Abstract
Aims/hypothesis
The adipose tissue-derived hormone leptin plays an important role in the maintenance of body weight and glucose homeostasis. Leptin mediates its effects by interaction with leptin receptors (LepRb), which are highly expressed in the hypothalamus and other brain centres, and at lower levels in the periphery. Previous studies have used relatively promiscuous or inefficient Cre deleter strains, respectively, to explore the roles of LepR in pancreatic β and α cells. Here, we use two newly-developed Cre lines to explore the role of leptin signalling in insulin and proglucagon-expressing cells.
Methods
Leptin receptor expression was measured in isolated mouse islets and highly-purified islet cells by RNASeq and quantitative RT-PCR. Mice lacking leptin signalling in pancreatic β, or in α and other proglucagon-expressing cells, were generated using Ins1Cre- or iGluCre-mediated recombination respectively of flox'd leptin receptor alleles. In vivo glucose homeostasis, changes in body weight, pancreatic histology and hormone secretion from isolated islets were assessed using standard techniques.
Results
Leptin receptor mRNA levels were at or below the level of detection in wild-type adult mouse isolated islets and purified cells, and leptin signalling to Stat3 phosphorylation was undetectable. Whereas male mice further deleted for leptin receptors in β cells exhibited no abnormalities in glucose tolerance up to 16 weeks of age, females transiently displayed improved glucose tolerance at 8 weeks (11.2 ± 3.2% decrease in area under curve; p < 0.05), and improved (39.0 ± 13.0%, P < 0.05) glucose-stimulated insulin secretion in vitro. No differences were seen between genotypes in body weight, fasting glucose or β/α cell ratio. Deletion of LepR from α-cells, a minority of β cells, and a subset of proglucagon-expressing cells in the brain, exerted no effects on body weight, glucose or insulin tolerance, nor on pancreatic hormone secretion assessed in vivo and in vitro.
Conclusions/interpretation
The use here of a highly selective Cre recombinase indicates that leptin signalling plays a relatively minor, age- and sex-dependent role in the control of β cell function in the mouse. No in vivo role for leptin receptors on α cells, nor in other proglucagon-expressing cells, was detected in this study.
Keywords: Leptin, Insulin, Glucagon, GLP-1, Diabetes, β cell, α cell, L-cell
Abbreviations: AUC, area under the curve; [Ca2+]i, intracellular free Ca2+ concentration; KATP, ATP-sensitive K+ channel; GTT, ITT, glucose and insulin tolerance test, respectively; IP, intraperitoneal; NTS, nucleus tractus solitarius
Highlights
-
•
β cell leptin receptors play a minor role in glucose homeostasis.
-
•
α cell leptin receptors are not involved in responses to hypoglycaemia.
-
•
Body weight maintenance does not require leptin action on GLP-1-secreting cells.
1. Introduction
Type 2 Diabetes mellitus (T2D) currently affects approximately 380 million individuals worldwide [1] and is characterized by elevated blood glucose levels. The treatment of T2D complications, which include cardiovascular disease, retinopathy and peripheral nerve damage, typically consumes 10–20% of the health care budgets of westernized societies [2]. Whilst insulin, secreted from pancreatic islet β cells, acts to lower blood glucose levels, glucagon, secreted by pancreatic α cells, increases glycaemia. Defects in the release or actions of either hormone can thus contribute to the disease [3].
Obesity, which affects ∼1 in 4 adults in the UK (www.hscic.gov.uk/catalogue/PUB10364), is an important risk factor for T2D and promotes both insulin resistance and β cell failure [4]. The adipose tissue-derived hormone leptin is an important satiety factor which acts on the feeding centres in the brain to suppress appetite [5]. Human mutations in either the leptin (obese, or ob) [6,7] or the leptin receptor (LepR) [8] gene lead to severe obesity from an early age. Demonstrating a conserved role for the hormone across mammalian species, mice carrying mutations in the homologous genes (ob/ob [9] or db/db) [10] display severe hyperphagia, obesity and disturbed glucose homeostasis.
In addition to the central actions of leptin, roles for the hormone have also been suggested in the periphery. Thus, LepR expression has been described in the endocrine pancreas [11,12], and several studies [12–18] have reported an action of leptin to inhibit insulin secretion. Furthermore, over-expression of insulin receptors in the islets of LepR-deficient Zucker diabetic fatty (ZDF) rats restores glucose-stimulated insulin secretion (GSIS) [19]. Whether physiological concentrations of leptin (≤1 nM) also inhibit secretion, however, has been contested [20].
Recent work [21] suggests that the actions of leptin on insulin release involve the stimulation of AMPK-activation protein kinase (AMPK) and trafficking of ATP-sensitive K+ (KATP) channels to the plasma membrane. These changes are thus expected to impede the glucose-dependent closure of KATP channels, voltage-gated Ca2+ channel opening and Ca2+ influx that trigger insulin secretion in response to glucose [22].
Using a Cre deleter strain driven by the rat insulin 2 promoter (RIP2) Covey et al. [23] deleted critical flox'd exons in the gene encoding the active, long form of the leptin receptor (LepRb) in vivo in murine pancreatic β cells and in a specific population of neurons in the hypothalamus (RIP2Cre neurons). These animals developed obesity, fasting hyperinsulinaemia, impaired glucose-stimulated insulin release and glucose intolerance. On the other hand, Morioka et al. [24] disrupted the leptin receptor in all pancreatic cells using a Pdx1 promoter-driven Cre and these mice, in contrast, exhibited normal body weight, improved glucose tolerance and elevated plasma insulin. However, when challenged with high fat diet, Pdx1Cre:LepRF/F mice became glucose intolerant.
