Abstract
Objective
To investigate whether the immunohistochemical expression of p53, p63 and her2/neu is correlated with the prognosis of tumour recurrence and progression in patients with non-muscle invasive (NMI) bladder cancer.
Patients and methods
In all, 88 patients diagnosed with NMI transitional cell carcinoma of the bladder in a Urology Department from May 2009 to April 2014 were included in the study. Paraffin-embedded specimens were obtained by transurethral resection of the bladder tumours. Sections on haematoxylin and eosin-stained slides were examined histologically and tumour grade was classified according to the World Health Organisation system (2004) Mostofi classification. The sections were evaluated using p63, p53 and her2/neu immunohistochemical staining before and after immunotherapy with bacille Calmette–Guerin (BCG), and patients were followed up for 36 months in the Urology Department.
Results
For tumour grade there was a significant relationship with the overexpression of p53 (P = 0.010), her2 (P = 0.025) and negativity of p63 (P = 0.025). There was no significant relationship between p53 or her2/neu overexpression and tumour stage. However, there was a significant correlation (P = 0.005) between p63 negativity and tumour stage. There was a significant relationship between p53 (P = 0.01), her2/neu (P = 0.025) overexpression and p63 negativity (P = 0.005) and tumour recurrence and progression.
Conclusion
Patients with transitional cell carcinoma who are selected for BCG treatment should preferably be positively immunoreactive for p63, but negative for both p53 and her2/neu. These patients were less susceptible to recurrence and/or progression after BCG adjuvant therapy. Further studies are needed to investigate the relationship between these three markers and treatment with anti-her2/neu therapies.
Abbreviations: NMI, non-muscle invasive; TURBT, transurethral resection of the bladder tumour; H&E, haematoxylin and eosin; Cis, carcinoma in situ
Keywords: Bladder cancer; P53, P63, Her2; BCG
Introduction
The management of non-muscle-invasive (NMI) TCC of the urinary bladder is a major problem for clinicians in deciding whether transurethral resection of the bladder tumour (TURBT) alone is sufficient, or if adjuvant immunotherapy e.g., with BCG, is useful. The choice of further intravesical adjuvant therapy depends on the patient’s risk of recurrence and progression. European and American urological guidelines consider the intravesical instillation of BCG after TURBT to be the first-line treatment for stage T1, grade 3 (T1G3) bladder cancer [1,2]. Cormio et al. [3], in a study of 153 patients with T1G3 disease, stated that there was no recurrence or progression in a third of patients, deferred cystectomy was required in a third and the remaining third eventually died from the disease.
It was reported that p53 nuclear accumulation was found in higher tumour stages and grades [4,5]. However, the prognostic value of p53 for NMI bladder tumours treated with BCG remains controversial [6–9]. P63 is a member of the p53 family located on chromosome 3q27–28 [3,4]. It plays a key role in regulating epithelial differentiation and proliferation, rather than tumour suppression [10]. Advanced stages of human bladder carcinoma are associated with alterations and loss of p63 expression [11–13], but their clinical significance requires investigation. Human epidermal growth factor receptor-2 (her2), contributes to the physiological mechanisms of cell proliferation by intrinsic tyrosine kinase activity. An assessment of the her2 status is crucial for both the prognosis and prediction of the response to targeted therapies for managing breast cancer. In urothelial bladder carcinoma, immunohistochemical her2 expression varies among different studies, at 9–81% [14–18]. In the present study we explored the role of immunohistochemical p53, p63 and her2/neu expression in selecting patients with NMI bladder cancer for BCG treatment.
