Abstract
OBJECTIVES: Several cytokines secreted from breast cancer tissues are suggested to be related to disease prognosis. We examined Th1/Th2/Th17 cytokines produced from three-dimensionally cultured breast cancer tissues and related them with patient clinical profiles. METHODS: 21 tumor tissues and 9 normal tissues surgically resected from breast cancer patients were cultured in thermoreversible gelatin polymer–containing medium. Tissue growth and Th1/Th2/Th17 cytokine concentrations in the culture medium were analyzed and were related with hormone receptor expressions and patient clinical profiles. RESULTS: IL-6 and IL-10 were expressed highly in culture medium of both cancer and normal tissues. However, IFN-γ, TNF-α, IL-2, and IL-17A were not detected in the supernatant of the three-dimensionally cultured normal mammary gland and are seemed to be specific to breast cancer tissues. The growth abilities of hormone receptor–negative cancer tissues were significantly higher than those of receptor-positive tissues (P = 0.0383). Cancer tissues of stage ≥ IIB patients expressed significantly higher TNF-α levels as compared with those of patients with stage < IIB (P = 0.0096). CONCLUSIONS: The tumor tissues resected from breast cancer patients can grow in the three-dimensional thermoreversible gelatin polymer culture system and produce Th1/Th2/Th17 cytokines. Hormone receptor–positive cancer tissues showed less growth ability. TNF-α is suggested to be a biomarker for the cancer stage.
Introduction
Individualized pharmacotherapeutic approaches in breast cancer patients have been carried out according to the histological findings of tumors and the difference of the stages. Hormone receptors and HER2 could be efficient targets for the individualized breast cancer pharmacotherapy. However, in case of the patients negative with these targets, the effectiveness of cytotoxic chemotherapy largely influences the patient outcome.
Many kinds of immune cells are known to work to exclude cancer cells [1], whereas it has been suggested that the cancer cells secrete cytokines to escape from the tumor immunity [2]. The cytokine network made by cancer cells contributes to growth and development of tumors and inhibits host immunity [3] by disturbing the effector-cell responses [4]. Th2 cytokines are reported to increase in tumor tissues, which can act to suppress tumor immunity [5]. Indeed, many kinds of cytokines [6], such as IL-6, IL-8, and IL-10 [7,8], have been reported to be upregulated in tumor tissues. Moreover, increase in some kinds of cytokines in breast cancer is relevant to clinical disease stage and progression of cancer [9]. Thus, it is suggested that cytokines play important roles in the pathology or prognosis of cancer.
Many types of gels have been used for three-dimensional culture of mammalian cells and tissues, which include spinner cultivation technique [10], alginate beans [11], agarose [12], soft agarose, Matrigel [13], or collagen matrix gel [14]. However, cytotoxic enzyme use is required for culturing the cells or tissues in these culture systems. The three-dimensional culture system using thermoreversible gelatin polymer (TGP) [15] is simple and easy for culturing cells or tissues and collecting culture superatant without effecting damages on the cells. TGP is a heat-reversible hydrogel constituted by block copolymer of hydrophilic polymer and temperature-sensitive polymer. TGP has a unique property as a gel in high temperature and a sol state in low temperature reversibly, with a transition temperature of 22°C. The cell toxicity of TGP is extremely low so that cells can be cultured for long time in this gel system. Moreover, fibroblasts are hard to grow, whereas cancer cells can easily proliferate, in the TGP gel.
In this study, we three-dimensionally cultured breast cancer and normal mammary tissues, surgically resected from patients, by using TGP and investigated the growth ability of the tissues or Th1/Th2/Th17 cytokines secreted from the tissues. These biomarkers are also related with patient clinical profiles, including the hormone receptors and the stage of tumor.
Materials and Methods
Patients’ Characteristics
This study was approved as "The study for cytokines secreted from breast cancer tissues" by the Ethics Committee of the Cancer Institute Hospital of JFCR in June 2012, and informed consent was obtained from all the patients before enrolling participants in this study. We evaluated a total of 21 patients diagnosed as having breast cancer in the Department of Breast Cancer Oncology of The Cancer Institute Hospital of JFCR (all of them are women, and their mean age was 53.9 years) (Table 1). Paclitaxel, docetaxel, herceptin, cyclophosphamide/epirubicin/5-FU, docetaxel/adriamycin/cyclophosphamide, and cyclophosphamide/adriamycin/5-FU were used as adjuvant chemotherapy in 6 out of 21 patients. Statuses for estrogen receptor (ER), progesterone receptor (PgR), and HER2 in cancer tissues were examined by immunohistochemical analysis. The breast cancer tissues were surgically resected from these patients for the purpose of their therapy, and 12 tissues resected from 12 patients out of the 21 patients were cultured in The Cancer Institute Hospital, whereas the other 9 tissues from 9 patients were cultured in Tokyo University of Pharmacy and Life Sciences. The nine normal mammary tissues from the latter nine patients were also provided and cultured, and eight of them were used for cytokine measurement.
Table 1.