The difference between these studies is thus likely to be the result of using different Cre lines and hence deletion in partially overlapping, but different, cell types: crucially, in neither case was the leptin receptor deleted exclusively in pancreatic β cells. Thus, the RIP2Cre promoter used by Covey et al. is active in β cells and also in several areas of the brain, particularly in the hypothalamus [25]. By contrast, the Pdx1Cre used by Morioka et al. is expressed in adult β-cells and also deletes in glucagon-secreting pancreatic α cells as well intestinal glucagon-like peptide 1- (GLP-1) secreting L-cells and several other cell types with roles in glucose homeostasis [26]. The Pdx1Cre line also deletes in neuronal populations responsive to leptin [25].
Our first aim here, therefore, was to examine the effects of deleting the long form of the leptin receptor (LepRb) highly selectively in pancreatic β cells using the novel Ins1Cre deleter strain [27,28]. Selectivity with this strain is achieved firstly by the use of the Insulin I promoter, whose expression is essentially confined to pancreatic β cells [29]. By contrast, the Insulin II gene, which drives RIP2Cre promoter expression, is expressed in multiple brain regions [25,30]. Secondly, precise developmental and spatial control of Cre expression by Ins1Cre is enhanced by its targeted insertion (knock-in) at the Ins1 locus. Finally, this line also avoids risks associated with the use of other insulin promoter-driven Cres [31] of Cre-independent actions due to co-expression of growth hormone. The present strategy is expected, therefore, to distinguish between the actions of leptin on glucose homeostasis via the pancreatic β cell and those acting via the brain and elsewhere.
The possibility has also recently been explored that leptin receptors play a role in pancreatic α cells to control glucagon secretion [32]. However, whilst the latter studies failed to identify any defects in glucose homeostasis after LepR deletion using a Cre expressed under the control of a short fragment of the glucagon promoter [33], recombination was only achieved in ∼43% of α cells. Correspondingly, we [34] and others [35,36] have similarly found that the latter Cre deletes in only a minority (13–45%) of α cells. This leaves open the possibility that the effects of deletion described by Tuduri et al. [32] might be masked by changes in the remaining, non-recombined α cell population. To test this hypothesis, our second aim here was to use an alternative glucagon promoter-driven Cre, iGluCre [37], based on a much longer (∼100 kB) region upstream of the proglucagon gene, which drives recombination in the vast majority of α cells. However, Cre is also expressed with this line at other sites of proglucagon expression including the nucleus tractus solitarius (NTS) of the hindbrain [38], and in intestinal L-cells, both of which express leptin receptors [37,39]. Both of these cell types secrete the incretin glucagon-like peptide-1 (GLP-1) and thus impaired leptin receptor signalling, particularly in the neuronal population [40], might affect body weight, glucose homeostasis, or both.
2. Materials and methods
2.1. Generation of mice
Mice bearing LepRF alleles on an FVB background were kindly provided by Dr Streamson Chua (Columbia University) and, after backcrossing twice to C57BL/6 mice, bred to Ins1Cre [28] or iGluCre [37] mice (C57BL6 background) as indicated. Further crosses to Rosa26tdRFP were performed with iGluCreLepRF/F mice to allow labelling of recombined cells. Animals (2–5) were maintained in separately ventilated cages in a specific pathogen-free environment. All procedures were approved by the U.K. Home Office and were compliant with the Animals (Scientific Procedures) Act 1986 of the United Kingdom (PPL 70/7349). Genotyping was performed as described ([41] and see Results). Primer sequences are given in Table 1.
Table 1.
Primer sequences.
| Target | Forward primer sequence 5′-3′ | Reverse primer sequence 5′-3′ |
|---|---|---|
| Tissue control | FER2-Q2: accttcagaccttggcgttggagg | FER2-R2: cctgaggttcctgttgctgtgactcc |
| Ins1Cre | Cre jva rv 2: gccagattacgtatatcctggcag | Cre jva fw 2: ttcactggttatgcggcgg |
| LepRF | 103: tgagttccctcatgattctgg | See below |
| LepRF | 104: cagccgaccaatgcttatt | 105: acaggcttgagaacatgaacac |
| iCre | Cre002: gacaggcaggccttctctgaa | Cre003: cttctccacaccagctgtgga |
| β-catenin | RM41: aaggtagagtgatgaaagttgtt | RM42: caccatgtcctctgtctattc |
| tdRFP | Anti: ctacaggaacaggtggtgg | Sense: ctgttcctggggcatggc |
2.2. Studies of glucose homeostasis and hormone release in vivo
Intraperitoneal glucose (1 g/kg) or insulin (0.75 U/mL) tolerance tests were performed on mice fasted for 16 h or 5 h, respectively, as described [42], using an automated glucometer (Accucheck, Roche, Burgess Hill, U.K.). Plasma insulin and glucagon levels were measured with ELISA kits from Cis Bio (Bagnols-sur-Cèze, France) and Mercodia (Uppsala, Sweden), respectively.