Patients and methods
This study was carried out in the Urology, Pathology and Oncology Departments of the Faculty of Medicine, Zagazig University, Egypt. During the period from May 2009 to April 2014, 88 patients with NMI bladder cancer (76 men and 12 women; mean age 60.5 years, range 45–76) were included. The patients’ symptoms included haematuria, burning on voiding and storage symptoms (LUTS). Each patient had a history taken and a physical examination, and urine analysis, ultrasonography and CT were done before TURBT. We selected patients who had NMI bladder cancer with or without carcinoma in situ (Cis) after a pathological examination. Tumours were staged and graded according to the TNM and Mostofi classifications [19,20]. Patients were followed up in the Urology Department. Urine cytology was carried out before TURBT and the resected tumours were examined histologically. BCG was instilled for six consecutive weeks (90 mg of Connaught Immucyst1 strain Egyptian Co. for Production Of Vaccines, Sera and Drugs, one of the affiliated companies of VACSERA) at 2–4 weeks after the last TURBT. At 3–4 weeks after the last BCG instillation, urine cytology and diagnostic cystoscopy were performed. Randomised cold biopsies were taken from the tumour site, adjacent to the tumour, bladder dome, trigone, the opposite bladder wall, and the prostatic urethra. Patients with negative urine cytology and biopsies received a maintenance therapy of BCG instillation (3-weekly instillations, given at 3, 6, 12, 18, 24, 30 and 36 months). Patients were followed up every 3 months for 2 years, then twice yearly for another year, in the absence of recurrence or progression by urine cytology and diagnostic cystoscopy. The assessment of the response depended on the results of histopathology and urine cytology, and was considered a complete response if they were negative. A positive biopsy was considered to be tumour recurrence regardless of any stage progression. Tumour stage progression, muscle involvement or metastasis was considered to be tumour progression, requiring a change in treatment regimen or a radical cystectomy [21].
Immunohistochemistry
After TURBT specimens were immediately fixed in 10% neutral buffered formalin for up to 12 h, then dehydrated in ascending grades of alcohol and embedded in paraffin. Paraffin sections were cut, deparaffinised, hydrated in descending grades of alcohol, stained with haematoxylin and eosin (H&E), examined by light microscopy, and classified according to the TNM and Mostofi classifications. Patients with NMI bladder cancer (Ta, T1) were selected for the immunohistochemical staining and BCG instillation. Paraffin-embedded sections were immunostained for p53, p63 and her2, essentially as described previously [22], and scored for nuclear p53, p63 and her2 membranous immunoreactivity in tumour cells by manually counting the number of stained cells. Staining of >20% tumour cells was considered overexpression, according to Lacombe et al. [23], leading to the categorisation of patients as p53, p63 or her2-negative or -positive [22].
The variables analysed statistically were age, sex, tumour stage, grade, multifocality, tumour size, associated Cis, recurrence, progression and death, in relation to p53, p63, and her2/neu immunoreactivity.
Results
The 88 patients had NMI bladder tumours, either associated with Cis or not, and with a tumour size of 24–30 mm. Radiological investigations showed a bladder mass in 52 patients by ultrasonography and in 88 by CT.
There was no significant relationship between p53 or her2/neu overexpression and tumour stage (Fig. 1A), but there was a significant correlation (P = 0.005) between p63 overexpression and tumour stage (Fig. 1B). For tumour grading there was a significant relationship with p53 (P = 0.010), p63 (P = 0.025) and her2 (P = 0.025) overexpression. There was no significant correlation between p53, p63 or her2 overexpression and cytological examination before BCG, multifocal tumours, and association with Cis or previous tumour before BCG (Table 1). There was a significant correlation between tumour recurrence and progression and p53 (P = 0.01), p63 (P = 0.005) and her2/neu (P = 0.025) overexpression (Fig. 2A and B). Fifteen patients had a recurrence before 6 months and 12 after 6 months; only 12 had progression, three of whom died later (Table 2).
Fig. 1.