Patient Characteristics
| Case Number | Age | Chemotherapy | Menopause | ER | PR | HER2 | FISH | NG | Tissue Type | Stage |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 57 | − | Post | − | − | 3 + | Unknown | 1 | a3 | Unknown |
| Case 2 | 42 | − | Pre | − | − | 0 | Unknown | Unknown | a3 | IIIC |
| Case 3 | 41 | + | Pre | − | − | 1 + | Unknown | 3 | a2 | IIA |
| Case 4 | 61 | − | Post | + | − | 2 + | − | 2 | a2 | IIIC |
| Case 5 | 43 | − | Pre | + | + | 0 | Unknown | Unknown | a3 | IIIC |
| Case 6 | 48 | − | Pre | + | + | 0 | Unknown | 2 | a3 | IIIA |
| Case 7 | 74 | + | Post | − | − | 0 | Unknown | 3 | a2 | IIA |
| Case 8 | 46 | + | Pre | + | + | 0 | Unknown | 1 | a3 | IIA |
| Case 9 | 34 | − | Pre | − | − | 0 | Unknown | Unknown | a3 | IV |
| Case 10 | 51 | + | Pre | + | − | 1 + | Unknown | 1 | a3 | IIA |
| Case 11 | 64 | + | Post | + | + | 0 | Unknown | 3 | a3 | IIA |
| Case 12 | 43 | + | Pre | − | − | 1 + | Unknown | 3 | a2 | IIA |
| Case 13 | 62 | + | Post | + | − | 1 + | Unknown | 1 | a3 | IIA |
| Case 14 | 67 | + | Post | + | + | 2 + | − | 3 | a2 | IIA |
| Case 15 | 47 | + | Pre | + | + | 2 + | + | 2 | a3 | IIA |
| Case 16 | 62 | + | Post | + | + | 0 | Unknown | 2 | a3 | IIA |
| Case 17 | 45 | + | Pre | + | + | 0 | Unknown | 2 | a1 | IIA |
| Case 18 | 48 | + | Pre | + | + | 1 + | Unknown | 2 | a3 | IIA |
| Case 19 | 59 | + | Post | − | − | 1 + | Unknown | 3 | a3 | IIA |
| Case 20 | 57 | + | Post | + | + | 0 | Unknown | 2 | a3 | IIA |
| Case 21 | 81 | + | Post | − | − | 2 + | − | 2 | a3 | IV |
Twenty-one breast cancer patients were included. ER, PgR, and HER2 in cancer tissue were detected by immunohistochemical analysis. In the case for HER2 2 +, fluorescence in situ hybridization was performed. Nuclear grade (NG) and tissue type were performed by pathologic examination.
Three-Dimensional Culture of Resected Tissues
Breast cancer tissues and normal mammary tissues were obtained by surgery or biopsy from 21 patients. The tumor size in all of the patients was more than 3 cm at their diagnosis. Each tissue was immersed in a tissue carry solution, Hanks’ Balanced Salt Solution (Sigma-Aldrich, USA), including penicillin, streptomycin, and amphotericin B. The tissues were washed by the solution more than three times to prevent bacterial infection. After removal of blood and fat, the tissues were cut into pieces to sizes less than 0.5 mm3. Two to three pieces of the tissue were seeded in a well of DSeA-3D Plate (IFTL Inc., JAPAN) filled with TGP (IFTL Inc.). After incubating in 5% CO2 for 30 minutes at 37°C, 325 μl/well of RPMI1640 including 16% FBS (SAFC Biosciences, Inc., USA) was added, and the plate was warmed to 37°C. Then, 25 μl/well of PBS (GIBCO Inc., USA) was added, and the plate was incubated in 5% CO2 at 37°C for 6 days. After the incubation, 250 μl/well of the supernatant was collected and stored at − 80°C. Under the same condition, a blank well that included no tissue was prepared. The supernatant after 6 days of incubation was collected in the same manner. All of these experiments were carried out axenically.
WST-8 Assay for Three-Dimensionally Cultured Tissue
A total of 25 μl/well Cell Counting Kit-8 reagent (DOJINDO Inc., Japan) was added to each well of DSeA-3D plate after incubation of each tissue for 6 days at 37°C under 5% CO2. After incubation in 5% CO2 at 37°C for 2 hours, the absorbance at 450 nm in each well was measured using the microplate reader SAFIRE (TECAN Inc., JAPAN). The data subtracted from the value of blank well from each sample well were calculated.
Th1/Th2/Th17 Cytokine Measurements
The supernatant of three-dimensionally cultured breast cancer tissue and normal mammary tissue as prepared above was diluted four times with Wash Buffer (BD Inc., USA). The Wash Buffer was filtrated by 0.2μm membrane before use. Then, the concentrations of seven cytokines, IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL-10, and IL-17A, in the supernatant were measured using BD Cytometric Beads Array (CBA) Human Th1/Th2/Th17 Cytokine Kit (BD Inc., USA), followed by flow cytometry. The detection limits for these cytokines were 2.6, 4.9, 2.4, 4.5, 3.8, 3.7, and 18.9 pg/ml for IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, and IL-17A, respectively. The concentration of each cytokine less than the detection limits was recognized as 0 pg/ml. These procedures followed the manufacturer’s instruction, and the data were analyzed with BD CBA software.
Statistical Analysis
Statistical significances for the difference of the WST-8 assay values and the concentrations of cytokines in the culture supernatant between the three-dimensionally cultured breast cancer tissues and the normal mammary tissues were analyzed using Wilcoxon signed rank test. The χ2 test was performed for analyzing differences in the frequencies of IL-6 and IL-10 expression between normal and cancer tissue cultures. The Mann-Whitney U test was used to compare the cytokine concentrations between patient subgroups that underwent chemotherapy and those without chemotherapy. The correlations between the WST-8 assay values of cancer or normal mammary tissues and the concentrations of each cytokine in the supernatant were analyzed by the Spearman or Pearson correlation coefficient. Statistical significances for the differences of the WST-8 assay values and the concentrations of seven cytokines between patients with ER positive (ER +) and those with ER negative (ER −) cancer tissues were analyzed by Mann-Whitney U test. χ2 tests were performed for analyzing differences in the frequencies of patients with cancer tissues exhibiting relatively higher WST-8 assay values or concentrations of seven cytokines produced into culture medium between ER + and ER − patients. Statistical significance for the differences of the WST-8 assay values or the concentrations of seven cytokines between patients with PgR positive (PgR +) and those with negative (PgR −) tissues was analyzed by unpaired t test with Welch correction. χ2 tests were performed for analyzing differences in the frequencies of patients with cancer tissues exhibiting relatively higher WST-8 assay values or concentrations of seven cytokines produced into culture medium between PgR + and PgR − patients. Mann-Whitney U test was used to compare concentrations of each cytokine in culture medium between patients with stage IIA and those with stages later than IIB. χ2 tests were used to analyze differences in the frequencies of patients with cancer tissues exhibiting relatively higher concentrations of seven cytokines in culture medium between patients with stage IIA and those with stages later than IIB. P values less than 0.05 were estimated to be statistically significant. All statistical analyses were carried out by GraphPad PRISM 4.0 (GraphPrism Software Inc., USA).
