Abstract
Highly active and recoverable nanobioreactors prepared by immobilizing rat liver microsomes on magnetic nanoparticles (LMMNPs) were utilized in metabolic study of Angelica dahurica extracts. Five metabolites were detected in the incubation solution of the extracts and LMMNPs, which were identified by means of HPLC-MS as trans-imperatorin hydroxylate (M1), cis-imperatorin hydroxylate (M2), imperatorin epoxide (M3), trans-isoimperatorin hydroxylate (M1’), and cis-isoimperatorin hydroxylate (speculated M2’), respectively. Comparing to the metabolisms of imperatorin and isoimperatorin, it was found that those five metabolites were all transformed from these two major compounds presented in the plant. Since no study on isoimperatorin metabolism by liver microsomal enzyme system has been reported so far, its metabolites (M1’ and M3’) were isolated by preparative HPLC for structure elucidation by 1H-NMR and MS2 analysis. M3’ was identified as isoimperatorin epoxide, which is a new compound as far as its chemical structure is concerned. But interestingly, M3’ was not detected in the metabolism of the whole plant extract. In addition, study with known chemical inhibitors on individual isozymes of the microsomal enzyme family revealed that CYP1A2 was involved in metabolisms of both isoimperatorin and imperatorin, while CYP3A4 only in that of isoimperatorin.
Keywords: Angelica dahurica, furocoumarins, metabolites, immobilized liver microsomes, magnetic nanoparticles, HPLC-MS
Introduction
Metabolization of the active ingredients of herbal medicines as well as phytochemical research is important to understand the herbs’ therapeutic effects and the action mechanisms. However, to identify the metabolites and elucidate the metabolic pathways of herbal extracts remain a challenge because of their complicated chemical compositions (Li et al., 2013; Xin et al., 2011). Over the past years, metabolic study of herbal extracts has been carried out mainly in vivo. In these studies, metabolite profiles in sample matrices such as urine, plasma, and tissues were determined after giving oral administrations to experimental animals (Liang, et al., 2013; Liu et al., 2013). As is well known, the profiles obtained can be incomplete because metabolites may be produced at tiny amounts and not detectable in these complex matrices. In vitro studies, liver microsomes or microbials have been reported as enzymatic systems (Fan et al., 2009). These studies mimicking only a part of the body simplify the tests and data interpretation, and normally produce more repeatable and comprehensive results because the experimental conditions are controllable. However, in most in vitro metabolic studies of herbal medicines reported so far, only one or several active ingredients instead of the whole herbal extract were investigated (Li et al., 2006; Jahn et al., 2011; Hackenmueller et al., 2012). This has been due to the difficulties with metabolite identification in complex sample matrices and a lack of enzymatic systems for an effective metabolism of whole herbal extracts.
Various types of enzyme functionalized bioreactors have been developed for in vitro metabolic study of drugs (Ginai et al., 2013; Nicoli et al., 2008). Recently, magnetic nanoparticles (MNPs) have attracted increasing interest as solid phase support for enzymes to confer them with improved activity and stability since they provide large enzyme loading capacity and increase the collision frequency between immobilized enzyme molecules and substrate molecules due to Brownian motion of magnetic nanoparticles (Gupta et al., 2011). Their special nanostructures and magnetic properties also offer practical advantages including easy magnetic solid-liquid separation and good reusability which make them very usable for biomedical analysis as well as food analysis (Liu et al., 2014; Qing et al., 2010; Safarikova et al., 1999). Biological macromolecules such as proteins, enzymes, and DNA immobilized on MNPs can serve as excellent solid phase extraction absorbents to screen and isolate active ingredients from herbal medicines. We previously reported the use of α-glucosidase–MNP and protein tyrosine phosphatase 1B-MNPs to screen for enzyme inhibitors from the extract of Granati pericarpium (Qing et al., 2012). Furthermore, metabolic enzymes immobilized onto the surface of MNPs have been proved to be a powerful tool for in vitro metabolism study of drugs (Xue et al., 2013).
Dried rhizomes of A. dahurica have been used as an herbal medicine for treatments of acne, ulcer, carbuncle, rheumatism, headache and toothache (Zhang et al., 2009; Kang et al., 2008). The major bioactive components present in this herb are coumarins, which exhibit various biological activities including anti-cancer, anti-inflammation, antibacterial, and anti-dermatosis (Luo et al., 2011; Kang et al., 2009; Kleiner et al., 2008). Two linear furocoumarins, i.e. imperatorin and isoimperatorin, are predominant components in this plant. They have been found to show potent anti-inflammatory, anti-tumor and β-secretase inhibitory activities (Ban et al., 2003; Kawaii et al., 2001; Marumoto et al., 2010). Pharmacokinetic profiles of imperatorin and isoimperatorin obtained from a study with rats indicated a rapid absorption and an extensive metabolic elimination after administration (Yang et al., 2010). It was reported that less than 0.01% of imperatorin and less than 1% of isoimperatorin given as parent drugs were recovered from rat bile and urine (Liu et al., 2010), indicating that they were apt to be metabolized in vivo and excreted mainly as metabolites. Two oxidation metabolites of imperatorin were reported in a previous study (Wang et al., 2012), and the metabolic pathways were recently proposed (Qiao et al., 2013). Metabolisms of isoimperatorin in rat and in cunninghamella blakesleana AS 3.970, a strain of fungi, were investigated (Shi et al., 2013). To the best of our knowledge, no in vitro metabolic study of imperatorin or isoimperatorin with liver microsomal enzymes has been reported so far, not to say that of the whole extract of A. dahurica.
