Abstract
Background
Posttraumatic osteoarthritis (OA) is a variant of OA that can develop after articular injury. Although the mechanism(s) of posttraumatic OA are uncertain, the presence and impact of postinjury proteolytic enzymes on articular cartilage remain unknown. To our knowledge, there are no studies that evaluate the presence of matrix metalloproteinases (MMPs) or aggrecan degradation after articular fracture.
Questions/purposes
(1) Are MMP concentrations and aggrecan degradation elevated after intraarticular fracture? (2) Are MMP concentrations and aggrecan degradation greater in high-energy injuries compared with low-energy injuries? (3) Do the concentrations of these biomarkers remain elevated at a secondary aspiration?
Methods
Between December 2011 and June 2013, we prospectively enrolled patients older than 18 years of age with acute tibial plateau fracture. Exclusion criteria included age older than 60 years, preexisting knee OA, injury greater than 24 hours before evaluation, contralateral knee injury, history of autoimmune disease, open fracture, and non-English-speaking patients. During the enrollment period, we enrolled 45 of the 91 (49%) tibial plateau fractures treated at our facility. Knee synovial fluid aspirations were obtained from both the injured and uninjured knees; two patients received aspirations in the emergency department and the remaining patients received aspirations in the operating room. Twenty patients who underwent spanning external fixator followed by definitive fixation were aspirated during both surgical procedures. MMP-1, -2, -3, -7, -9, -10, -12, and -13 concentrations were quantified using multiplex assays. Aggrecan degradation was quantified using sandwich enzyme-linked immunosorbent assay.
Results
There were higher concentrations of MMP-1 (3.89 ng/mL [95% confidence interval {CI}, 2.37–6.37] versus 0.37 ng/mL [95% CI, 0.23–0.61], p < 0.001), MMP-3 (457.35 ng/mL [95% CI, 274.5–762.01] versus 129.17 ng/mL [95% CI, 77.01–216.66], p < 0.001), MMP-9 (6.52 ng/mL [95% CI, 3.86–11.03] versus 0.96 ng/mL [95% CI, 0.56–1.64], p < 0.001), MMP-10 (0.52 ng/mL [95% CI, 0.40–0.69] versus 0.23 ng/mL [95% CI, 0.17–0.30], p < 0.001), and MMP-12 (0.18 ng/mL [95% CI, 0.14–0.23] versus 0.10 ng/mL [95% CI, 0.0.081–0.14], p = 0.005) in injured knees compared with uninjured knees. There was not a detectable difference in MMP concentrations or aggrecan degradation between high- and low-energy injuries. MMP-1 (53.25 versus 3.89 ng/mL, p < 0.001), MMP-2 (76.04 versus 0.37 ng/mL, p < 0.001), MMP-3 (1250.62 versus 457.35 ng/mL, p = 0.002), MMP-12 (1.37 versus 0.18, p < 0.001), MMP-13 (0.98 versus 0.032 ng/mL, p < 0.001), and aggrecan degradation (0.58 versus 0.053, p < 0.001) were increased at the second procedure (mean, 9.5 days; range, 3–21 days) as compared with the initial procedure.
Conclusions
Because MMPs and aggrecan degradation are elevated after articular fracture, future studies are necessary to evaluate the impact of elevated MMPs and aggrecan degradation on human articular cartilage.
Clinical Relevance
If further clinical followup can demonstrate a relationship between posttraumatic OA and elevated MMPs and aggrecan degradation, they may provide potential for therapeutic targets to prevent or delay the destruction of the joint. Additionally, these markers may offer prognostic information for patients.
Introduction
Posttraumatic osteoarthritis (OA) is a variant of OA that can develop after articular injury. Despite improvements in surgical management of articular injuries, the incidence of posttraumatic OA has remained relatively unchanged over the last several decades [36]. Although the exact mechanism(s) that lead to development of posttraumatic OA remain unknown, there has been increased interest in the role of the postinjury inflammatory response leading to chondrocyte apoptosis and synovitis [32, 36].
Cartilage degeneration is a primary feature in the development of OA, and it involves the loss of extracellular matrix components including aggrecan and Type II collagen. Aggrecan is the major proteoglycan found in articular cartilage. Aggrecan breakdown in degenerative joints is typically the result of proteolytic cleavage, and aggrecanases and matrix metalloproteinases (MMPs) are the primary proteases responsible for aggrecan cleavage in pathologic conditions [40]. Additionally, MMPs are capable of degrading intact Type II collagen into large fragments that can then be further degraded by gelatinases [42].
Several animal and human studies have demonstrated elevated concentrations of MMPs and aggrecan degradation in OA and posttraumatic OA models. A variety of animal OA models with ACL transection have shown upregulation of genes coding for aggrecanases and MMPs as well as increased concentrations of these destructive proteases [1, 10, 20, 47]. Most human studies demonstrate elevated MMPs and aggrecan degradation in patients with OA as compared with healthy control subjects and that these proteases are more elevated in patients with end-stage OA compared with early OA [4, 21, 23, 28, 34]. In an effort to look specifically at the presence of proteolytic enzymes after traumatic injury, several authors have shown elevated MMPs and aggrecan degradation after ACL tear. However, the time from ACL injury to joint aspiration had considerable variability in these studies [5, 7, 26, 30]. There are currently no human studies of which we are aware that quantify the concentrations of MMPs and/or aggrecan degradation acutely after articular fracture. The purpose of our study was to characterize MMP production and aggrecan degradation in a joint after acute articular fracture.
Specifically, we set out to answer the following questions: (1) Are MMP concentrations and aggrecan degradation elevated after intraarticular fracture? (2) Are MMP concentrations and aggrecan degradation greater in high-energy injuries compared with low-energy injuries? (3) Do the concentrations of these biomarkers remain elevated at a secondary aspiration?