2.3. Assay of insulin and glucagon secretion from isolated islets
Islet isolation by collagenase injection and pancreatic distension was performed as described [43]. Islets were cultured for 24–36 h in RPMI medium supplemented with 10% foetal calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin. Hormone release was measured during 30 or 60 min (insulin or glucagon, respectively) static incubation of batches of six (insulin) or 20 (glucagon) islets in 0.5 mL modified Kreb's Ringer medium comprising (mM): 120 NaCl, 4.8 KCl, 24 NaHCO3, 0.5 Na2HPO4, 5 HEPES, 2.5 CaCl2, 1.2 MgCl2 and 5 d-glucose, pH 7.4, at 37 °C. Secreted and total hormone levels were measured by homogeneous time-resolved fluorescence-based (HTRF) assay (Cisbio).
2.4. Intracellular free Ca2+ imaging and connectivity analysis
Imaging was performed at 34–36 °C essentially as described [44]. Briefly, islets were loaded with Fluo2 (Teflab) and Ca2+ signals captured with a Nipkow spinning disk coupled to a Zeiss Axiovert M200 microscope fitted with x10–x20/0.3–0.5 numerical aperture (NA) objectives (EC Plan-Neofluar, Zeiss). Cells were illuminated at 491 nm using a solid state laser (Cobalt) with emitted light detected from 500 to 550 nm using a 16-bit back-illuminated EM-CCD (Hamamatsu C9100-13). Connectivity analysis was performed as described [44].
2.5. Immunocytochemical analysis
2.5.1. tdRFP and glucagon
Animals were anaesthetised with ketamine and transcardially perfused-fixed with 4% PFA before post-fixation for 1 h and embedding in paraffin and sectioning. Sections were labelled with anti-RFP (Rockland, 600-401-379, 1:50 dilution, secondary-Alexa 568 1:250) and anti-glucagon (1:500 dilution, secondary-Alexa 488 1:250) and anti-glucagon (1:500 dilution, secondary-Alexa 488 1:250) antibodies and sealed using Vectashield hardset mounting medium with DAPI (Vector Laboratories).
2.5.2. Insulin and glucagon
Mouse pancreata were extracted and fixed in 10% (v/v) neutral buffered formalin at 4 °C for 18 h before paraffin embedding and sectioning. Sections were labelled with polyclonal anti-insulin (Dako A4056, 1:200 dilution, Secondary-Alexa 488 1:1,000) or anti-glucagon (Sigma, G2654 or Santa Cruz, sc-13091, 1:200 dilution, Secondary-Alexa 568 1:500) antibodies and sealed using Vectashield hardset mounting medium with DAPI (Vector Laboratories). Fluorophores were excited using a Zeiss Observer Vivatome and a 20x/NA 0.8 x objective and 365 nm, 470-nm and 540–580-nm LEDs. Emission was detected using Semrock filters centred on 377/50 nm, 472/30 nm, and 534/20 nm for DAPI, Alexa 488, and Alexa 568, respectively.
2.6. Islet isolation and FACS sorting
Transgenic mice (males and females, >16 weeks) expressing the fluorescent proteins Venus under the control of the proglucagon promoter [45], or EYFP under the control of the prosomatostatin [46] promoter (>8 week old mice) were euthanized by cervical dislocation and the pancreas injected immediately with 5 ml of collagenase V (0.5 mg/ml) in Ca2+ and Mg2+-free HBSS. Pancreata were digested and the islets hand-picked into HBSS containing 0.1% fatty acid-free bovine serum albumin (BSA) and 11 mM glucose. Islets were disrupted into single cells by trituration following incubation for 1 min in Ca2+-free HBSS containing 0.1x trypsin/EDTA and 0.1% BSA. Cells were immediately sorted by flow cytometry using a BD Influx cell sorter (BD Biosciences, San Jose, CA, USA) equipped with a 488 laser for excitation of Venus or EYFP. To collect alpha and beta cells, Venus-positive and negative cells were collected, with the latter further subdivided into a population that was large (according to side and forward scatter) and with high background autofluorescence at 530 and 580 nm. To collect delta cells, only EYFP positive cells were collected. Cells were collected into RLT lysis buffer (QIAGEN) and frozen on dry ice.
2.7. Massive parallel sequencing (RNASeq)
Details of RNASeq for whole islets, and subsequent data analysis, are provided in [27]. For studies on individual islet cell types, total RNA was extracted using an RNeasy Micro Plus kit (Qiagen, Manchester, UK) according to the manufacturer's instructions. RNA was amplified using Ovation RNA-seq System V2 (PN 7102, NuGEN) whereby 2.5 ng of RNA for each sample was used (3, 2, and 4 replicates were used each for α, β, and δ cells, respectively, totalling 9 samples). To prepare the RNA-seq library, the amplified cDNA (3 μg per sample) was fragmented to 200 bp using the Bioruptor Sonicator (Diagenode) and barcode ligation and end repair were achieved using the Ovation Rapid DR Library System (PN 0319,0320, NuGEN). The barcoded libraries were combined and sent for SE50 sequencing using an Illumina HiSeq 2500 system at the Genomics Core Facility, Cambridge Institute, CRUK.
2.8. Quantitative RT-PCR
Total RNA was extracted using an RNeasy Micro kit (QIAGEN, UK), according to the manufacturer's protocol. Superscript III (Life technologies) reverse transcriptase was used to synthesize cDNA.