Results
Growth Ability of Three-Dimensionally Cultured Breast Cancer Tissues and Normal Mammary Tissues
Growth abilities of three-dimensionally cultured breast cancer tissues (n = 21) and normal mammary tissues (n = 9) were analyzed by WST assay. Whereas cancer tissues tended to show higher growth potential than normal tissues, there was no significant difference (P = 0.3125) (Figure 1A). Growth abilities of breast cancer tissues resected from patients under chemotherapy (n = 6) seemed to be higher than those from the patients without chemotherapy (n = 15), but there was no significant difference (P = 0.1291) (Figure 1B).
Figure 1.
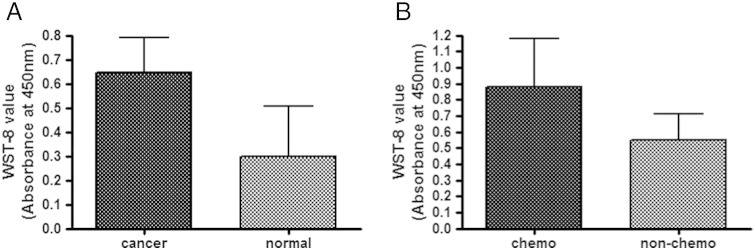
Growth abilities of three-dimensionally cultured breast cancer tissues. The growth abilities of the tissues three-dimensionally cultured in TGP gel were assessed by WST-8 assay (absorbance at 450 nm; see Materials and Methods) after the culture. (A) Comparison of the growth abilities between breast cancer tissues (n = 21) and normal breast tissues (n = 9). No significant difference was observed as analyzed by Wilcoxon signed rank test (P = 0.3125). (B) Comparison between cancer tissues from patients treated by chemotherapy (n = 6) and those from patients who were not treated by chemotherapy (n = 15). No significant difference was observed as analyzed by Mann-Whitney test (P = 0.1291).
Characterization of Th1/Th2/Th17 Cytokines in Supernatant of Three-Dimensionally Cultured Tissues
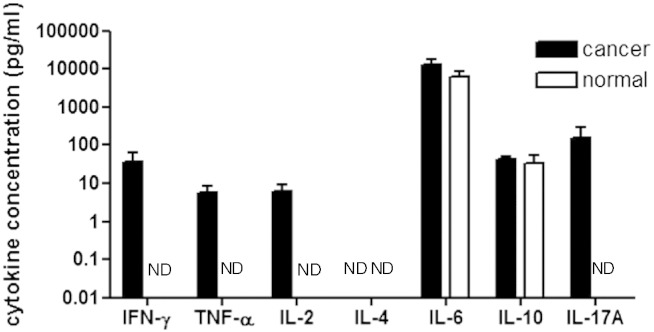
We measured the concentrations of IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL-10, and IL-17A secreted in the supernatant of three-dimensionally cultured breast cancer tissues (n = 21) and normal mammary tissues (n = 8). Both of the cancer and the normal mammary tissues secreted IL-6 and IL-10 in the supernatant (Figure 2). However, IFN-γ, TNF-α, IL-2, and IL-17A were only detected in the supernatant of cancer tissues (Figure 2). IL-4 was not detected in each culture supernatant (Figure 2). There were no significant differences in IL-6 and IL-10 concentrations between cancer and normal tissues. The frequency for the excretion of IFN-γ into the culture supernatant of breast cancer tissues was statistically higher than that of the normal mammary tissues (P = 0.0052) (Table 2). Thus, IFN-γ is considered to be specific to cancer tissues.
Figure 2.
Comparison of cytokine concentrations in supernatant of three-dimensionally cultured breast cancer and normal tissues. Breast cancer tissues (cancer tissues) or normal tissues of breast cancer patients (normal tissues) were cultured for 6 days in TGP gel, respectively, and the cytokine concentrations in supernatant were determined by CBA assay procedures. Twenty-one cancer tissues and eight normal tissues were examined. The mean ± SD concentrations were indicated. The cytokine concentrations did not significantly differ between cancer tissues and normal tissues. The frequencies of the expression of IFN-γ were significantly different between the cancer tissues and the normal tissues as analyzed by χ2 test (P = 0.0052). ND (not detected): The cytokine concentrations were under each detection limit.
Table 2.
Differences for the Incidence of Each Cytokine Expression between Breast Cancer Tissues and Normal Tissues
| Cytokine Cutoff Value (pg/mL) | Expression | Cancer | Normal | Total | P Value |
|---|---|---|---|---|---|
| IFN-γ (3.7) | + | 12⁎ | 0 | 12 | ⁎P = 0.0052 |
| − | 9 | 8 | 17 | ||
| TNF-α (3.8) | + | 6 | 0 | 6 | P = 0.0896 |
| − | 15 | 8 | 23 | ||
| IL-2 (2.6) | + | 7 | 0 | 7 | P = 0.0608 |
| − | 14 | 8 | 22 | ||
| IL-4 (4.9) | + | 0 | 0 | 0 | Not analyzed |
| − | 21 | 8 | 29 | ||
| IL-6 (2.4) | + | 19 | 6 | 25 | P = 0.2800 |
| − | 2 | 2 | 4 | ||
| IL-10 (4.5) | + | 16 | 4 | 20 | P = 0.1730 |
| − | 5 | 4 | 9 | ||
| IL-17A (18.9) | + | 2 | 0 | 2 | P = 0.3657 |
| − | 19 | 8 | 27 |
The resected tissue samples were grouped into those expressing and not expressing each cytokine, and the difference for the incidence of cytokine production between cancer and normal tissues was analyzed. The cutoff points of cytokine expressions were the detection limit of each cytokine concentration shown in the table. Statistical analysis was performed by χ2 test. The incidence for the expression of IFN-γ was significantly higher in cancer tissues (⁎P = 0.0052).