Patients and Methods
After receiving institutional review board approval, we prospectively enrolled patients older than 18 years of age who presented to our Level I trauma center meeting inclusion criteria and identified with a tibial plateau fracture December 1, 2011, through June 30, 2013.
Inclusion criteria included any patient older than 18 years of age with acute (within 24 hours of injury) tibial plateau fracture. Exclusion criteria included any patient older than 60 years of age; any history of preexisting knee OA based on previous diagnosis or radiographic findings; regular use of nonsteroidal antiinflammatory drugs; any history of autoimmune disease; acute contralateral intraarticular knee injury; open fracture; non-English-speaking patient; and injury greater than 24 hours before evaluation. During the study period, 91 tibial plateau fractures were treated at our facility and 45 patients were enrolled in the study (Fig. 1). Basic patient demographic data were obtained including age, gender, and comorbidities. Fractures were classified based on the AO/OTA classification system and the Schatzker classification system [27, 35]. Injury Severity Scores (ISS) were recorded for all patients [2]. Nonosteopenic plateau fractures that were classified as Schatzker IV, V, and VI patterns were also classified as high-energy injuries. All patients were treated by one of two fellowship-trained orthopaedic traumatologists.
Fig. 1.
Flowchart illustrates patient recruitment during the study enrollment period.
Patients undergoing either spanning external fixation or open reduction and internal fixation in the first 24 hours after injury had the knee aspiration from the injured and uninjured extremities obtained in the operating room. Otherwise, the aspiration was performed in the emergency department. Patients who required spanning external fixator followed by definitive fixation were aspirated at the beginning of both procedures, after the patient had undergone general anesthesia. During the surgical placement of the external fixator, the knee was not violated. Twenty patients received spanning external fixator followed by definitive fixation, thus providing two time points for analysis. Patients received definitive fixation a mean of 9.5 days (range, 3–21 days) after the initial injury. After thorough sterile preparation of the skin, both the injured and control knees were injected with 5 mL sterile saline and as much fluid as possible was withdrawn. A dilutional factor was calculated to account for differences in knee fluid volume in injured and uninjured knees (ie, presence of hemarthrosis in the injured knee). The synovial fluid was transferred to tubes and combined with a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). The samples were then centrifuged at 2500 rpm for 15 minutes. The supernatant was aspirated, placed in 2-mL cryopreservation tubes, and frozen at −80° C until subsequent analysis.
MMP Analysis
The concentrations of eight MMPs were quantified (MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, MMP-12, and MMP-13) using a human MMP panel with the Millipore multiplex system (Millipore, Billerica, MA, USA) following the manufacturer’s protocol in a 96-well plate format. Twenty-five microliters of sample were used in each well of the assay plate. The assays use antibodies linked to magnetic beads, and the relative concentration of each sample is analyzed compared with the concentrations of the standard controls provided by the manufacturer. The assays were performed blinded to patient injury classification and whether the sample was from an injured or uninjured knee.
Aggrecan Breakdown Analysis
The concentration of aggrecan degradation, based on the ARGS neoepitope, was quantified using a sandwich enzyme-linked immunosorbent assay (ELISA) (BC3-C2) as previously described [41]. Briefly, the BC3-C2 antibody was used as the capture antibody, and the HRP-conjugated α-HABR antibody was used as the detection antibody. The BC3-C2 antibody was immobilized on ELISA plates overnight at a concentration of 1 μg/mL. Synovial fluid samples were then added to the plate and the HRP-HABR antibody (1:8000 dilution) was added to the plate to capture the ARGS containing fragments. The ELISA signal was measured using the Supersignal ELISA Femto Maximum Sensitivity Substrate (Pierce Biotechnology, Rockford, IL, USA) and read using a Victor luminometer. The assay was performed blinded to patient injury classification and whether the sample was from the injured or uninjured knee.
Statistical Analysis
Because we were analyzing highly skewed data, which is often encountered in biomarker analysis, analyses were conducted using log transformation of the variables. The results were then back-transformed to the scale of the original data to allow for better understanding of the results. A repeated-measures mixed-effects analysis of variance was performed to compare mean concentrations of all MMPs and aggrecan degradation between each patient’s injured and uninjured knees. The profile of the uninjured knee should provide some baseline for comparison to the injured knee, hoping to control for systemic inflammatory response. A repeated-measures mixed-effects analysis of variance was performed to test for the effects of the injury status of the knee, the surgery time, and the interaction of the injury status and surgery time. Specifically, the same biomarker was measured at the first and second surgery as well as for the injured and uninjured knees. Because the response would be expected to be more uniform within an individual than across individuals, a repeated-measures analysis of variance was used for these comparisons. For concentration comparisons between high- and low-energy injuries within 24 hours of injury, the mean concentration difference between injured and uninjured knees was used. To adjust for the testing of nine biomarkers, the Bonferroni correction was used so that the adjusted alpha level for significance was 0.05/9 or p < 0.0055.
The study cohort consisted of 45 patients (31 men, 14 women) with an average age of 42.3 years (range, 20–60 years). There were 24 low-energy (Schatzker 1–3, all AO/OTA 41B) tibial plateau injuries and 21 high-energy (Schatzker 4–6, AO/OTA Type C) tibial plateau injuries. Of the high-energy fractures, five were AO/OTA 41B3 and nine were AO/OTA 41C. The average ISS was 5.5, and there were only two patients with ISS greater than 18.