Analysis was performed with a 7900 HT Fast Real-Time PCR system (Applied Biosystems). The PCR reaction mix consisted of first-strand cDNA template, primer pairs for LepR, forward primer binding exon junction 16–17 (GTTTCACCAAAGATGCTATCGAC) and reverse primer binding exon 17 (GAGCAGTAGGACACAAGAGG), and PCR Master mix (Applied Biosystems). Expression of selected targets was compared with that of Cyclophilin measured on the same sample in parallel on the same plate.
2.9. Immunocytochemical analysis of Stat3 phosphorylation
Islets were incubated for 30 min in Kreb's Ringer bicarbonate buffer followed by incubation with either leptin (10 nM), IL1-β (50 ng/mL) and TNF-α (1000 pg/mL) or control conditions at 37 °C. Islets were fixed in 4% PFA for 1 h at 4 °C.
Antigen retrieval steps were performed by incubating islets in 0.01 M Tris-HCl for 5 min at 90 °C, before cooling to room temperature and incubation in 100% methanol at −20 °C for 10 min. Free-floating islets were stained immunohistochemically with anti-pSTAT3 (pTyr-705; NEB, 4113S, 1:100 dilution, secondary-Alexa 488 1:500) and anti-insulin (1:200 dilution, secondary-Alexa 488 1:500) antibodies, and mounted onto glass slides, as above. Images were obtained by using a Zeiss LSM-780 microscope and a 60x/NA 0.8 x objective.
2.10. Statistical analysis
Statistical significance was assessed using Student's t-test with Bonferroni correction, or two-way ANOVA, as indicated, using GraphPad Prism 6.0. P-values < 0.05 were considered significant. Values are presented as means ± S.E.M.
3. Results
3.1. LepR mRNA levels are low in isolated mouse islets and in purified islet cells
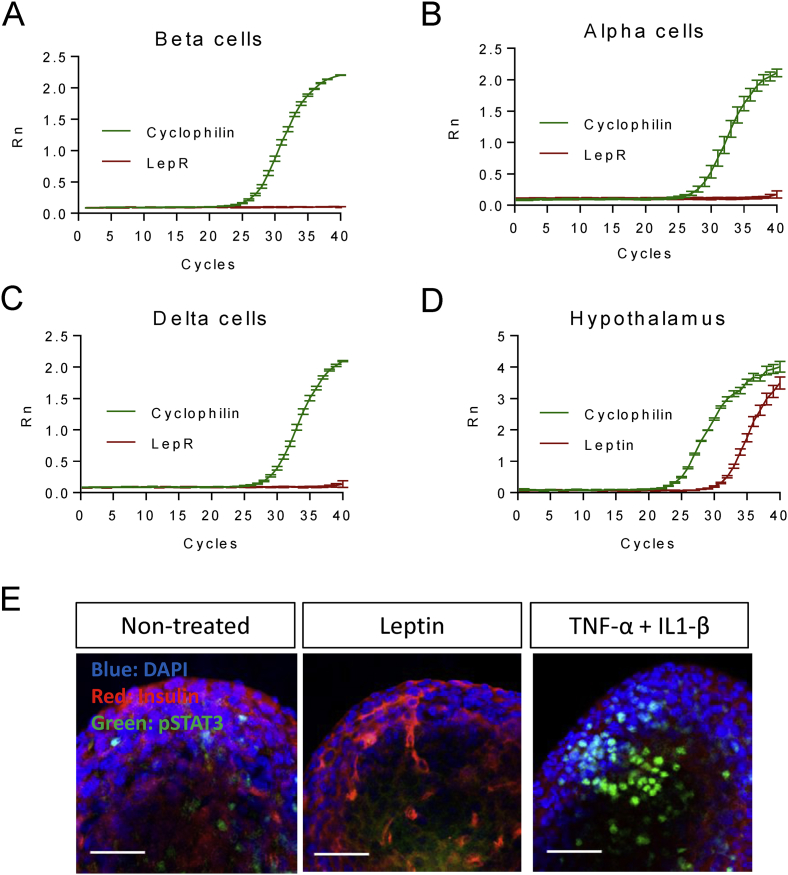
We first assessed expression of leptin receptors in isolated islets and highly purified preparations of islet cells from wild-type mice (Materials and Methods) using RNASeq and quantitative RT-PCR. LepR mRNA was detected at 0.2 RPKM in whole islets [27], at the lower 39th centile of all messages, and 0.23, 0.036 and 0 RPKM in purified β, δ and α and cells, respectively (n = 3, 2 and 4 separate preparations, respectively). Correspondingly, whereas qRT-PCR amplified LepR mRNA from whole islets (not shown), this approach failed to amplify a product from any of the three isolated cell preparations (Figure 1A–C). By contrast, the primers used efficiently amplified LepR mRNA from hypothalamus-derived polyA + mRNA (Figure 1D). Consistent with low levels of LepR protein in islets, staining of islets with fluorescently-labelled Cy3-leptin [12] failed to reveal consistent signals above background (results not shown). To further test the hypothesis that LepR signalling is low or undetectable in pancreatic islet cells, we exposed isolated islets from wild type C57BL6 mice to either leptin, or to a cytokine mixture (IL1-β and TNF-α) as a positive control, and monitored Stat3 phosphorylation on Tyr 705 using immunocytochemistry and laser-scanning confocal imaging. Whereas the cytokine mixture caused a robust increase in Stat3 phosphorylation as anticipated, no effects were observed upon addition of leptin, even at the highest dose (10 nM) tested (Figure 1E).
