductions in MPTP-induced AIMs were found to occur in conjunction with suppression of calpain 1 expression in the ST and the SN of MPTP-lesioned mice (Farah et al., 2013). Calpains are widely expressed in the central nervous system and are essential for many physiological neuronal functions other than abnormally enhanced cdk5 activity (Kusakawa et al., 2000). The MPTP injection has been reported to up-regulate calpains, particularly calpain-1 in the SNc, which is translationally regulated, probably not transcriptionally (Kanagaraj et al., 2014). Calpains are known to cleave its co-activator p35 to the p25 isoform, which is involved in activating cdk5 and cell death (Camins et al., 2009). Activated cdk5 can in turn phosphorylate the transcription factor myocyte enhancer factor 2 (MEF2), making it inactive for protective transcriptional signals (Crespo-Biel et al., 2009; Chagniel et al., 2012). The phosphorylated form of MEF2D on Ser444 normally functions in the activation of genes involved in neuronal death and functional loss, including dopaminergic neurons (Smith et al., 2006). Further, a potent MEF-2D activator, B3C prevented the MPTP-induced TH signal loss in the SN (Yao et al., 2012). Lithium has been shown to inhibit calpain and cdk5 activity in primary cell cultures and in vivo (Jorda et al., 2005). Based on these data, we hypothesize that lithium may act as a suppressor in the upstream of the calpain-p35-p25/cdk5-mediated pathway for MEF2 activation (Camins et al., 2009) (Fig. 8). The up-regulation of TH may be mediated via calpain 1 inhibition, but reduced 20S proteasomal activity or enhanced neurogenesis may also play a role in reducing AIMs (data not shown) (Rice and Sartorelli, 2001; Alvira et al., 2008; Crespo-Biel et al., 2009). In addition, we recently reported that lithium inhibits glial activation, increased by chronic insults in striatum (Lieu et al., 2014).
Figure 8.
A schematic view of putative mechanisms involved in lithium-induced dopamine synthesis/cytoprotection. Red arrows indicate the up/down-regulation by MPTP and blue arrows indicate the regulation by lithium. Abb.: cdk5: cyclin-dependent kinase-5; MEF2: myocyte enhancer factor 2; TH: tyrosine hydroxylase.
An important post-translational histone modification impacting on gene transcription is the reversible process of lysine acetylation. The enzyme responsible for this acetylation event is histone acetyltransferase (HAT); histone deacetylase (HDAC) catalyzes the reverse reaction. In the normal cellular environment, HAT and HDAC protein concentrations and activity remain in a balanced state, which is considered to provide neuronal homeostasis, and are responsible for controlled gene expression (Saha and Pahan, 2006). However, histone acetylation levels tend to decrease in a variety of neurodegenerative diseases in relation to HDAC activity, thereby unbalancing the acetylation homeostasis (Rouaux et al., 2003). In Parkinson’s disease, it has been shown that alpha-synuclein binds directly to histones, reducing levels of acetylated histone H3, and inhibiting HAT-mediated acetyltransferase activity associated with gene expression (Kontopoulos et al., 2006; Siddiqui et al., 2012). Here we found that 2 mM LiCl treatment induced a four-fold increase in HAT activity, although the subtype(s) involved were not elucidated. These results suggest that lithium stimulates HAT expression, which may result in increased level of transcription factors such as MEF-2A or 2D which in turn may be implicated in the up-regulation of TH gene expression for cellular differentiation and survival (Jorda et al., 2005; Crespo-Biel et al., 2009; Riadh et al., 2011). While an imbalance of HAT/HDAC expression can lead to disease states, there is evidence that the down-regulation of HDAC activity relative to that of HAT induces the expression of multiple downstream targets that may function collectively to provoke neuroprotective effects (Abel and Zukin, 2008; McColl et al., 2008). In our previous report, we demonstrated that lithium prevented or degraded paraquat/maneb-induced alpha-synuclein aggregation in the brainstem and the striatum in mice (Kim et al., 2011a). Here we found that lithium reduces MPTP-induced AIMs in a mouse model, probably via a pathway mediated by calpain 1 inhibition, cdk5-mediated MEF2 increase, and TH up-regulation in both the ST and the SN (Fig. 8). In addition, lithium also substantially increased the HAT activity, compared with moderate increase of HDAC activity. Based on the presented data, we propose that combining lithium treatment with the widely used PD drug L-Dopa/Carbidopa may induce not only neuroprotective effects but also reduce AIMs and possibly other potential side effects associated with L-Dopa/Carbidopa treatment.
