Abstract
BACKGROUND: C-arm cone-beam computed tomography (CBCT) is a comparatively novel modality for guiding percutaneous transthoracic lung biopsies (PTLBs), and despite its potential advantages over conventional computed tomography (CCT), a head-to-head comparison of the two techniques has yet to be reported in the literature. This study aims to evaluate the diagnostic value and safety of CBCT-guided PTLB compared to CCT-guided biopsy, with cases performed in a single hospital. METHODS: A total of 104 PTLB patients were retrospectively analyzed in this study. 35 PTLBs were performed under CBCT guidance, and 69 PTLBs were performed under CCT guidance. Diagnostic accuracy, sensitivity, and specificity for malignancy as well as procedure time, radiation dose of patients, and complication rate in the two groups were compared. RESULTS: Total procedure time was significantly lower in the CBCT group (32 ± 11 minutes) compared to the CCT group (38 ± 9.7 minutes; P = .009), especially among patients ≥ 70 years of age (CBCT: 33 ± 12 minutes, CCT: 42 ± 13, P = .022). For lesions in the lower lobes, the CBCT-guided group received significantly reduced effective radiation dose (2.9 ± 1.6 mSv) than CCT-guided patients (3.7 ± 0.80; P = .042). Diagnostic accuracy, sensitivity, and specificity for malignancy were comparable between the two groups, as were post-biopsy complication rates. CONCLUSION: CBCT guidance significantly reduces the procedure time and radiation exposure for PTLBs compared with CCT, and should be considered in clinical settings that may be difficult or time-consuming to perform under CCT.
Introduction
Upon the detection of a suspicious pulmonary mass by any imaging modality, tissue samples are usually sought as part of the clinical assessment. Bronchoscopy is often the first step, as it allows both direct observation of the lung mass to evaluate its characteristics and extent and also to conduct tissue sampling. Although diagnostic rates of up to 80% have been reported depending on the method used to acquire tissues, bronchoscopic biopsy is limited to centrally located tumors that are visible from within the airways [1].
For lesions that cannot be approached through bronchoscopy, a percutaneous lung biopsy is usually performed under image guidance. Ultrasound and computed tomography (CT) are the two most commonly used guidance techniques, each with its own advantages and disadvantages. While ultrasound provides real-time feedback for relatively quick and inexpensive biopsy, its utility is limited to either pleural-based masses or lesions located within a short distance of the pleura [2]. CT-guided procedures are the current standard for transthoracic needle biopsy of pulmonary masses and enjoy high diagnostic accuracy and widespread availability [3,5]. Spatial resolution is high and serious complications are rare in the hands of experienced practitioners [4]. Its primary drawback lies in the absence of real-time visualization during needle insertion, which is readily available in conventional fluoroscopy guidance systems. For deep pulmonary masses requiring oblique needle angles (for example, to avoid major vessels, ribs, or airways) and in older patients who have difficulty holding their breath, the procedure time and subsequent radiation doses are often increased [4]. Additionally, owing to the ever-expanding role of CT in both diagnosis and screening [5], sharing valuable scanning time with potentially time-consuming biopsy procedures creates both an administrative burden in terms of patient scheduling and a financial burden in terms of reduced CT scans.
Initially used for neurovascular imaging, flat panel cone-beam CT systems have in recent years been applied in image-guided percutaneous procedures [6]. By combining a C-arm gantry with cone-beam X-ray tube and flat panel detectors, C-arm cone-beam CT (CBCT) incorporates the imaging resolution of conventional CT (CCT) with the real-time needle guiding capability of fluoroscopic systems. [7] With the aid of path-planning software and the rotational capability of a C-arm, CBCT allows an operator to approach lesions that are difficult to reach under CCT with greater confidence. Recent studies have given promising initial descriptions on the accuracy and safety of CBCT-guided biopsies performed on pulmonary masses, which are comparable to previously reported results for CCT [7,8,11].
Despite its potential for reducing procedure times and radiation doses in the biopsy of pulmonary masses [8,11,14], CBCT-guided biopsy has yet to be directly compared to CCT. In this study, we retrospectively compare the diagnostic success, procedure time, radiation exposure, and safety of lung mass biopsies performed within a single hospital, under either cone-beam or CCT guidance.
Materials and Methods
Study Population and Patient Selection
From March to December of 2013, 104 consecutive patients who received percutaneous transthoracic lung biopsies (PTLBs) were retrospectively included in this study. PTLBs were scheduled under CBCT or CCT imaging guidance according to operating room availability. 35 PTLBs were performed under CBCT guidance (20 males and 15 females; mean age, 69 ± 12 years; age range, 42-89 years; group A) by one experienced thoracic radiologist (S.-H.T.), and 69 PTLBs were performed under CCT guidance (35 males and 34 females; mean age, 62 ± 13 years; age range, 31-89 years; group B) by two experienced thoracic radiologists (S.-H.T. with 4 years of experience and Y.-C.C. with 5 years of experience).