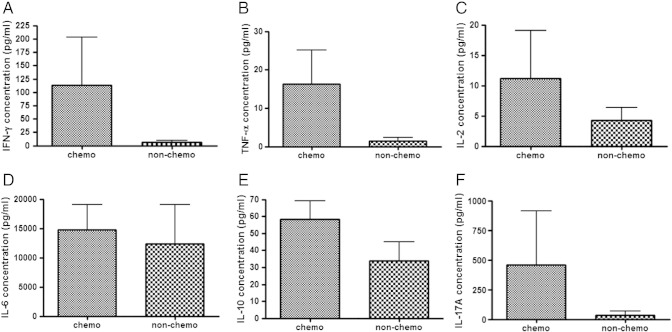
The concentrations of IFN-γ and IL-10 in the supernatant of cancer tissues obtained from the patients who underwent chemotherapy (n = 6) tended to be higher than those in the supernatant of cancer tissues obtained from the patients without chemotherapy (n = 15), although the differences were not statistically significant (Figure 3). There was no difference in IFN-γ, TNF-α, IL-2, IL-6, IL-10, and IL-17A concentrations between these patient subgroups (Figure 3).
Figure 3.
Comparison of each cytokine concentration in supernatant of three-dimensionally cultured tissues obtained from patients with and without chemotherapy. IFN-γ, TNF-α, IL-2, IL-6, IL-10, and IL-17A in the supernatant of three-dimensionally cultured tissues obtained from patients with (n = 6) or without (n = 15) chemotherapy were compared. There was no difference in IFN-γ (A; P = 0.0662), TNF-α (B; P = 0.1612), IL-2 (C; P = 0.4254), IL-6 (D; P = 0.1106), IL-10 (E; P = 0.0674), and IL-17A (F; P = 0.7086) concentrations between these patient subgroups, as analyzed by Mann-Whitney U test.
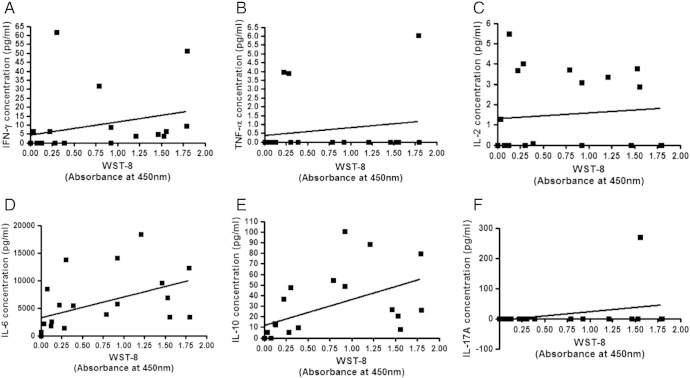
The growth abilities of cancer tissues as assessed by WST-8 assay significantly correlated positively with concentrations of IFN-γ (A; P = 0.0031 by Pearson), IL-6 (D; P = 0.0274 by Pearson and P = 0.0009 by Spearman), and IL-10 (E; P = 0.0158 by Pearson and P = 0.0003 by Spearman), whereas those of TNF-α (B), IL-2 (C), and IL-17A (F) did not (Figure 4). The growth abilities of normal mammary tissues were significantly correlated positively with concentrations of IL-10 (P = 0.0458) (Figure 5B); however, there was no significant correlation between normal tissue growth and IL-6 concentration (Figure 5A).
Figure 4.
Correlation between cytokine concentrations in supernatant of three-dimensionally cultured breast cancer tissues and WST-8 assay values of the cultured tissues. The growth abilities of the tissues three-dimensionally cultured in TGP gel were assessed by WST-8 assay (absorbance at 450 nm) after the culture. Correlations were evaluated by Pearson correlation coefficient test and Spearman correlation coefficient test (n = 21). Concentrations for IFN-γ (A; P = 0.0031 by Pearson), IL-6 (D; P = 0.0274 by Pearson and P = 0.0009 by Spearman), and IL-10 (E; P = 0.0158 by Pearson and P = 0.0003 by Spearman) in the culture supernatant were significantly correlated with the growth abilities of the tissues as evaluated by the WST-8 assay, whereas those of TNF-α (B), IL-2 (C), and IL-17A (F) were not.
Figure 5.
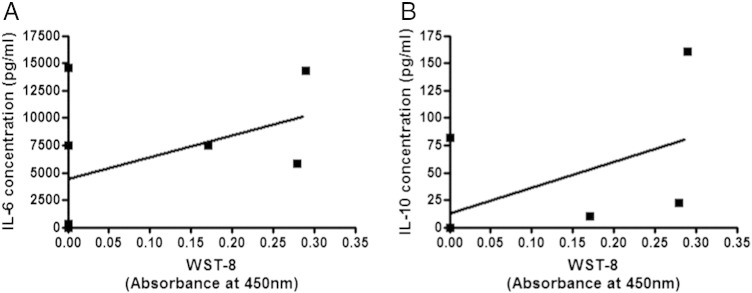
Correlation between cytokine concentrations in supernatant of three-dimensionally cultured normal tissues and the WST-8 assay values of cultured tissues. Correlation was evaluated by Pearson correlation coefficient test and Spearman correlation coefficient test (n = 8). Concentrations for IL-10 (B) in the culture supernatant was significantly correlated with the WST-8 assay values of the tissues (P = 0.0458 by Spearman); however, there was no significant correlation between normal tissue growth and IL-6 concentration (A).