4. Experimental procedures
4.1. MPTP treatment and lithium feeding
All animal protocols were conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals; all procedures were approved by the Buck Institute Animal Care and Use Committee. All efforts were made to minimize animal numbers and distress. C57BL/6J male mice (8 months of age) were obtained from Charles River Laboratory (Wilmington, MA) and intraperitoneally injected with 25 mg/kg 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, Sigma, St. Louis, MO, n=40) or saline (n=8) daily on 5 consecutive days. Three days following the last MPTP injection, MPTP-treated and age-matched control littermates were both fed either mouse chow (PicoLab Rodent Diet 20, Purina 5053, Richmond, IN) containing 0.127% lithium chloride (Kim et al., 2011a) or the same chow without LiCl (n=20 in each group) for 2 months. In addition, half of those mice in each group were also intraperitoneally injected with L-Dopa/Carbidopa (Sinemet®: 100 mg/kg levodopa and 25 mg/kg Carbidopa) or vehicle (saline) daily (6 times a week) starting at 9 months of age (Shin et al., 2009). Groups included: 1) No MPTP with vehicle injection, 2) MPTP injection, followed by regular food and vehicle injection, 3) MPTP injection, followed by 0.127% LiCl feeding with vehicle injection, 4) MPTP injection, followed by daily L-Dopa/Carbidopa injection, or 5) MPTP injection, followed by lithium feeding and L-Dopa/Carbidopa injection (Fig. 1A). Body weight was monitored once a week; no mice experienced more than a 10% loss of body weight after the 2nd week.
4.2. Hindlimb clasping test
To assess animals for alterations in motor behavior, we used the hindlimb clasping test. Animals were picked up 2 cm from the tip of tail for 15 seconds, ~20 cm above the floor on 3 consecutive days. Recorded videos were analyzed by a rater who was blind to the status of each mouse to measure severity of clasping (0: no clasping, 1: mild, and 2: severe) and to count the average numbers of clasps (one leg clasping only: 0.5 and two legs clasping simultaneously: 1.0) over a 10 second period (Fernagut et al., 2002).
4.3. Immunohistochemistry of tyrosine hydroxylase (TH) and G protein-activated inward rectifier potassium channel 2 (Girk 2) in the striatum and the substantia nigra
For immunohistochemical analyses, brains perfused in 150 mM NaCl/70% ethanol were post-fixed in 4% paraformaldehyde overnight, followed by incubation in 30% sucrose for 2 days before embedding in OCT (optimum cutting temperature, Tissue Tek, Sakura Finetek USA, Torrance, CA); samples were kept at −80 °C until sectioning. Tissues from each experimental group were sectioned at 40 µm using a cryostat on the same set of slides to minimize slide variability. Rabbit poly-clonal anti-TH (1:200 dilution, Millipore, Temecula, CA) or rabbit anti-Girk2 (1:200 diluted poly-clonal, ab30738, Abcam, Cambridge, MA) antibodies were incubated at 4 °C overnight, followed by incubation in 1:200 diluted anti-rabbit biotinylated secondary antibody (Chemicon, Temecula, CA) for both. Label intensity was enhanced via incubation in 1:250 dilution of Vectastain ABC kit solution (Vector Laboratories inc., Burlingame, CA) for 60 minutes followed by development in diaminobenzidine (DAB, Chemicon).