Hemograms and coagulation profiles were assessed for all patients before biopsy, and only patients with a platelet count of 10,000/ml or higher and international normalized ratio of 1.4 or less went on to receive percutaneous biopsies. Any anticoagulants or platelet inhibitors regularly taken by patients were withheld for at least 3 days before PTLB. After the procedure, all patients were kept under observation at our hospital, and an erect chest radiograph was arranged 4 hours later to detect potential late complications. In cases of marked changes in vital signs or clinical status, repeat pulmonary radiographs were taken.
Overview of Biopsy Procedure
All PTLBs were performed under local anesthesia, using either a 64-detector CT scanner (Brilliance 64; Philips Healthcare, Best, The Netherlands) or a CBCT virtual navigation guidance system (XperCT and XperGuide software, AlluraXperFD20; Philips Healthcare). A coaxial cutting needle technique was employed, using an 18-gauge cutting needle and a 17-gauge coaxial introducer needle (Temno Biopsy Device). Patients were placed in either supine or prone positions depending on their lesion location and the presence of overlying ribs or large blood vessels. Before each biopsy, we performed a pre-procedural CT or CBCT scan of the entire lung to identify the safest and most accessible route to reach the target nodule(s), while avoiding obstacles and minimizing pleural contact and the distance traveled by the needle through lung parenchyma. For CBCT procedures, angulation of the biopsy needle was kept to the vertical plane of rotation as much as possible so as to reduce complexity of needle course planning and increase accuracy. Each CT or CBCT scan was performed during a single breath-hold in either inspiration or expiration, as best tolerated by each patient. Post-procedural CT or CBCT images were acquired after biopsy completion to identify procedure-related complications.
Protocol for CCT Guidance
For each patient before PTLB, we obtained low-dose axial CT scan on a 64-detector scanner (Brilliance 64; Philips Healthcare) under the following imaging parameters: 120 kVp, 30 mA per slice, 0.75-second rotation time, and collimation of 8 × 5 mm. The window center and width were 0 and 2800 HU, respectively, which allowed simultaneous visualization of vessels, tumor, pneumothorax, bone, muscle, and fat. Once a skin entry site had been determined and the coaxial introducer needle inserted into the subcutaneous tissue, additional CT scans were performed before each needle course correction so as to precisely position the needle tip within the target lesion. Each procedure was performed with a “move off and scan” approach to minimize radiation exposure to the operator [9].
Protocol for CBCT Guidance
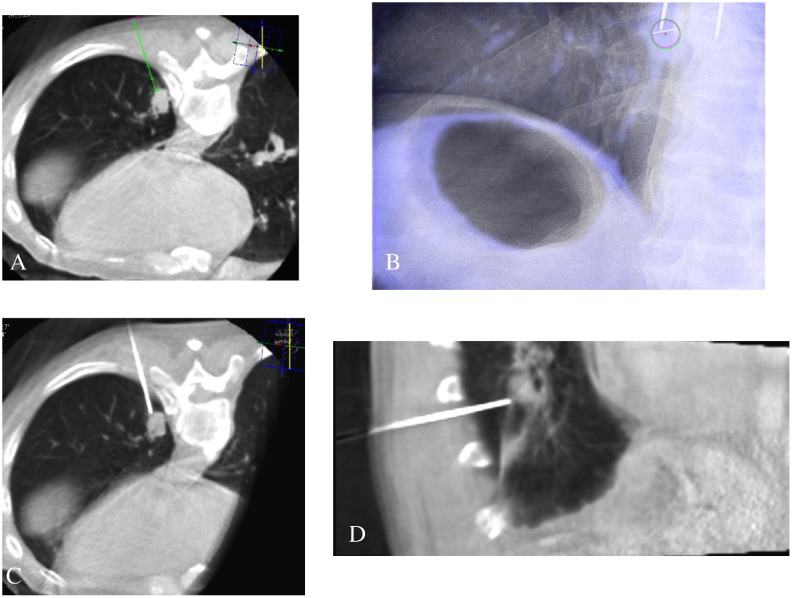
CBCT was performed with the C-arm rotating 240° in 4 seconds, generating 242 images in a 512 × 512 matrix. The acquired CBCT images were transferred to commercially available dedicated medical imaging workstations, where the safest and most effective skin entry site and needle pathway to reach target lesion were determined (Figure 1A). Distances from needle skin entry site to lesion and skin to pleura were also measured and recorded. Virtual guidance (XperGuide; Philips Healthcare) with automatic angle alignment from the skin entry site to the target lesion (bull’s-eye view) was then used along with virtual color overlay on the fluoroscopic image (Figure 1B) to guide insertion of a 17-gauge coaxial introducer needle into the target nodule. At this point, a procedural cone-beam CT scan was performed to check the exact needle tip location (Figure 1, C and D). If the introducer needle was correctly situated with tip adjacent to the target lesion, an 18-gauge semi-automated cutting needle was inserted into the target lesion through the introducer and a tissue sample was obtained. After sufficient samples were obtained, the coaxial introducer was removed.
Figure 1.
