Abstract
Pediatric intestinal motility disorders affect many children and thus not only impose a significant impact on pediatric health care in general but also on the quality of life of the affected patient. Furthermore, some of these conditions might also have implications for adulthood. Pediatric intestinal motility disorders frequently present as chronic constipation in toddler age children. Most of these conditions are functional, meaning that constipation does not have an organic etiology, but in 5% of the cases, an underlying, clearly organic disorder can be identified. Patients with organic causes for intestinal motility disorders usually present in early infancy or even right after birth. The most striking clinical feature of children with severe intestinal motility disorders is the delayed passage of meconium in the newborn period. This sign is highly indicative of the presence of Hirschsprung disease (HD), which is the most frequent congenital disorder of intestinal motility. HD is a rare but important congenital disease and the most significant entity of pediatric intestinal motility disorders. The etiology and pathogenesis of HD have been extensively studied over the last several decades. A defect in neural crest derived cell migration has been proven as an underlying cause of HD, leading to an aganglionic distal end of the gut. Numerous basic science and clinical research related studies have been conducted to better diagnose and treat HD. Resection of the aganglionic bowel remains the gold standard for treatment of HD. Most recent studies show, at least experimentally, the possibility of a stem cell based therapy for HD. This editorial also includes rare causes of pediatric intestinal motility disorders such as hypoganglionosis, dysganglionosis, chronic intestinal pseudo-obstruction and ganglioneuromatosis in multiple endocrine metaplasia. Underlying organic pathologies are rare in pediatric intestinal motility disorders but must be recognized as early as possible.
Keywords: Intestinal motility disorder, Children, Hirschsprung disease, Chronic constipation, Delayed passage of meconium, Rectal biopsy, Stem cell based treatment, Multiple endocrine metaplasia
Core tip: Intestinal motility disorders are frequent in early childhood. Despite the fact that most of these patients suffer from functional problems it is of major importance to recognize the cases with severe underlying organic causes. Pediatric patients with intestinal motility disorders require a standardized diagnostic and if necessary therapeutic approach. Functional constipation is the most frequent condition in toddlers and preschool age, which requires demystification, diet and concomitant laxative treatment. Functional constipation carries a very good prognosis. Organic causes are rare in intestinal motility disorders and require therefore meticulous diagnostics and adequate surgical treatment. Hirschsprung disease is the most relevant organic cause for pediatric intestinal motility disorders.
EPIDEMIOLOGY
Defecation disorder is among the most common complaints in pediatric patients. Chronic constipation accounts for 3%-5% of consultations to pediatricians and for 10%-25% of referrals to pediatric gastroenterologists[1,2]. Nevertheless, less than 5% of the affected children have an underlying organic cause for this condition.
Possible congenital anomalies of the intestinal motor functions must be detected as early as possible.
The most common congenital disease presenting with intestinal motor dysfunction is Hirschsprung disease (HD). Its incidence varies between 1:5000 and 1:10000 live births. Hirschsprung disease is characterized by the congenital absence of ganglion cells starting distally from the inner circular muscle with a varying extent towards the proximal bowel. The most frequent localization of the congenital aganglionosis is confined to the rectosigmoid colon[3].
Hypoganglionosis is an abnormality that is usually observed in conjunction with HD in the transitional zone proximal to the aganglionosis. Isolated hypoganglionosis is extremely rare and has only been reported in approximately 100 cases[4,5].
Dysganglionosis of the enteric nervous system (ENS) has previously been described as intestinal neuronal dysplasia (IND). This entity may not really exist as a clinical condition because, despite the described histopathological changes, almost all affected patients improve with time and conservative treatment. Currently it is assumed that the so-called IND is a normal age-related variant[6].
Ganglioneuromatosis usually presents with an intestinal motility disorder and is an important and mostly primary feature of the multiple endocrine neoplasia (MEN) syndrome type 2B[7].
Chronic intestinal pseudo-obstruction is a rare but severe condition, which is characterized by repetitive episodes or continuous symptoms of intestinal obstruction without a fixed obstructive bowel lesion. This disease frequently presents with radiological signs of bowel obstruction[8].
CLINICAL PRESENTATION
Functional constipation has been discussed within several consensus conferences for functional gastrointestinal disorders (FGIDs) and was revised in 2006, leading to the ROME III child and adolescent criteria. Functional constipation usually presents with a decreased frequency of bowel motions per week. At least two of the following criteria must be present for more than two months to be regarded as functional constipation: (1) two or fewer defecations per week; (2) at least one episode of fecal incontinence per week; (3) retentive posturing or excessive volitional stool retention; (4) painful or hard bowel movements; (5) presence of a large fecal mass in the rectum; and (6) large diameter stools that may obstruct the toilet[9].