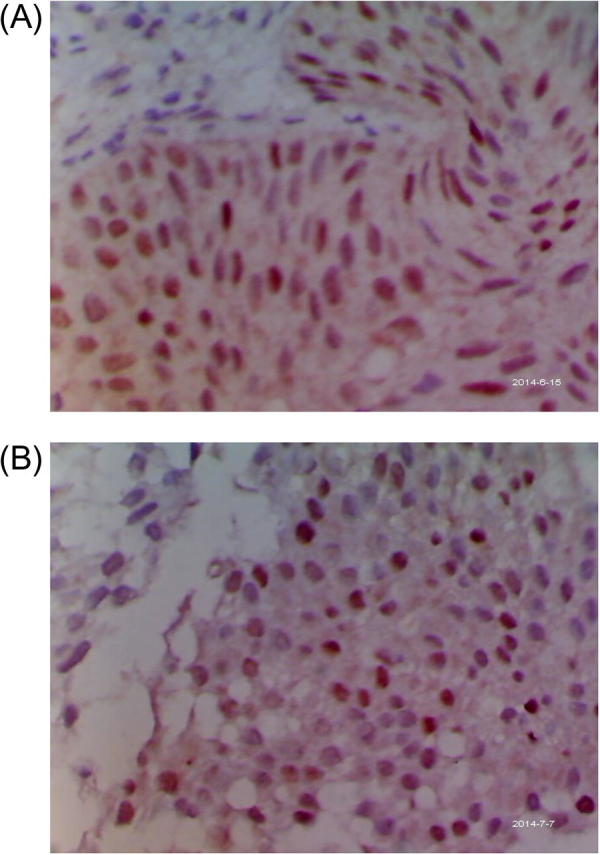
(A) A case of T1G2, showing a strong nuclear reaction to p53; ×400. (B): A case of T1G2, showing a weak nuclear reaction to p63; ×400.
Table 1.
Clinical patient data in relation to the immunohistochemical profile of p53, p63, and her2.
| Variable (n) | p53 |
p63 |
Her2/neu |
|||
|---|---|---|---|---|---|---|
| +/– | P | +/– | P | +/– | P | |
| Tumour stage | ||||||
| High risk (44) | 24/20 | NS | 12/32 | 0.005 | 26/18 | 0.010 |
| (Ta/T1 G3, Cis) | ||||||
| Intermediate risk (44) | 20/24 | 22/22 | 24/20 | |||
| (Ta/T1 G1/G2) | ||||||
| Grade (n) | ||||||
| G1/G2 (64) | 35/29 | 0.010 | 42/22 | 0.025 | 30/34 | 0.025 |
| G3 (24) | 20/4 | 8/16 | 18/6 | |||
| Cytology before BCG | ||||||
| Positive (45) | 10/35 | NS | 12/33 | NS | 23/22 | NS |
| Suspicious (23) | –/– | –/– | –/– | |||
| Negative (20) | –/– | –/– | –/– | |||
| Multifocal tumours | ||||||
| Yes (56) | 30/26 | NS | 23/33 | NS | 37/19 | NS |
| No (32) | 15/17 | 14/18 | 12/20 | |||
| Associated Cis | ||||||
| Yes (46) | 38/8 | NS | 11/35 | NS | 30/16 | NS |
| No (42) | 22/20 | 19/23 | 20/22 | |||
| Recurrence | ||||||
| <6 months (15) | 12/3 | <0.01 | 3/12 | <0.025 | 10/5 | <0.05 |
| >6 months (12) | 8/4 | 8/4 | 3/9 | |||
| Progression | ||||||
| Yes (12) | 6/6 | <0.01 | 3/9 | 0.005 | 7/5 | 0.025 |
| No (76) | 26/50 | 28/48 | 28/48 | |||
| Death | ||||||
| Yes (3) | 2/1 | 0.010 | 0/3 | 0.010 | 2/1 | 0.005 |
| No (85) | 52/33 | 51/34 | 29/56 | |||
Fig. 2.