Cone-beam CT-guided transthoracic lung biopsy of a 1.5-cm left lower lobe nodule in an 80-year-old man. (A) Selection of skin entry site after initial planning CBCT scan, with placement of needle along an inclined axis as aided by the path-guiding software. The skin-to-pleura and skin-to-lesion distances were recorded during planning. (B) Bull’s-eye view of a pulmonary nodule, with red and green circles to help align the biopsy needle with the target lesion. Real-time graphics overlay (in blue) displays the original position of a patient’s target lesion during planning relative to its current position as it changes owing to patient motion. (C and D) Axial and sagittal views as shown during CBCT-guided needle insertion, allowing vertical angle adjustments that facilitate avoidance of intervening obstacles such as ribs and vascular structures.
Data Collection and Interpretation
For each patient enrolled in this study, all relevant medical history, radiology reports, and histopathology data from biopsy and subsequent surgery (if any) were retrospectively collected, followed by immediate de-identification of all patient information. Final diagnosis of target lung lesions was confirmed in the following ways. Pathologic report of malignancy from a PTLB sample was accepted as true-positive unless there was no evidence of malignancy during examination of subsequent surgically resected specimen, in which case it was counted as false-positive. A benign PTLB result was considered true-negative if the target lesion remained stable in size or regressed 20% or more during follow-up CT examination (6 months) in the absence of medical treatment [17], or if surgical resection revealed a specific benign pathology such as tuberculosis or hamartoma. Conversely, if the presence of malignant pathology was confirmed in a surgical specimen following PTLB result of benign lesion, the case was considered false-negative. Using these criteria, the sensitivity, specificity, and diagnostic accuracy of CBCT- and CCT-guided biopsy procedures were calculated.
Sizes and depths of target lesions were evaluated during pre-biopsy CBCT or CCT scan for each patient. Nodule size was defined as the longest diameter of the lesion on lung-window CT images, while lesion depth was calculated by subtracting skin-to-pleura distance from skin-to-lesion distance on CT or CBCT planning view. The distance from pleura to lung nodule was selected over skin-to-lesion distance for two reasons: first, because the depth of needle passage into lung parenchyma is more likely to influence the incidence of pneumothorax and hemoptysis [21] and, second, to reduce the confounding factor of patient’s physical build that affects skin-to-pleura distance. We also recorded the following factors for each PTLB: the number of biopsies performed, the patient’s relative position during biopsy, the number of CCT or CBCT scans, total procedure time, and whether any complications developed. Total procedure time was defined as the time interval from start of first pre-biopsy CT scan to the last post-procedure scan, and complications were defined according to the guidelines described by Manhire et al. [26] The number of CT scans performed for needle adjustment in each patient was also calculated on the basis of the total number of CT scans subtracted by the number of pre-procedural and post-procedural scans. Procedures with complications that did not require invasive interventions were defined as minor complications, including hemoptysis and pneumothorax that resolved without pig-tail or chest tube drainage. A complication was considered major if additional treatment was needed (e.g., tube placement for a large pneumothorax and intubation for post-procedure respiratory distress). A pneumothorax detected on post-biopsy chest plain film was graded as small if no chest tube placement was necessary, and large if tube placement was performed.
Patient radiation exposure data during each PTLB procedure was also collected for both CBCT and CCT cases. Absorbed radiation doses for CBCT-guided biopsies were measured in terms of dose area product (DAP, mGy cm2), while the dose length product (mGy cm) was documented for procedures done under CCT guidance. DAP, defined as the sum of absorbed radiation by fluoroscopy and CBCT acquisition during the entire procedure, was converted into effective dose using a conversion factor of 0.183 mSv/Gy cm2 as previous determined by Braak et al. under the same CBCT system used in this study [8]. Dose length product was likewise converted into effective dose using a factor of 0.02 mSv/mGy cm [25].
Statistical Analysis
Owing to the nonparametric distribution of data collected in this study, comparisons between CBCT and CCT groups were performed with Chi-squared test for qualitative variables and with Wilcoxon rank-sum test for quantitative variables. For all analyses, a two-sided P value smaller than .05 was considered statistically significant. Statistical analysis was performed with SPSS software (version 22.0; SPSS, Chicago, IL).
Results
Demographic and Lesion Characteristics of the Study Groups
Selected baseline characteristics for the CBCT and CCT groups are shown in Table 1. Mean age for the 35 patients who underwent CBCT-guided biopsy was higher than in the CCT biopsy group (CBCT = 69 years, CCT = 63 years, P < .01), while gender distribution was relatively even (F:M = 15:20 for CBCT, F:M = 35:34 for CCT). Mean body weight of the patients across the two groups were likewise similar (CBCT = 63 kg, CCT = 64 kg).
Table 1.
Characteristics of the Patients and Pulmonary Lesions in Cone-Beam and CCT Groups
| CBCT (n = 35) | CT (n = 69) | P Value | |
|---|---|---|---|
| Mean age ± SD (years) | 69 ± 2⁎ | 62 ± 1.5 | .009† |
| < 70 | 58 ± 6.2 | 55 ± 7.4 | .21† |
| ≥ 70 | 79 ± 4.8 | 77 ± 5.4 | .52† |
| Mean body weight ± SD (kg) | 63 ± 12 | 64 ± 12 | .68† |
| Gender | |||
| Female | 15 (43) | 35 (51) | .54‡ |
| Male | 20 (57) | 34 (49) | |
| Lesion size (mm) | |||
| < 10 | 1 (3) | 4 (6) | |
| 10-19 | 5 (14) | 8 (11) | |
| 20-29 | 16 (46) | 20 (29) | |
| 30-39 | 4 (11) | 14 (20) | |
| 40-49 | 5 (14) | 10 (14) | |
| ≥ 50 | 4 (11) | 13 (19) | |
| Mean size ± SD | 30 ± 14 | 35 ± 19 | .16† |
| Mean pleura-to-lesion depth ± SD (mm) | 43 ± 17 | 37 ± 13 | .08† |
P < .05.