Relationship between Growth Abilities or Cytokine Amounts in Cultured Tissues and Female Hormone Receptor Expression
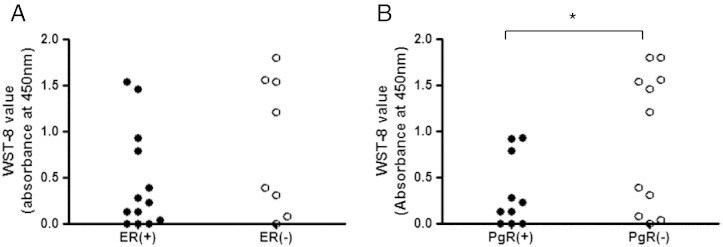
We investigated differences of the growth abilities of cancer tissues as assessed by WST-8 assay or seven cytokine concentrations in supernatant of three-dimensionally cultured cancer tissues, between ER + and ER −, PgR + and PgR −, or HER2 + and HER2 − tissues, respectively. There were no significant differences in IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL-10, and IL-17A concentrations between the ER + and ER −, PgR + and PgR −, or HER2 + and HER2 − tissues (n = 21) (data not shown). On the other hand, there was a significant difference in the WST-8 assay values of breast cancer tissues between the PgR + and the PgR − patient subgroups (P = 0.0383) (Figure 6B), whereas the WST-8 assay values were not significantly different between the ER + and the ER − patient subgroups (P = 0.1282) (Figure 6A). Then, 21 breast cancer tissues were grouped into the high– or low–growth ability subgroup by the median of the WST-8 values (0.3026 read by absorbance at 450 nm), and the frequencies for the hormone receptor expression in cancer tissues were compared. The ER − tissues showed significantly higher incidence of the high-WST-8 value than the ER + tissues by χ2 test (P = 0.0306), whereas there was no significant difference in the incidence of the high-WST-8 value between the PgR + and the PgR − tissues (P = 0.0502) (Table 3).
Figure 6.
Comparison for the WST-8 assay values of three-dimensionally cultured tissues between hormone receptor–positive and –negative breast cancer tissues. (A) Comparison between ER(+) tissues (n = 13) and ER(−) tissues (n = 8). (B) Comparison between PgR(+) tissues (n = 10) and PgR(−) tissues (n = 11). A significant difference for the WST-8 indices was observed between PgR(+) and PgR(−) tissues as analyzed by unpaired t test with Welch correction (P = 0.0383).
Table 3.
Relationship between ER or PgR Expression Incidence and the WST-8 Assay Value (Index for Tumor Growth) of Three-Dimensionally Cultured Breast Cancer Tissues
| WST-8 Assay Value for 3D Cultured Sample |
||||
|---|---|---|---|---|
| Higher | Lower | Total | ||
| ER | + | 5 | 8 | 13 |
| − | 6⁎ | 2 | 8 | |
| Total | 11 | 10 | 21 | |
| PgR | + | 3 | 7 | 10 |
| − | 8 | 3 | 11 | |
| Total | 11 | 10 | 21 | |
The patients were grouped into the higher and lower subjects expressing higher and lower WST-8 assay values, respectively, by the median score (0.3026 read by absorbance at 450 nm) for the results of WST-8 assay of 3D cultured sample. On the other hand, the patients were divided into the ER positive (+) and ER negative (−) or PgR positive (+) and PgR negative (−) groups. ER and PgR of patients’ cancer tissue were detected by immunohistochemical analysis. Statistical analysis for the incidence of subjects with cancer tissues exhibiting the high WST-8 values in vitro was performed by χ2 test. The ER(−) tissues exhibited a significantly high incidence of the greater WST-8 assay-values (⁎P = 0.0306).
Relationship between Stages of Cancer and Growth Activities or Cytokine Concentrations in Supernatant in Three-Dimensionally Cultured Cancer Tissues
All patients were divided into the IIA-stage group (n = 14) and the IIB-or-later-stage group (n = 6), and the WST-8 assay values of the cultured tissues or the concentrations of IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL-10, and IL-17A in supernatant were compared. There were no significant differences in the cytokine concentrations and the WST-8 values between the two stage groups (data not shown). We also compared the incidence of each cytokine secretion between the stages. Eight out of 21 cancer tissues secreted TNF-α into the culture supernatant. The cancer tissues obtained from the patients with stages IIB or later showed significantly high incidence of TNF-α secretion as compared with the tissues obtained from the stage IIA patients (P = 0.0096) (Table 4).
Table 4.
Relationship between the TNM Stages and the Incidences of Cytokine Production in Culture Medium of Three-Dimensionally Cultured Breast Cancer Tissues
| The Incidence of Cytokine in Cultured Cancer Tissue | TNM Stage |
P Value | |||
|---|---|---|---|---|---|
| Stage IIA | Over Stage IIB | Total | |||
| IFN-γ | Positive | 7 | 4 | 11 | P > 0.05 |
| Negative | 7 | 2 | 9 | ||
| TNF-α | Positive | 3 | 5⁎ | 8 | ⁎P = 0.0096 |
| Negative | 11 | 1 | 12 | ||
| IL-2 | Positive | 4 | 3 | 7 | P > 0.05 |
| Negative | 10 | 3 | 13 | ||
| IL-4 | Positive | 0 | 0 | 0 | Not analyzed |
| Negative | 14 | 6 | 20 | ||
| IL-6 | Positive | 5 | 5 | 10 | P = 0.051 |
| Negative | 9 | 1 | 10 | ||
| IL-10 | Positive | 10 | 5 | 15 | P > 0.05 |
| Negative | 4 | 1 | 5 | ||
| IL-17A | Positive | 1 | 1 | 2 | P > 0.05 |
| Negative | 13 | 5 | 18 | ||
| Total | 14 | 6 | 20 | ||
The patients were divided into positive and negative groups for the cytokine production in culture medium of three-dimensionally cultured breast cancer tissues. The cutoff limits of cytokine expressions were the median of each cytokine concentration (all median was 0 pg/ml).
On the other hand, the patients were divided into the stage-IIA and over-stage-IIB groups. Then, the statistical analysis was performed by χ2 test for the incidence of cytokine production from their three-dimensionally cultured cancer tissues into culture medium. The patients with stages over IIB of TNM stage exhibited significantly higher incidence for the TNF-α production in their three-dimensionally cultured tissues.
P = .0096.
Discussion
We showed in this study that tumor tissues, as well as normal mammary tissues, resected from breast cancer patients are able to grow in a three-dimensional culture system using TGP and produce several kinds of cytokines into the culture medium.
