Abstract
BACKGROUND: An elevated preoperative C-reactive protein/albumin (CRP/Alb) ratio has been reported to be associated with a poor prognosis for hepatocellular carcinoma. The aim of the present study was to investigate the prognostic value of the preoperative CRP/Alb ratio and compare it with other systemic inflammatory response markers in patients with gastric cancer (GC). METHODS: A retrospective study was performed in 455 patients with GC undergoing curative resection. We investigated the correlations between the preoperative CRP/Alb ratio and overall survival (OS). Kaplan-Meier and Cox regression models were used to assess independent prognostic factors. The area under the curve was used to compare the prognostic value of different markers. RESULTS: On multivariate analysis, the CRP/Alb ratio were independently associated with OS in patients with GC (hazard ratio: 1.626; 95% confidence interval: 1.191-2.219; P = .002), along with age (P = .003), preoperative body weight loss (P = .001), tumor location (P = .008), metastatic lymph node ratio (P < .001), and seventh tumor-nodes-metastasis stage (American Joint Committee on Cancer) (P = .007). However, several other systemic inflammation–based prognostic scores (neutrophil lymphocyte ratio, platelet lymphocyte ratio and systemic immune-inflammation index, Glasgow Prognostic Score, modified Glasgow prognostic score, and high-sensitivity modified Glasgow prognostic score) were not. In addition, the CRP/Alb ratio had a higher area under the curve value (0.625) compared with several other systemic inflammation–based prognostic scores (P < .001). CONCLUSION: The preoperative CRP/Alb ratio, a system inflammation-based prognostic score, is a superior predictor of OS in patients undergoing curative resection for GC and may help to identify the high-risk patients for treatment decisions.
Introduction
Gastric cancer (GC), one of the most common malignant tumors in the digestive tract, is the second most frequent cause of mortality worldwide [1]. Over the past couple of decades, although advances have been seen in surgical techniques and adjuvant chemotherapy, the prognosis of patients with GC still remains unsatisfactory [2]. Therefore, it is of important significance to identify promising prognostic factors to select patients for tailor treatment.
It is now increasingly recognized that the systemic inflammatory response plays an important role in carcinogenesis and tumor progression [3–5]. A number of studies have demonstrated that the systemic inflammatory response is associated with a poor prognosis in various types of cancer. Several common inflammation-based prognostic scores, including the Glasgow Prognostic Score (GPS), modified Glasgow prognostic score (mGPS), high-sensitivity modified Glasgow prognostic score (HS-mGPS), neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), and systemic immune-inflammation index (SII), have been reported to have prognostic significance in many types of cancer [6–12]. Recently, a new prognostic index, preoperative C-reactive protein/albumin (CRP/Alb) ratio, has also been reported as an independent prognostic marker in hepatocellular carcinoma [13]. However, compared with other established inflammation-based prognostic scores, the prognostic value of the CRP/Alb ratio in patients with GC is still unclear.
In this study, we performed a large-scale retrospective cohort analysis to investigate the prognostic value of the preoperative CRP/Alb ratio. We also compared it with several other markers of the systemic inflammatory response in patients undergoing curative resection for GC.
Material and Methods
A total of 455 consecutive GC patients who received D2 gastrectomy with R0 resection at Cancer Center of Sun Yat-sen University between January 2005 and December 2010 were enrolled. D2 lymphadenectomy was performed following the Japanese Research Society for Gastric Cancer guidelines [14]. All patients were histologically confirmed as having stage I to III adenocarcinoma of the stomach depending on postoperative histological specimen. Our study complied with the standards of the Declaration of Helsinki and was approved by the Sun Yat-sen University Cancer Center research ethics committee. Every patient provided written informed consent before inclusion.
Data regarding potential prognostic factors were gathered, including age, sex, preoperative laboratory measurements, postoperative tumor characteristic, and survival times. Blood samples were obtained within 1 week before surgery to measure the neutrophil, lymphocyte, and platelet counts; Alb; and CRP. Tumor was staged according to the seventh edition of the American Joint Committee on Cancer tumor-nodes-metastasis (TNM) staging [15]. Patients were excluded if they had infection or inflammatory disease for nearly 1 month, immunity disease, and absent data regarding potential prognostic factors. Moreover, patients who received neoadjuvant chemotherapy or radiotherapy were excluded from the study. Patients were followed up carefully after surgery at 6- to 12-month intervals. A dynamic computed tomogram and a gastroscope examination were performed every 6 months. The last follow-up date was April 30, 2014. Overall survival (OS) was calculated from the date of surgery to the date of death or last follow-up.