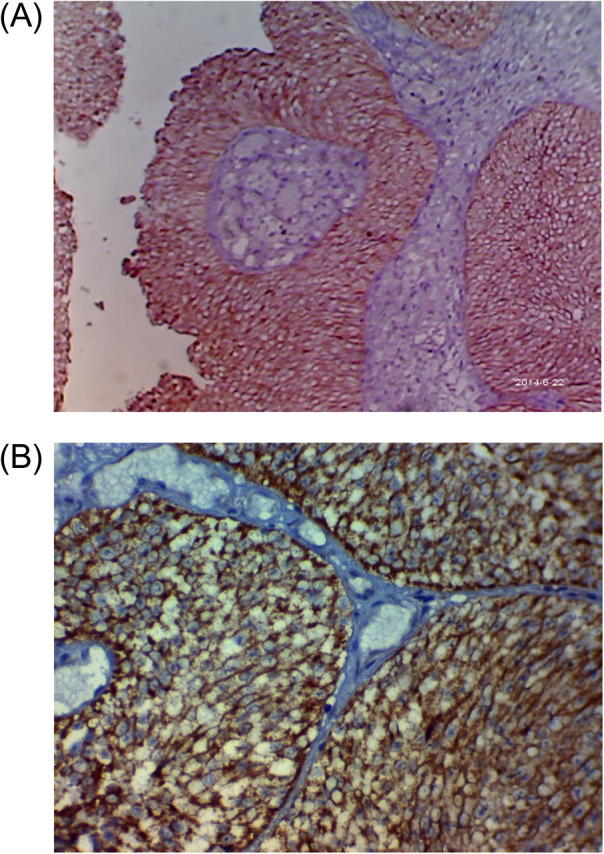
(A) A case of T1G2, showing a strong membranous reaction to her2/neu; ×100. (B): A case of T1G3, showing a strong membranous reaction to her2/neu; ×200.
Table 2.
Patients with progression; stage, grade and immunohistochemical profile for p53, p63 and her2.
| Patient | Stage before BCG | p53 | P63 | Her2 | Progression | Death (month) |
|---|---|---|---|---|---|---|
| 1 | T1 G3 | +ve | −ve | +ve | T2G3 | 30 |
| 2 | Ta G3 | +ve | +ve | +ve | T1G2 | – |
| 3 | Ta G3 | −ve | +ve | +ve | T2G2 | – |
| 4 | T1 G3 | +ve | +ve | +ve | T2G3 | – |
| 5 | Ta G2 | +ve | −ve | −ve | T1G3? | 32 |
| 6 | T1 G2 | −ve | −ve | −ve | T3G2 | – |
| 7 | T1 G3 | −ve | −ve | +ve | T3G3 | – |
| 8 | T1 G2 | −ve | −ve | −ve | T2G3 | – |
| 9 | T1 G2 | +ve | −ve | −ve | T3G2 | – |
| 10 | T1 G2 | −ve | −ve | −ve | T3G3 | – |
| 11 | Ta G3 | +ve | −ve | +ve | T2G3 | – |
| 12 | T1 G1 | −ve | −ve | +ve | T3G3 | 20 |
Discussion
We investigated the immunohistochemical profile of p53, p63 and her2/neu in patients with NMI urinary bladder TCC. Wild-type p53 helps to limit tumour growth by preventing the neovacularisation induced by the overproduction of endogenous vascular endothelial growth factor and basic fibroblast growth factor [24]. Mutated p53 loses this important regulatory function and thus there is uninhibited neovascularisation, allowing tumour development and progression to continue. Adjuvant intravesical BCG is frequently given to patients with recurrent or high-grade NMI bladder cancer (with or without Cis) [22]. The prognostic value of nuclear p53 immunoreactivity before and after BCG therapy has been assessed in several studies. Lacombe et al. [23] reported a correlation between pre-treatment p53 overexpression and disease progression after BCG therapy, but others found no such correlation [6–9]. Nevertheless, using a yeast functional assay, in patients with mutated p53, the treatment with BCG failed [25]. The p53 overexpression in patients whose tumours failed to respond to BCG therapy was correlated with disease progression and poor survival [6,22,23,26]. To date, whether p53 tumour status is an independent predictive factor for the response to BCG remains in debate. For p63 expression many studies [11,13,27] showed nuclear immunoreactivity in non-neoplastic urothelium, except for the umbrella cells. This correlates p63 physiological function with the morphogenesis and differentiation of transitional epithelia [10,27,28]. Morgan et al. [29] stated that BCG adjuvant therapy for NMI urinary bladder tumours reduced the incidence of her2/neu expression. For tumour staging, the present results showed an enhanced immunohistochemical reactivity with higher stage, which was significant for p63 (P = 0.005), but insignificant for p53 and her2/neu. Others [30] reported an insignificant relationship with her2/neu. However, Charfia et al. [31] showed a significant relationship between p53, p63 and her2/neu and tumour staging, because in that study they assessed both NMI and MI bladder cancer. For tumour grading in the present study, there was a significant relationship with the overexpression of p53, p63 