During the enrollment period, 91 tibial plateau fractures were treated at our facility. Forty-six patients were excluded from enrollment because they did not meet the entry criteria. Thirteen patients were excluded as a result of age older than 60 years, and three patients were excluded for preexisting knee OA. Seventeen patients were excluded as a result of injury greater than 24 hours from initial hospital evaluation. Three patients were excluded for contralateral injury, three patients had a history of autoimmune disease, four patients refused to participate, two patients were non-English-speaking, and one patient had an open tibial plateau fracture. All enrolled patients had a knee aspiration performed within 24 hours of injury, and there were no dry aspiration attempts.
Results
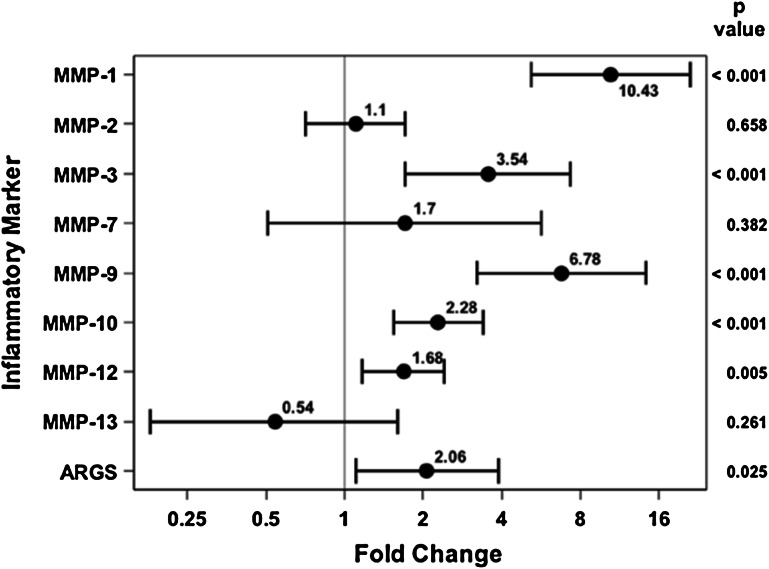
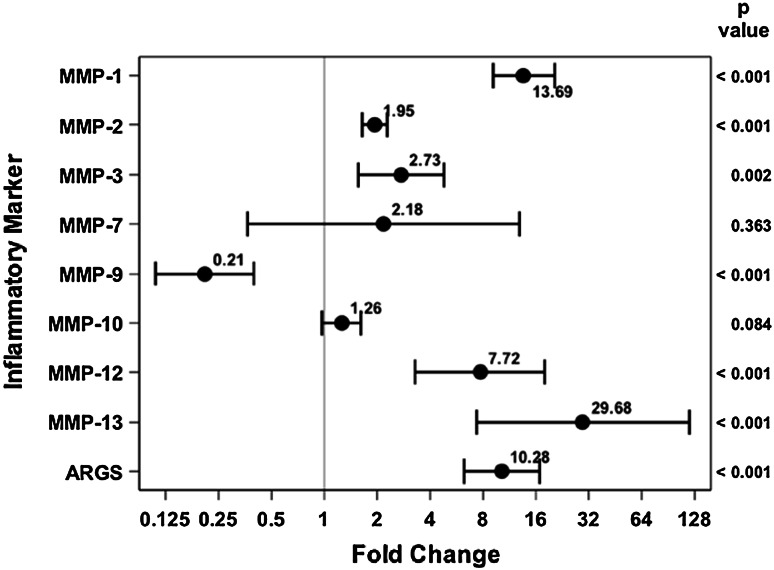
In comparing injured knee aspirates with uninjured knee aspirates within 24 hours of injury, several of the proteases were elevated on the injured side. MMP-1 (3.89 ng/mL [95% confidence interval {CI}, 2.37–6.37] versus 0.37 ng/mL [95% CI, 0.23–0.61], p < 0.001), MMP-3 (457.35 ng/mL [95% CI, 274.5–762.01] versus 129.17 ng/mL [95% CI, 77.01–216.66], p < 0.001), MMP-9 (6.52 ng/mL [95% CI, 3.86–11.03] versus 0.96 ng/mL [95% CI, 0.56–1.64], p < 0.001), MMP-10 (0.52 ng/mL [95% CI, 0.40–0.69] versus 0.23 ng/mL [95% CI, 0.17–0.30], p < 0.001), and MMP-12 (0.18 ng/mL [95% CI, 0.14–0.23] versus 0.10 ng/mL [95% CI, 0.081–0.14], p = 0.005) were all found to be elevated in the injured knee compared with the uninjured knee (Fig. 2). . With the numbers of comparisons we had, MMP-2 (38.97 ng/mL [95% CI, 28.64–53.02] versus 35.34 ng/mL [95% CI, 25.87–48.28], p = 0.658), MMP-7 (2.40 ng/mL [95% CI, 1.03–5.59] versus 1.41 ng/mL [95% CI, 0.60–3.32], p = 0.382), MMP-13 0.032 ng/mL [95% CI, 0.014–0.07] versus 0.061 ng/mL [95% CI, 0.027–0.132], p = 0.261), and aggrecan degradation (0.053 ng/mL [95% CI, 0.034–0.084] versus 0.025 ng/mL [95% CI, 0.016–0.04], p = 0.025) were not different between the injured and uninjured joints.
Fig. 2.
Fold change and 95% CIs demonstrate elevated MMP concentrations for the acutely injured extremity as compared with the uninjured extremity.