Based on previous studies, preoperative body weight loss was defined as “no, or limited” (≤ 10%) or “severe” (> 10%), and body mass index (BMI) was defined according to the following categories: < 18.5 kg/m2, ≥ 18.5 to < 23 kg/m2, ≥ 23 to < 25.0 kg/m2, or ≥ 25.0 kg/m2 [16,17]. The NLR and PLR were defined as the absolute neutrophil count and platelet count divided by the absolute lymphocyte count [18]. The SII was calculated as follows: SII = P × N/L, where P, N, and L were the preoperative absolute platelet, neutrophil, and lymphocyte counts, respectively [9]. The GPS was calculated by CRP and Alb using standard thresholds (> 10 mg/l for CRP and < 35 g/l for Alb). Patients with both a CRP level > 10 mg/l and an Alb level < 35 g/l were categorized as having a score of 2. Patients with only one of these abnormalities were categorized as having a score of 1. Patients with neither of these abnormalities were categorized as having a score of 0 [19]. The mGPS was determined as previously described [12]. Patients with an elevated CRP level (> 0.5 mg/dl) and hypoalbuminemia (< 3.8 g/dl) were assigned an mGPS of 2, those with only one abnormality were assigned an mGPS of 1, and those with neither of these abnormalities were assigned an mGPS of 0. The HS-mGPS was determined as follows: patients with an elevated CRP level (> 0.3 mg/dl) and hypoalbuminemia (< 3.5 mg/dl) were assigned an HS-mGPS of 2, those with an abnormality in the CRP level were assigned an HS-mGPS of 1, and those with neither abnormality were assigned an HS-mGPS of 0 [11]. The CRP/Alb ratio was defined as the serum CRP level divided by the serum Alb level [20].
Statistical Analysis
Descriptive statistics of patient characteristics were presented as mean and 95% confidence intervals (CIs). Comparisons between groups were performed using the χ2 test for categorical variables. If continuous variable was proven for the assumption of linearity in the logit, the variable was categorized by generating receiver operating characteristics (ROC) curve to identify the optimal cutoff value. Survival analysis and curves were performed according to the Kaplan-Meier method and compared by the log-rank test. A Cox proportional-hazard model for multivariable analysis was applied for variables that proved to be significant in the univariate analysis. If variables were significantly associated with other variables, they were excluded from the final multivariable analysis. Multivariate P values were used to characterize the independence and significance of these factors. The discriminatory ability of the factors to predict OS was assessed using the area under the curve (AUC).
Statistical analyses were performed using IBM SPSS 19.0 software (IBM Corporation, Armonk, NY). Differences at P < .05 were considered to be significant in all statistical analyses.
Results
The clinicopathological characteristics of 455 GC patients and their association with OS were summarized in Table 1. The median age of the patients was 59 years (range, 19-86). Overall, 314 (69%) cases were males and 141 (31%) were females. The median follow-up period was 25 months (range, 1-76). There were 268 (58.9%) cases confirmed dead, and 187 (41.1%) were alive at last follow-up.
Table 1.
Clinical and Laboratory Characteristics of 455 GC Patients Associated with OS
| No. of Patients (%) | OS (Months) Mean (95% CI) | P Value⁎ | |
|---|---|---|---|
| Patient-related factors | |||
| Sex | .895 | ||
| Male | 314 (69.0%) | 39.8 (36.5-43.0) | |
| Female | 141 (31.0%) | 40.1 (35.1-45.0) | |
| Age (years) | .002 | ||
| < 60 | 252 (55.4%) | 43.9 (40.2-47.5) | |
| ≥ 60 | 203 (44.6%) | 34.9 (31.0-38.8) | |
| Preoperative body weight loss | < .001 | ||
| No | 220 (48.4%) | 46.3 (42.3-50.3) | |