In this work, metabolism of imperatorin, isoimperatorin, and A. dahurica extract were comparatively studied using highly active and recoverable nanobioreactors in combination with HPLC/MS and MNR techniques. The nanobioreactors were prepared by immobilizing rat liver microsomes onto magnetic nanoparticles (LMMNPs). Biological activity of LMMNPs was assessed using isoimperatorin as the test compound. All the metabolites produced from incubating the samples with LMMNPs were characterized by HPLC-UV-MS. Since isoimperatorin metabolism has not been studied in vitro by liver microsomal enzymes before, semi-preparative HPLC was used to prepare enough amounts of the metabolites for structure elucidation by MS/MS and 1H-NMR. Finally, the microsomal isozymes responsible for the metabolism of imperatorin and isoimperatorin were also investigated.
Experimental
Chemicals and reagents
A. dahurica was obtained from local markets in Chengdu, Sichuan province, China. Imperatorin and isoimperatorin were isolated from A. dahurica in our lab. Their chemical structures (Fig.1) were elucidated by MS and 1H-NMR. β-Naphthoflavone, polydiallyldimethylammonium chloride (PDDA), β-nicotinamide adenine dinucleotide phosphate hydrate(NADP), glucose-6-phosphate and yeast glucose-6-phosphate dehydrogenase were purchased from Sigma (MO, USA). Acetonitrile (Fisher, Fair Lawn, USA) was HPLC grade and the water was from a Milli-Q water system (Millipore Corp, Bedford, MA, USA). Tetraethyl orthosilicate (TEOS), α-naphthoflavone, quinidine and ketoconazole were purchased from TCI (Tokyo, Japan). Other chemicals and solvents were of analytical reagent grade.
Fig 1.
Chemical structures of imperatorin, isoimperatorin, and the proposed metabolites. M1, trans-imperatorin hydroxylate; M2, cis-imperatorin hydroxylate; M3, isoimperatorin epoxide; M1’, trans-isoimperatorin hydroxylate; M2’, cis-isoimperatorin hydroxylate; and M3’, isoimperatorin epoxide.
Preparation and characterization of rat liver microsomes
Rat liver microsomes were prepared as previously reported (Xue et al., 2013). Briefly, male Sprague Dawley rats were fed normally and acclimatized at 12 h light/dark cycle for 7 days before experiment. β-naphthoflavone, dissolved in vegetable oil at 8 mg/mL was given to the rats by intraperitoneal injection for three days to induce hepatic cytochrome P450 enzymes from the eighth day. The rats were fasted for 12 h before being used. Then the rats were sacrificed and fresh livers were removed immediately, weighted and homogenized with 3× volumes of potassium phosphate buffer (PH 7.4). The homogenate was centrifuged at 10,000 g for 20 min. The supernatant was mixed with 88 mM CaCl2. The mixture was centrifuged at 27,000 g for 30 min. Then the precipitate was washed in Tris-HCl buffer (PH 7.4). Finally, the precipitate was resuspended in 0.1 M potassium phosphate buffer (PH 7.4) containing 0.25 M glycerol and aliquoted and stored at −80°C before use. The temperature was keep at 4°C during the whole period of preparation, and all solutions were stored at 4°C before using. Protein concentrations of the prepared microsomes were calculated using the methods of Bradford assay with the bovine serum albumin (BSA) as standard (Bradford, 1976).
The animal study was approved by the Animal Ethics Committee of Chengdu Institute of Biology, Chinese Academy of Sciences.
Immobilization of liver microsomes on magnetic nanoparticles
Firstly, Fe3O4 magnetic nanoparticles (MNPs) were prepared via co-precipitation of Fe2+ and Fe3+ with ammonia water under vigorous stirring, and the resultant MNPs were then coated with SiO2 with TEOS (Qing et al., 2010). Immobilization of rat liver microsomes onto MNPs by the electrostatic layer-by-layer self-assembling technique was carried out following a procedure reported previously (Bajrami et al., 2008). Briefly, the SiO2-coated Fe3O4 were dispersed in a 2 mg/mL PDDA solution and incubated for 20 min. PDDA-MNPs were then separated by an external magnet and washed with water for three times before being dispersed in a microsomal suspension. The mixture of PDDA-MNPs and microsomes was incubated at room temperature for 30 min. Then LMMNPs were separated and washed twice with potassium phosphate buffer (0.1 M, PH 7.4). The amount of immobilized microsomes was calculated by comparing the UV absorption of the supernatant before and after immobilization at the wavelength of 595 nm by Bradford assay.
Preparation of A. dahurica extract
Dried rhizomes of A. dahurica were powdered, and an aliquot of 5 g powder was ultrasonic extracted with 60 mL of 95% ethanol for 40 min and then filtered. The filtrate was then dried using a rotary evaporator and the residue was resolved in 4 mL of methanol and stored at 4 °C until HPLC-MS analysis and metabolization.
Metabolism of imperatorin, isoimperatorin and A. dahurica extract
One hundred μL of LMMNP suspension, 10 μL imperatorin solution (1 mg imperatorin in 500 μL methanol) and 150 μL incubation buffer (0.1 M potassium phosphate buffer, PH 7.4) were transferred to a 1.5 mL Eppendorf tube. The final incubation solution contained 10 mM magnesium chloride, 1.3 mM NADP, 3.3 mM glucose-6-phosphate, 1 U/mL yeast glucose-6-phosphate dehydrogenase, and 74 μM imperatorin. The mixture was incubated at 37 °C for 30 min. The reaction was terminated by magnetic separation of LMMNPs from the incubation solution. LMMNPs were then washed with 0.1 M potassium phosphate buffer three times before being reused.
