Abstract
Background
Spinal cord injury is a rare complication after lower extremity surgery in children with skeletal dysplasia and thoracic kyphosis. We encountered two patients who had this complication, from among 51 (39 from Nemours/Alfred I. duPont Hospital for Children and 12 from Seattle Children’s Hospital) who underwent lower extremity surgery during an 8.5-year period (June 2004 to December 2012). Because spinal cord injury is a devastating complication likely not known to most physicians treating patients with skeletal dysplasias, we sought to examine factors that may contribute to this rare complication.
Case Description
We performed a retrospective review of two patients with skeletal dysplasia who had paraplegia develop after extremity surgery. Outcome measures included operative time, vital signs, and postsurgery recovery of neurologic deficit. MR images were reviewed. Two patients were found—an 8.5-year-old boy with spondyloepiphyseal dysplasia congenita with a 76°-thoracic kyphosis apex at T4 and a 6.5-year-old boy with mucopolysaccharidosis type 1-H with an 80°-thoracic kyphosis apex at T2. Bilateral proximal femoral osteotomies or bilateral innominate and proximal femoral osteotomies had been performed. The spinal cord injuries occurred at the apex of the kyphosis as determined by clinical examination and MRI assessment. In both patients, the mean arterial blood pressure decreased below 50 mm Hg and might be a factor in the etiology of the paralysis. The first patient recovered motor function in 5 months; the second had no recovery.
Literature Review
Paraplegia is extremely rare after nonspine operations. Many factors contribute to the risk for a spinal cord event: low mean arterial pressure, duration of the surgery, position on the operating table, the kyphotic spine deformity, or unappreciated vascular disease. Motor-evoked potentials and somatosensory-evoked potentials together potentially provide high sensitivity and specificity for predicting a postoperative neurologic deficit.
Clinical Relevance
Based on our two patients with skeletal dysplasia and a literature review of patients with hyperkyphosis undergoing extremity surgery, the surgeon must be aware of the risk of spinal cord injury. Careful preoperative assessment possibly including MRI of the spine is recommended. Mean arterial pressure should be maintained at a safe level; neuromonitoring should be considered.
Introduction
Sagittal and coronal deformities of the spine are common in skeletal dysplasia, including spondyloepiphyseal dysplasia and mucopolysaccharidosis. These dysplasias are a nonhomogeneous group of growth disorders characterized by shortening of the trunk and extremities [13]. Patients with spondyloepiphyseal dysplasia are characterized not only by disproportionate short posture (dwarfism), but also by progressive involvement of the spine and epiphyses of the long bones, resulting in kyphoscoliosis and limb malalignment with progressive hip deformities [8, 17, 22]. In patients with mucopolysaccharidosis, glycosaminoglycan accumulation affects skeletal structure, connective tissues, and other organs, which may lead to joint contractures and limb and spine deformities. Atlantoaxial instability, spinal stenosis, and progressive kyphoscoliosis are of particular importance and may be associated with spinal cord compression or myelopathy in both types of dysplasias [5, 8, 17, 25, 26]. We identified two patients with skeletal dysplasia who experienced a neurologic complication and present their cases, with a review of the relevant literature.
Case Report
No institutional review board approval was needed for a case report of two patients. We reviewed the cases of two patients: one with mucopolysaccharidosis and one with spondyloepiphyseal dysplasia, who had neurologic injury after lower extremity surgery. Our outcome measures included operative time, anesthesia time, intraoperative complications, estimated blood loss, O2 saturation, mean arterial pressure (MAP), urine output, patient positioning, time for manifestation of deficit, and neurologic recovery. Postoperative acuity of care and neurologic history also were considered. Pre- and postdeficit neuraxis MR images were compared and radiographic images correlated to deficits were determined. The followup was a minimum of 2 years after surgery.
Two patients with skeletal dysplasia who had a new neurologic deficit with confirmatory neuroimaging after extremity surgery were identified. There were no other complications noted. Blood work including a complete blood count and coagulation test results were normal, and neither patient had a family history of thrombotic disease (Table 1).
Table 1.