Wilcoxon signed-rank test.
χ2 test.
The mean pleura-to-lesion distance in the CBCT group (43 ± 17 mm) was similar to that for patients in the CCT group (37 ± 13 mm), with the size of each lesion biopsied being displayed in Table 1. Approximately 50% of lesions were in the size range of 20 to 39 mm in both groups. There was also no significant difference in the distribution of lesion locations between the two groups, as listed in Table 2.
Table 2.
Location of Pulmonary Lesions and Patient Orientation during Procedure
| CBCT (n = 35) | CT (n = 69) | P Value | |
|---|---|---|---|
| Lesion location | .3⁎ | ||
| RUL | 15 (43) | 21 (30) | |
| RML | 3 (9) | 2 (3) | |
| RLL | 8 (23) | 16 (23) | |
| LUL | 5 (14) | 18 (26) | |
| LLL | 4 (11) | 12 (17) | |
| Patient position | .7⁎ | ||
| Supine | 16 (46) | 27 (39) | |
| Prone | 18 (51) | 41 (59) | |
| Oblique | 1 (3) | 1 (2) |
Numbers in parentheses indicate percentage within each group.
RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
χ2 test.
Procedure Duration and Radiation Dose
Total procedure time was significantly shorter for CBCT group patients (32 ± 11 minutes; range 20-70 minutes; Table 1) than for the CCT group (38 ± 9.7 minutes; range 24-80 minutes; P < .01). Further subgroup analysis revealed that procedure time difference between the two guidance methods was greater in patients 70 years or older (33 ± 12 minutes for CBCT, 42 ± 13 minutes for CCT, P = .022). The mean number of CT scans performed for needle adjustment during CBCT biopsy was also much lower than in the CCT group (1.8 ± 1.0 times for CBCT, 10 ± 3.8 for CCT, P < .001), although the total scan time was comparable (CBCT = 7.1 ± 3.7 seconds, CCT = 7.6 ± 2.8 seconds, P = .42) because of rotational speed limitations of the C-arm in cone-beam systems.
Mean effective dose was lower in the CBCT group (3.4 ± 2.1 mSv) compared to the CCT group (3.9 ± 0.79 mSv), and although there was no statistical significance (P = .07) for the overall study groups, dose was significantly lower for lesions in the lower lobes (2.9 ± 1.6 mSv for CBCT, 3.7 ± 0.80 mSv for CCT, P = .042). Despite shorter total procedure times and reduced patient dose, the mean number of cutting needle passes performed during each biopsy was still significantly higher in CBCT patients than for CCT patients (CBCT = 4.3 ± 0.9, CCT = 3.7 ± 1.2, P = .009), which was valuable for acquiring adequate tissues for pathologic analysis.
Safety and Accuracy of Procedure
There was no statistically appreciable difference in post-biopsy complication rates between the two different guidance methods, although the cone-beam guidance method had proportionally less complications overall (Table 3). Minor complications arose in 10 of 35 patients (29%) in the CBCT group and 16 of 69 patients (23%) in the CCT group, with a significance level of P = .72. Major complications involve post-procedure pneumothorax patients for whom chest tube had to be placed, with only one patient from each group falling under this category. The patient from the CBCT group was a 65-year-old woman with a medical history of chronic obstructive pulmonary disease, who underwent biopsy for a 3.4-cm right middle lobe nodule. She suffered from hemoptysis and parenchymal hemorrhage with progressive dyspnea soon after biopsy, and eventually required endotracheal intubation with assisted ventilation. She was safely discharged after 2 weeks of additional treatment at our hospital. Her counterpart in the CCT group was a 75-year-old woman who had to receive chest tube drainage for 2 days after biopsy, also because of progressive dyspnea. Further analysis of the time spent in hospital as a result of post-biopsy pneumothorax revealed minimal difference between CBCT (11 patients; 3.3 ± 2.6 days) and CCT (17 patients; 3.2 ± 2.0 days) groups.
Table 3.