The procedure for treating isoimperatorin or A. dahurica extract was the same as described above. Isoimperatorin or A. dahurica extract (10 μL) was added to the incubation system. The supernatant was then filtrated through a 0.45 μm filter prior to HPLC analysis.
Assessment of the activity and the reusability of LMMNPs
Isoimperatorin was used as the test compound to assess the activity and the reusability of LMMNPs. LMMNPs were washed with 0.1 M potassium phosphate buffer three times before being reused. And the equivalent reaction condition was adopted during each metabolic test. Six times of metabolism of isoimperatorin were conducted using the same LMMNPs.
Instrumentation
HPLC analysis
HPLC analysis was performed using a liquid chromatograph (Shimadzu, Kyoto, Japan) equipped with a binary HPLC pump (LC-20AD), a dual wavelength detector (SPD-20A), a manual injector (7725i-049) and a LC Solution Station for data collection and handling. Chromatographic separation was achieved on an Agilent zorbax SB C18 column (4.6 mm × 250 mm, 5 μ m) with a similar guard column (4.6 mm ×10 mm). The column compartment was set at 35 °C. The mobile phase consisted of water (A) and acetonitrile (B). The flow rate was 0.8 mL/min. The gradient elution was as follows: 20% to 40% B at 0 – 3 min, 40% to 55% B at 3 -15 min, 55% to 95% B at 15-20 min and 95% B at 30 min. The wavelength was set at 300 nm.
HPLC–MS/MS analysis
An Agilent 1100 system equipped with a microTOF-QII mass spectrometer (Bruker, Billerica, MA, USA) was used. Data were acquired and processed by using Bruker Compass Data Analysis 4.0 software. The mobile phase, column and other conditions were the same as the HPLC analysis described above, and 20 μL of each sample was injected for analysis. The mass spectrometer was operated in the positive ion mode by using ESI under the following general conditions: the capillary voltage was 4.5 kV, the collision cell RF was 150 Vpp. The source and desolvation temperature were set at 100 °C and 180 °C. Nitrogen was used as the desolvation gas at a flow rate of 8.0 L/min. The argon was used as collision gas at a flow rate of 0.15 mL/min. The collision energy was set at 10 eV.
Semi-preparative HPLC isolation of isoimperatorin metabolites
Metabolization of isoimperatorin was scaled up by the same process as described above. After the incubation with and removing of LMMNPs, the metabolic solution was extracted with ethyl acetate in a 100 mL separating funnel for three times. The organic phase was dried using a rotary evaporator at 50 °C. The residue was re-dissolved in 6 mL of methanol and stored at 4 °C until metabolite isolation.
The isolation of metabolites of isoimperatorin was performed using a semi-preparative HPLC system (Waters 2545). Separation was performed on a Kromasil C18 column( 10 mm × 250 mm, 5 μm) with a mobile phase consisting of methanol and H2O (60:40, v/v) at 35 °C and a constant flow rate of 4 mL/min. The injection volume was 500 μL, and the UV detector was set at a wavelength of 254 nm. The fraction of effluent between 8.75-9.50 min was collected as M1’ and 10.80-11.50 min collected as M3’. They were dried by rotary evaporator at 50 °C and stored at 4 °C before analysis.
1H-NMR measurement of the isolated metabolites
1H-NMR spectra were measured on a Bruker AV 600 MHz spectrometer, and chemical shifts were reported in ppm downfield relative to tetramethylsilane. The isolated compound M1’ was dissolved in methanol-d4 and M3’ were dissolved in chloroform-d1.
Results and discussion
Preparation of LMMNP nanobioreactors
The nanobioreactors were prepared by using the layer-by-layer (LBL) technique previously reported. The loading capability of SiO2-coated magnetic nanoparticles for microsomes was determined by measuring protein contents in the microsomal suspension before and after immobilization. The microsomes immobilized on the nanoparticles were calculated about 65 μg/mg nanoparticle. It was noted that the suspendability of LMMNPs was much better as compared with that of the precursor MNPs. A diluted suspension of LMMNPs was stable for several days in terms of its appearance. These nanoparticles showed no significant changes in their metabolic bioactivity after being kept in a refrigerator for one month at 4 °C.