4.4. Tissue extraction and Western blot analysis of TH and calpain 1 & 2 using region-specifically isolated tissues
Brain stem, striatal, and the whole brain tissues were isolated from one half of each brain for protein extraction, and the other half of each brain was used for immunohistochemistry (see below). All procedures for tissue extraction were performed as previously described (Kim et al., 2011b). Brain tissues were extracted in the lysis buffer (1× PBS, 1% Triton X-100, 0.1% NP-40, pH 7.4, without protease inhibitors for the calpain activity assay), and 20 µg (TH) or 60 µg (calpains) of total proteins from each sample were loaded onto 10% SDS-PAGE gels (BioRad, Hercules, CA) and transferred to PVDF membranes (Millipore, Billerica, MA) at 4 °C overnight for TH and calpain Western blots. Primary antibodies used include rabbit anti-TH (1:1,000 dilution, Millipore: AB152, Temecula, CA), mouse anti-calpain 1 (1:1,000 dilution, Millipore: MAB 3104), goat anti-calpain 2 (1:400 dilution, Santa Cruz Biotechnology: sc-7533, Santa Cruz, CA) and mouse anti-β-actin (1:12,000 dilution, Millipore: MAB 1501), the latter as a loading control. We used different secondary antibodies such as anti-mouse or -rabbit IgG-HRP (1:1,000 for TH and calpain-1, 1:500 for calpain-2 and 1:12,000 dilutions for β-actin) for detecting primary antibodies. Membranes were developed as described previously (Kim et al., 2011b). The band intensity in Western blots were quantified using Image J, after calibrating the background and loading controls.
4.5. Calpain and histone acetyltransferase (HAT)/histone deacetylase (HDAC) activity assays
Rat dopaminergic N27 parental cells were cultured in 0.5, 1, 2, 4, or 6 mM LiCl versus 1 mM NaCl or untreated controls in the cell culture media (10% FBS in RPMI 1640, Invitrogen, Carlsbad, CA) for 48 hours, followed by cell extraction. We added 40 µg total proteins from the in vitro cell extracts and 80 µg from in vivo the whole brain samples to 2 mM CaCl2 for the Calpain-Glo protease activity assay ; the assay is a homogeneous, luminescent assay that measures calpain 1 and 2 activities and other steps were followed as recommended by the manufacturer (Promega G8501, Madison, WI). Due to the limit of collecting high concentration of total protein from the midbrain and the striatum, we used the whole brain tissue except cerebellum for measuring Calpain enzyme activities without adding protease inhibitors. Following cell extraction, nuclear fraction isolation was performed using the NE-PER Nuclear and Cytoplasmic Extraction Reagent (Thermo Scientific, cat. no. 78833); steps were performed as recommended by the manufacturer. The nuclear extract was used to measure histone acetyltransferase (HAT) and histone deacetylase (HDAC) activities using the PromoKine HAT Activity Assay Kit (cat. no. RK-CA577-K332, Promega) and the HDAC Fluorescent Assay Kit (cat. no. 56200, Active Motif), respectively. The protein samples were incubated at 37 °C for 3 hours. Both assays were performed as recommended by the manufacturers.
4.6. Stereological cell count analyses
Following TH or Girk2 staining, dopaminergic neurons were stereologically counted using the optical fractionator method (Stereo Investigator, MBF Bioscience, Williston, VT) to quantitatively compare numbers after MPTP versus drug-treated samples as previously described (Kim et al., 2011a). For density measurement of TH+ neurites in the striatum (ST) and TH+ cells in the SNpc, all stereological cell counts/measurements were performed by a rater who was blind to the treatments; they are presented as mean labeled cell number (or density) ± SEM (n=6–8 per group).