| Limited | 170 (37.4%) | 36.8 (32.6-40.9) | |
| Severe | 65 (14.3%) | 26.6 (20.8-32.5) | |
| BMI (kg/m2) | .108 | ||
| < 18.5 | 71 (15.6%) | 32.2 (26.0-38.5) | |
| 18.5 ≤ < 23.0 | 223 (49.0%) | 41.2 (37.3-45.1) | |
| 23.0 ≤ < 25.0 | 90 (19.8%) | 40.0 (33.7-46.0) | |
| 25.0 ≤ | 71 (15.6%) | 42.7 (36.1-49.4) | |
| Neutrophils (× 109/L) | .002 | ||
| < 7.5 | 417 (91.6%) | 41.1 (38.3-44.0) | |
| ≥ 7.5 | 38 (8.4%) | 26.1 (19.1-33.1) | |
| Lymphocytes (× 109/L) | .510 | ||
| < 3 | 431 (94.7%) | 40.2 (37.4-43.0) | |
| ≥ 3 | 24 (5.3%) | 34.1 (23.8-44.4) | |
| Platelet (× 109/L) | .478 | ||
| < 400 | 416 (91.4%) | 40.3 (37.4-43.1) | |
| ≥ 400 | 39 (8.6%) | 35.3 (26.7-43.8) | |
| CRP (mg/L) | .002 | ||
| ≤ 10 | 388 (85.3%) | 41.6 (38.6-44.6) | |
| > 10 | 67 (14.7%) | 29.8 (23.8-35.8) | |
| Alb (g/L) | < .001 | ||
| < 35 | 51 (11.2%) | 24.2 (18.7-29.8) | |
| ≥ 35 | 404 (88.8%) | 41.7 (38.9-44.6) | |
| NLR | < .001 | ||
| < 2.3 | 269 (59.1%) | 44.6 (41.0-48.2) | |
| ≥ 2.3 | 186 (40.9%) | 33.3 (29.4-37.2) | |
| PLR | < .001 | ||
| < 180 | 336 (73.8%) | 42.8 (39.6-46.0) | |
| ≥ 180 | 119 (26.2%) | 31.3 (26.6-35.9) | |
| SII | .001 | ||
| < 660 | 302 (66.4%) | 43.1 (39.7-46.5) | |
| ≥ 660 | 153 (33.6%) | 33.5 (29.3-37.7) | |
| GPS | < .001 | ||
| 0 | 350 (76.9%) | 43.6 (40.5-46.7) | |
| 1 | 92 (20.2%) | 26.8 (21.9-31.6) | |
| 2 | 13 (2.9%) | 29.9 (19.2-40.5) | |
| mGPS | .001 | ||
| 0 | 274 (60.2%) | 44.1 (40.6-47.7) | |
| 1 | 145 (31.9%) | 33.9 (29.5-38.4) | |
| 2 | 36 (7.9%) | 31.8 (22.9-40.7) | |
| HS-mGPS | .006 | ||
| 0 | 295 (64.8%) | 42.7 (39.3-46.1) | |
| 1 | 135 (29.7%) | 35.8 (31.0-40.7) | |
| 2 | 25 (5.5%) | 24.5 (17.5-31.5) | |
| CRP/Alb ratio | < .001 | ||
| < 0.25 | 153 (33.6%) | 51.7 (47.0-56.3) | |
| ≥ 0.25 | 302 (66.4%) | 34.2 (31.0-37.3) | |
| Tumor-related factors | |||
| Tumor location Upper third | 178 (39.1%) | 32.1 (28.3-36.0) | < .001 |
| Middle third | 100 (22.0%) | 38.8 (32.9-44.7) | |
| Lower third | 177 (38.9%) | 48.4 (44.0-52.8) | |
| Tumor size (cm) | < .001 | ||
| < 3 | 155 (34.1%) | 49.3 (44.5-54.1) | |
| ≥ 3 | 300 (65.9%) | 35.0 (31.9-38.2) | |
| Metastatic lymph node ratio | < .001 | ||
| < 0.17 | 195 (42.9%) | 56.6 (52.7-60.4) | |
| ≥ 0.17 | 260 (57.1%) | 27.4 (24.5-30.2) | |
| TNM stage | < .001 | ||
| I | 60 (13.2%) | 67.1 (61.6-72.5) | |
| II | 95 (20.9%) | 53.6 (47.9-59.3) | |
| III | 300 (65.9%) | 30.0 (27.1-32.9) |
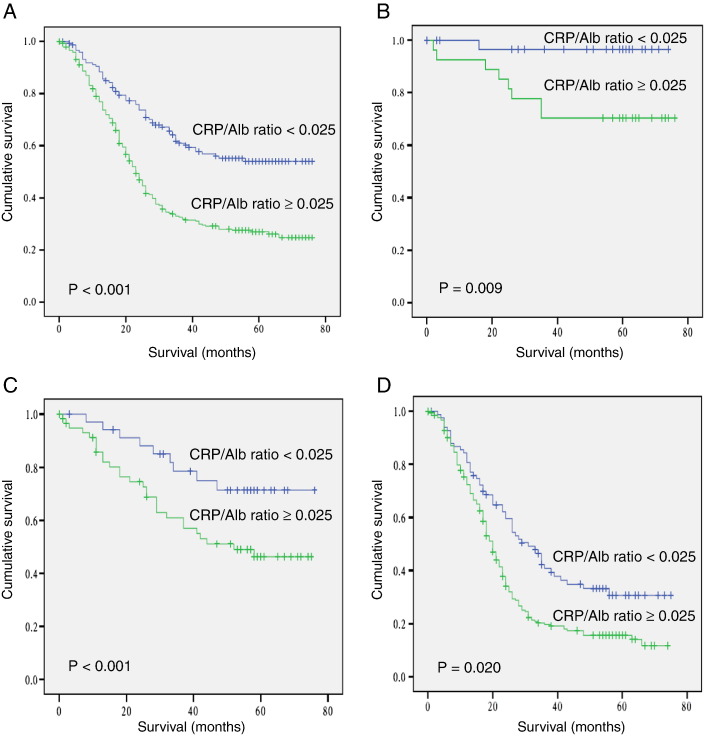
Kaplan-Meier survival analysis.
Based on previous studies, the accepted optimal cutoff value was determined for some variables, including preoperative body weight loss, BMI, neutrophil, lymphocyte, and platelet counts [16,17,21,22]. Besides the variables, ROC curve analysis was used to determine the optimal cutoff value for others. All the optimal cutoff values for variables were shown in Table 1. The optimal cutoff value for the CRP/Alb ratio was 0.025 for the OS. The sensitivity and specificity were 76.8% and 48.1%, respectively. According to the cutoff level, patients were divided into two groups (≤ 0.025, n = 153; > 0.025, n = 402). The 1-, 3-, and 5-year OS rates in patients with a CRP/Alb ratio ≤ 0.025 were 88.3%, 60.7%, and 54.5%, respectively. The survival rates were significantly higher than those in patients with a CRP/Alb ratio > 0.025, which were 76.9%, 32.6%, and 26.9%, respectively (P < .001; Figure 1A). In addition, when stratified by TNM stage, the prognostic significance of CRP/Alb ratio was still maintained in stage I to III (P = .009, P = .020, and P < .001, respectively; Figure 1, B–D). A low CRP/Alb ratio was associated with longer median OS in stage I (72.0 vs 59.6 months), stage II (62.9 vs 47.9 months), and stage III (38.6 vs 26.4 months), respectively.