and her2/neu, and these results were in line with others [30,31]. From our results the three markers were important prognostically, and showed some integration, i.e., if one marker failed the other took its place. From the cytological analysis before biopsy, followed by the immunocytochemical assay, 10/35, 12/33 and 23/22 samples were positive for p53, p63 and her2/neu, respectively, which is relatively low, possibly because of the low sensitivity of the cytological assessment. However, in multifocal tumour, and in association with areas of Cis, there was an increase in the immunoreactivity of p53 and her2/neu associated with a decrease in p63 expression. Moreover, 15 patients had recurrences before 6 months, 12 of whom had progression and three of whom eventually died. These patients had enhanced immunoreactivity for p53 and her2/neu but attenuated immunoreactivity for p63. Notably, patients who will benefit from BCG should be negative for p53 and her2/neu, and/or positive for p63.
In conclusion, patients with TCC who are selected for BCG treatment should preferably be positively immunoreactive for p63 but negative for both p53 and her2/neu. These patients are likely to be less susceptible to recurrence and/or progression after BCG adjuvant therapy. Further studies are recommended to investigate the relationship between these three markers and treatment with anti-her2/neu therapies.
Conflict of interest
None.
Source of funding
None.
UROSCIENCE
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Oosterlinck W., Lobel B., Jakse G., Malmstrom P.-U., Stockle M., Sternberg C. European Association of Urology; Arnhem: 2003. Guidelines on Bladder Cancer. [DOI] [PubMed] [Google Scholar]
- 2.Hall M.C., Chang S.S., Dalbagni G., Seigne J.D., Skinner E.C. The Bladder Cancer Clinical Guideline Update Panel. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis) J Urol. 2007;2007(178):2314–2330. doi: 10.1016/j.juro.2007.09.003. Update. [DOI] [PubMed] [Google Scholar]
- 3.Cormio L., Tolve I., Annese P., Saracino A., Zamparese R., Sanguedolce F. Altered p53 and pRb expression is predictive of response to BCG treatment in T1G3 bladder cancer. Anticancer Res. 2009;29:4201–4204. [PubMed] [Google Scholar]
- 4.Nakopoulou L., Vourlakou C., Zervas A., Tzonou A., Gakiopoulou H., Dimopoulos M.A. The prevalence of bcl-2, 53, and Ki-67 immunoreactivity in transitional cell bladder carcinoma and their clinicopathologic correlates. Hum Pathol. 1998;29:146–154. doi: 10.1016/s0046-8177(98)90225-8. [DOI] [PubMed] [Google Scholar]
- 5.Popov Z., Hoznek A., Colombel M., Bastuji-Garin S., Lefrere-Belda M.A., Bellot J. The prognostic value of p53 nuclear overexpression and MIB-1 as a proliferative marker in transitional cell carcinoma of the bladder. Cancer. 1997;80:1472–1481. [PubMed] [Google Scholar]
- 6.Ovesen H., Horn T., Steven K. Long-term efficacy of intravesical Bacillus Calmette–Guerin for carcinoma in situ: relationship of progression to histological response and p53 nuclear accumulation. J Urol. 1997;157:1655–1659. [PubMed] [Google Scholar]