Figure 1.
Low levels of LepR mRNA and receptor signalling in purified islet cells and whole islets, respectively. Amplification plot of LepR (red) or Ppia (cyclophilin, green) using mRNA purified from mouse islet (A) β (B) α or (C) δ cells or (D) hypothalamus. (E) Immunofluorescence staining of pStat3 in whole islets after treatment with either leptin (10 nM), TNF-α and IL1-β, or control non-treated islets. Data are representative of two further experiments. Scale bar 52.5 μm. See the Materials and Methods section for further details.
The above findings suggest that leptin receptor levels are vanishingly low (β, δ) or zero (α) in key islet cell types and limit the actions of the hormone on this tissue.
3.2. Gender and age-dependent effects of LepR deletion from pancreatic β cells
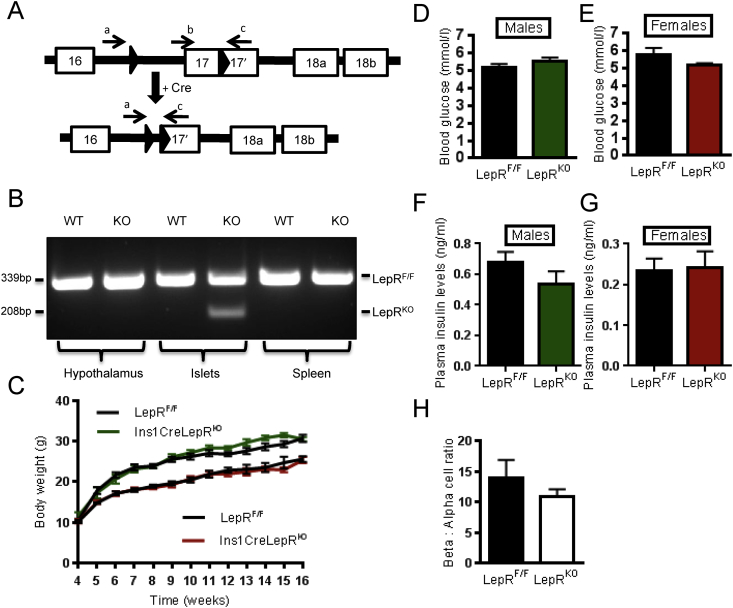
To ablate residual LepRb function selectively from pancreatic β cells, animals bearing alleles in which LoxP sites flank exon 17 of the leptin receptor gene [41] were crossed with animals harbouring the Ins1Cre transgene. Exon 17 includes the BOX1 domain required for leptin-induced JAK-STAT signalling [41]. Based on the use of reporter strains [27], this approach is expected to lead to recombination in the majority (>94%) of β cells, with <2% recombination in other islet cell types [27], in the resulting Ins1CreLepRKO mice. To confirm Cre-mediated excision of exon 17 (LepRbΔ17), genomic DNA was isolated from several tissues and PCR performed with two sets of primers as indicated in Figure 2A [41]. PCR amplification of DNA from islets derived from LepRbF/F or Ins1CreLepRKO animals generated a product of 339 bp and a band of 208 bp where Cre-mediated excision of exon 17 had occurred (Figure 2B). Whilst attempts were made to confirm deletion by measuring full-length and truncated LepRb mRNA, levels these were below the level of reliable quantitation both in LepRbF/F and Ins1CreLepRKO mice, as discussed above, and in line with the absence of a functional effect of leptin on islet pStat3 phosphorylation (Figure 1E).
Figure 2.
Ins1Cre expression results in excision of the flox'd region of LepRb alleles selectively in islets. Genomic DNA was harvested from Ins1CreLepRKO and control LepRF/F animals and used as a template for PCR with the primers indicated (A). Predicted product sizes are 339 bp for the flox'd allele (primers b,c) and 207 bp for the excised allele (primers a,c) (B). Ins1CreLepRKO animals displayed a normal progression in bodyweight. Ins1CreLepRKO males (green line) and LepRF/F (black line) and below Ins1CreLepRKO females (red line) and LepRF/F (black line). (C). Blood glucose concentration after overnight fast in males (D) (Ins1CreLepRKO, n = 8, LepRF/F, n = 7) and females at 8 weeks (E) (Ins1CreLepRKO, n = 13, LepRF/F, n = 9). Plasma insulin levels in male mice (F) Ins1CreLepRKO, n = 10, LepRF/F n = 7, and in females (G) Ins1CreLepRKO, n = 9, LepRF/F, n = 7. β to α cell ratio in Ins1CreLepRKO and wild-type mice (H).
Consistent with the absence of recombination and deletion of LepRb in satiety centres in the hypothalamus, loss of LepRb mediated by Ins1Cre in the β cell exerted no effect on body mass in Ins1CreLepRKO mice (Figure 2C). Neither fasting glucose (Figure 2D,E) nor plasma insulin (Figure 2F,G) was different between wild-type and null mice. Since both the previously-described leptin receptor-deficient mice generated by recombination with RIP2Cre [23] or Pdx1Cre [24] animals display an abnormal β cell area, β to α cell ratio was examined in Ins1CreLepRKO animals. No changes in this parameter were apparent (Figure 2H).