Patient characteristics
| Patient | Age; sex | Diagnosis/apex of kyphosis | Unrelated surgery | EBL (cc) | Operative time/anesthesia (minutes) | Time to deficit | Postoperative MRI findings |
|---|---|---|---|---|---|---|---|
| 1 | 8 years, 6 months; male | SED, hyperkyphosis, coxa vara bilateral Kyphosis 76° (T1–T7, T4 apex) |
Bilateral femoral valgization osteotomies | 200 | 293/398 | Immediate after surgery | Spinal cord signal abnormality on T2 at apex of kyphoscoliosis |
| 2 | 6 years, 6 months; male | MPS, hyperkyphosis, bilateral hip dysplasia Kyphosis 80° (C7–T4, T2 apex) |
Bilateral hip open reduction and innominate osteotomy | 400 | 252/473 | Seen at 4 hours postoperative | Increased T2/STIR signal |
| Level of paralysis | Treatment | Lowest MAP at surgery | Postoperative care (PICU vs floor) | Time to recovery | Last MRI |
|---|---|---|---|---|---|
| T4-T5 | Solu-Medrol® 30 mg/kg IV bolus, 5.4 mg/kg for 23 hours | 40 mm Hg | PICU | 5 months, patient returned to near baseline strength | Increased signal at T4-T5. The cord appears slightly narrowed, 6months postoperative |
| T2 | Methyl-prednisolone IV | 48 mm Hg | PICU | None at 2 years, 3 months followup | Atrophy of cord at 2-years, 3-months postoperative |
EBL = estimated blood loss; MAP = mean arterial pressure; PICU = pediatric intensive care unit; SED = spondyloepiphyseal dysplasia; IV = intravenous; MPS = mucopolysaccharidosis; STIR = Short tau inversion recovery; Solu-Medrol® (Pfizer Inc, Peapack, NJ, USA).
Patient 1
An 8.5-year-old boy with spondyloepiphyseal dysplasia congenita and severe coxa vara bilaterally underwent bilateral proximal femoral realignment osteotomies at one surgery. Preoperative neurologic examination was normal. Scoliosis and midthoracic kyphosis were 115° and 76°, respectively. The thoracic kyphosis apex was T4–T5 (Table 1).
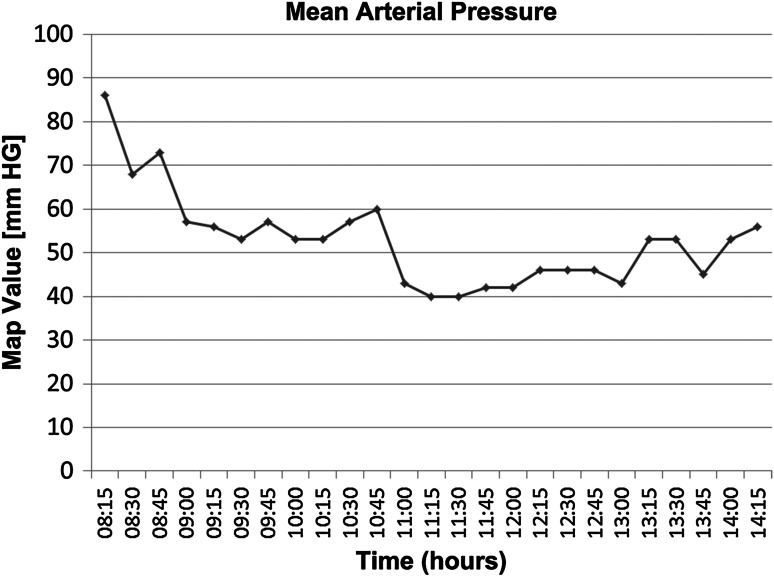
General anesthesia was administered with the patient in the supine position. Total anesthesia and surgery time were 398 and 293 minutes, respectively. Neuromonitoring was not used. Estimated blood loss was 200 cc with maintenance of O2 saturation at 95%. Low MAP values (40 mm Hg) occurred twice for 15 minutes (Fig. 1). Urine output was within normal limits (1.2–1.7 mL/kg/hour).
Fig. 1.
The intraoperative mean arterial pressure (mm Hg) levels of Patient 1 in relation to time of surgery are shown.
Dense paraplegia of both lower extremities was observed postoperatively but both showed nondermatomal paresthesia and no discernible sensory level loss. Five-hour postoperative MR images showed a T2-signal change in the dorsal spinal cord at T4–T5 (apex of kyphosis), without evidence of compression at any level (Fig. 2).
Fig. 2A–B.