Procedure Times, Radiation Exposure, and Safety of Lung Biopsy Guided by CBCT Compared to CCT
| CBCT | CT | P Value | |
|---|---|---|---|
| Mean total procedure time ± SD (minutes) | 32 ± 11⁎ | 38 ± 9.7 | .009† |
| < 70 years | 31 ± 13 | 37 ± 13 | .075† |
| ≥ 70 years | 33 ± 12⁎ | 42 ± 13 | .022† |
| Mean effective dose ± SD (mSv) | 3.4 ± 2.1 | 3.9 ± 0.79 | .074† |
| Lower lobes | 2.9 ± 1.6⁎ | 3.7 ± 0.80 | .042† |
| Upper/middle lobe | 3.6 ± 2.3 | 4.0 ± 0.79 | .44 |
| Mean number of CT scans per biopsy# | 1.8 ± 1.0 | 10 ± 3.8 | < .001 |
| Mean needle passes per biopsy | 4.3 ± 0.9⁎ | 3.7 ± 1.2 | .011† |
| Post-biopsy complications | |||
| Minor§ | 10 (28) | 16 (23) | .72‡ |
| Major§ | 1 (3) | 1 (1) | |
| Hemoptysis | 4 (11) | 5 (7) | .47‡ |
| Pneumothorax | 7 (20) | 14 (20) | .23‡ |
| Mean hospitalization time (days)¶ | 3.3 ± 2.6 | 3.2 ± 2.0 | .99† |
Numbers in parentheses indicate percentage within each group.
P < .05.
Wilcoxon signed-rank test.
χ2 test.
Minor, post-biopsy hemoptysis or pneumothorax that was spontaneously resolved. Major, post-biopsy pneumothorax requiring additional treatment, including chest tube and intubation.
Days in hospital because of post-biopsy pneumothorax.
Excluding routine pre-procedural and post-procedural CT scans.
Sensitivity and specificity of biopsy results for the CBCT and CCT groups compared to their final diagnosis are listed as follows. There were 33 malignant and 2 benign cases in the CBCT group, with biopsy demonstrating only chronic inflammation in 1 of the cases that turned out to be invasive adenocarcinoma. The sensitivity and specificity for malignant lesions in this group were 97% and 100%, respectively, with an accuracy of 97%. For the CCT group, there were 54 malignant and 15 benign cases, with biopsy sensitivity, specificity, and accuracy of 100%.
Discussion
Flat-panel CBCT systems designed specifically for guiding percutaneous needle procedures have only recently become commercially available, after initial use in the field of interventional angiography [10]. Its primary advantage over existing CT-guided techniques is the addition of real-time imaging during needle insertion, which simplifies needle path planning and increases the chance of reaching target lesions. Additionally, there is potential for reducing procedure times and thus radiation doses for patients [11,12]. A recent study has shown favorable results in using CBCT to direct percutaneous bone biopsies [13], with reduced radiation doses to the patient and operator. Although previous authors have established that CBCT is a valuable technique in image-guided biopsies, a direct comparison of its accuracy, safety, and radiation exposure to the current standard of CT guidance has yet to be performed for pulmonary lesions, which almost always requires a biopsy before further surgical or medical management. This study provides a head-to-head assessment of CBCT- and CCT-guided lung biopsies in terms of clinically important parameters, using cases performed within a single hospital by the same team of interventional radiologists.
In this study, we demonstrated that CBCT guidance with real-time imaging was a highly accurate diagnostic method for biopsy of lung nodules, with reduced procedure time compared to CCT. Diagnostic accuracy was comparable between CBCT and CCT groups, with only one false-negative result under CBCT guidance. CCT-guided percutaneous biopsies have been extensively shown in various studies to be reliable in the hands of experienced operators, with accuracy ranging from 74% to 83.9% in earlier investigations [15,16] to 90% in a more recent study [3]. A higher mean diagnostic accuracy for CBCT-guided percutaneous lung biopsy has been reported by previous authors, ranging from 92% to 98% [14,17,18], and results from our patients compared favorably to these studies. A factor in the low diagnostic accuracy of CCT guidance may be the difficulty of approaching small lesions in the lower lobes, which are more affected by respiratory movements [22,24]. This problem is compounded in patients for whom respiratory control is difficult or impossible. With the addition of real-time visualization during needle insertion, operators of CBCT-guided systems may be able to biopsy lesions in the lower lobes and in elderly patients with greater speed and reduced radiation exposure, as was demonstrated in our study (Table 3). This was consistent with earlier investigations that found advantages in terms of reduced procedure time and dose when using conventional fluoroscopy guidance for pulmonary biopsies over CT, since cone-beam CT is, in essence, a combination of fluoroscopy and CT [23,27].
Although Choo et al. [19] reported a reduction in total procedure time (defined as time from local anesthesia to completion of post-biopsy CBCT) compared to published data on CT-guided biopsy, they did not compare patients performed at a single institution. In the present study, total procedure time was defined as the interval from start of first planning CT scan to the last post-procedure scan to take into account any potential time difference in planning needle insertion and path to target between CBCT and CCT systems. By using the internal clocks of the imaging systems themselves to record time, more accurate and reproducible interval measurements can also be obtained. A consistent and statistically significant difference was found in the mean procedure time, and a higher number of successful needle passes can be maintained during this shorter time period using the real-time visualization provided by CBCT. The potential for greater flexibility in selection of skin entry site and reduced dependence on patient’s physical condition are of great value for both operators and patients, as is the ability to avoid overlying ribs and other structures [24]. Another aspect of performing biopsies with cone-beam CT that may be advantageous is the reduced room time used for biopsies in CCT systems, which may decrease waiting time and simplify scheduling for patients requiring diagnostic CT scans. Since CBCT is normally used for neuroradiology and angiographic examinations and interventions, scheduling biopsy patients during downtimes between examinations may also prove economical in terms of asset utilization.