The most striking symptom of severe intestinal motility disorder is the delayed passage of meconium. Healthy newborn babies usually evacuate meconium in the first hours after birth, at the latest 24-48 h postnatally. Delayed passage of meconium combined with defecation problems in infancy is clinically very suggestive of Hirschsprung disease[10].
Patients suffering from hypoganglionosis or ganglioneuromatosis usually present with severe chronic constipation and ongoing defecation problems or occasional bowel obstruction mimicking HD[11].
Patients with ganglioneuromatosis in MEN2b may reveal intestinal problems characterized by constipation, bowel obstruction or diarrhea up to 7 years before the final diagnosis is made (own unpublished data).
The clinical presentation of chronic intestinal pseudo-obstruction (CIPO) comprises symptoms and signs of severe bowel dysmotility that frequently lead to vomiting and repetitive bowel obstruction.
DIAGNOSIS
Pediatric intestinal motility disorders should be diagnosed by the standardized approach of taking the patient’s history and conducting clinical investigations, imaging and biopsy[12,13].
Coordinated intestinal motility (peristalsis) is a result of the interaction of the ENS, interstitial cells of Cajal (ICCs) and intestinal smooth muscles[14,15].
Functional, radiological and histological investigations are applied in pediatric motility disorders to diagnose the potentially underlying disease[16,17].
First step is usually a radiographic investigation, i.e., a contrast enema to reveal a suspected narrow rectosigmoid segment in Hirschsprung disease. Contrast enema has a sensitivity of 76% and a specificity of 97% to diagnose HD[18]. Furthermore contrast enema is usually not conclusive in the attempt to clearly estimate the length of the transition zone in HD[19].
Anorectal manometry (ARM) could be performed in each age group to assess the rectoanal inhibitory reflex. Absent rectoanal inhibitory reflex indicates HD. Sensitivity and specificity of ARM is 91% and 94%[18].
Rectal biopsies are the gold standard for the final diagnosis of severe motility disorders, especially Hirschsprung disease[20]. Routine staining methods are hematoxylin-eosin (HE) and Acetylcholine-Esterase-Histochemistry (AChE). Recent studies revealed that several new immunohistochemical markers, i.e., calretinin, peripherin and S-100, are useful for the diagnostic of rectal biopsies[21,22] Especially calretinin allows the staining of formaldehyde fixed specimen which enables reference pathology on the respective specimen.
Typical and therefore pathognomonic findings at the specimen are the absence of ganglion cells and the presence of hypertrophic AChE-positive fibers within the submucosal and mucosal layers of rectal biopsies in patients with HD (Figure 1)[6]. Serial biopsies are required to define the length of the aganglionic bowel segment. Rectosigmoid disease is the most frequent form of HD (up to 90%). Rarely, patients suffer from total colonic or even total intestinal aganglionosis. At the present time, genetic investigations do not play an important role in the clinical management of HD but point towards further valuable research.
Figure 1.
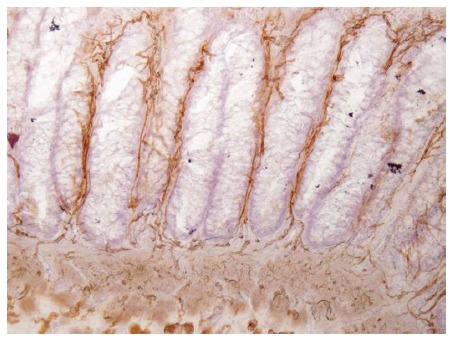
Acetylcholine-Esterase-Histochemistry-staining of a rectal biopsy in a patient with Hirschsprung disease. Note absence of ganglion cells and presence of hypertrophic cholinergic fibres within the mucosal layer (original magnification × 40).
Hypoganglionosis is a disease that is not easily diagnosed, even in rectal biopsies. The use of full-thickness biopsies and the careful interpretation by an experienced pediatric pathologist are necessary[6]. Severe cases of hypoganglionosis that possibly require segmental resection must be evaluated using serial biopsies.
A histological finding of ganglioneuromatosis is a hyperplastic submucosal and myenteric plexus containing an increased number of ganglion cells, glial cells and nerve fibres (Figure 2)[23]. The diagnosis of MEN type 2b should to be evaluated by a genetic investigation, and the analysis should show the specific mutations of the codon 918 in RET exon 16 (95% of MEN2B patients)[24].
Figure 2.

Acetylcholine-Esterase-Histochemistry-staining of a rectal biopsy in a patient with ganglioneuromatosis in MEN2b. Note presence of hyperplastic submucosal ganglions and hypertrophic cholinergic fibres (original magnification × 20).
Clinical symptoms and typical radiological signs usually make the diagnosis of CIPO. Previous histological studies have revealed distinct abnormalities in the enteric nervous system, intestinal smooth muscles (visceral neuropathy) and ICCs, which might also be a secondary phenomenon[1].