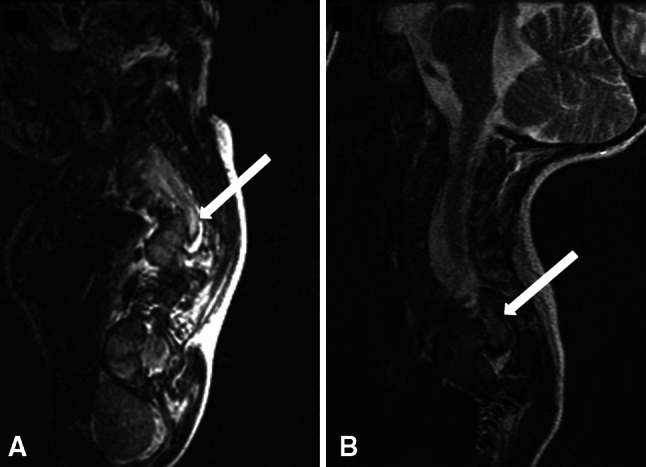
(A) An MR image taken 30 minutes after manifestation of clinical signs of paralysis in Patient 1 shows the T2 signal changes in the spinal cord at the kyphosis level (arrow). (B) This MR image shows the same zone (arrow) with the heterogeneous signal 6 months later.
Postoperatively, the patient was started on methylprednisolone (30 mg/kg bolus, then 5.4 mg/kg/hour × 23 hours infusion). The patient had spontaneous movement of his left great toe on postoperative Day 3. He had voluntary urine voiding on the sixth day after surgery after initial neurogenic bladder with urinary retention. Motor strength was 4 of 5 and there was no clinical evidence of myelopathy at his 5-month postoperative visit. MR images of the spine indicated a decrease in T2 signal abnormality with slight loss of cord volume at the corresponding region. The patient is able to complete activities of daily living to his satisfaction 5 years postoperatively.
Patient 2
A 6-year-old boy with mucopolysaccharidosis 1-H underwent bilateral innominate osteotomies and proximal femoral varus osteotomies during one surgical event. Cervicothoracic kyphosis was 80°. The apex of the thoracic kyphosis was T2–T3 (Table 1). His preoperative neurologic examination was normal. General anesthesia was administered with the patient in the supine position. Total anesthesia and surgical time were 473 and 252 minutes, respectively. Three additional procedures were performed: dental restorations, MRI, and bilateral medial tibial hemiepiphysiodesis. For pediatric hip reconstruction, the surgeons did not deem any dental procedure as a contraindication. No neuromonitoring was done during surgery. Estimated blood loss was 400 cc and O2 saturation level was greater than 95% during surgery. Urine output was within normal limits (1.3–1.7 mL/kg/hour). The patient’s MAP decreased to 48 mm Hg 30 minutes before the end of the procedure and persisted for 2 hours.
Flaccid paralysis was discovered 4 hours postoperatively as the patient started to recover from the epidural anesthesia. The entire case was done with the patient receiving epidural anesthesia and sedation. Deep tendon reflexes and movement were absent. Skin sensation was present. MR images obtained the following morning showed ischemia of the spinal cord at the T2 level. High-dose, intravenous corticosteroid therapy and pharmacologic treatment for hypertension were started. Discharge occurred on Day 52 postoperatively with the patient having continued complete flaccid paraplegia and bladder incontinence. At the last followup 27 months after surgery, paralysis of both lower extremities with complete sensory and motor loss and bladder incontinence were still present. MR images obtained 3 months and 27 months postoperatively showed atrophy of the spinal cord.
Discussion
Patients with skeletal dysplasia may require operative procedures for long bone or hip reconstruction [4, 24]. Children with skeletal dysplasia also may have associated atlantoaxial instability, spinal stenosis, or kyphoscoliosis.
There were several limitations to this study. First, we had only two patients and therefore a statistical analysis for potential causes of paraplegia were not possible; however, as this complication is not well appreciated by treating physicians, it is worth noting that it can occur. We explored many plausible explanations for the cause of the neurologic injury. We believe that the decrease in MAP during the procedure might be a cause but we cannot document that maintaining MAP at a consistent level would have prevented the injuries. The actual cause or causes of the neurologic injury, other than the spinal cord ischemia observed on MR images, remain unknown in these two patients. We also cannot comment on the frequency of this complication because we could not accurately assess every similar patient with skeletal dysplasia and spine deformity who underwent lower extremity surgery at our institutions, but we think there were 52 during an 8½-year period.