Comparison of radiation DAP values between CBCT systems can usually be made directly since the amount of radiation output is accurately recorded by the imaging system itself. [20] The mean DAP in our CBCT group was 18757 ± 11300 mGy cm2 (not shown in results), which was modest compared to the 10,717.78 ± 11,043 to 50,794.7 ± 28,251.4 mGy cm2 range of DAP values reported in several recent studies [14,17,19,23,24]. Although DAP is convenient for comparison between CBCT systems, many investigators in recent studies eschew this parametric in favor of effective dose, which allows better correlation with CT-guided procedures. Varying levels of effective radiation dose for CBCT guidance have been reported by different investigators, with some reporting a lower dose (CBCT: 5.72 mSv and CCT: 11.05 mSv by Choo et al. [19], CBCT: 4.6 mSv and CCT: 10 mSv by Hwang et al. [20]), while others saw higher doses (8.6 mSv by Choi et al. [14]). The CBCT conversion factor used to obtain effective dose from measured DAP in this study was 0.183 mSv/Gy cm2, as cited from Braak et al. [8] who used the same CBCT imaging system in their 2011 study. The corresponding factor used for CCT was 0.02 mSv/mGy cm [25]. Effective radiation dose in our study was 3.4 mSv for the CBCT group and 3.9 mSv for the CCT group, which compare favorably with these studies. The markedly lower CT dose in the present study may be explained by the low-dose mode used during biopsy planning and post-procedure.
Despite patients being on average 7 years older than in the CCT group, CBCT-guided biopsies in our study were performed more quickly. Especially striking was the time difference for patients older than 70 years of age, which averaged around 9 minutes faster for CBCT cases. Given that higher age did not correspond to increased complication rates, there may be significant benefits to considering CBCT for pulmonary biopsies in elderly patients. Additionally, it may be noted that mean nodule size for the CBCT group was smaller (30 mm to 35 mm) and lesion depth was greater (43 mm to 37 mm), both of which are factors that have been previously shown to cause diagnostic failures for CCT-guided biopsies [15,28]. Indeed, while a diagnostic accuracy of 77.2% was reported for nodules smaller than 20 mm [28], our own results indicated a 100% accuracy for lesions of this size and smaller biopsied under CBCT. Lee et al. also noted in their study that the real-time imaging guidance capability and high flexibility in entry site selection of cone-beam CT systems contributes to high diagnostic accuracy in small lesions [24]. Of course, very small lesions can still pose problems for accuracy and post-biopsy complications even under CBCT guidance, and in the same study, it was found that a lesion size of 1 cm or less is a significant independent risk factor for diagnostic failure. Although an earlier investigation by Choi et al. did not show any factors that significantly decreased diagnostic accuracy with CBCT, ground-glass nodules were found to be associated with increased risk of post-biopsy hemoptysis [14]. Despite these potential caveats, the fact that small and difficult to access nodules were biopsied in less time and with decreased dose (in the case of lower lobe lesions, as shown in Table 3) suggested a potential role for recruiting patients at high risk of biopsy failure with CCT to cone-beam CT systems for biopsy, provided that radiologists experienced with CBCT-guided procedures are available.
Pneumothorax and hemoptysis are the most common complications of percutaneous needle biopsy for lung lesions [21,22]. Patients at increased risk of pneumothorax include those with preexisting lung disease, increased number of needle passes, greater lesion depth, patient age, and needle size [5,15,21,22]. Pneumothorax rate in our series of CBCT-guided needle biopsy was equal to the CCT biopsy group at 20%, which fell within previously reported ranges for CT-guided lung biopsy, 13% to 45% [4,5,15,16,22]. It was also comparable to several recent CBCT studies that reported a range of 15.4% to 31.8% [7,9,14,17–19]. Patients who suffered from post-biopsy pneumothorax in the cone-beam group had a mean age of 62 years, target lesion size of 2.8 cm, and pleura-to-lesion depth of 4.6 cm (for CCT: 62 years old, lesion size of 3.6 cm and lesion depth of 4.0 cm). It must be noted that despite having almost all of the risks for increased pneumothorax, CBCT-guided biopsy patients in the present study did not suffer from increased post-procedure complications compared to their CCT counterparts. This fact is certainly aided by the ability of cone-beam CT systems to visualize relative positions of biopsy needle and target lesion in real time. Hemoptysis is the next most significant post-biopsy complication after pneumothorax, with incidence rates that tend to vary significantly in the literature from 0% to 14% depending on patient condition and biopsy protocol. [4,5,9,17–19]. While immediate post-biopsy hemoptysis occurred slightly more frequently in our CBCT-FNB group (CBCT: 11%, CCT: 7%, P = .47), it may be explained by the generally more difficult biopsy conditions in the CBCT group as noted above, especially greater lesion depth [24]. It should also be noted that more passes were made with the cutting needle in the CBCT group (four passes on average, compared to 2.6 times in the CCT group).