HPLC-MS/MS identification of imperatorin and isoimperatorin metabolites with LMMNPs
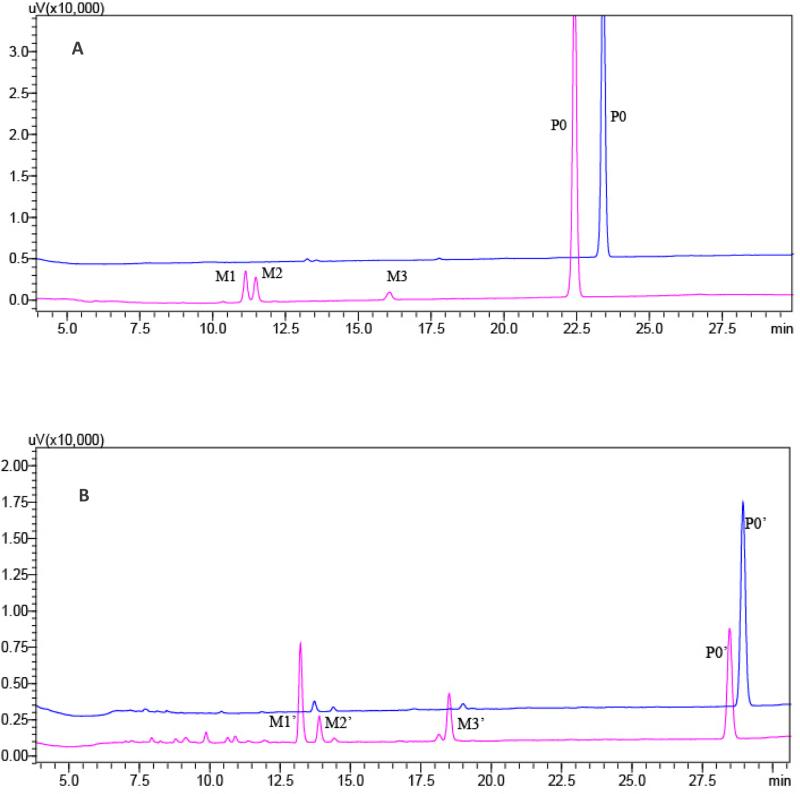
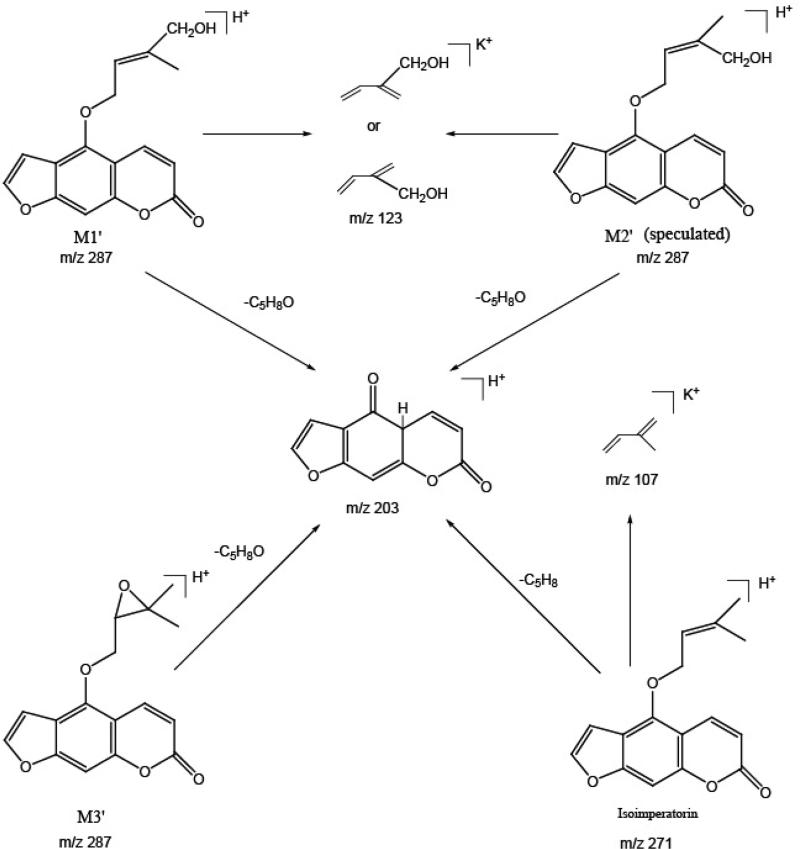
The two major active ingredients in A. dahurica extract, imperatorin and isoimperatorin, were individually incubated with LMMNPs in order to simply the metabolite identification. The metabolic solutions were analyzed by HPLC-UV-MS. From HPLC-UV chromatograms obtained (Fig.2), incubation of imperatorin or isoimperatorin with LMMNPs for 30 min produced three metabolites respectively. The six metabolites were well separated under the HPLC conditions established in this work. Surprisingly, MS detection of these metabolite peaks revealed that they were from the same two ions i.e. m/z 325 and 287. It was noted that the mass difference suggested a pair of [M+K]+ and [M+H]+. In the MS2 spectra of these metabolites, certain common product ions, including m/z 203 and m/z 123 were observed. Based on these m/z values and other characteristic product ions detected (see table 1 for details), the six metabolites were identified as the oxidation products of the parent drugs. A previous in vivo study of imperatorin identified two metabolites, i.e. cis-imperatorin hydroxylate and imperatorin epoxide (Wang et al., 2012). The MS2 data of M2 and M3 were found to be in consistent with those two metabolites (shown in Fig. 3) and therefore, identified as cis-imperatorin hydroxylate and imperatorin epoxide. Since the HPLC retention time of M1 was very close to that of M2, and both compounds possess very similar mass spectrometric properties, M1 was deduced to be trans-imperatorin hydroxylate. Similarly, the three metabolites produced from isoimperatorin were identified as trans-isoimperatoirn hydroxylate (M1’), cis-isoimperatorin hydroxylate (M2’) and isoimperatorin epoxide (M3’), respectively. Their MS2 spectrometric properties are showed in Fig 4. To our knowledge, there has been no report on the in vitro phase I metabolites of isoimperatorin in a liver microsomal system. Furthermore, we isolated M1’ and M3’ from the isoimperatorin-LMMNPs incubation solution using a semi-preparative HPLC system, and their structures were confirmed by 1H-NMR. The amount of M2’ in the incubation solution was so small that it was hard to collect enough for an NMR analysis. However, it could be speculated as cis-isoimperatorin hydroxylate from its HPLC chromatographic behavior and the biogenesis point of view.