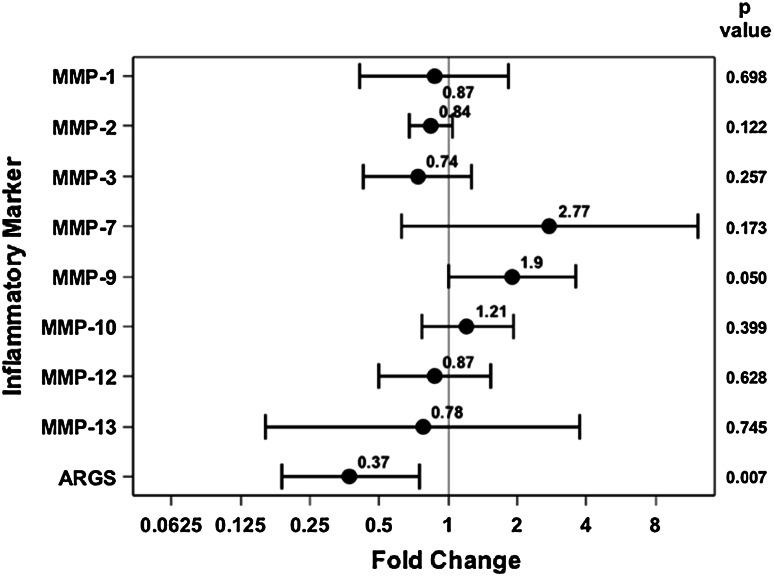
With the numbers available, we were unable to detect a difference in MMP concentrations or aggrecan degradation between high- and low-energy injuries. In comparing high-energy versus low-energy fractures, MMP-1 (3.61 ng/mL [95% CI, 2.11–6.18] versus 4.17 ng/mL [95% CI, 2.47–7.05], p = 0.698), MMP-2 (35.70 ng/mL [95% CI, 30.53–41.74] versus 42.35 ng/mL [95% CI, 36.36–49.33], p = 0.122), MMP-3 (390.62 ng/mL [95% CI, 265.18–575.39] versus 531.287 ng/mL [95% CI, 364.23–774.96], p = 0.257), MMP-7 (4.053 ng/mL [95% CI, 1.40–11.76] versus 1.46 ng/mL [95% CI, 0.52–4.13], p = 0.173), MMP-9 (9.06 ng/mL [95% CI, 5.73–14.33] versus 4.77 ng/mL [95% CI, 3.05–7.46], p = 0.05), MMP-10 (0.57 ng/mL [95% CI, 0.41–0.80] versus 0.47 ng/mL [95% CI, 0.34–0.66], p = 0.399), MMP-12 (0.17 ng/mL [95% CI, 0.11–0.25] versus 0.19 ng/mL [95% CI, 0.13–0.28], p = 0.628), MMP-13 (0.028 ng/mL [95% CI, 0.008–0.089] versus 0.037 ng/mL [95% CI, 0.012–0.11], p = 0.745), and aggrecan degradation (0.031 ng/mL [95% CI, 0.018–0.053] versus 0.085 ng/mL [95% CI, 0.052–0.14], p = 0.007) were not different (Fig. 3).
Fig. 3.
Fold change and 95% CIs demonstrate no difference in MMP concentration or aggrecan degradation between high-energy and low-energy fractures.
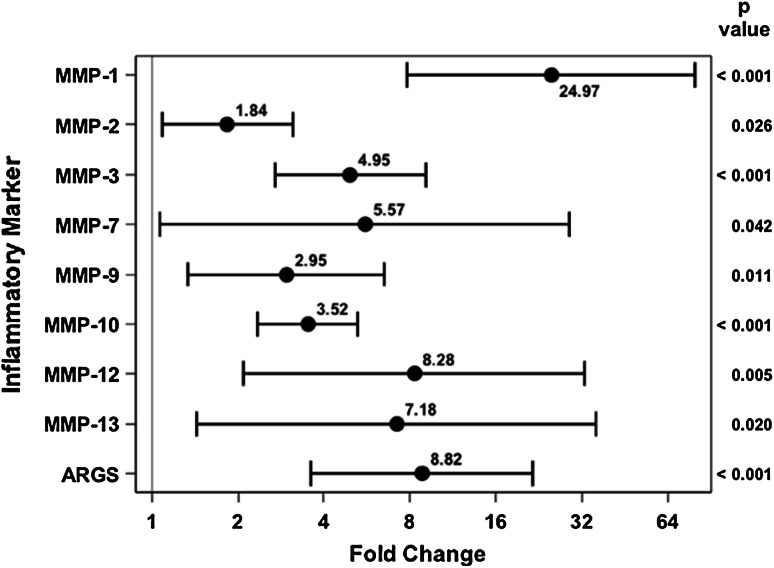
In the 20 patients who received spanning external fixation followed by definitive fixation, the concentrations of several MMPs increased in the injured knee at the time of the secondary procedure, which occurred a mean of 9.5 days (range, 3–21 days) after initial injury. The concentrations of MMP-1 (53.25 ng/mL [95% CI, 32.99–85.96] versus 2.03 ng/mL [95% CI, 0.71–5.79], p < 0.001), MMP-3 (1250.62 ng/mL [95% CI, 756.86–2066.48] versus 228.01 ng/mL [95% CI, 79.44–654.40], p < 0.001), MMP-10 (0.66 ng/mL [95% CI, 0.49–0.88] versus 0.18 ng/mL [95% CI, 0.11–0.29], p < 0.001), MMP-12 (1.37 ng/mL [95% CI, 0.65] versus 0.15 ng/mL [95% CI, 0.10–0.24], p = 0.005), and aggrecan degradation (0.56 ng/mL [95% CI, 0.34–0.91] versus 0.055 ng/mL [95% CI, 0.023–0.13], p < 0.001) were greater at the time of the second procedure when comparing injured with uninjured joints; this was compared with the normal knee at the time of the second aspiration (Fig. 4). MMP-2 (76.04 ng/mL [95% CI, 64.52–89.60] versus 39.35 ng/mL [95% CI, 19.85–77.99], p = 0.026), MMP-7 (5.25 ng/mL [95% CI, 1.21–22.72] versus 0.62 ng/mL [95% CI, 0.12–3.09], p = 0.042), MMP-9 (1.36 ng/mL [95% CI, 0.75–2.46] versus 0.59 ng/mL [95% CI, 0.21–1.65], p = 0.011), and MMP-13 (0.98 ng/mL [95% CI, 0.27–3.62] versus 0.12 ng/mL [95% CI, 0.034–0.39], p < 0.02) were not different at the time of the second procedure when comparing injured with uninjured joints. In the injured knee, MMP-1 (53.25 ng/mL [95% CI, 32.99–85.96] versus 3.89 ng/mL [95% CI, 2.67–5.66], p < 0.001), MMP-2 (76.04 ng/mL [95% CI, 64.52–89.60] versus 38.97 ng/mL [95% CI, 34.74–43.70], p < 0.001), MMP-3 (1250.62 ng/mL [95% CI, 756.86–2066.48] versus 457.35 ng/mL [95% CI, 330.36–633.16], p = 0.002), MMP-12 (1.37 ng/mL [95% CI, 0.65–2.90] versus 0.18 ng/mL [95% CI, 0.11–0.29], p < 0.001), MMP-13 (0.98 ng/mL [95% CI, 0.27–3.62] versus 0.032 ng/mL [95% CI, 0.013–0.078], p < 0.001), and aggrecan degradation (0.56 ng/mL [95% CI, 0.34–0.91] versus 0.053 ng/mL [95% CI, 0.037–0.076], p < 0.001) were all increased at the time of the second procedure as compared with the first procedure. In contrast, the concentration of MMP-9 (1.36 ng/mL [95% CI, 0.75–2.46] versus 6.52 ng/mL [95% CI, 4.44–9.58], p < 0.001) was less in the injured joint at the time of the second surgery compared with the first surgery. With the numbers we had, we could not detect a difference in MMP-10 (0.66 ng/mL [95% CI, 0.49–0.88] versus 0.52 ng/mL [95% CI, 0.42–0.65], p = 0.084) and MMP-7 (5.25 ng/mL [95% CI, 1.21–22.72] versus 2.40 ng/mL [95% CI, 0.94–6.15], p = 0.363) at the second time point compared with the initial injury (Fig. 5).
Fig. 4.