Figure 1.
Overall survival based on the preoperative CRP/Alb ratio. Overall survival based on the preoperative CRP/Alb ratio in patients with stage I to III (A), stage I (B), stage II (C), and stage III (D) GC, respectively.
The relationship between the CRP/Alb ratio and clinicopathologic characteristics in GC patients was shown in Table 3. Patients with an elevated CRP/Alb ratio were more likely found in males (P = .005) and were associated with age ≥ 60 years (P < .001), higher neutrophil counts (P = .040), higher platelet counts (P = .001), elevated NLR (P < .001), elevated PLR (P < .001), elevated SII (P < .001), higher GPS score (P < .001), higher mGPS score (P < .001), higher HS-mGPS score (P < .001), tumor location (upper third) (P = .013), larger tumor size (P < .001), elevated metastatic lymph node ratio (P = .005), and higher TNM stage (P < .001).
Table 3.
Relationship between the CRP/Alb Ratio and Clinicopathologic Characteristics
| CRP/Alb Ratio < 0.25 |
CRP/Alb Ratio ≥ 0.25 |
P Value | |
|---|---|---|---|
| (n = 153) | (n = 302) | ||
| Patient-related factors | |||
| Sex | .005 | ||
| Male | 93 | 222 | |
| Female | 60 | 80 | |
| Age (years) | < .001 | ||
| < 60 | 103 | 150 | |
| ≥ 60 | 50 | 152 | |
| Preoperative body weight loss | .363 | ||
| No | 81 | 139 | |
| Limited | 53 | 117 | |
| Severe | 19 | 46 | |
| BMI (kg/m2) | .315 | ||
| < 18.5 | 19 | 52 | |
| 18.5 ≤ < 23.0 | 81 | 142 | |
| 23.0 ≤ < 25.0 | 33 | 57 | |
| 25.0 ≤ | 20 | 51 | |
| Neutrophils (× 109/L) | .040 | ||
| < 7.5 | 146 | 271 | |
| ≥ 7.5 × 109/L | 7 | 31 | |
| Lymphocytes (× 109/L) | .688 | ||
| < 3 | 144 | 287 | |
| ≥ 3 | 9 | 15 | |
| Platelet (× 109/L) | .001 | ||
| < 400 | 149 | 267 | |
| ≥ 400 | 4 | 35 | |
| NLR | < .001 | ||
| < 2.3 | 118 | 151 | |
| ≥ 2.3 | 35 | 151 | |
| PLR | < .001 | ||
| < 180 | 130 | 206 | |
| < 180 | 23 | 96 | |
| SII | < .001 | ||
| < 660 | 121 | 182 | |
| < 660 | 32 | 120 | |
| GPS | < .001 | ||
| 0 | 148 | 201 | |
| 1 | 4 | 88 | |
| 2 | 0 | 13 | |
| mGPS | < .001 | ||
| 0 | 133 | 141 | |
| 1 | 20 | 125 | |
| 2 | 0 | 36 | |
| HS-mGPS | < .001 | ||
| 0 | 153 | 142 | |
| 1 | 0 | 135 | |
| 2 | 0 | 25 | |
| Tumor-related factors | |||
| Tumor location | .013 | ||
| Upper third | 48 | 130 | |
| Middle third | 32 | 68 | |
| Lower third | 73 | 104 | |
| Tumor size (cm) | < .001 | ||
| < 3 | 78 | 78 | |
| ≥ 3 | 75 | 224 | |
| Metastatic lymph node ratio | .005 | ||
| < 0.17 | 80 | 115 | |
| ≥ 0.17 | 173 | 187 | |
| TNM stage | < .001 | ||
| I | 34 | 27 | |
| II | 34 | 60 | |
| III | 85 | 215 |
The results of the univariate and multivariate analysis were shown in Table 2. After excluding the related variables, the significant variables (age, tumor location, metastatic lymph node ratio, tumor size, TNM stage, NLR, PLR, SII, GPS, and CRP/Alb ratio) were tested in the multivariate analysis. The multivariate analysis indicated that the age [hazard ratio (HR): 1.447; 95% CI: 1.130-1.853; P = .003], preoperative body weight loss (HR: 1.324; 95% CI: 1.116-1.571; P = .001), CRP/Alb ratio (HR: 1.626; 95% CI: 1.191-2.219; P = .002), tumor location (HR: 0.824; 95% CI: 0.713-0.951; P = .008), metastatic lymph node ratio (HR: 2.511; 95% CI: 1.715-3.676; P < .001), and seventh TNM stage (American Joint Committee on Cancer) (HR: 1.608; 95% CI: 1.138-2.273; P = .007) were independent prognostic factors for OS. When GPS was replaced by mGPS and HS-mGPS, respectively, the results of multivariate analysis did not change significantly (Supplement Table 1).