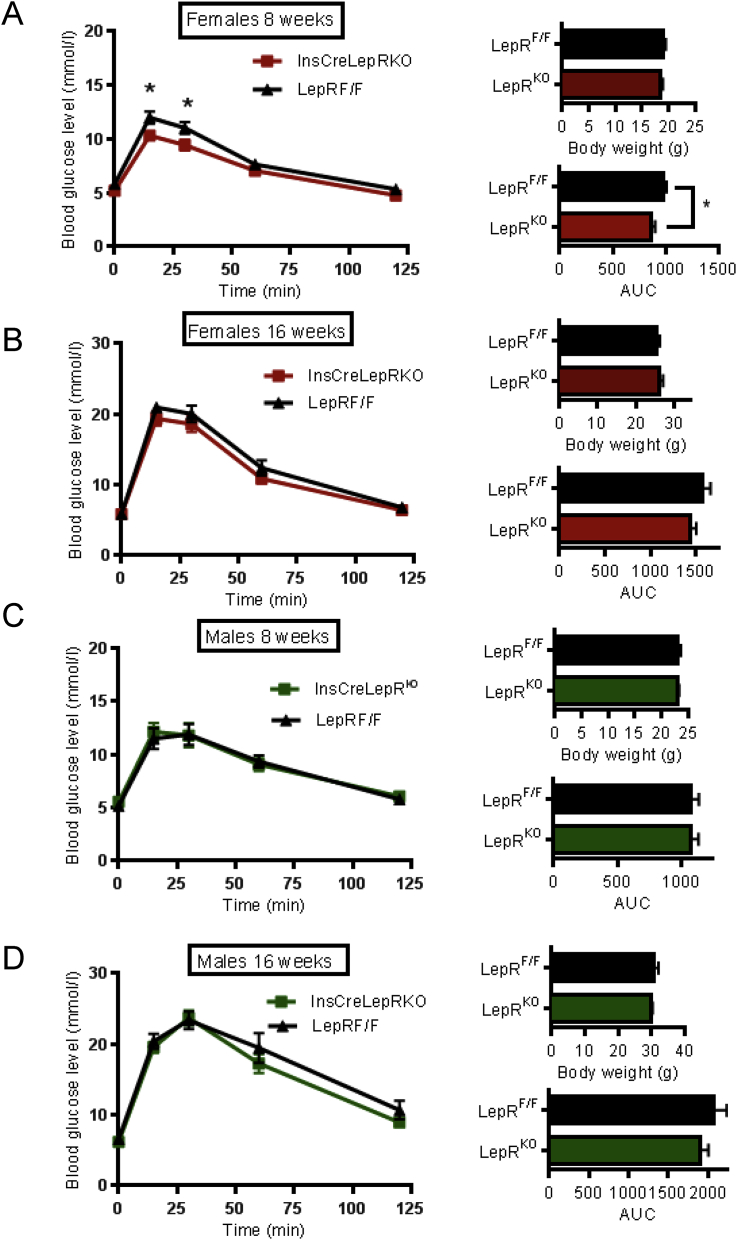
To examine in more detail whether disruption of leptin signalling in β cells affected glucose metabolism, animals aged 8, 12 and 16 weeks were subjected to intraperitoneal glucose tolerance tests (IPGTT). At 8 weeks, female Ins1CreLepRKO null mice showed transiently improved glucose tolerance, which reversed by 16 weeks (Figure 3A,B). No glycaemic phenotype was identified in male Ins1CreLepRKO at any of the ages examined (Figure 3C,D). Ins1CreLepRKO mice also displayed normal insulin sensitivity (Figure 4A).
Figure 3.
Ins1CreLepRKOfemales display improved glucose tolerance at the age of 8 weeks. (A) Blood glucose concentration following IPGTT in Ins1CreLepRKO (square symbol) and LepRF/F littermate controls (triangles) in 8-week old females: Ins1CreLepRKO, n = 13, LepRF/F, n = 9, (B) 16-week old females: Ins1CreLepRKO, n = 15, LepRF/F, n = 17, (C) 8-weeks old males: Ins1CreLepRKO, n = 8, LepRF/F, n = 7, (D) 16-week old males: Ins1CreLepRKO, n = 13, LepRF/F, n = 10. For all panels, the corresponding body weight and area under curve (AUC) are inset. Data are expressed as the average ± SEM. Statistical analysis was performed using two-way ANOVA *P < 0.05.