Fig 2.
HPLC-UV chromatograms obtained from the metabolic solutions of imperatorin (A) and isoimperatorin (B) at incubation times of 0 and 30 min, respectively.
Table 1.
MS properties of imperatorin, isoimperatorin and their metabolites
| Compound | [M+K]+ | [M+H]+ | Major fragment ions |
|---|---|---|---|
| imperatorin | 309 | 271 | 107, 203 |
| M1 | 325 | 287 | 123, 203, 241, 269 |
| M2 | 325 | 287 | 123, 203 |
| M3 | 325 | 287 | 123, 203, 269 |
| Isoimperatorin | 309 | 271 | 107, 176, 203 |
| M1’ | 325 | 287 | 122, 203 |
| M2’ | 325 | 287 | 123, 203 |
| M3’ | 325 | 287 | 123, 203 |
Fig 3.
MS2 properties of the imperatorin metabolites detected in the metabolic solution with LMMNPs for 30 min.
Fig 4.
MS2 spectrometric properties of the isoimperatorin metabolites detected in the metabolic solution with LMMNPs for 30 min.
Reusability of LMMNP nanoparticle bioreactors
A significant advantage of using the proposed liver microsomal nanobioreactors in a metabolic study is that they can be easily recovered from the incubation solution and can be reused for many times. In addition, the subsequent chemical analysis is greatly simplified due to the absence of proteins in the solution. To evaluate the reusability of the LMMNPs, six consecutive incubation experiments were performed using a same sample of LMMNPs. As can be seen from Fig. 5, the LMMNPs were reusable for at least six times. No obvious changes in the metabolite concentrations were observed after the sixth time.
Fig 5.
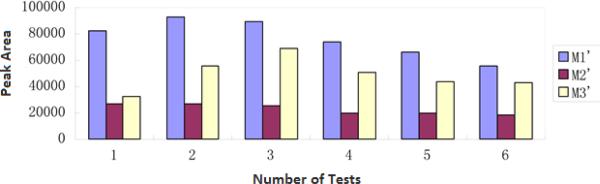
The reusability of LMMNPs using isoimperatorin as the test compound.
NMR analysis of the two metabolites of isoimperatorin
The 1H-NMR spectrum (600 MHz, methanol-d4) data of M1’ was as follows: δ 8.28 (d, 1H, J = 12 Hz, H-9), 7.80 (d, 1H, J = 3.0Hz, H-4), 7.21 (s, 1H, H-8), 7.19 (d, 1H, J = 1.5 Hz, H-3), 6.29 (d, 1H, J = 12 Hz, H-10), and 5.84 (t, 1H, J = 6 Hz, H-2’), 5.10 (d, 2H, J = 6 Hz, H-1’), 3.98 (s, 2H, H-5’), 1.70 (s, 3H, H-4’), 4.54 (s, 1H, OH-4’). Based on the MS data described above, NMR analysis results, and the information in literature (Shi et al., 2013; Bajrami et al., 2008), M1’ was identified as trans-isoimperatorin hydroxylate.
The 1H NMR (600 MHz, chloroform-d1) data of M3’ was as follows: δ 8.21 (d, 1H, J = 10.2 Hz, H-9), 7.61 (d, 1H, J = 2.4 Hz, H-4), 7.20 (s, 1H, H-8), 6.95 (d, 1H, J=1.2 Hz, H-3), 6.32 (d, 1H, J=9.6 Hz, H-10), 4.58 (dd, 1H, J = 10.8 and 4.2 Hz, H-1’), 4.43 (dd, 1H, J = 10.8 and 6 Hz, H-1’), 3.23 (dd, 1H, J = 6.6 and 4.8 Hz, H-2’), 1.41(s, 3H, H-5’), 1.33 (s, 3H, H- 4’). Based on the MS data described above and NMR analysis results, and M3’ was identified as isoimperatorin epoxide metabolite. It's worth mentioning that M3’ was identified as a metabolites of isoimperatorin for the first time in this work.
Identification of the components in A. dahurica extract
The A. dahurica extract was subjected to HPLC-MS/MS analysis. The structures of the eight major compounds appeared in the HPLC chromatogram were identified by comparison of their retention times, molecular weights and MS2 data with the authentic samples as well as those reported in literatures (Franke et al., 2001; Kang et al., 2008; Liu et al .2004).
The HPLC chromatogram of A. dahurica extract is shown in Figure 6 (trace d). The chromatographic and MS data of the main coumarins are listed in Table 2. Imperatorin and isoimperatorin were the main compounds present in the extract, and were quantitatively determined using the calibration curves. The calibration curve of imperatorin and isoimperatorin showed a good linearity over the concentration range of 0.5-10 μg/mL. The regression equation of imperatorin was y = 0.3138x + 0.0278 and the correlation coefficient (r2) was 0.9993. The regression equation of isoimperatorin was y = 0.3971x + 0.0428 and the correlation coefficient (r2) was 0.9994. The concentration of imperatorin and isoimperatorin in A. dahurica extract was calculated as 6.99 μg/mL and 4.75 μg/mL, respectively.