Table 2.
Univariate and Multivariate Analyses of OS
| Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Patient-related factors | ||||
| Sex | 0.983 (0.757-1.276) | .896 | ||
| Male | ||||
| Female | ||||
| Age (years) | 1.461 (1.149-1.856) | .002 | 1.447 (1.130-1.853) | .003 |
| < 60 | ||||
| ≥ 60 | ||||
| Preoperative body weight loss | 1.533 (1.301-1.806) | < .001 | 1.324 (1.116-1.571) | .001 |
| No | ||||
| Limited | ||||
| Severe | ||||
| BMI (kg/m2) | 0.888 (0.777-1.016) | .083 | ||
| < 18.5 | ||||
| 18.5 ≤ < 23.0 | ||||
| 23.0 ≤ < 25.0 | ||||
| 25.0 ≤ | ||||
| Neutrophils (× 109/L) | 1.826 (1.241-2.686) | .002 | ||
| < 7.5 | ||||
| ≥ 7.5 | ||||
| Lymphocytes (× 109/L) | 1.183 (0.713-1.960) | .515 | ||
| < 3 | ||||
| ≥ 3 | ||||
| Platelet (× 109/L) | 1.165 (0.759-1.787) | .484 | ||
| < 400 | ||||
| ≥ 400 | ||||
| CRP (mg/L) | 1.632 (1.193-2.232) | .002 | ||
| ≤ 10 | ||||
| > 10 | ||||
| Alb (g/L) | 0.507 (0.362-0.710) | < .001 | ||
| < 35 | ||||
| ≥ 35 | ||||
| NLR | 1.634 (1.285-2.078) | < .001 | 1.095 (0.797-1.503) | .576 |
| < 2.3 | ||||
| ≥ 2.3 | ||||
| PLR | 1.588 (1.227-2.057) | < .001 | 1.204 (0.879-1.650) | .247 |
| < 180 | ||||
| ≥ 180 | ||||
| SII | 1.493 (1.168-1.907) | .001 | 1.036 (0.723-1.485) | .846 |
| < 660 | ||||
| ≥ 660 | ||||
| GPS | 1.634 (1.324-2.015) | < .001 | 1.160 (0.910-1.478) | .230 |
| 0 | ||||
| 1 | ||||
| 2 | ||||
| mGPS | 1.394 (1.166-1.668) | < .001 | ||
| 0 | ||||
| 1 | ||||
| 2 | ||||
| HS-mGPS | 1.361 (1.119-1.654) | .002 | ||
| 0 | ||||
| 1 | ||||
| 2 | ||||
| CRP/Alb ratio | 2.225 (1.673-2.960) | < .001 | 1.626 (1.191-2.219) | .002 |
| < 0.25 | ||||
| ≥ 0.25 | ||||
| Tumor-related factors | ||||
| Tumor location | 0.706 (0.615-0.810) | < .001 | 0.824 (0.713-0.951) | .008 |
| Upper third | ||||
| Middle third | ||||
| Lower third | ||||
| Tumor size (cm) | 1.900 (1.440-2.507) | < .001 | 0.944 (0.698-1.277) | .710 |
| < 3 | ||||
| ≥ 3 | ||||
| Metastatic lymph node ratio | 4.011 (3.021-5.325) | < .001 | 2.511 (1.715-3.676) | < .001 |
| < 0.17 | ||||
| ≥ 0.17 | ||||
| TNM stage | 2.979 (2.317-3.831) | < .001 | 1.608 (1.138-2.273) | .007 |
| I | ||||
| II | ||||
| III | ||||
As CRP/Alb ratio was significantly associated with other variables (age, tumor location, metastatic lymph node ratio, and seventh TNM stage), subgroup analyses were further performed to evaluate the prognostic value of CRP/Alb ratio more comprehensively. Of note, the prognostic significance was still maintained when stratified by age (< 60: P < .001; ≥ 60: P < .001), tumor location (upper third: P = .001; middle third: P = .004; lower third: P = .007), and metastatic lymph node ratio (< 0.17: P < .001; ≥ 0.17: P < .001).
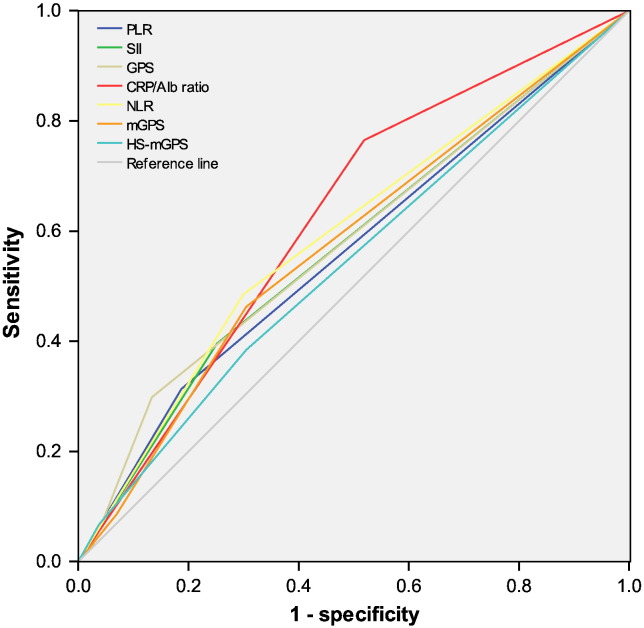
Considering correlations among the markers of the systemic inflammatory response, we constructed ROC curves to further assess their discrimination ability (Figure 2). The discrimination ability of the CRP/Alb ratio, as assessed by AUC, was 0.625 (95% CI: 0.572-0.678; P < .001), which was higher than that of other markers (Table 4).