- 7.Lebret T., Becette V., Barbagelatta M., Herve J.M., Gaudez F., Barre P. Correlation between p53 over expression and response to Bacillus Calmette–Guerin therapy in a high risk select population of patients with T1G3 bladder cancer. J Urol. 1998;159:788–791. [PubMed] [Google Scholar]
- 8.Zlotta A.R., Noel J.C., Fayt I., Drowart A., Van Vooren J.P., Huygen K. Correlation and prognostic significance of p53, 21WAF1/CIP1and Ki-67 expressions in patients with non muscle invasive bladder tumors treated with Bacillus Calmette-Guerin intravesical therapy. J Urol. 1999;161:792–798. [PubMed] [Google Scholar]
- 9.Pages F., Flam T.A., Vieillefond A., Abeille X., Lazar V. P53 status does not predict initial clinical response to Bacillus Calmette–Guerin intravesical therapy in T1 bladder tumors. J Urol. 1998;159:1079–1084. [PubMed] [Google Scholar]
- 10.Yang A., Schweitzer R., Sun D., Kaghad M., Walker N., Bronson R.T. P63 is essential for regenerative proliferation in limb, craniofacial, and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 11.Urist M.J., Di Como C.J., Lu M.L., Charytonowicz E., Verbel D., Crum C.P. Loss of p63 expression is associated with tumor progression in bladder cancer. Am J Pathol. 2002;161:1199–1206. doi: 10.1016/S0002-9440(10)64396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park B.J., Lee S.J., Kim J.I., Lee S.J., Lee C.H., Chang S.G. Frequent alteration of p63 expression in human primary bladder carcinomas. Cancer Res. 2000;60:3370–3374. [PubMed] [Google Scholar]
- 13.Koga F., Kawakami S., Kumagai J., Takizawa T., Ando N., Arai G. Impaired DeltaNp63 expression associates with reduced beta-catenin. An aggressive phenotype of urothelial neoplasms. Br J Cancer. 2003;88:740–747. doi: 10.1038/sj.bjc.6600764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsubara H., Yamada Y., Naruse K., Nakamura K., Aoki S., Taki T. Potential for HER-2/neu molecular targeted therapy for invasive bladder carcinoma comparative study of immunohistochemistry and fluorescent in situ hybridization. Oncol Rep. 2008;19:57–63. [PubMed] [Google Scholar]
- 15.Naruse K., Yamada Y., Nakamura K., Aoki S., Taki T., Zennami K. Potential of molecular targeted therapy of HER-2 and Cox-2 for invasive transitional cell carcinoma of the urinary bladder. Oncology Rep. 2010;23:1577–1583. doi: 10.3892/or_00000798. [DOI] [PubMed] [Google Scholar]
- 16.Olsson H., Fyhr I.M., Hultman P., Jahnson S. HER2 status in primary stage T1 urothelial cell carcinoma of the urinary bladder. Scand J Urol Nephrol. 2012;46:102–107. doi: 10.3109/00365599.2011.637955. [DOI] [PubMed] [Google Scholar]
- 17.Simonetti S., Russo R., Ciancia G., Altieri V., De Rosa G., Insabato L. Role of polysomy 17 in transitional cell carcinoma of the bladder: immunohistochemical study of HER2/neu expression and fish analysis of c-erbB-2 gene and chromosome 17. Int J Surg Pathol. 2009;17:198–205. doi: 10.1177/1066896909333415. [DOI] [PubMed] [Google Scholar]
- 18.Skagias L., Politi E., Karameris A., Sambaziotis D., Archondakis A., Vasou O. Prognostic impact of HER2/neu protein in urothelial bladder cancer. Survival analysis of 80 cases and an overview of almost 20 years’ research. J BUON. 2009;14:457–462. [PubMed] [Google Scholar]