Fold change and 95% CIs demonstrate elevated MMP concentrations and aggrecan degradation for the injured extremity as compared with the uninjured extremity at the second aspiration.
Fig. 5.
Fold change and 95% CIs demonstrate increased MMP concentrations and aggrecan degradation in the injured extremity at the second aspiration compared with the first aspiration.
Discussion
MMPs and aggrecanases are thought to play a role in cartilage degradation and the progression of OA. Aggrecan is the major proteoglycan in articular cartilage, and aggrecan depletion is believed to impair joint function and to be involved in the progression to end-stage arthritis [22–24]. MMPs are enzymes that play a role in both physiologic and pathologic remodeling of chondrocyte extracellular matrix. MMP-1 and -13 are capable of directly degrading intact Type II collagen, which is one of the main components of articular cartilage [18, 29, 45]. MMP-13 has been shown to be five times to 10 times more active in digesting Type II collagen than other collagenases, and MMP-13 overexpression in mice has been associated with advanced joint degeneration [3, 17, 25, 31]. Prior human studies have demonstrated elevated MMP concentrations in arthritic joint synovial fluid [4, 21, 23, 28, 34]. However, there were no prior studies evaluating for the presence of MMPs after articular fracture, which is the clinical setting with the most articular damage and the highest rates of posttraumatic OA. In the current study, we have demonstrated elevated concentrations of MMPs and increased aggrecan degradation in the knee acutely after tibial plateau fracture and that several of these factors increase in concentration during the first 2 weeks. This would suggest that the articular cartilage continues to interact with degradative proteases long after initial injury and these proteases could play a role in the development of posttraumatic OA.
Limitations of the study include the relatively small number of patients that received a second aspiration and the large time range (3–21 days) when the second aspiration was obtained. This makes formulating conclusions based on the relationship of MMPs/aggrecan degradation with time more difficult. Additionally, we did not validate the results of our multiplex system or ARGS neoepitope ELISA with a separate ELISA. We did not feel this was necessary, because both the multiplex system and ARGS fragment ELISA have been previously validated using human synovial fluid [8, 9, 41]. Finally, we do not have long-term joint aspirations or patient followup to demonstrate if these proteases continue to be present in injured joints and/or lead to posttraumatic OA. .We did not compare our aspiration results with serum results, which has been done in other studies because these studies failed to show good correlations between serum and synovial fluid concentrations [7, 14, 16]. Also it is possible that our dilution methodology, especially when aspirating joint fluid from a normal joint, could have altered the true levels of degradative products and enzymes in our study. In contrast to most other studies analyzing synovial fluid where experimental aspirates are compared with aspirates from different, “healthy” control patients, the authors chose to compare the uninjured and injured knees from the same patient to account for any potential biomarker elevation resulting from acute systemic trauma [21, 26, 30, 42, 43] . With the patient in the supine position, it is difficult to obtain synovial fluid from the normal knee because the fluid likely collects along the posterior capsule. Unlike other studies in which patients can modify their position (seated position, etc) to assist with synovial fluid aspiration from a normal joint, patients in this study remained supine as a result of the tibial plateau fracture. Because strong correlations between serum and synovial fluid biomarkers after trauma have not been demonstrated and the authors’ decision that “healthy” control patients were an inadequate comparison, dilution of the samples could impact comparisons between this study and previous studies.