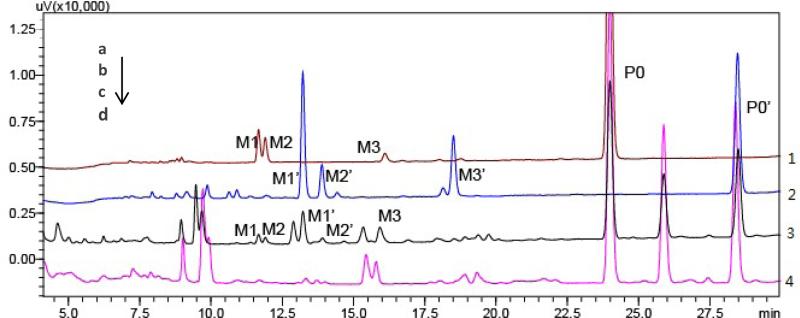
Fig 6.
Overlaid HPLC-UV chromatograms obtained from imperatorin-LMMNP (trace a), isoimperatorin-LMMNP (trace b), A. dahurica extract-LMMNP (trace c) incubation solutions, and A. dahurica extract (trace d).
Table 2.
The analysis results of the chemical constituents in the extracts of Angelica dahurica by HPLC-MS/MS
| tR(min) | Identification | Formula | Mass | MS | MS/MS |
|---|---|---|---|---|---|
| 9.02 | Xanthotol | C11H6O4 | 202 | 201 | 173, 156 |
| 9.75 | Oxypeucedanin hydrate | C16H16O6 | 304 | 305 | 203 |
| 9.99 | Byakangelicin | C17H18O7 | 334 | 335 | 317, 233 |
| 15.48 | Bergapten | C12H8O4 | 216 | 217 | 202 |
| 15.80 | Pabulenol | C16H14O5 | 286 | 287 | 203, 147 |
| 24.02 | Imperatorin | C16H14O4 | 270 | 271 | 203, 146 |
| 25.98 | Phelloptorin | C17H16O5 | 300 | 301 | 233, 217 |
| 28.45 | Isoimperatorin | C16H14O4 | 270 | 271 | 203, 146 |
Metabolism of A. dahurica extract
Metabolism of A. dahurica extract by LMMNPs was subsequently performed and the incubation solution was analyzed by HPLC-UV-MS. The HPLC-UV chromatogram obtained was very complex with many peaks, which made the identification of metabolites difficult. However, by comparing the HPLC-UV chromatograms obtained from the incubation solution of A. dahurica extract and those of imperatorin and isoimperatorin (Fig. 6), the metabolites produced from A. dahurica extract were identified. Five of the six metabolites described above were detected in the incubation solution, among which three were from imperatorin and two from isoimperatorin. Isoimperatorin epoxide was not detected.
Determination of CYP isozymes involved in the metabolism
Since immobilized liver microsomes were used, the CYP isozymes responsible for the metabolism of imperatorin and isoimperatorin were investigated to examine if any changes in metabolic mechanisms were involved. In this work, we determined the acting CYP isozymes by using isozyme inhibitors. It has been reported that α-naphthoflavone, quinidine and ketoconazole are the inhibitors of CYP1A2, CYP2D6 and CYP3A4, respectively (Dasgupta et al., 2010). Therefore, these isozyme inhibitors were used. Table 3 shows the test results. In these tests, incubation solutions were all prepared in parallel except that different isozyme inhibitors were added in the respective experiment groups. From comparing the relative amount of metabolites of imperatorin and isoimperatorin between experiment groups and blank groups obtained from HPLC-UV analysis, the metabolic CYP isozymes were determined. The results suggested that CYP1A2 was the metabolic isozyme for both isoimperatorin and imperatorin, but CYP3A4 metabolized only isoimperatorin effectively.
Table 3.
Results of inhibition tests on CYP isozymes in the metabolism of imperatorin and isoimperatorin
| The compound |
Imperatorin | Isoimperatorin |
|---|---|---|
| Inhibitor | ||
| Blank control | / | / |
| α-naphthoflavone | + | + |
| quinidine | - | - |
| ketoconazole | - | + |
Note : “+” represents that the inhibitor has an inhibitory effect on the metabolism, while “-” indicates no effects.
Conclusions
By using a highly effective and reusable liver microsomal nanobioreactor and HPLC-UV-MS/MS in combination with NMR analysis, in vitro microsomal metabolism of A. dahurica extracts was investigated for the first time. The nanobioreactors were prepared by immobilizing rat liver microsomes on SiO2-coated Fe3O4 magnetic nanoparticles. They were easily recovered from the incubation solution by means of an external magnet, making the incubation solution enzyme free for a convenient HPLC analysis, which turned out to be a powerful tool for in vitro metabolic study for plant extracts. By means of HPLC-MS and/or NMR, five metabolites were identified as trans-imperatorin hydroxylate, cis-imperatorin hydroxylate, imperatorin epoxide, trans-isoimperatorin hydroxylate, and cis-isoimperatorin hydroxylate. Comparing the respective metabolites of imperatorin and isoimperatorin, all of them were found to be the oxidation products transformed from these two major compounds present in the plant extracts. In addition, metabolic study of isoimperatorin revealed a new compound, i.e. isoimperatorin epoxide, which is interestingly not detected in the metabolites of the plant extracts. Furthermore, CYP1A2 was proved to be the metabolic isozyme responsible for both isoimperatorin and imperatorin, while only CYP3A4 for isoimperatorin.