Cancer tissues tended to grow more than normal mammary tissues, but there was no significant difference. There are generally significant growth differences found between normal and cancer cells. In the present study, we used TGP culture system, in which cells other than cancer cells such as fibroblasts can hardly grow. Furthermore, we used clinically resected tissue samples obtained from cancer patients, which is another difference from the culture system of the previous researchers using cell lines. Thus, the growth of clinically resected tissues in TGP gel is suggested to be relatively slow as compared with the growth of cells derived from cancer cell lines in other three-dimensional culture system. It may also be considered that the small sample size for normal tissues in the present study influenced the statistical analysis power. These points in combination are suggested to be implicated in the controversial observation between our present data and those of other researchers.
Chemotherapy did not influence significantly the in vitro growth of cancer tissues after surgical resection in this three-dimensional culture system. The production of IFN-γ, TNF-α, IL-2, IL-10, and IL-17 from the three-dimensionally cultured tissues into medium appeared to be greater in cancer tissues obtained from the patients under chemotherapy than those from the patients without chemotherapy, but the differences were not statistically significant. In the study “Breast Invasive Carcinoma” in the public domain The Cancer Genome Atlas, the patients administered adjuvant postoperative pharmaceutical therapy indicator express higher IFN-γ mRNA than those without the therapy [16]. Hormone positive cancer tissues showed significantly less ability to grow in this culture system. IFN-γ, TNF-α, and IL-2 are likely to be specific to cancer tissue, and TNF-α is suggested to be a biomarker for the stage of breast cancer.
The three-dimensionally cultured breast cancer tissues secreted markedly high amounts of IL-6 and IL-10. IL-6 has been suggested to have multifaceted action to breast cancer cells in vitro. There is another report that suggests that IL-6 is a growth factor of three-dimensionally cultured ER(+) breast cancer cells in vitro and in vivo [17]. It has been reported that there was no significant difference in the distribution of the IL-6 572G/C polymorphisms between lung cancer patients and healthy controls [18]. From these observations of other researchers, some of DNA encoding IL-6 and IL-10 might be expressed in both cancer and normal tissues.
Cancer tissue consists of various phenotypes, and some of them are cancer stem cells, which have oncogenicity and accelerate tumor progression [19]. Cancer stem cells have the ability to grow as sphere, called mammosphere. IL-6 has been reported to control the balance of reversible change between cancer stem cells and noncancer stem cells [20]. Cancer stem cells are known to produce a larger amount of IL-6 as compared with noncancer stem cells [20]. Breast cancer is suggested to be one of the stem cell diseases [21], and the growth of cancer depends on the growth of cancer stem cells and the ability of self-renewal [22–24]. It has been reported that, in human carcinoma or mammosphere derived from normal mammary gland, IL-6 induces malignant progression [25]. In our study, we observed high concentrations of IL-6 in the supernatant of three-dimensionally cultured normal mammary tissues as well as those of breast cancer tissues, suggesting that malignant progression is induced in normal mammary tissue.
In the present study, IL-10 was detected almost equally in cancer and normal tissues. It may be explained that IL-10 secreted from cancer tissue plays a role as the growth activator, whereas IL-10 secreted from normal mammary gland may act as the growth inhibitor. Recent findings suggest that regulatory B (Breg) cells inhibit autoimmune diseases [26]. Breg cells have been reported to produce IL-10 [27]. Breg cells are known to be induced from B cells of primary breast cancer patients [28]. Hence, it may be possible to consider that the three-dimensionally cultured breast cancer tissues contain Breg cells and those cells produce IL-10. From this point of view, further study is needed to define from where each cytokine was produced.
In this study, IL-17A was also detected with high concentration in the supernatant of three-dimensionally cultured breast cancer tissues, whereas it was not detected in normal tissue. IL-17 is produced from various T-cell subsets such as CD4+ T cells, CD8+ T cells, NKT cells, and γδT cells [29–32]. Cancer tissues are suggested to express IL-17 [33,34], and IL-17 promotes cancer angiogenesis resulting in cancer advance [35].
Th2 cytokines produced in cancer microenvironment are suggested to play a role in inhibition of cellular immunity against cancer [36]. Th1 cytokines were lower, whereas Th2 cytokines were higher, in gastric cancer patients who have lymph node metastasis [37]. In the present study, we observed that the supernatants of breast cancer tissues contain high amounts of Th2 cytokines such as IL-6 and IL-10 rather than Th1 cytokines such as IFN-γ and TNF-α. These results are consistent with other observations described above. IL-6 and IL-10 were detected in the supernatant of both cancer and normal tissues, whereas others were not detected in normal tissues.
The production of IFN-γ, TNF-α, IL-2, IL-6, IL-10, and IL-17A in cancer patients treated by chemotherapy tended to increase compared with the patients without chemotherapy (Figure 3). Especially, there was a big difference between absolute IL-17A concentrations. There was a report describing that IL-17–mediated paracrine network promotes resistance of cancer tissues to antiangiogenic therapy [38]. IL-17 is also known to promote tumor resistance to VEGF inhibitory therapy [39]. In general, inflammation is initiated by chemotherapeutic drug. High concentrations of IL-1β and IL-6 were observed in patients with poor response speed performance and were perceived cognitive disturbances [40]. From the viewpoint stated above, it is considered that the breast cancer tissues from the patients with chemotherapy may secrete several cytokines, which make the tumor to be resistant to chemotherapy.
It is known that IL-6 promotes the growth of breast cancer cells [41], and thus, it can be considered that the IL-6 secreted from breast cancer tissue promotes through an autocrine mechanism. Whereas IL-10 inhibits Th1 immunity and it may conversely promote proliferation of cancer potentially, it also inhibits cancer growth indirectly through antiangiogenic mechanism [42]. IL-10 is known as not only immunosuppressive but also inducible of inflammation, and it augments cytotoxic activity and growth of CD8+ T cells [43]. In this study, concentrations of IFN-γ, IL-6, and IL-10 in the supernatant of three-dimensionally cultured breast cancer tissues correlated positively with the growth of the tissues, as analyzed by WST-8 assay. Serum concentrations of IFN-γ, IL-2, IL-6, and granulocyte-macrophage colony-stimulating factor have been reported to increase, whereas serum concentrations of IL-1 and TNF-α decrease, in cancer patients treated by docetaxel or paclitaxel [44]. In our study, only 3 out of 21 patients examined were treated by taxane before extirpation of cancer tissue sample, and thus, the effect of taxane on serum cytokine concentration has not been clarified.