There are few studies that quantify MMP concentration after articular injury. Catterall et al. [7] reported MMP-3 concentration of 29.02 ng/mL in synovial fluid obtained within 3 weeks of an ACL injury. Similarly, Tchetverikov [42] reported MMP-1 concentration of 1.12 ng/mL and MMP-3 concentration of 50.8 ng/mL within 4 days of ligamentous knee injury. Our reported MMP-1 concentration of 3.9 ng/mL and MMP-3 concentration of 457 ng/mL in the joint with articular fracture are severalfold higher than levels that have been previously published. This may be related to our study including only patients with articular fracture and only patients within 24 hours of injury. Although the concentrations of MMP-1, -3, -9, -10, and -12 were all acutely elevated, the concentration of MMP-13 was not, contrary to what we would have expected. However, at the time of the second aspiration, the concentration of MMP-13 was greater than the uninjured knee and greater than the initial aspiration. In agreement with other studies, this suggests that MMP-13 may rely on other inflammatory cytokines and MMPs for upregulation and may not be a major factor immediately after injury but rather with increasing time [13, 17]. Two studies have demonstrated elevated levels of aggrecan degradation acutely after ACL injury that remained elevated up to 1 year after injury as compared with healthy control patients [24, 26]. However, in both studies, acute injury was defined as within 4 weeks of injury and aggrecan degradation was compared with the levels of healthy control subjects. In the current study, we demonstrated that several MMPs were greater in acutely injured knees as compared with uninjured knees. Given that MMP-13 is the most aggressive collagenase and its presence was delayed after injury, this may be a protease that could be targeted early after injury to prevent its accumulation in the joint.
Perhaps the most surprising finding was the poor association between elevated MMP concentrations/aggrecan degradation and presumed energy of injury. It is assumed that increasing energy during the injury would be associated with a worse fracture pattern and increased local soft tissue and bone damage. The Schatzker classification has been used to classify plateau fractures into high- and low-energy injuries, and prior studies have demonstrated higher rates of posttraumatic OA in bicondylar as compared with unicondylar tibial plateau fractures [6, 11, 46]. We were unable to detect a difference in MMP concentration or aggrecan degradation when comparing high- and low-energy injuries. Although this is contrary to what is known regarding fracture energy and development of posttraumatic OA, these outcomes are consistent with recent prior findings from our group [19]. Prior research on inflammatory cytokine concentrations between high- and low-energy injuries demonstrated no difference [19]. In a study using CT-based metrics, Thomas et al. [44]found that both fracture energy and articular comminution combined served as a better predictor of eventual development of posttraumatic OA than each individual component alone. The Schatzker classification does not account for articular comminution, and the presence of articular comminution may be more indicative of some elements of fracture energy than condylar involvement. The final possibility is that the groups are not adequately powered to detect a difference.
Nearly all quantified MMP concentrations and aggrecan degradation were elevated a mean of 10 days after injury in the injured knee. Prior studies have suggested that the greatest elevation in MMP concentration and aggrecan degradation occurs within the first 2 weeks and then gradually returns closer to a normal level [26, 39, 42]. However, these prior studies all compare different patients at different stages of injury, whereas this study has multiple time points for the same patient. Additionally, MMP-1, -2, -3, -12, -13, and aggrecan degradation were all increased at the second procedure as compared with the first procedure in the injured knee. This suggests that the articular cartilage in injured joints continues to be exposed to these potentially destructive aggrecanases and undergo breakdown over 1 week after injury. The impact of having these proteases in contact with articular cartilage remains unknown.
An interesting finding in addition to our research questions was the elevated MMPs and aggrecan degradation in the normal, uninjured knee. There are few studies that report knee synovial fluid MMP concentrations. In a study comparing synovial fluid from normal, control knees with synovial fluid from patients with OA and those with rheumatoid arthritis, Tchetverikov et al reported that MMP-1, -8, -2, -9, and -13 were not elevated in the normal knee [43]. Other studies have reported similar results [21, 26]. The difference is that our samples were obtained from the normal knee in a patient who sustained a traumatic injury. This possibly indicates that the systemic inflammatory response upregulates the expression of MMPs and aggrecan degradation in the joint.
In conclusion, the present study is the first to our knowledge to report the concentrations of MMPs and aggrecan degradation present in synovial fluid after an acute intraarticular fracture compared with an uninjured joint in the same patient. MMP-1, MMP-3, MMP-9, MMP-10, and MMP-12 were elevated in acutely injured knees compared with uninjured knees. Additionally, there was a poor association of elevated MMP concentration with fracture energy, suggesting that this classification may not be helpful in quantifying local acute inflammatory response or that the presence, but not the magnitude of, the response is the most important aspect after trauma. Finally, several MMPs and aggrecan degradation remained elevated at a secondary time point suggesting that these MMPs and aggrecanases may play a role in chronic inflammation and the development of posttraumatic OA. That elevations were also present in the normal joint may suggest a systemic response to injury. Before drawing conclusions regarding the role of these proteases in the development of posttraumatic OA, these patients will need to be followed over time to evaluate if these MMPs and aggrecan degradation are more strongly correlated with the ultimate development of posttraumatic OA. Prior studies have suggested various cell-signaling mechanisms that may play a role in the development of posttraumatic OA including transforming growth factor-β, endothelin-1, interleukin-1β, and NF-κB [12, 15, 22, 33, 37, 38]. Our current study should draw attention to the postinjury presence of MMPs and aggrecan degradation as potentially playing a role in progression to OA as well. If further study shows a correlation between elevated MMPs and aggrecanases and the development of posttraumatic OA, it may provide a potential therapeutic target for preventing or at least delaying the development of posttraumatic OA.