To our knowledge, our study is the first report of spinal cord injury associated with extremity surgery in patients with skeletal dysplasia. Neurologic sequelae related to the spinal cord are rare after nonspinal operations, with the overall incidence estimated to be 0.08% [2, 19].
Many factors contribute to a patient’s risk for a spinal cord event: low MAP, low O2 saturation, duration of the operation, positioning on the operating table, and the presence of a spine deformity, or unappreciated small vessel vascular disease unique to patients with skeletal dysplasias. We know of no evidence that a synergistic action occurs between these many factors; however, MAP may be the main factor. Both patients in our study had kyphosis (76° and 80°) at the time of the procedure but neither showed any spinal or peripheral nerve dysfunction preoperatively. These kyphosis measurements are not exceptional for patients with skeletal dysplasia.
Blood Pressure and MAP
Both patients had their MAP measured on a continuous basis during surgery. Low MAP (< 60 mm Hg) is an important risk factor for spinal cord injury intra- and postoperatively [6]. Data regarding spinal ischemia injury in patients in the supine position are limited [19]. Blood pressure changes and heart rate instability may not be sensitive enough to caution the surgical team regarding low cardiac output [25]. In a young, healthy adult patient, a MAP of 50 mm Hg to 60 mm Hg is well tolerated, but higher pressures may be required in patients with cardiovascular disease. According to Haque and Zaritsky [11], the MAP for patients at age 6 years should be 70 ± 20 mm Hg.
Spinal Cord Ischemia
The diagnosis of spinal cord ischemic injury was made with MRI in both children. Most often, the clinical presentation of spinal cord ischemia is anterior spinal syndrome, with paraplegia and loss of pain, temperature, and touch sensation (but with relative sparing of pressure, vibration, and proprioception sensation). Urinary and fecal incontinence, impotence, and spinal dysautonomia also may occur [12]. Specific risk factors, such as spinal stenosis, vascular disease, intraoperative hypotension, or the use of epinephrine in the local anesthetic solution may cause spinal cord ischemia [1, 18]. In a case report of spinal cord ischemia after esophagectomy, the patient had a thoracic epidural catheter placed for postoperative pain control [21]. The spinal catheter was used in our second patient but at the lower lumbar level. Tong et al. [25] showed the upper thoracic level (T2–T4) to be a region at particular risk for ischemic injury, which corresponds to the watershed region of the spinal cord and therefore is most susceptible to changes in blood flow. The anterior spinal artery arises from the intercostal arteries and is one of three arteries associated discontinuously along the spinal cord. The anterior spinal artery supplies the anterior 2/3 of the spinal cord [16]. The posterior spinal arteries are fed by segmental arteries that arise from the aorta. There are more segmental arteries supplying the posterior spinal arteries than the anterior arteries, which accounts for anterior spinal artery syndrome being more frequently observed [19]. The diameter of spinal vessels may influence the blood flow through the spinal cord [15, 19]. In a dynamic flexion cadaveric study, Farley et al. [9] found that, in thoracic kyphosis exceeding 63°, spinal cord intramedullary pressure increased significantly compared with less substantial curves in the same specimen. However, a change of intramedullary pressure with progressive increases in the kyphosis angle on specimens did not correlate with spinal cord stenosis in the thoracic spine [9].
Positioning on the Operating Table
There are numerous reports of reduction of evoked potentials during patient positioning for surgery [6, 14, 21, 23, 25]. All but one of the patients in these studies sustained a spinal cord injury while they were in the prone position, whereas our patients were in the supine position [1, 3, 19–21]. Paraplegia attributable to a thoracic spinal cord infarction was described in a 16-year-old patient with Morquio syndrome (mucopolysaccharidosis IV) [25]. The child had surgery on the foramen magnum and atlantal decompression led to paraplegia because of a thoracic spinal cord infarction while the patient was in the prone position [25].
Intraoperative Monitoring
Neither of our study patients had neuromonitoring during surgery. It might be an important consideration when performing lower extremity procedures on patients with skeletal dysplasia, but neuromonitoring presents some challenges in maintaining a sterile field. We now use sterile neuromonitoring leads with adhesive tape, and pass over the end of the table after prepping the patient. The sterile field was maintained and allowed for manipulation of the lower extremity as needed.