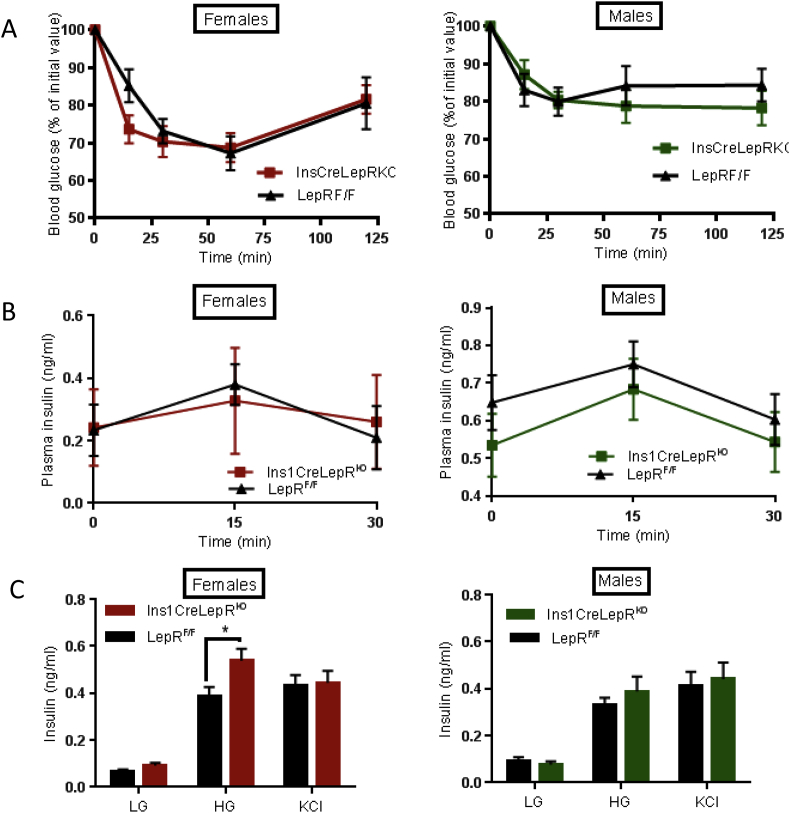
Figure 4.
Enhanced glucose-stimulated insulin secretion in Ins1CreLepRKOfemales. (A) Blood glucose after IP injection of insulin in Ins1CreLepRKO (square symbol) and LepRF/F littermate controls (triangles) in males and females (n = 9–10 mice per genotype). (B) Plasma insulin levels after IP injection of glucose in males and females: Ins1CreLepRKO, n = 10, LepRF/F, n = 6. (C) Insulin secretion as assessed from isolated islet in response to glucose (LG = 3 mM, HG = 17 mM) and KCl (30 mM) in 30-week old female and male mice. n = 3 separate experiments involving 2–3 mice per genotype/experiment. Statistical analysis was performed using two-way ANOVA *P < 0.05.
3.3. Impact of LepRb deletion on β cell glucose signalling and insulin secretion
Given the reported role of leptin to inhibit insulin secretion [23,24], we measured insulin secretion in Ins1CreLepRKO mice in vivo and in vitro. No differences in the excursions in plasma insulin concentration were observed during intraperitoneal glucose tolerance tests between Ins1CreLepRKO mice and littermate controls (Figure 4B). Glucose- (16.7 vs 3 mM) stimulated insulin secretion was significantly potentiated in vitro in LepRb-deleted islets from 30 week old female, but not male Ins1CreLepRKO mice (Figure 4C). These changes, which may reflect the lower variability of measurements performed in isolated islets, are consistent with the observed small improvements in glucose tolerance (Figure 3A).
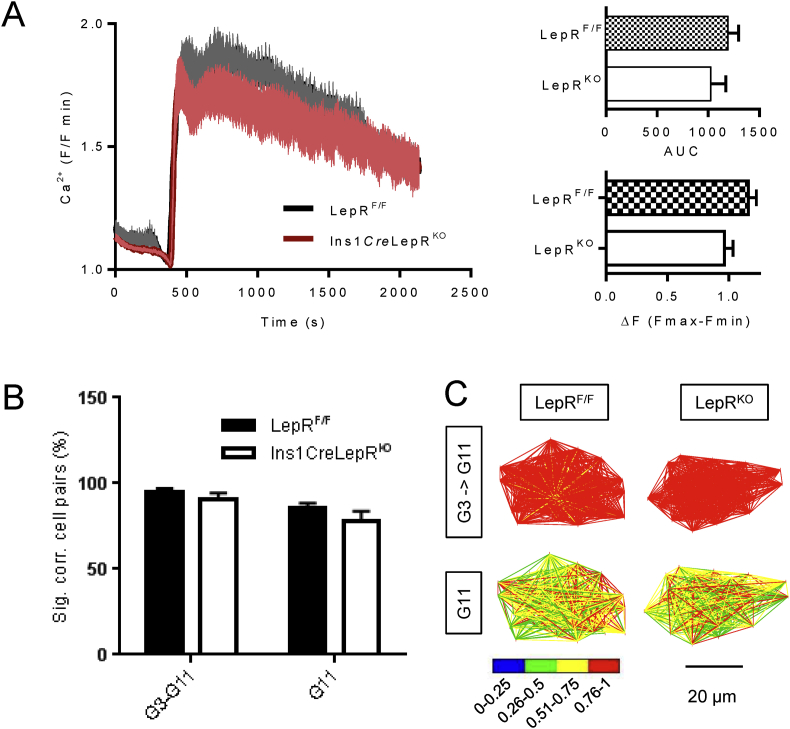
Deletion of LepRb from β-cells using a RIP2Cre has been reported to impair glucose-dependent Ca2+ signals [47]. However, this observation was ascribed partly to the effects of the RIP2Cre transgene itself [31], as well as to the loss of LepRb. Contrary to the latter findings, deletion of LepRb with Pdx1.Cre potentiated glucose-induced Ca2+ oscillations. To examine the effect of LepRb deletion more selectively in the β cell we therefore monitored changes in intracellular free Ca2+ ([Ca2+]i) in response to high glucose, or depolarisation with KCl, in islets from 8 weeks old female Ins1CreLepRKO or control mouse islets. Islets lacking LepRb in β cells displayed a tendency towards lower [Ca2+]i increases in response to high glucose (Figure 5A), though this difference was not significant when hundreds of single cells were individually assessed within each micro-organ to exclude outliers which confound signal analysis (i.e. glucose non-responsive cells, and those cells displaying large and sustained increases indicative of apoptosis). Likewise, no changes in response to stimulation with KCl (not shown), or in β cell-β cell connectivity (Figure 5B,C), [44], were observed in Ins1CreLepRKO mice versus control littermates suggesting that β cell population responses to glucose remain coordinated even in the absence of LepRb signalling.