4.7. Statistical analyses
The hindlimb clasping tests such as severity index and average instances were statistically evaluated between pre- and post-treatment using unpaired t-test and displayed in mean ± SEM (Fig. 1B and C, n=6–10 per group). One-way ANOVA Tukey’s multiple comparison was adopted in Figure 6. In the analyses of TH density, TH+ cell counts in the ST and SNpc and the HAT and HDAC activity assays, one-way ANOVA followed with the post hoc analysis Dunnett’s t-test was used for significance, compared with MPTP-sal or the 1 mM NaCl control (#) in Figures 2, 3, 4, 5 and 7 (n=4–7). For all studies, p<0.05 was considered statistically significant; GraphPad Prism 5 software was used for all data analyses and display.
Supplementary Material
Lithium reduces MPTP-induced abnormal involuntary movements (AIMs) in a mouse model
Lithium’s effect is mediated by calpain 1 inhibition
Lithium increases the HAT activity and tyrosine hydroxylase expression
Combining lithium with L-Dopa/Carbidopa may be a viable option for PD
Acknowledgments
We thank Dr. Alan Hubbard at the University of California, Berkeley for statistical discussion for this paper. This study was supported by NIH-5P20GM103653-02 (YHK) and NIH-RL1 NS062415 (JKA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008 Feb;8(1):57–64. doi: 10.1016/j.coph.2007.12.002. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Alvira D, Ferrer I, Gutierrez-Cuesta J, Garcia-Castro B, Pallas M, et al. Activation of the calpain/cdk5/p25 pathway in the girus cinguli in Parkinson's disease. Parkinsonism Relat Disord. 2008;14:309–313. doi: 10.1016/j.parkreldis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Blunt SB, Jenner P, Marsden CD. The effect of L-dopa and carbidopa treatment on the survival of rat fetal dopamine grafts assessed by tyrosine hydroxylase immunohistochemistry and [3H]mazindol autoradiography. Neuroscience. 1991;43(1):95–110. doi: 10.1016/0306-4522(91)90420-s. [DOI] [PubMed] [Google Scholar]
- Bogaerts V, Theuns J, van Broeckhoven C. Genetic findings in Parkinson's disease and translation into treatment: a leading role for mitochondria? Genes Brain Behav. 2008;7:129–151. doi: 10.1111/j.1601-183X.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt-Christensen M, Kvist K, Nilsson FM, Andersen PK, Kessing LV. Treatment with antidepressants and lithium is associated with increased risk of treatment with antiparkinson drugs: a pharmacoepidemiological study. J Neurol Neurosurg Psychiatry. 2006;77:781–783. doi: 10.1136/jnnp.2005.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camins A, Crespo-Biel N, Junyent F, Verdaguer E, Canudas AM, Pallàs M. Calpains as a target for therapy of neurodegenerative diseases: putative role of lithium. Curr Drug Metab. 2009 Jun;10(5):433–447. doi: 10.2174/138920009788898028. Review. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Wanamaker WM. L-dopa-carbidopa: combined therapy for the treatment of Parkinson's disease. Dis Nerv Syst. 1976 Mar;37(3):123–125. [PubMed] [Google Scholar]
- Chagniel L, Robitaille C, Lebel M, Cyr M. Striatal inhibition of calpains prevents levodopa-induced neurochemical changes and abnormal involuntary movements in the hemiparkinsonian rat model. Neurobiol Dis. 2012;45:645–655. doi: 10.1016/j.nbd.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Ross DR, Ferren EL, Sullivan JL, Olanow CW. Treatment of the "on-off" phenomenon in Parkinsonism with lithium carbonate. Ann Neurol. 1982;12:375–379. doi: 10.1002/ana.410120410. [DOI] [PubMed] [Google Scholar]
- Crespo-Biel N, Camins A, Pallas M, Canudas AM. Evidence of calpain/cdk5 pathway inhibition by lithium in 3-nitropropionic acid toxicity in vivo and in vitro. Neuropharmacology. 2009;56:422–428. doi: 10.1016/j.neuropharm.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, et al. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J Neurosci. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, et al. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54:S21–S23. [PubMed] [Google Scholar]