We report a major complication rate of 3% in our CBCT group, a single patient who required endotracheal intubation because of progressive breathing difficulty and hypoxia. After reviewing his medical history, we believe that the chronic obstructive pulmonary disease, which he had been treated for more than 20 years, and his subsequently diminished baseline pulmonary function (FEV1 was 34% of predicted) were major contributing factors in his post-procedural respiratory failure. According to Manhire et al. [26], patients with FEV1 < 35% of predicted should not undergo percutaneous needle biopsy without careful risk assessment. Adhering to this recommendation may have prevented the serious post-biopsy complications experienced by the patient in our present study.
There were some limitations to this study. First, no randomized comparison was made between CBCT and CCT guidance, although this was mitigated to a certain extent by similar demographic and physical characteristics of the patients enrolled in this study. A prospective randomized study between these two techniques would still be of great value in better judging their respective benefits and drawbacks. Second, although using established conversion parameters allow a reasonably accurate comparison between CBCT and CCT imaging platforms, a more robust and universally accepted framework on calculating effective radiation dose would certainly be of exceptional value in future investigations. Lastly, a larger number of patients in the CBCT group would add to the generalizability of this study. However, given the consistently high accuracy and safety of CBCT-guided systems along with ease of operation and real-time feedback, we believe they can be of great benefit in clinical practice.
In conclusion, CBCT guidance with real-time guiding software appears to significantly reduce procedure times for patients receiving PTLBs, while maintaining high accuracy and safety in technically challenging situations compared to CCT systems. Our study demonstrated that under CBCT guidance, biopsy duration may be safely reduced in elderly patients even with small nodules at greater depth than CCT. There was also a notable reduction in radiation dose in locations such as the lower lobes where respiration control may be difficult to achieve. For clinicians considering whether a difficult-to-access pulmonary nodule is feasible for transthoracic biopsy, CBCT provides a valuable alternative to CCT while at the same time freeing up CT systems for diagnostic examinations.
Acknowledgement
We thank all of the radiologic technologists and nurses involved in CT and CBCT-guided biopsies for their excellent assistance.
References
- 1.Herth FJF, Eberhardt R, Ernst A. The future of bronchoscopy in diagnosing, staging and treatment of lung cancer. Respiration. 2006;73:399–409. doi: 10.1159/000093369. [DOI] [PubMed] [Google Scholar]
- 2.Diacon AH, Theron J, Schubert P, Brundyn K, Louw M, Wright CA, Bolliger CT. Ultrasound-assisted transthoracic biopsy: fine-needle aspiration or cutting-needle biopsy? Eur Respir J. 2007;29:357–362. doi: 10.1183/09031936.00077706. [DOI] [PubMed] [Google Scholar]
- 3.Hiraki T, Mimura H, Gobara H, Iguchi T, Fujiwara H, Sakurai J, Matsui Y, Inoue D, Toyooka S, Sano Y. CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles: diagnostic yield and risk factors for diagnostic failure. Chest. 2009;136:1612–1617. doi: 10.1378/chest.09-0370. [DOI] [PubMed] [Google Scholar]
- 4.Heck SL, Blom P, Berstad A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur Radiol. 2006;16:1387–1392. doi: 10.1007/s00330-006-0152-2. [DOI] [PubMed] [Google Scholar]
- 5.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 6.Heautot JF, Chabert E, Gandon Y, Croci S, Romeas R, Campagnolo R, Chereul B, Scarabin JM, Carsin M. Analysis of cerebrovascular diseases by a new 3-dimensional computerised X-ray angiography system. Neuroradiology. 1998;40:203–209. doi: 10.1007/s002340050568. [DOI] [PubMed] [Google Scholar]
- 7.Braak SJ, van Strijen MJ, van Leersum M, van Es HW, van Heesewijk JP. Real-time 3D fluoroscopy guidance during needle interventions: technique, accuracy, and feasibility. Am J Roentgenol. 2010;194:W445–W451. doi: 10.2214/AJR.09.3647. [DOI] [PubMed] [Google Scholar]
- 8.Braak SJ, Herder GJM, van Heesewijk JPM, van Strijen MJL. Pulmonary masses: initial results of cone-beam CT guidance with needle planning software for percutaneous lung biopsy. Cardiovasc Intervent Radiol. 2012;35:1414–1421. doi: 10.1007/s00270-011-0302-z. [DOI] [PubMed] [Google Scholar]
- 9.Tsai IC, Tsai WL, Chen MC, Chang GC, Tzeng WS, Chan SW, Chen CC. CT-guided core biopsy of lung lesions: a primer. Am J Roentgenol. 2009;193:1228–1235. doi: 10.2214/AJR.08.2113. [DOI] [PubMed] [Google Scholar]