Motor evoked potentials and somatosensory evoked potentials usually are performed to monitor spinal cord function of patients during spine surgeries. The somatosensory evoked potentials may identify 90% of clinically relevant neurologic events [25]. The motor evoked and somatosensory evoked potentials together obtain almost 100% sensitivity and specificity [7, 10, 25].
Some patients with severe thoracic kyphoscoliosis are at risk for ischemic cord injury during lower extremity surgery. In our series, the ischemic injury was located near the apex of the thoracic kyphotic deformity. The avoidance of low MAP intraoperatively and during postoperative care may be important for children with substantial thoracic kyphosis in the watershed area. Surgeons should consider neuromonitoring whenever possible in these patients, although this presents some logistic challenges in maintaining a sterile field with the placement of monitoring electrodes.
Acknowledgments
We thank Amir Ahmadian MD (Department of Neurosurgery, University of South Florida Morsani School of Medicine, Tampa, FL, USA), for his involvement preparing the manuscript and neurosurgical point of view. We thank Dustin Samples BS (Nemours/Alfred I. DuPont Hospital for Children. Wilmington, DE, USA) for comments and edits during manuscript preparation and submission.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the reporting of this case and that all investigations were conducted in conformity with ethical principles of research.
The work was performed at Nemours/Alfred I. duPont Hospital for Children, Department of Orthopaedics, Wilmington, DE, USA.
References
- 1.Alexianu D, Skolnick ET, Pinto AC, Ohkawa S, Roye DP, Jr, Solowiejczyk DE, Hyman JE, Sun LS. Severe hypotension in the prone position in a child with neurofibromatosis, scoliosis and pectus excavatum presenting for posterior spinal fusion. Anesth Analg. 2004;98:334–335. doi: 10.1213/01.ANE.0000096187.58714.B6. [DOI] [PubMed] [Google Scholar]
- 2.Attar S, Hankins JR, Turney SZ, Krasna MJ, McLaughlin JS. Paraplegia after thoracotomy: report of five cases and review of the literature. Ann Thorac Surg. 1995;59:1410–1415. doi: 10.1016/0003-4975(95)00196-R. [DOI] [PubMed] [Google Scholar]
- 3.Bafus BT, Chiravuri D, van der Velde ME, Chu BI, Hirshl R, Farley FA. Severe hypotension associated with the prone position in a child with scoliosis and pectus excavatum undergoing posterior spinal fusion. J Spinal Disord Tech. 2008;21:451–454. doi: 10.1097/BSD.0b013e31815725f2. [DOI] [PubMed] [Google Scholar]
- 4.Bethem D, Winter RB, Lutter L, Moe JH, Bradford DS, Lonstein JE, Langer LO. Spinal disorders of dwarfism: review of the literature and report of eighty cases. J Bone Joint Surg Am. 1981;63:1412–1425. [PubMed] [Google Scholar]
- 5.Chen H. Skeletal dysplasia treatment & management. Available at: http://emedicine.medscape.com/article/943343-treatment#a1128. Accessed January 15, 2014.
- 6.Dharmavaram S, Jellish WS, Nockels RP, Shea J, Mehmood R, Ghanayem A, Kleinman B, Jacobs W. Effect of prone positioning systems on hemodynamic and cardiac function during lumbar spine surgery: an echocardiographic study. Spine (Phila Pa 1976). 2006;31:1388–1393. [DOI] [PubMed]
- 7.Drake J, Zeller R, Kulkarni AV, Strantzas S, Holmes L. Intraoperative neurophysiological monitoring during complex spinal deformity cases in pediatric patients: methodology, utility, prognostication, and outcome. Childs Nerv Syst. 2010;26:523–544. doi: 10.1007/s00381-010-1115-0. [DOI] [PubMed] [Google Scholar]
- 8.Erol B, Dormans JP, States L, Kaplan FS. Skeletal dysplasias and metabolic disorders of bone. In: Dormans JP, editor. Pediatric Orthopaedics and Sports Medicine: The Requisites in Pediatrics. 1. St Louis, MO: Mosby; 2004. pp. 111–146. [Google Scholar]
- 9.Farley CW, Curt BA, Pettigrew DB, Holtz JR, Dollin N, Kuntz C 4th. Spinal cord intramedullary pressure in thoracic kyphotic deformity: a cadaveric study. Spine (Phila Pa 1976). 2012;37:E224–E230. [DOI] [PubMed]
- 10.Fehlings MG, Brodke DS, Norvell DC, Dettori JR. The evidence for intraoperative neurophysiological monitoring in spine surgery: does it make a difference? Spine (Phila Pa 1976). 2010;35(9 suppl):S37–S46. [DOI] [PubMed]
- 11.Haque IU, Zaritsky AL. Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children. Pediatr Crit Care Med. 2007;8:138–144. doi: 10.1097/01.PCC.0000257039.32593.DC. [DOI] [PubMed] [Google Scholar]
- 12.Hobai IA, Bittner EA, Grecu L. Perioperative spinal cord infarction in nonaortic surgery: report of three cases and review of the literature. J Clin Anesth. 2008;20:307–312. doi: 10.1016/j.jclinane.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Horton WA, Hecht JT. Disorders involving cartilage matrix proteins. In: Kliegman RM, Stanton BM, St. Geme J, Schor N, Behrman RE, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier; 2011:2424–2425.