Acknowledgments
We thank Nousheen Alasti for her assistance in collecting patient samples and arranging logistics for the study. Additionally, we acknowledge Sihem Boudina, Shaobo Pei, and the Metabolic Phenotyping Core facility at the University of Utah for assistance in running study assays.
Footnotes
The institution of one or more of the authors (JMH, TFH) has received peer-reviewed research grant funding from the Orthopedic Trauma Association, LS Peery Foundation, and AO North America for this project. One of the authors certifies that he (TFH), or a member of his immediate family, has signed an agreement with DePuy Synthes (Warsaw, IN, USA) for consulting activities not related to the current study, and has not received payments or benefits, during the study period. One or more of the authors (CAS, KT) are employed at Eli Lilly (Indianapolis, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved or waived approval for the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
This project was performed at the University of Utah, Salt Lake City, UT, USA.
References
- 1.Backus JD, Furman BD, Swimmer T, Kent CL, McNulty AL, DeFrate LE, Guilak F, Olson SA. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. J Orthop Res. 2011;29:501–510. doi: 10.1002/jor.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker SP, O’Neill B. The injury severity score: an update. J Trauma. 1976;16:882–885. doi: 10.1097/00005373-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobacz K, Maier R, Fialka C, Ekhart H, Woloszczuk W, Geyer G, Erlacher L, Smolen J, Graninger WB. Is pro-matrix metalloproteinase-3 a marker for posttraumatic cartilage degradation? Osteoarthritis Cartilage. 2003;11:665–672. doi: 10.1016/S1063-4584(03)00159-6. [DOI] [PubMed] [Google Scholar]
- 5.Brophy RH, Rai MF, Zhang Z, Torgomyan A, Sandell LJ. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J Bone Joint Surg Am. 2012;94:385–393. doi: 10.2106/JBJS.K.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canadian Orthopaedic Trauma Society Open reduction and internal fixation compared with circular fixator application for bicondylar tibial plateau fractures. Results of a multicenter, prospective, randomized clinical trial. J Bone Joint Surg Am. 2006;88:2613–2623. doi: 10.2106/JBJS.E.01416. [DOI] [PubMed] [Google Scholar]
- 7.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis Res Ther. 2010;12:R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuellar JM, Scuderi GJ, Cuellar VG, Golish SR, Yeomans DC. Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. J Bone Joint Surg Am. 2009;91:2313–2320. doi: 10.2106/JBJS.H.00835. [DOI] [PubMed] [Google Scholar]
- 9.Cuellar VG, Cuellar JM, Golish SR, Yeomans DC, Scuderi GJ. Cytokine profiling in acute anterior cruciate ligament injury. Arthroscopy. 2010;26:1296–1301. doi: 10.1016/j.arthro.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 10.de Grauw JC, van de Lest CHA, van Weeren PR. Inflammatory mediators and cartilage biomarkers in synovial fluid after a single inflammatory insult: a longitudinal experimental study. Arthritis Res Ther. 2009;11:R35. doi: 10.1186/ar2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egol KA, Tejwani NC, Capla EL, Wolinsky PL, Koval KJ. Staged management of high-energy proximal tibia fractures (OTA types 41): the results of a prospective, standardized protocol. J Orthop Trauma. 2005;19:448–455; discussion 456. [DOI] [PubMed]
- 12.Fan Z, Söder S, Oehler S, Fundel K, Aigner T. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am J Pathol. 2007;171:938–946. [DOI] [PMC free article] [PubMed]
- 13.Fosang AJ, Last K, Maciewicz RA. Aggrecan is degraded by matrix metalloproteinases in human arthritis. Evidence that matrix metalloproteinase and aggrecanase activities can be independent. J Clin Invest. 1996;98:2292–2299. doi: 10.1172/JCI119040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furman BD, Kimmerling KA, Zura RD, Reilly RM, Zlowodzki MP, Huebner JL, Kraus VB, Guilak F, Olson SA. Brief report: articular ankle fracture results in increased synovitis, synovial macrophage infiltration, and synovial fluid concentrations of inflammatory cytokines and chemokines. Arthritis Rheumatol. 2015;67:1234–1239. doi: 10.1002/art.39064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH, Huebner JL, Kraus VB, Guilak F, Olson SA. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther. 2014;16:R134. doi: 10.1186/ar4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Germaschewski FM, Matheny CJ, Larkin J, Liu F, Thomas LR, Saunders JS, Sully K, Whittall C, Boyle Y, Peters G, Graham NM. Quantitation of ARGS aggrecan fragments in synovial fluid, serum and urine from osteoarthritis patients. Osteoarthritis Cartilage. 2014;22:690–697. doi: 10.1016/j.joca.2014.02.930. [DOI] [PubMed] [Google Scholar]
- 17.Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì RM, Marcu KB. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–220. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427(Suppl):S27–36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 19.Haller JM, McFadden M, Kubiak EN, Higgins TF. Inflammatory cytokine response following acute tibial plateau fracture. J Bone Joint Surg Am. 2015;97:478–483. doi: 10.2106/JBJS.N.00200. [DOI] [PubMed] [Google Scholar]