MANAGEMENT
Management of chronic functional constipation is conservative and, in nearly all cases, successful. The major components of the treatment are de-mystification, toilet training, diet and the careful use of laxatives[9].
The treatment of choice in HD is resection of the aganglionic bowel segment with preservation of the anal sphincter muscles. Recently established less invasive transanal surgical procedures might lead to better postoperative results and improved long-term outcome[25].
Severe cases of hypoganglionosis also require surgical treatment comparable to that of HD.
The diagnosis of ganglioneuromatosis in rectal or other intestinal biopsies requires additional studies to rule out MEN2B. Proven cases of MEN2B require an intermediate thyroidectomy. The intestinal symptoms of MEN2B should be treated conservatively as long as possible. Bowel resection and/or stoma creation are very rarely indicated[24].
Treatment of CIPO is primarily conservative, but surgery might be required. The surgical procedures carried out might be the creation of a catheterizeable stoma for antegrade bowel irrigation or other enterostomata. Furthermore, parenteral nutrition or even small bowel transplantation is required in severe cases[26].
OUTCOME
Appropriately treated functional constipation has a good prognosis, but it has to be taken into account that up to 20% of patients with chronic constipation continue to have symptoms until adulthood[27].
The classical form of rectosigmoid HD is amenable to a straightforward surgical correction. Intra- and/or postoperative complications are rare. Nevertheless, the long-term outcome after surgical treatment of HD is compromised in 20%-25% of patients still suffering from chronic constipation and/or enterocolitis even after complete resection of the aganglionic bowel including the transitional zone. Several conditions might be responsible for that phenomenon such as reduced numbers of ICCs in the ganglionic bowel[28].
CIPO is a chronic disease that produces a severe impact on the quality of life. The underlying malignant disease mainly determines the outcome of MEN2b. The gastrointestinal symptoms usually improve with time.
CONCLUSION
Pediatric intestinal motility disorders require careful and meticulous diagnostic measures to rule out significant underlying organic disease. There have been numerous advances in diagnostic methods and surgical treatment options, leading to a better outcome for the affected children. Nevertheless, further research in needed in the field of genetics for long-term treatment and transition of these diseases.
Footnotes
Conflict-of-interest statement: No conflict of interest for Gfroerer S and Rolle U.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 29, 2015
First decision: April 27, 2015
Article in press: July 3, 2015
P- Reviewer: Han-Geurts IJM, Kwon S, Lourencao PLTD, Ueno T S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.van den Berg MM, Di Lorenzo C, Mousa HM, Benninga MA, Boeckxstaens GE, Luquette M. Morphological changes of the enteric nervous system, interstitial cells of cajal, and smooth muscle in children with colonic motility disorders. J Pediatr Gastroenterol Nutr. 2009;48:22–29. doi: 10.1097/MPG.0b013e318173293b. [DOI] [PubMed] [Google Scholar]
- 2.Peeters B, Benninga MA, Hennekam RC. Childhood constipation; an overview of genetic studies and associated syndromes. Best Pract Res Clin Gastroenterol. 2011;25:73–88. doi: 10.1016/j.bpg.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Wetherill C, Sutcliffe J. Hirschsprung disease and anorectal malformation. Early Hum Dev. 2014;90:927–932. doi: 10.1016/j.earlhumdev.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Puri P, Rolle U. Variant Hirschsprung’s disease. Semin Pediatr Surg. 2004;13:293–299. doi: 10.1053/j.sempedsurg.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Dingemann J, Puri P. Isolated hypoganglionosis: systematic review of a rare intestinal innervation defect. Pediatr Surg Int. 2010;26:1111–1115. doi: 10.1007/s00383-010-2693-3. [DOI] [PubMed] [Google Scholar]
- 6.Schäppi MG, Staiano A, Milla PJ, Smith VV, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, et al. A practical guide for the diagnosis of primary enteric nervous system disorders. J Pediatr Gastroenterol Nutr. 2013;57:677–686. doi: 10.1097/MPG.0b013e3182a8bb50. [DOI] [PubMed] [Google Scholar]
- 7.Lee NC, Norton JA. Multiple endocrine neoplasia type 2B--genetic basis and clinical expression. Surg Oncol. 2000;9:111–118. doi: 10.1016/s0960-7404(00)00038-4. [DOI] [PubMed] [Google Scholar]