The role of IL-17 in cancer starts from the initial stages of tumorigenesis having already been established as having a role in the earliest formation of a tumor by its increased presence within the tumor microenvironment [45]. It has been demonstrated that IL-17–producing Th17 and IL-17–producing CD8+ T (Tc17) cells were induced from naive CD4+ and CD8+ T cells in the presence of TGF-β and IL-6, which are highly produced in the tumor microenvironment [46,47]. In our result, it is possible to consider that IL-17–producing cells such as Tc17 cells induced by Th1 cytokines secreted by surrounding cancer tissues produce IL-17.
Finally, we demonstrated that the WST-8 assay values of three-dimensionally cultured human breast cancer tissues have relationships with cytokine concentrations in supernatant, stages of patients, and clinical data. We observed that the ER − cancer tissues exhibited significantly high incidence of greater growth ability in the absence of ER than that of ER + tissues (Table 3). In the study “Breast Invasive Carcinoma” in The Cancer Genome Atlas, TNFAIP3 gene, which strongly inhibits NFκB signaling, and ESR1 gene encoding ER have significant tendency towards co-occurrence (P = 0.027, log odds ratio: 0.931) [16]. It means that NFκB signaling is inhibited by TNFAIP3 expression in breast cancer patients expressing ESR1. Growth of ER + breast tumor is well known to be promoted by estradiol, and therefore the patients with ER + breast tumor are sensitive to endocrine therapy such as antiestrogen, aromatase inhibitor, or LH-RH analog. The expression of PgR has been reported to influence the therapeutic effect of tamoxifen significantly [48]. In a retrospective cohort study dealing with more than 14,000 breast cancer patients, the survival rate in ER +/PgR − or ER −/PgR − patients decreased as compared with that in ER +/PgR + patients [49]. The tumor-proliferative indices, WST-8 assay values, of hormone receptor–negative breast cancer tissues were significantly higher than those of hormone receptor–positive tissues; the proliferation of positive one is higher than negative one (Table 3). These observations suggest that the hormone receptor–negative breast cancer tissues can progressively grow in our culture system, which may be related to their malignancy. On the other hand, the ER-positive cancer tissues could not extensively grow without estrogen in the present TGP culture conditions.
In our result, the higher the stage of cancer was, the more the cancer tissues frequently secrete TNF-α into supernatant of three-dimensional culture (Table 4). TNF-α serum concentration was significantly higher in colorectal cancer patients than in controls. The highest TNF-α level was found in stage IV patients and was significantly elevated as compared with patients in earlier stages of colorectal cancer and controls. The survival rate of colorectal patients with low TNF-α serum concentration was significantly higher than that of patients with high levels of TNF-α [50]. It is also reported that plasma TNF-α concentrations were significantly elevated in benign and malignant breast masses than healthy controls and were also higher in malignant masses than in benign breast masses [51]. Thus, our results that the tissues of advanced breast cancer frequently secrete TNF-α did not conflict with the observations of these reports.
Our present study, in conclusion, showed that the three-dimensionally cultured human breast cancer tissues using TGP secreted relatively high amounts of Th2 cytokines such as IL-6 and IL-10 into the culture medium. These cytokines possibly suppress tumor immunity related to Th1 cell immunity and promote the proliferation of cancer. IFN-γ, TNF-α, IL-2, and IL-17A were not detected in the supernatant of the three-dimensionally cultured normal mammary gland and seemed to be specific to breast cancer tissues. Hormone-positive cancer tissues showed less ability to grow in this culture system. TNF-α is suggested to be a biomarker for the stage of breast cancer.
Conflict of Interest
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgements
We thank Yukinori Hara, Minami Sagae, and Keita Misago, Department of Clinical Pharmacology, Tokyo University of Pharmacy and Life Sciences, for their technical assistance.
Contributor Information
Anna Kiyomi, Email: y064076@toyaku.ac.jp.
Masujiro Makita, Email: m-makita@nms.ac.jp.
Tomoko Ozeki, Email: y094055@toyaku.ac.jp.
Na Li, Email: y094227@toyaku.ac.jp.
Aiko Satomura, Email: aiko_satomura@yahoo.co.jp.
Sachiko Tanaka, Email: sachiko@toyaku.ac.jp.
Kenji Onda, Email: knjond@toyaku.ac.jp.
Kentaro Sugiyama, Email: sugiyama@toyaku.ac.jp.
Takuji Iwase, Email: takuji.iwase@jfcr.or.jp.