- Farah R, Khamisy-Farah R, Amit T, Youdim MB, Arraf Z. Lithium's gene expression profile, relevance to neuroprotection A cDNA microarray study. Cell Mol Neurobiol. 2013 Apr;33(3):411–420. doi: 10.1007/s10571-013-9907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Stefanova N, Biran M, Wenning GK, et al. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: behavioural and histopathological characterisation. Neuroscience. 2002;114:1005–1017. doi: 10.1016/s0306-4522(02)00205-1. [DOI] [PubMed] [Google Scholar]
- Ferrucci M, Spalloni A, Bartalucci A, Cantafora E, Fulceri F, et al. A systematic study of brainstem motor nuclei in a mouse model of ALS, the effects of lithium. Neurobiol Dis. 2010;37:370–383. doi: 10.1016/j.nbd.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Jorda EG, Verdaguer E, Canudas AM, Jimenez A, Garcia de Arriba S, et al. Implication of cyclin-dependent kinase 5 in the neuroprotective properties of lithium. Neuroscience. 2005;134:1001–1011. doi: 10.1016/j.neuroscience.2005.04.061. [DOI] [PubMed] [Google Scholar]
- Kanagaraj N, Beiping H, Dheen ST, Tay SS. Downregulation of miR-124 in MPTP-treated mouse model of Parkinson's disease and MPP iodide-treated MN9D cells modulates the expression of the calpain/cdk5 pathway proteins. Neuroscience. 2014;11:272, 167–179. doi: 10.1016/j.neuroscience.2014.04.039. [DOI] [PubMed] [Google Scholar]
- Kim YH, Rane A, Lussier S, Andersen JK. Lithium protects against oxidative stress-mediated cell death in alpha-synuclein-overexpressing in vitro and in vivo models of Parkinson's disease. J Neurosci Res. 2011a;89:1666–1675. doi: 10.1002/jnr.22700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Lussier S, Rane A, Choi SW, Andersen JK. Inducible dopaminergic glutathione depletion in an alpha-synuclein transgenic mouse model results in age-related olfactory dysfunction. Neuroscience. 2011b;172:379–386. doi: 10.1016/j.neuroscience.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SH, Song C, Noh MY, Kim HY, Lee KY, et al. Inhibition of glycogen synthase kinase-3 reduces L-DOPA-induced neurotoxicity. Toxicology. 2008;247:112–118. doi: 10.1016/j.tox.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kontopoulos E, Parvin JD, Feany MB. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet. 2006 Oct 15;15(20):3012–3023. doi: 10.1093/hmg/ddl243. [DOI] [PubMed] [Google Scholar]
- Kusakawa G, Saito T, Onuki R, Ishiguro K, Kishimoto T, et al. Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem. 2000;275:17166–17172. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- Li X, Bijur GN, Jope RS. Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection. Bipolar Disord. 2002;4:137–144. doi: 10.1034/j.1399-5618.2002.40201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu CA, Dewey CM, Chinta SJ, Rane A, Rajagopalan S, Batir S, Kim YH, Andersen JK. Lithium prevents parkinsonian behavioral and striatal phenotypes in an aged parkin mutant transgenic mouse model. Brain Res. 2014;3:1591, 111–117. doi: 10.1016/j.brainres.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl G, Killilea DW, Hubbard AE, Vantipalli MC, Melov S, Lithgow GJ. Pharmacogenetic analysis of lithium-induced delayed aging in Caenorhabditis elegans. J Biol Chem. 2008;4(1):350–357. doi: 10.1074/jbc.M705028200. 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouatt-Prigent A, Karlsson JO, Agid Y, Hirsch EC. Increased M-calpain expression in the mesencephalon of patients with Parkinson's disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience. 1996;73:979–987. doi: 10.1016/0306-4522(96)00100-5. [DOI] [PubMed] [Google Scholar]