Acknowledgements
Financial supports from National Natural Science Foundation of China (21072184 and 81173536 to XL) and U.S. National Institutes of Health (GM 089557 to YML) are gratefully acknowledged.
References
- Bajrami B, Hvastkovs EG, Jensen GC, Schenkman JB, Rusling JF. Enzyme-DNA biocolloids for DNA adduct and reactive metabolite detection by chromatography-mass Spectrometry. Anal Chem. 2008;80:922–932. doi: 10.1021/ac702025f. [DOI] [PubMed] [Google Scholar]
- Ban HS, Lim SS, Suzuki K, Jung SH, Lee S, Lee YS, Shin KH, Ohuchi K. Inhibitory effects of furanocoumarins isolated from the roots of Angelica dahurica on prostaglandin E-2 production. Planta Med. 2003;69:408–412. doi: 10.1055/s-2003-39702. [DOI] [PubMed] [Google Scholar]
- Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dasgupta D, Tang W, Caldwell GW, Yan Z. Use of stable isotope labeled probes to facilitate liquid chromatography/mass spectrometry based high-throughput screening of time-dependent CYP inhibitors. Rapid Commun Mass Spectrom. 2010;24:2177–2185. doi: 10.1002/rcm.4610. [DOI] [PubMed] [Google Scholar]
- Fan Y, Schreiber EM, Day BW. Human liver microsomal metabolism of (+)-discodermolide. J Nat Prod. 2009;72:1748–1754. doi: 10.1021/np900245k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K, Porzel A, Masaoud M, Adam G, Schmidt J. Furanocoumarins from Dorstenia gigas. Phytochemistry. 2001;56:611–621. doi: 10.1016/s0031-9422(00)00419-2. [DOI] [PubMed] [Google Scholar]
- Ginai M, Elsby R, Hewitt CJ, Surry D, Fenner K, Coopman K. The use of bioreactors as in vitro models in pharmaceutical research. Drug Discov Today. 2013;18:922–935. doi: 10.1016/j.drudis.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Gupta MN, Kaloti M, Kapoor M, Solanki K. Nanomaterials as matrices for enzyme immobilization. Artificial Cells, Blood Substitutes and Biotechnology. 2011;39:98–109. doi: 10.3109/10731199.2010.516259. [DOI] [PubMed] [Google Scholar]
- Hackenmueller SA, Scanlan TS. Identification and quantification of 3-iodothyronamine metabolites in mouse serum using liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2012;1256:89–97. doi: 10.1016/j.chroma.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn S, Baumann A, Roscher J, Hense K, Zazzeroni R, Karst U. Investigation of the biotransformation pathway of verapamil using electrochemistry/liquid chromatography/mass spectrometry-A comparative study with liver cell microsomes. J Chromatogr A. 2011;1218:9210–9220. doi: 10.1016/j.chroma.2011.10.052. [DOI] [PubMed] [Google Scholar]
- Kang J, Zhou L, Sun J, Han J, Guo DA. Chromatographic fingerprint analysis and characterization of furocoumarins in the roots of Angelica dahurica by HPLC/DAD/ESI-MSn technique. J Pharm Biomed Anal. 2008;47:778–785. doi: 10.1016/j.jpba.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Kang KH, Kong CS, Seo Y, Kim MM, Kim SK. Anti-inflammatory effect of coumarins isolated from Corydalis heterocarpa in HT-29 human colon carcinoma cells. Food Chem Toxicol. 2009;47:2129–2134. doi: 10.1016/j.fct.2009.05.036. [DOI] [PubMed] [Google Scholar]
- Kawaii S, Tomono Y, Ogawa K, Sugiura M, Yano M, Yoshizawa Y, Ito C, Furukawa H. Antiproliferative effect of isopentenylated coumarins on several cancer cell lines. Anticancer Res. 2001;21:1905–1911. [PubMed] [Google Scholar]
- Kleiner HE, Xia X, Sonoda J, Zhang J, Pontius E, Abey J, Evans RM, Moore DD, Digiovanni J. Effects of naturally occurring coumarins on hepatic drug-metabolizing enzymes in mice. Toxicol Appl Pharmacol. 2008;232:337–350. doi: 10.1016/j.taap.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wang GJ, Li J, Hao HP, Zheng CN. Identification of tanshinone IIA metabolites in rat liver microsomes by liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2006;1104:366–369. doi: 10.1016/j.chroma.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Li SG, Chen HY, Ou-Yang CS, Wang XX, Yang ZJ, Tong Y, Cho WC. PloS One. 2013;8:e57604. doi: 10.1371/journal.pone.0057604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Xu F, Zhang YZ, Huang S, Zang XY, Zhao X, Zhang L, Shang MY, Yang DH, Wang X. The profiling and identification of the absorbed constituents and metabolites of Paeoniae Radix Rubra decoction in rat plasma and urine by the HPLC–DAD–ESI-IT-TOF-MSn technique: A novel strategy for the systematic screening and identification of absorbed constituents and metabolites from traditional Chinese medicines. J Pharm Biomed Anal. 2013;83:108–121. doi: 10.1016/j.jpba.2013.04.029. [DOI] [PubMed] [Google Scholar]
- Liu M, Shi X, Yang W, Liu S, Wang N, Shi R, Qiao S, Wang Q, Wang Y. Quantitative analysis of nine coumarins in rat urine and bile after oral administration of Radix Glehniae extract by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Biomedical Chromatography. 2011;25:783–793. doi: 10.1002/bmc.1517. [DOI] [PubMed] [Google Scholar]