Figure 2.
The predictive ability of the seven inflammation-based prognostic scores was compared by ROC curves.
Table 4.
Comparison of the AUCs for the Seven Inflammation-Based Prognostic Scores
| AUC | 95% CI | P Value | |
|---|---|---|---|
| CRP/Alb ratio | 0.625 | (0.572-0.678) | < .001 |
| NLR | 0.594 | (0.541-0.646) | .001 |
| PLR | 0.564 | (0.511-0.617) | .021 |
| SII | 0.571 | (0.518-0.624) | .010 |
| GPS | 0.581 | (0.528-0.633) | .003 |
| mGPS | 0.576 | (0.523-0.629) | .006 |
| HS-mGPS | 0.543 | (0.489-0.596) | .012 |
Discussion
It is well known that the systemic inflammatory response plays an important role in carcinogenesis and tumor progression [23,24]. With proinflammatory cytokines releasing and the formation of an inflammatory microenvironment, inflammatory cells are powerful tumor promoters [25,26]. Inflammatory responses should also be thought of as antitumor, but cancer patients are often defective in inflammatory responses. To date, although the prognostic value of common inflammation-based prognostic scores, including the GPS, mGPS, HS-mGPS, NLR, PLR, SII, and CRP/Alb ratio, has been given great concern, the mechanisms underlying the relationship between inflammation and cancer survival have not been elucidated completely. It may be associated with malnutrition, immune dysfunction, upregulation of growth factors, angiogenesis, etc. [27,28]. In recent years, studies have demonstrated that several important inflammation-based prognostic scores are associated with various cancer survival outcomes [6–10].
Serum CRP, an acute-phase protein, is produced in hepatocytes, predominantly under the control of IL-6. It was shown to be very sensitive prognosis indicator of inflammation in a variety of primary malignancies, including GC [29–31]. On the other hand, low serum Alb levels, as a state of malnutrition, have also been considered to be associated with various cancer survival outcomes, for example, gastrointestinal, colorectal, lung, and ovarian cancer [32–35]. Furthermore, we can speculate that CRP/Alb ratio, which is based on both elevated serum CRP concentrations and hypoalbuminemia, may enable a better appreciation of the outcome of the malignancy. In fact, Akiyoshi et al. have proved that the CRP/Alb ratio is an independent prognostic marker in hepatocellular carcinoma [13].
Currently, we first investigate the prognostic significance of these markers in patients with GC. Of the inflammation-based prognostic scores, only the CRP/Alb ratio was significantly associated with OS and had prognostic value independent of TNM stage. More importantly, we found that its prognostic significance was substantially stable in stage I to III patients undergoing curative resection for GC. In hepatocellular carcinoma, Akiyoshi et al. also found that an elevated CRP/Alb ratio was associated with a higher Cancer of the Liver Italian Program score, Barcelona Clinic Liver Cancer stage, and Child-Pugh grade. The novel inflammation-based prognostic score had comparable prognostic ability to other inflammation-based prognostic scores. They demonstrated that an elevated CRP/Alb ratio was significantly parallel to tumor progression. Obviously, their conclusion was consistent with our study [13]. Moreover, we further explored the association between CRP/Alb ratio and nutritional status. We found that it was not significantly associated with preoperative body weight loss and BMI, although CRP/Alb ratio reflected not only inflammation but also nutritional status of cancer patients. The data suggested, in terms of CRP/Alb ratio, that systemic inflammatory response exerted more potent prognostic effect than nutritional status. In addition, a study from Crumley et al. demonstrated that the relation between hypoalbuminemia and poor outcome was secondary to that of the systemic inflammatory response, which might also explain part of the reason [32].
In our study, the multivariate analysis revealed that only the CRP/Alb ratio, rather than other inflammation-based prognostic scores, was independently associated with OS. Considering that preoperative systemic inflammation might be associated with postoperative infection that led to poor clinical outcome, we rechecked follow-up data to further explore whether CRP/Alb ratio adversely affected postoperative short-term survival. We found that 25 cases died within half a year after surgery and that none of them died of postoperative infection. Therefore, CRP/Alb ratio was considered ideal to evaluate prognosis in GC. More importantly, we found that the prognostic significance, regardless of TNM stage, was substantially stable. This may be useful for postoperative evaluation of recurrence after curative surgery, especially in early-stage patients in whom tumor relapse is difficult to predict.