- Nciri R, Desmoulin F, Allagui MS, Murat JC, Feki AE, et al. Neuroprotective effects of chronic exposure of SH-SY5Y to low lithium concentration involve glycolysis stimulation, extracellular pyruvate accumulation and resistance to oxidative stress. Int J Neuropsychopharmacol. 2012:1–12. doi: 10.1017/S1461145712000132. [DOI] [PubMed] [Google Scholar]
- Pies R. Have we undersold lithium for bipolar disorder? J Clin Psychopharmacol. 2002;22:445–449. doi: 10.1097/00004714-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Riadh N, Allagui MS, Bourogaa E, Vincent C, Croute F, et al. Neuroprotective and neurotrophic effects of long term lithium treatment in mouse brain. Biometals. 2011;24:747–757. doi: 10.1007/s10534-011-9433-6. [DOI] [PubMed] [Google Scholar]
- Rice AM, Sartorelli AC. Inhibition of 20 S and 26 S proteasome activity by lithium chloride: impact on the differentiation of leukemia cells by all-trans retinoic acid. J Biol Chem. 2001;276:42722–42727. doi: 10.1074/jbc.M106583200. [DOI] [PubMed] [Google Scholar]
- Riverol M, Ordóñez C, Collantes M, DiCaudo C, Peñuelas I, Arbizu J, Marcilla I, Luquin MR. Levodopa induces long-lasting modification in the functional activity of the nigrostriatal pathway. Neurobiol Dis. 2014 Feb;62:250–259. doi: 10.1016/j.nbd.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Rouaux C, Jokic N, Mbebi C, Boutillier S, Loeffler JP, Boutillier AL. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. EMBO J. 2003 Dec 15;22(24):6537–6549. doi: 10.1093/emboj/cdg615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ. 2006 Apr;13(4):539–550. doi: 10.1038/sj.cdd.4401769. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC, Li JY, Lo YK. Lithium intoxication-induced acute parkinsonism complicated with hyperparathyroidism and nephrogenic diabetes insipidus: report of a case. Acta Neurol Taiwan. 2007;16:231–233. [PubMed] [Google Scholar]
- Shin JY, Park HJ, Ahn YH, Lee PH. Neuroprotective effect of L-dopa on dopaminergic neurons is comparable to pramipexol in MPTP-treated animal model of Parkinson's disease: a direct comparison study. J Neurochem. 2009;111:1042–1050. doi: 10.1111/j.1471-4159.2009.06381.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Chinta SJ, Mallajosyula JK, Rajagopolan S, Hanson I, Rane A, Melov S, Andersen JK. Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson's disease. Free Radic Biol Med. 2012 Aug 15;53(4):993–1003. doi: 10.1016/j.freeradbiomed.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF. Antagonistic effect of lithium chloride on l-dopa-induced locomotor activity in rats. Pharmacol Res Commun. 1976;8:575–579. doi: 10.1016/0031-6989(76)90049-7. [DOI] [PubMed] [Google Scholar]
- Smith PD, Mount MP, Shree R, Callaghan S, Slack RS, Anisman H, Vincent I, Wang X, Mao Z, Park DS. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2006 Jan 11;26(2):440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajsbort J, Dorner A, Wajsbort E. A comparative clinical investigation of the therapeutic effect of levodopa alone and in combination with a decarboxylase inhibitor (carbidopa) in cases of Parkinson's disease. Curr Med Res Opin. 1978;5(9):695–708. doi: 10.1185/03007997809110209. [DOI] [PubMed] [Google Scholar]
- Yao L, Li W, She H, Dou J, Jia L, He Y, Yang Q, Zhu J, Cápiro NL, Walker DI, Pennell KD, Pang Y, Liu Y, Han Y, Mao Z. Activation of transcription factor MEF2D by bis(3)-cognitin protects dopaminergic neurons and ameliorates Parkinsonian motor defects. J Biol Chem. 2012;287(41):34246–34255. doi: 10.1074/jbc.M112.367540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Arraf Z. Prevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and Bax. Neuropharmacology. 2004;46:1130–1140. doi: 10.1016/j.neuropharm.2004.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.