Toshihiko Hirano, Email: hiranot@toyaku.ac.jp.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Wilson J, Balkwill F. The role of cytokines in the epithelial cancer microenvironment. Semin Cancer Biol. 2002;12:113–120. doi: 10.1006/scbi.2001.0419. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 4.Venetsanakos E, Beckman I, Bradley J, Skinner JM. High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. Br J Cancer. 1997;75:1826–1830. doi: 10.1038/bjc.1997.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura K, Modlin R, Wollman J, Dubinett SM. Human non–small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995;55:3847–3853. [PubMed] [Google Scholar]
- 6.Green AR, Green VL, White MC, Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer. 1997;72:937–941. doi: 10.1002/(sici)1097-0215(19970917)72:6<937::aid-ijc3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, Dirix LY. Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer. 2002;2:311–315. doi: 10.3816/cbc.2002.n.008. [DOI] [PubMed] [Google Scholar]
- 8.Kozłowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- 9.Knüpfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer. Review. Breast Cancer Res Treat. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 10.Moscona A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- 11.O’Keane JC, Kupchik HZ, Schroy PC, Andry CD, Collins E, O’Brien MJ. A three-dimensional system for long-term culture of human colorectal adenomas. Am J Pathol. 1990;137:1539–1547. [PMC free article] [PubMed] [Google Scholar]
- 12.Carlsson J, Nilsson K, Westarmark B, Ponten J, Sundstrom C, Larsson E, Bergh J, Pahlman S, Busch C, Collins VP. Formation and growth of multicellular spheroids of human origin. Int J Cancer. 1983;31:523–553. doi: 10.1002/ijc.2910310502. [DOI] [PubMed] [Google Scholar]
- 13.Kleinman HK, McGarvey ML, Hassel JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 14.Lawler E, Miller FR, Heppner GH. Significance of three-dimensional growth patterns of mammary tissues in collagen gels. In Vitro. 1983;19:600–610. doi: 10.1007/BF02619573. [DOI] [PubMed] [Google Scholar]
- 15.Tsukikawa S, Matsuoka H, Kurahashi Y, Konno Y, Satoh K, Satoh R, Isogai A, Kimura K, Watanabe Y, Nakano S. A new method to prepare multicellular spheroids in cancer cell lines using a thermo-reversible gelation polymer. Artif Organs. 2003;27:598–604. doi: 10.1046/j.1525-1594.2003.07131.x. [DOI] [PubMed] [Google Scholar]
- 16.The Cancer Genome Atlas. http://www.cbioportal.org/index.do
- 17.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;13:3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YM, Mao YM, Sun YX. Genetic polymorphisms of IL-6 and IL-10 genes correlate with lung cancer in never-smoking Han population in China. Int J Clin Exp Med. 2015;8(1):1051–1058. [PMC free article] [PubMed] [Google Scholar]
- 19.Filipova A, Seifrtova M, Mokry J, Dvorak J, Rezacova M, Filip S, Diaz-Garcia D. Breast cancer and cancer stem cells: a mini-review. Tumori. 2014;100(4):363–369. doi: 10.1700/1636.17886. [DOI] [PubMed] [Google Scholar]
- 20.Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A. 2011;108:1397–1402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dontu G, Liu S, Wicha MS. Stem cells in mammary development and carcinogenesis: implications for prevention and treatment. Stem Cell Rev. 2005;1:207–213. doi: 10.1385/SCR:1:3:207. [DOI] [PubMed] [Google Scholar]
- 22.Fillmore C, Kuperwasser C. Human breast cancer stem cell markers CD44 and CD24: enriching for cells with functional properties in mice or in man? Breast Cancer Res. 2007;9:303. doi: 10.1186/bcr1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 24.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R, Jr., Badve S, Nakshatri H. CD44 +/CD24 − breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;12:3660–3663. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita T. Regulatory B cell and autoimmune disease. Jpn J Clin Immunol. 2010;33:234–241. doi: 10.2177/jsci.33.234. [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Kim AR, Nam ST, Kim HW, Park YH, Lee D, Lee MB, Park YM, Kim HS, Kim YM. Autocrine stimulation of IL-10 is critical to the enrichment of IL-10-producing CD40hiCD5 + regulatory B cells in vitro and in vivo. BMB Rep. 2015;48(1):54–59. doi: 10.5483/BMBRep.2015.48.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17–producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 30.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17–producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tajima M, Wakita D, Noguchi D, Chamoto K, Yue Z, Fugo K, Ishigame H, Iwakura Y, Kitamura H, Nishimura T. IL-6–dependent spontaneous proliferation is required for the induction of colitogenic IL-17–producing CD81T cells. J Exp Med. 2008;205:1019–1027. doi: 10.1084/jem.20071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 33.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK. IL-17 enhances the net angiogenic activity and in vivo growth of human non–small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 34.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 35.Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, Kitamura H, Nishimura T. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40:1927–1937. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- 36.Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell. 2008;13:7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 37.Cao Z, Xu X, Luo X, Li L, Huang B, Li X, Tao D, Hu J, Gong J. Role of RANTES and its receptor in gastric cancer metastasis. J Huazhong Univ Sci Technolog Med Sci. 2011;31:342–347. doi: 10.1007/s11596-011-0378-3. [DOI] [PubMed] [Google Scholar]
- 38.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV. An interleukin-17–mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19(9):1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 39.Sakata H, Murakami S, Hirayama R. Serum soluble interleukin-2 receptor (IL-2R) and immunohistochemical staining of IL-2R/Tac antigen in colorectal cancer. Int J Clin Oncol. 2002;7(5):312–317. doi: 10.1007/s101470200046. [DOI] [PubMed] [Google Scholar]
- 40.Cheung YT, Ng T, Shwe M, Ho HK, Foo KM, Cham MT, Lee JA, Fan G, Tan YP, Yong WS. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol. 2015;26(7):1446–1451. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2014;15(7):848–862. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howell WM, Rose-Zerilli MJ. Interleukin-10 polymorphisms, cancer susceptibility and prognosis. Fam Cancer. 2006;5:143–149. doi: 10.1007/s10689-005-0072-3. [DOI] [PubMed] [Google Scholar]
- 43.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8(+) T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26(5):623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183(7):4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 46.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 47.Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, Carter RD, Rivkin SE, Borst JR, Belt RJ. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective southwest oncology group study. J Clin Oncol. 1992;10:1284–1291. doi: 10.1200/JCO.1992.10.8.1284. [DOI] [PubMed] [Google Scholar]
- 49.Bardou V, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 50.Stanilov N, Miteva L, Dobreva Z, Stanilova S. Colorectal cancer severity and survival in correlation with tumor necrosis factor-alpha. Biotechnol Biotechnol Equip. 2014;28(5):911–917. doi: 10.1080/13102818.2014.965047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamed EA, Zakhary MM, Maximous DW. Apoptosis, angiogenesis, inflammation, and oxidative stress: basic interactions in patients with early and metastatic breast cancer. J Cancer Res Clin Oncol. 2012;138(6):999–1009. doi: 10.1007/s00432-012-1176-4. [DOI] [PubMed] [Google Scholar]