To further evaluate the discrimination ability of the inflammation-based prognostic scores, ROC curves were performed to compare the AUC value. The CRP/Alb ratio had a higher AUC value than other markers (0.625; P < .001). A study from Xu et al. indicated that the GPS was superior to the NLR and PLR and was associated with OS in patients undergoing potential curative resection for stage III GC [36]. Takeno et al. revealed that the HS-mGPS was a superior prognostic predictor compared with the mGPS in patients with GC [11]. Hu et al. also showed that the SII could be a more objective index than the NLR and PLR in hepatocellular carcinoma [9]. As far as we know, this is the first to include the SII in GC. Our study proved that the CRP/Alb ratio, in terms of its prognostic ability, was superior to other inflammation-based prognostic scores, including the GPS, mGPS, HS-mGPS, NLR, PLR, and SII. Although the GPS, mGPS, and HS-mGPS use the same variables to estimate the scores, they may have the potential for underestimation (a lower CRP level) or overestimation (a lower Alb level) in patients with GC. However, the CRP/Alb ratio reduces these potential by using the ratio of the CRP and Alb, which may be superior to them in GC.
It is worth noting that our findings may have important value in the therapy of GC. With an elevated CRP/Alb ratio, early-stage patients may need closer follow-up, and local advanced patients may require more aggressive adjuvant chemotherapy or additional active therapy. In addition, patients with an elevated CRP/Alb ratio may benefit from anti-inflammatory therapy or nutritional support [37–39]. Future studies, especially prospective multicenter clinical trials, are warranted as validation studies.
We acknowledge several potential limitations in present study. First, it was a retrospective, single-institution, and small-sample-size study. However, the surgical procedures (R0 resection plus D2 lymphadenectomy), laboratory examinations, and follow-up were uniform throughout the entire study period. Second, different postoperative therapy might induce confounding. Third, we lacked the data of disease-free survival, although OS was the standard indicator for cancer prognosis study.
Conclusions
To our knowledge, we first demonstrate that preoperative CRP/Alb ratio is an independent prognostic marker in patients after curative resection for GC. Moreover, its prognostic ability is superior to other inflammation-based prognostic scores. Due to its simplicity, cheapness, and availability, it is of important significance to help clinicians identify the high-risk patients and enable postoperative targeted rational therapy.
The following are the supplementary data related to this article.
Multivariate Analyses of OS
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
L.X.C. and S.X.W. contributed equally to this work. X.D.Z. and S.X.W. contributed to the conception and design of the study. L.J.J., C.S.X., and K.P.F. performed literature search, data extraction, quality assessment, and statistical analyses. L.X.C. composed the first draft of the manuscript. S.X.W. and Z.Y.Q. read and critically revised the manuscript.
Acknowledgements
We thank all the people who gave help in this study.
Contributor Information
Xuechao Liu, Email: liuxch@sysucc.org.cn.
Xiaowei Sun, Email: sunxw@sysucc.org.cn.
Jianjun Liu, Email: liujj@sysucc.org.cn.
Pengfei Kong, Email: kongpf@sysucc.org.cn.
Shangxiang Chen, Email: chenshx@sysucc.org.cn.
Youqing Zhan, Email: zhanyq@sysucc.org.cn.
Dazhi Xu, Email: xudzh@sysucc.org.cn.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Sasako M., Sano T., Yamamoto S., Kurokawa Y., Nashimoto A., Kurita A., Hiratsuka M., Tsujinaka T., Kinoshita T., Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359(5):453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Bromberg J., Wang T.C. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15(2):79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 6.Gao Y., Huang D. The value of the systematic inflammation-based Glasgow Prognostic Score in patients with gastric cancer: a literature review. J Cancer Res Ther. 2014;10(4):799–804. doi: 10.4103/0973-1482.146054. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Zhang W., Feng L.J. Prognostic significance of neutrophil lymphocyte ratio in patients with gastric cancer: a meta-analysis. PLoS One. 2014;9(11):e111906. doi: 10.1371/journal.pone.0111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J.J., Hu Z.G., Shi W.X., Deng T., He S.Q., Yuan S.G. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21(9):2807–2815. doi: 10.3748/wjg.v21.i9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B., Yang X.R., Xu Y., Sun Y.F., Sun C., Guo W., Zhang X., Wang W.M., Qiu S.J., Zhou J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 10.Feng J.F., Huang Y., Chen Q.X. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. doi: 10.1186/1477-7819-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeno S., Hashimoto T., Shibata R., Maki K., Shiwaku H., Yamana I., Yamashita R., Yamashita Y. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor in patients with resectable gastric cancer. Oncology. 2014;87(4):205–214. doi: 10.1159/000362601. [DOI] [PubMed] [Google Scholar]