- 8.Ambartsumyan L, Rodriguez L. Gastrointestinal motility disorders in children. Gastroenterol Hepatol (N Y) 2014;10:16–26. [PMC free article] [PubMed] [Google Scholar]
- 9.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keckler SJ, St Peter SD, Spilde TL, Tsao K, Ostlie DJ, Holcomb GW, Snyder CL. Current significance of meconium plug syndrome. J Pediatr Surg. 2008;43:896–898. doi: 10.1016/j.jpedsurg.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedmacher F, Puri P. Classification and diagnostic criteria of variants of Hirschsprung’s disease. Pediatr Surg Int. 2013;29:855–872. doi: 10.1007/s00383-013-3351-3. [DOI] [PubMed] [Google Scholar]
- 12.Rolle U, Till H. [Therapeutic strategies for chronic constipation in childhood: pediatric gastroenterological and surgical aspects] Pathologe. 2007;28:155–160. doi: 10.1007/s00292-007-0893-y. [DOI] [PubMed] [Google Scholar]
- 13.Di Lorenzo C, Youssef NN. Diagnosis and management of intestinal motility disorders. Semin Pediatr Surg. 2010;19:50–58. doi: 10.1053/j.sempedsurg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Gfroerer S, Rolle U. Interstitial cells of Cajal in the normal human gut and in Hirschsprung disease. Pediatr Surg Int. 2013;29:889–897. doi: 10.1007/s00383-013-3364-y. [DOI] [PubMed] [Google Scholar]
- 15.Mazet B. Gastrointestinal motility and its enteric actors in mechanosensitivity: past and present. Pflugers Arch. 2015;467:191–200. doi: 10.1007/s00424-014-1635-7. [DOI] [PubMed] [Google Scholar]
- 16.Sutcliffe JR, King S, Hutson JM, Southwell B. What is new in radiology and pathology of motility disorders in children? Semin Pediatr Surg. 2010;19:81–85. doi: 10.1053/j.sempedsurg.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Raghunath N, Glassman MS, Halata MS, Berezin SH, Stewart JM, Medow MS. Anorectal motility abnormalities in children with encopresis and chronic constipation. J Pediatr. 2011;158:293–296. doi: 10.1016/j.jpeds.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 18.de Lorijn F, Kremer LC, Reitsma JB, Benninga MA. Diagnostic tests in Hirschsprung disease: a systematic review. J Pediatr Gastroenterol Nutr. 2006;42:496–505. doi: 10.1097/01.mpg.0000214164.90939.92. [DOI] [PubMed] [Google Scholar]
- 19.Muller CO, Mignot C, Belarbi N, Berrebi D, Bonnard A. Does the radiographic transition zone correlate with the level of aganglionosis on the specimen in Hirschsprung’s disease? Pediatr Surg Int. 2012;28:597–601. doi: 10.1007/s00383-012-3094-6. [DOI] [PubMed] [Google Scholar]
- 20.de Arruda Lourenção PL, Takegawa BK, Ortolan EV, Terra SA, Rodrigues MA. A useful panel for the diagnosis of Hirschsprung disease in rectal biopsies: calretinin immunostaining and acetylcholinesterase histochesmistry. Ann Diagn Pathol. 2013;17:352–356. doi: 10.1016/j.anndiagpath.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Holland SK, Hessler RB, Reid-Nicholson MD, Ramalingam P, Lee JR. Utilization of peripherin and S-100 immunohistochemistry in the diagnosis of Hirschsprung disease. Mod Pathol. 2010;23:1173–1179. doi: 10.1038/modpathol.2010.104. [DOI] [PubMed] [Google Scholar]
- 22.Montedonico S, Piotrowska AP, Rolle U, Puri P. Histochemical staining of rectal suction biopsies as the first investigation in patients with chronic constipation. Pediatr Surg Int. 2008;24:785–792. doi: 10.1007/s00383-008-2173-1. [DOI] [PubMed] [Google Scholar]
- 23.Feichter S, Meier-Ruge WA, Bruder E. The histopathology of gastrointestinal motility disorders in children. Semin Pediatr Surg. 2009;18:206–211. doi: 10.1053/j.sempedsurg.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Walls GV. Multiple endocrine neoplasia (MEN) syndromes. Semin Pediatr Surg. 2014;23:96–101. doi: 10.1053/j.sempedsurg.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Levitt MA, Hamrick MC, Eradi B, Bischoff A, Hall J, Peña A. Transanal, full-thickness, Swenson-like approach for Hirschsprung disease. J Pediatr Surg. 2013;48:2289–2295. doi: 10.1016/j.jpedsurg.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Chumpitazi B, Nurko S. Pediatric gastrointestinal motility disorders: challenges and a clinical update. Gastroenterol Hepatol (N Y) 2008;4:140–148. [PMC free article] [PubMed] [Google Scholar]
- 27.Nurko S, Scott SM. Coexistence of constipation and incontinence in children and adults. Best Pract Res Clin Gastroenterol. 2011;25:29–41. doi: 10.1016/j.bpg.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolle U, Piotrowska AP, Nemeth L, Puri P. Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch Pathol Lab Med. 2002;126:928–933. doi: 10.5858/2002-126-0928-ADOICO. [DOI] [PubMed] [Google Scholar]