- 19.Eble J.N., Sauter G., Epstein J.I., Sesterhenn I.A. IARC; Lyon: 2004. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. [Google Scholar]
- 20.Sobin L.H., Gospodarowicz M.K., Wittekind C. 7th ed. Wiley; Weinheim: 2009. TNM Classification of Malignant Tumours. [Google Scholar]
- 21.Herr H.W., Laudone V.P., Badalament R.A., Oettgen H.F., Pramod C.S., Freedman B.D. Bacillus Calmette–Guerin therapy alters the progression of non muscle invasive bladder cancer. J Clin Oncol. 1988;6:1450–1455. doi: 10.1200/JCO.1988.6.9.1450. [DOI] [PubMed] [Google Scholar]
- 22.Saint F., Le Frere Belda M., Quintela R., Hoznek A., Patard J.J., Bellot J. Retreatment p53 nuclear overexpression as a prognostic marker in superficial bladder cancer treated with bacillus Calmette–Guérin (BCG) Eur Urol. 2004;45:475–482. doi: 10.1016/j.eururo.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Lacombe L., Dalbagni G., Zhang Z.F., Cordon-Cardo C., Fair W.R., Her H.W. Overexpression of p53 protein in a high-risk population of patients with non muscle invasive bladder cancer before and after Bacillus Calmette–Guerin therapy: correlation to clinical outcome. J Clin Oncol. 1996;14:2646–2652. doi: 10.1200/JCO.1996.14.10.2646. [DOI] [PubMed] [Google Scholar]
- 24.Nishizaki M., Fujiwara T., Tanida T., Hizuta A., Nishimori H., Tokino T. Recombinant adenovirus expressing wild-type p53 is antiangiogenic: a proposed mechanisms for bystander effect. Clin Cancer Res. 1999;5:1015–1023. [PubMed] [Google Scholar]
- 25.Pfister C., Flaman J.M., Dunet F., Grise P., Frebourg T. P53 mutations in bladder tumors inactivate the transactivation of the P21 and BAX genes, and have a predictive value for the clinical outcome after Bacillus Calmette–Guerin. J Urol. 1999;162:69–73. doi: 10.1097/00005392-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Ick K., Schultz M., Stout P., Fan K. Significance of p53 overexpression in urinary bladder transitional cell carcinoma in situ before and after Bacillus Calmette–Guerin treatment. Urology. 1997;49:541–547. doi: 10.1016/s0090-4295(96)00624-3. [DOI] [PubMed] [Google Scholar]
- 27.Di Como C.J., Urist M.J., Babayan I., Drobnjak M., Hedvat C.V., Teruya-Feldstein J. P63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8:494–501. [PubMed] [Google Scholar]
- 28.Yang A., Kaghad M., Wang Y., Gillett E., Fleming M.D., Dötsch V. P63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 29.Morgan B.E., Salup R., Morgan M.B. Differential C-erbB-2 and VEGF expression following BCG immunotherapy in non muscle invasive papillary transitional cell carcinoma of the bladder. Urol Oncol. 2002;7:67–72. doi: 10.1016/s1078-1439(01)00153-3. [DOI] [PubMed] [Google Scholar]
- 30.Alexai A., Badercai F., Zahoi D., Lighezani R., Izvernariui D., Raicai M. Clinical significance of Her2/neu overexpression in urothelial carcinomas. Romanian J Morph Embryol. 2010;51:277–282. [PubMed] [Google Scholar]
- 31.Charfia S., Khabir A., Mnifa H., Ellouzea S., Mhirib M., Sellamia T. Immunohistochemical expression of HER2 in urothelial bladder carcinoma and its correlation with p53 and p63 expression. J Microsc Ultrastruct. 2013;1:17–21. [Google Scholar]