- 12.Hirashima K., Watanabe M., Shigaki H., Imamura Y., Ida S., Iwatsuki M., Ishimoto T., Iwagami S., Baba Y., Baba H. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014;49(6):1040–1046. doi: 10.1007/s00535-013-0855-5. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita A., Onoda H., Imai N., Iwaku A., Oishi M., Tanaka K., Fushiya N., Koike K., Nishino H., Matsushima M. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22(3):803–810. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 14.Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14(2):113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 15.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 16.Minami Y., Kawai M., Fujiya T., Suzuki M., Noguchi T., Yamanami H., Kakugawa Y., Nishino Y. Family history, body mass index and survival in Japanese patients with stomach cancer: a prospective study. Int J Cancer. 2015;136(2):411–424. doi: 10.1002/ijc.29001. [DOI] [PubMed] [Google Scholar]
- 17.van der Schaaf M.K., Tilanus H.W., van Lanschot J.J., Johar A.M., Lagergren P., Lagergren J., Wijnhoven B.P. The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. J Thorac Cardiovasc Surg. 2014;147(1):490–495. doi: 10.1016/j.jtcvs.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 18.Ying H.Q., Deng Q.W., He B.S., Pan Y.Q., Wang F., Sun H.L., Chen J., Liu X., Wang S.K. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31(12):305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 19.Forrest L.M., McMillan D.C., McArdle C.S., Angerson W.J., Dunlop D.J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non–small-cell lung cancer. Br J Cancer. 2003;89(6):1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairclough E., Cairns E., Hamilton J., Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med. 2009;9(1):30–33. doi: 10.7861/clinmedicine.9-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proctor M.J., Horgan P.G., Talwar D., Fletcher C.D., Morrison D.S., McMillan D.C. Optimization of the systemic inflammation-based Glasgow prognostic score: a Glasgow Inflammation Outcome Study. Cancer. 2013;119(12):2325–2332. doi: 10.1002/cncr.28018. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X., Hiki N., Nunobe S., Kumagai K., Kubota T., Aikou S., Sano T., Yamaguchi T. Prognostic importance of the inflammation-based Glasgow prognostic score in patients with gastric cancer. Br J Cancer. 2012;107(2):275–279. doi: 10.1038/bjc.2012.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 25.Wisastra R., Dekker F.J. Inflammation, cancer and oxidative lipoxygenase activity are intimately linked. Cancers. 2014;6(3):1500–1521. doi: 10.3390/cancers6031500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Visser K.E., Eichten A., Coussens L.M. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 27.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita A., Onoda H., Takano K., Imai N., Saeki C., Fushiya N., Miyakawa Y., Nishino H., Tajiri H. Pretreatment serum C-reactive protein level predicts poor prognosis in patients with hepatocellular carcinoma. Med Oncol. 2012;29(4):2800–2808. doi: 10.1007/s12032-012-0220-1. [DOI] [PubMed] [Google Scholar]
- 30.Wong V.K., Malik H.Z., Hamady Z.Z., Al-Mukhtar A., Gomez D., Prasad K.R., Toogood G.J., Lodge J.P. C-reactive protein as a predictor of prognosis following curative resection for colorectal liver metastases. Br J Cancer. 2007;96(2):222–225. doi: 10.1038/sj.bjc.6603558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishino Y., Saigusa S., Ohi M., Yasuda H., Tanaka K., Toiyama Y., Mohri Y., Kusunoki M. Preoperative C-reactive protein and operative blood loss predict poor prognosis in patients with gastric cancer after laparoscopy-assisted gastrectomy. Asian J Endosc Surg. 2014;7(4):287–294. doi: 10.1111/ases.12126. [DOI] [PubMed] [Google Scholar]
- 32.Crumley A.B., Stuart R.C., McKernan M., McMillan D.C. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer? World J Surg. 2010;34(10):2393–2398. doi: 10.1007/s00268-010-0641-y. [DOI] [PubMed] [Google Scholar]
- 33.Borda F., Borda A., Jimenez J., Zozaya J.M., Prieto C., Gomez M., Urman J., Ibanez B. Predictive value of pre-treatment hypoalbuminemia in prognosis of resected colorectal cancer. Gastroenterol Hepatol. 2014;37(5):289–295. doi: 10.1016/j.gastrohep.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Espinosa E., Feliu J., Zamora P., Gonzalez Baron M., Sanchez J.J., Ordon ez A., Espinosa J. Serum albumin and other prognostic factors related to response and survival in patients with advanced non–small cell lung cancer. Lung Cancer. 1995;12(1-2):67–76. doi: 10.1016/0169-5002(95)00407-r. [DOI] [PubMed] [Google Scholar]
- 35.Asher V., Lee J., Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol. 2012;29(3):2005–2009. doi: 10.1007/s12032-011-0019-5. [DOI] [PubMed] [Google Scholar]
- 36.Wang D.S., Ren C., Qiu M.Z., Luo H.Y., Wang Z.Q., Zhang D.S., Wang F.H., Li Y.H., Xu R.H. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour Biol. 2012;33(3):749–756. doi: 10.1007/s13277-011-0285-z. [DOI] [PubMed] [Google Scholar]
- 37.Langman M.J., Cheng K.K., Gilman E.A., Lancashire R.J. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ. 2000;320(7250):1642–1646. doi: 10.1136/bmj.320.7250.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez M.J., Robinson P., Madden T., Highbarger T. Nutritional support and prognosis in patients with head and neck cancer. J Surg Oncol. 1994;55(1):33–36. doi: 10.1002/jso.2930550110. [DOI] [PubMed] [Google Scholar]
- 39.Rizzo A., Cengel K.A. Anti-inflammatory therapy for pancreatic cancer: a sorely needed advance in therapeutics. Cancer Biol Ther. 2008;7(7):1051–1052. doi: 10.4161/cbt.7.7.6581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariate Analyses of OS