- Liu P, Duan JA, Guo JM, Shang EX, Qian DW, Su SL, Tang YP. Identificaiton of major chemical constituents and their metabolites in rat plasma and various organs after oral administration of effective xiang-fu-wu decoction fraction by UPLC-Q-TOF-MS and metabolynx. J Liq Chromatogr Relat Technol. 2013;36:1736–1749. [Google Scholar]
- Liu RM, Feng L, Sun AL, Kong LY. Preparative isolation and purification of coumarins from Cnidium monnieri (L.) cusson by high-speed counter-current chromatography. J Chromatogr. A. 2004;1055:71–76. doi: 10.1016/j.chroma.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Liu XY, Yu D, Yu YC, Ji SJ. Preparation of a magnetic molecularly imprinted polymer for selective recognition of rhodamine B. Applied Surface Science. 2014;320:138–145. [Google Scholar]
- Luo KW, Sun JG, Chan JW, Yang L, Wu SH, Fung KP, Liu FY. Anticancer effects of imperatorin isolated from Angelica dahurica: induction of apoptosis in HepG2 cells through both death-receptor- and mitochondria-mediated pathways. Chemotherapy. 2011;57:449–459. doi: 10.1159/000331641. [DOI] [PubMed] [Google Scholar]
- Marumoto S, Miyazawa M. Beta-secretase inhibitory effects of furanocoumarins from the root of Angelica dahurica. Phytother Res. 2010;24:510–513. doi: 10.1002/ptr.2967. [DOI] [PubMed] [Google Scholar]
- Nicoli R, Bartolini M, Rudaz S, Andrisano V, Veuthey JL. Development of immobilized enzyme reactors based on human recombinant cytochrome P450 enzymes for phase I drug metabolism studies. J Chromatogr. A. 2008;1206:2–10. doi: 10.1016/j.chroma.2008.05.080. [DOI] [PubMed] [Google Scholar]
- Qiao S, Shi X, Shi R, Liu M, Liu T, Zhang K, Wang Q, Yao M, Zhang L. Identification of urinary metabolites of imperatorin with a single run on an LC/Triple TOF system based on multiple mass defect filter data acquisition and multiple data mining techniques. Anal Bioanal Chem. 2013;405:6721–6738. doi: 10.1007/s00216-013-7132-6. [DOI] [PubMed] [Google Scholar]
- Qing LS, Tang N, Xue Y, Liang J, Liu YM, Liao X. Identification of enzyme inhibitors using therapeutic target protein-magnetic nanoparticle conjugates. Analytical Methods. 2012;4:1612–1615. doi: 10.1039/C2AY25320H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing LS, Xue Y, Zheng Y, Xiong J, Liao X, Ding LS, Li BG, Liu YM. Ligand fishing from Dioscorea nipponica extract using human serum albumin functionalized magnetic nanoparticles. J Chromatogr A. 2010;1217:4663–4668. doi: 10.1016/j.chroma.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safarikova M, Safarik I. Magnetic solid-phase extraction. J Magn Magn Mater. 1999;194:108–112. [Google Scholar]
- Shi X, Liu M, Zhang M, Zhang K, Liu S, Qiao S, Shi R, Jiang X, Wang Q. Identification of in vitro and in vivo metabolites of isoimperatorin using liquid chromatography/mass spectrometry. Food Chem. 2013;141:357–365. doi: 10.1016/j.foodchem.2013.02.068. [DOI] [PubMed] [Google Scholar]
- Wang L, Lu W, Shen Q, Wang S, Zhou H, Yu L, Wang S, Jiang H, He L, Zeng S. Simultaneous determination of imperatorin and its 2 metabolites in dog plasma by using liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal. 2012;70:640–646. doi: 10.1016/j.jpba.2012.06.034. [DOI] [PubMed] [Google Scholar]
- Xin GZ, Qi LW, Shi ZQ, Li P, Hao HP, Wang GJ, Shang J. Strategies for integral metabolism profile of multiple compounds in herbal medicines: pharmacokinetics, metabolites characterization and metabolic interactions. Curr Drug Metab. 2011;12:809–817. doi: 10.2174/138920011797470164. [DOI] [PubMed] [Google Scholar]
- Xue Y, Xiong J, Shi HL, Liu YM, Qing LS, Liao X. In vitro metabolic study of Rhizoma coptidis extract using liver microsomes immobilized on magnetic nanoparticles. Anal Bioanal Chem. 2013;405:8807–8817. doi: 10.1007/s00216-013-7303-5. [DOI] [PubMed] [Google Scholar]
- Yang W, Feng C, Kong D, Shi X, Cui Y, Liu M, Wang Q, Wang Y, Zhang L. Simultaneous and sensitive determination of xanthotoxin, psoralen, isoimpinellin and bergapten in rat plasma by liquid chromatography–electrospray ionization mass spectrometry. J Chromatogr B. 2010;878:575–582. doi: 10.1016/j.jchromb.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gong C, Lv L, Xu Y, Zhao L, Zhu Z, Chai Y, Zhang G. Rapid separation and identification of furocoumarins in Angelica dahurica by high-performance liquid chromatography with diode-array detection, time-of-flight mass spectrometry and quadrupole ion trap mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:2167–2175. doi: 10.1002/rcm.4123. [DOI] [PubMed] [Google Scholar]