- 20.Haslauer CM, Elsaid KA, Fleming BC, Proffen BL, Johnson VM, Murray MM. Loss of extracellular matrix from articular cartilage is mediated by the synovium and ligament after anterior cruciate ligament injury. Osteoarthritis Cartilage. 2013;21:1950–1957. doi: 10.1016/j.joca.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heard BJ, Martin L, Rattner JB, Frank CB, Hart DA, Krawetz RJ. Matrix metalloproteinase protein expression profiles cannot distinguish between normal and early osteoarthritic synovial fluid. BMC Musculoskelet Disord. 2012;13:126. doi: 10.1186/1471-2474-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier J-P, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2010;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 23.Larsson S, Englund M, Struglics A, Lohmander LS. Association between synovial fluid levels of aggrecan ARGS fragments and radiographic progression in knee osteoarthritis. Arthritis Res Ther. 2010;12:R230. doi: 10.1186/ar3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson S, Lohmander LS, Struglics A. Synovial fluid level of aggrecan ARGS fragments is a more sensitive marker of joint disease than glycosaminoglycan or aggrecan levels: a cross-sectional study. Arthritis Res Ther. 2009;11:R92. doi: 10.1186/ar2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohmander LS, Ionescu M, Jugessur H, Poole AR. Changes in joint cartilage aggrecan after knee injury and in osteoarthritis. Arthritis Rheum. 1999;42:534–544. doi: 10.1002/1529-0131(199904)42:3<534::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, Prokuski L, Sirkin MS, Ziran B, Henley B, Audige L. Fracture and dislocation classification compendium–2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21:S1–133. doi: 10.1097/00005131-200711101-00001. [DOI] [PubMed] [Google Scholar]
- 28.Misko TP, Radabaugh MR, Highkin M, Abrams M, Friese O, Gallavan R, Bramson C, Hellio Le Graverand MP, Lohmander LS, Roman D. Characterization of nitrotyrosine as a biomarker for arthritis and joint injury. Osteoarthritis Cartilage. 2013;21:151–156. [DOI] [PubMed]
- 29.Mix KS, Sporn MB, Brinckerhoff CE, Eyre D, Schurman DJ. Novel inhibitors of matrix metalloproteinase gene expression as potential therapies for arthritis. Clin Orthop Relat Res. 2004;427(Suppl):S129–37. doi: 10.1097/01.blo.0000144483.62033.8b. [DOI] [PubMed] [Google Scholar]
- 30.Nelson F, Billinghurst RC, Pidoux I, Reiner A, Langworthy M, McDermott M, Malogne T, Sitler DF, Kilambi NR, Lenczner E, Poole AR. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthritis Cartilage. 2006;14:114–119. doi: 10.1016/j.joca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson SA, Horne P, Furman B, Huebner J, Al-Rashid M, Kraus VB, Guilak F. The role of cytokines in posttraumatic arthritis. J Am Acad Orthop Surg. 2014;22:29–37. doi: 10.5435/JAAOS-22-01-29. [DOI] [PubMed] [Google Scholar]
- 33.Plaas A, Velasco J, Gorski DJ, Li J, Cole A, Christopherson K, Sandy JD. The relationship between fibrogenic TGFβ1 signaling in the joint and cartilage degradation in post-injury osteoarthritis. Osteoarthritis Cartilage. 2011;19:1081–1090. doi: 10.1016/j.joca.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Scanzello CR, Umoh E, Pessler F, Diaz-Torne C, Miles T, Dicarlo E, Potter HG, Mandl L, Marx R, Rodeo S, Goldring SR, Crow MK. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage. 2009;17:1040–1048. doi: 10.1016/j.joca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Schatzker J, McBroom R, Bruce D. The tibial plateau fracture. The Toronto experience 1968–1975. Clin Orthop Relat Res. 1979;138:94–104. [PubMed] [Google Scholar]
- 36.Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22:20–28. doi: 10.5435/JAAOS-22-01-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology (Oxford). 2008;47:584–590. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 38.Sin A, Tang W, Wen CY, Chung SK, Chiu KY. The emerging role of endothelin-1 in the pathogenesis of subchondral bone disturbance and osteoarthritis. Osteoarthritis Cartilage. 2015;23:516–524. doi: 10.1016/j.joca.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Struglics A, Hansson M, Lohmander LS. Human aggrecanase generated synovial fluid fragment levels are elevated directly after knee injuries due to proteolysis both in the inter globular and chondroitin sulfate domains. Osteoarthritis Cartilage. 2011;19:1047–1057. doi: 10.1016/j.joca.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Struglics A, Larsson S, Pratta MA, Kumar S, Lark MW, Lohmander LS. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthritis Cartilage. 2006;14:101–113. doi: 10.1016/j.joca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Swearingen CA, Carpenter JW, Siegel R, Brittain IJ, Dotzlaf J, Durham TB, Toth JL, Laska DA, Marimuthu J, Liu C, Brown DP, Carter QL, Wiley MR, Duffin KL, Mitchell PG, Thirunavukkarasu K. Development of a novel clinical biomarker assay to detect and quantify aggrecanase-generated aggrecan fragments in human synovial fluid, serum and urine. Osteoarthritis Cartilage. 2010;18:1150–1158. doi: 10.1016/j.joca.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Tchetverikov I. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64:694–698. doi: 10.1136/ard.2004.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tchetverikov I, Ronday HK, Van El B, Kiers GH, Verzijl N, TeKoppele JM, Huizinga TWJ, DeGroot J, Hanemaaijer R. MMP profile in paired serum and synovial fluid samples of patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:881–883. doi: 10.1136/ard.2003.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas TP, Anderson DD, Mosqueda TV, Van Hofwegen CJ, Hillis SL, Marsh JL, Brown TD. Objective CT-based metrics of articular fracture severity to assess risk for posttraumatic osteoarthritis. J Orthop Trauma. 2010;24:764–769. doi: 10.1097/BOT.0b013e3181d7a0aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 46.Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96:144–150. doi: 10.2106/JBJS.L.01691. [DOI] [PubMed] [Google Scholar]
- 47.Wei L, Fleming BC, Sun X, Teeple E, Wu W, Jay GD, Elsaid KA, Luo J, Machan JT, Chen Q. Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. J Orthop Res. 2010;28:900–906. doi: 10.1002/jor.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]