- 10.Racadio JM, Babic D, Homan R, Rampton JW, Patel MN, Racadio JM, Johnson ND. Live 3D guidance in the interventional radiology suite. Am J Roentgenol. 2007;189:W357–W364. doi: 10.2214/AJR.07.2469. [DOI] [PubMed] [Google Scholar]
- 11.Strocchi S, Colli V, Conte L. Multidetector CT fluoroscopy and cone-beam CT-guided percutaneous transthoracic biopsy: comparison based on patient doses. Radiat Prot Dosimetry. 2012;151:162–165. doi: 10.1093/rpd/ncr464. [DOI] [PubMed] [Google Scholar]
- 12.Kwok YM, Irani FG, Tay KH, Yang CC, Padre CG, Tan BS. Effective dose estimates for cone beam computed tomography in interventional radiology. Eur Radiol. 2013;23:3197–3204. doi: 10.1007/s00330-013-2934-7. [DOI] [PubMed] [Google Scholar]
- 13.Tselikas L, Joskin J, Roquet F, Farouil G, Dreuil S, Hakimé A, Teriitehau C, Auperin A, de Baere T, Deschamps F. Percutaneous bone biopsies: comparison between flat-panel cone-beam CT and CT-scan guidance. Cardiovasc Intervent Radiol. 2015;38:167–176. doi: 10.1007/s00270-014-0870-9. [DOI] [PubMed] [Google Scholar]
- 14.Choi JW, Park CM, Goo JM, Park YK, Sung W, Lee HJ, Lee SM, Ko JY, Shim MS. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of small (≤ 20 mm) lung nodules: diagnostic accuracy and complications in 161 patients. Am J Roentgenol. 2012;199:W322–W330. doi: 10.2214/AJR.11.7576. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Boiselle PM, Shepard JO, Trotman-Dickenson B, McLoud TC. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. Am J Roentgenol. 1996;167:105–109. doi: 10.2214/ajr.167.1.8659351. [DOI] [PubMed] [Google Scholar]
- 16.vanSonnenberg E, Casola G, Ho M, Neff CC, Varney RR, Wittich GR, Christensen R, Friedman PJ. Difficult thoracic lesions: CT-guided biopsy experience in 150 cases. Radiology. 1988;167:457–461. doi: 10.1148/radiology.167.2.3357956. [DOI] [PubMed] [Google Scholar]
- 17.Hwang HS, Chung MJ, Lee JW, Shin SW, Lee KS. C-arm cone-beam CT-guided percutaneous transthoracic lung biopsy: usefulness in evaluation of small pulmonary nodules. Am J Roentgenol. 2010;195:W400–W407. doi: 10.2214/AJR.09.3963. [DOI] [PubMed] [Google Scholar]
- 18.Choi MJ, Kim Y, Hong YS, Shim SS, Lim SM, Lee JK. Transthoracic needle biopsy using a C-arm cone-beam CT system: diagnostic accuracy and safety. Br J Radiol. 2012;85:e182–e187. doi: 10.1259/bjr/95413532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choo JY, Park CM, Lee NK, Lee SM, Lee HJ, Goo JM. Percutaneous transthoracic needle biopsy of small (≤ 1 cm) lung nodules under C-arm cone-beam CT virtual navigation guidance. Eur Radiol. 2013;23:712–719. doi: 10.1007/s00330-012-2644-6. [DOI] [PubMed] [Google Scholar]
- 20.Gupta R, Cheung AC, Bartling SH, Lisauskas J, Grasruck M, Leidecker C. Flat-panel volume CT: fundamental principles, technology, and applications. Radiographics. 2008;28:2009–2022. doi: 10.1148/rg.287085004. [DOI] [PubMed] [Google Scholar]
- 21.Ko JP, Shepard JO, Drucker EA, Aquino SL, Sharma A, Sabloff B, Halpern E, McLoud TC. Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology. 2001;218:491–496. doi: 10.1148/radiology.218.2.r01fe33491. [DOI] [PubMed] [Google Scholar]
- 22.Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW, Cheung YC, Chou AS. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126:748–754. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 23.Cheung JY, Kim Y, Shim SS, Lim SM. Combined fluoroscopy- and CT-guided transthoracic needle biopsy using a C-arm cone-beam CT system: comparison with fluoroscopy-guided biopsy. Korean J Radiol. 2011;12:89–96. doi: 10.3348/kjr.2011.12.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SM, Park CM, Lee KH, Bahn YE, Kim JI, Goo JM. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules: clinical experience in 1108 patients. Radiology. 2014;271:291–300. doi: 10.1148/radiol.13131265. [DOI] [PubMed] [Google Scholar]
- 25.Huda W, Mettler FA. Volume CT dose index and dose-length product displayed during CT: what good are they? Radiology. 2011;258:236–242. doi: 10.1148/radiol.10100297. [DOI] [PubMed] [Google Scholar]
- 26.Manhire A, Charig M, Clelland C, Gleeson F, Miller R, Moss H, Pointon K, Richardson C, Sawicka E. Guidelines for radiologically guided lung biopsy. Thorax. 2003;58:920–936. doi: 10.1136/thorax.58.11.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurban LA, Gomersall L, Weir J, Wade P. Fluoroscopy-guided percutaneous lung biopsy: a valuable alternative to computed tomography. Acta Radiol. 2008;49:876–882. doi: 10.1080/02841850802225893. [DOI] [PubMed] [Google Scholar]
- 28.Ohno Y, Hatabu H, Takenaka D, Higashino T, Watanabe H, Ohbayashi C, Sugimura K. CT-guided transthoracic needle aspiration biopsy of small (< or = 20 mm) solitary pulmonary nodules. Am J Roentgenol. 2003;180:1665–1669. doi: 10.2214/ajr.180.6.1801665. [DOI] [PubMed] [Google Scholar]