- 14.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–178. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 15.Mutch JA, Johansson JE. Occlusion of the artery of Adamkiewicz after hip and knee arthroplasty. J Arthroplasty. 2011;26:505–508. doi: 10.1016/j.arth.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Novy J. Spinal cord syndromes. Front Neurol Neurosci. 2012;30:195–198. doi: 10.1159/000333682. [DOI] [PubMed] [Google Scholar]
- 17.Oh CW, Thacker MM, Mackenzie WG, Riddle EC. Coxa vara: a novel measurement technique in skeletal dysplasias. Clin Orthop Relat Res. 2006;447:125–131. doi: 10.1097/01.blo.0000203476.81302.24. [DOI] [PubMed] [Google Scholar]
- 18.Othman Z, Lenke LG, Bolon SM, Padberg A. Hypotension-induced loss of intraoperative monitoring data during surgical correction of Scheuermann kyphosis: a case report. Spine (Phila Pa 1976). 2004;29:E258–E265. [DOI] [PubMed]
- 19.Shahi N, Asante-Siaw J, Marzouk JF. Paraplegia following oesophagectomy. BMJ Case Rep. 2010;2010. pii: bcr09.2009.2270. [DOI] [PMC free article] [PubMed]
- 20.Sudheer PS, Logan SW, Ateleanu B, Hall JE. Haemodynamic effects of the prone position: a comparison of propofol total intravenous and inhalation anaesthesia. Anaesthesia. 2006;61:138–141. doi: 10.1111/j.1365-2044.2005.04464.x. [DOI] [PubMed] [Google Scholar]
- 21.Tabara Y, Tachibana-Iimori R, Yamamoto M, Abe M, Kondo I, Miki T, Kohara K. Hypotension associated with prone body position: a possible overlooked postural hypotension. Hypertens Res. 2005;28:741–746. doi: 10.1291/hypres.28.741. [DOI] [PubMed] [Google Scholar]
- 22.Taylor C, Brady P, O’Meara A, Moore D, Dowling F, Fogarty E. Mobility in Hurler syndrome. J Pediatr Orthop. 2008;28:163–168. doi: 10.1097/BPO.0b013e3181649e25. [DOI] [PubMed] [Google Scholar]
- 23.Tetzlaff JE, O’Hara JF, Jr, Yoon HJ, Schubert A. Heart rate variability and the prone position under general versus spinal anesthesia. J Clin Anesth. 1998;10:656–659. doi: 10.1016/S0952-8180(98)00110-X. [DOI] [PubMed] [Google Scholar]
- 24.Tolo VT. Spinal deformity in short-stature syndromes. Instr Course Lect. 1990;39:399–405. [PubMed] [Google Scholar]
- 25.Tong CK, Chen JC, Cochrane DD. Spinal cord infarction remote from maximal compression in a patient with Morquio syndrome. J Neurosurg Pediatr. 2012;9:608–612. doi: 10.3171/2012.2.PEDS11522. [DOI] [PubMed] [Google Scholar]
- 26.Winter RB, Moe JH, Wang JF. Congenital kyphosis. Its natural history and treatment as observed in a study of one hundred and thirty patients. J Bone Joint Surg Am. 1973;55:223–256. [PubMed] [Google Scholar]