Figure 5.
Ins1CreLepRKOislets display impaired Ca2+responses to high glucose. Ca2+ recordings from control LepRF/F islets (black line) and Ins1CreLepRKO islets (red line) in response to either 11 mM glucose (A). Inset are area under the curve (AUC) and amplitudes (ΔF (Fmax-Fmin)) of the Ca2+ rises. Data are from 14 to 16 islets (3–4 mice) per genotype. (B,C) Connectivity analysis [44] showing the proportion (%) of significantly correlated cell pairs during a step change from 3 to 11 mM glucose (i.e. the activity onsets), or at steady state in the continued presence of 11 mM glucose. Pseudocolor plots in (C) show the strength of connections, determined using by Pearson Correlation (Pearson R). Data are expressed as mean ± SEM. Statistical analysis was performed using two-way ANOVA and Student's t-test. *P < 0.05.
3.4. Role of leptin receptors in pancreatic α- and other proglucagon-expressing cells
Leptin has been reported to exert a direct inhibitory effect on glucagon secretion from isolated mouse and human islets [48]. To generate animals deleted for the LepRb in pancreatic α cells and other proglucagon-expressing cells (including GLP-1-expressing intestinal L-cells and GLP-1-positive neurons in the brain), LepRb flox'd animals [41] were crossed with animals harbouring the iCre recombinase transgene under the control of ∼100 kB region immediately upstream of the proglucagon gene [37]. The resulting iGluCreLepRKO mice were further crossed to Rosa26.tdRFP reporter mice [49].
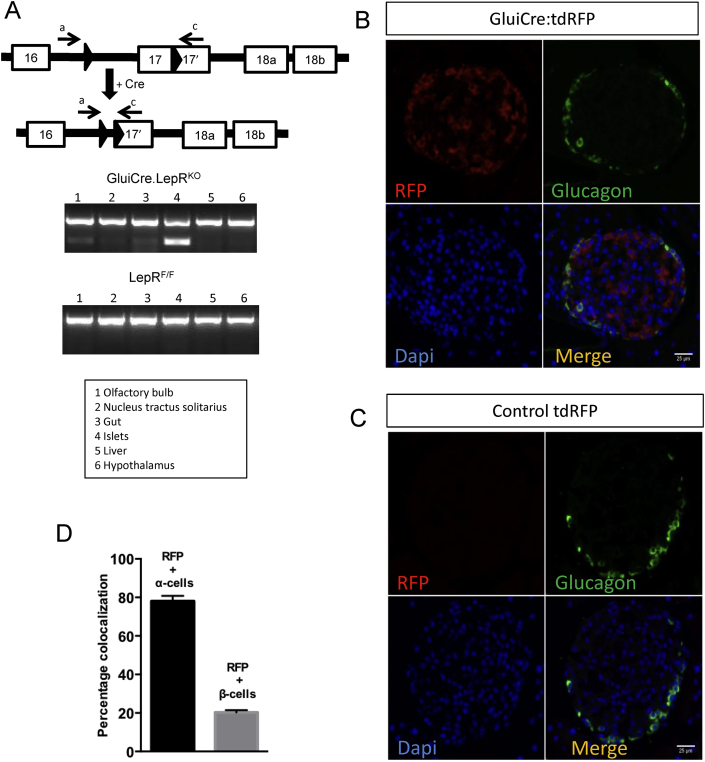
In order to confirm firstly the deletion of LepRb in target cells, genomic DNA was harvested from several different tissues and brain areas and PCR was performed using primers flanking the LoxP sites (Methods; Figure 6A). The presence of the recombined allele was clearly detected in islets, and to a lesser extent in the olfactory bulb, the nucleus tractus solitarius (NTS) and intestine. Correspondingly, in mice carrying the Rosa26.tdRFP reporter, red fluorescence was clearly apparent in the majority of glucagon-positive α cells (Figure 6B), but not in Rosa26.tdRFP mice lacking iGluCre (Figure 6C). In the presence of the Cre transgene, recombination occurred in the vast majority (∼80%) of glucagon-positive islet cells, but also in a minority (∼20%) of insulin-positive β cells (Figure 6B,D).
Figure 6.
iGluCre expression results in recombination of LepRb flox'd alleles in the olfactory bulb, nucleus tractus solitarius, intestine and pancreatic islets. Genomic DNA was harvested from the tissues indicated and used as a template for PCR with primer pair a and c (as indicated in Figure 1A). Predicted product sizes are 625 bp for the flox'd allele and 207 bp for the excised allele. (A) PCR transcript of the excised alleles in iGluCreLepRKO and LepRF/F controls. Immunohistochemical analysis of pancreas from iGluCre:tdRFPStopFlox mice, bearing wild type LepR alleles (B) stained for RFP (red), glucagon (green) and DAPI (blue), and in control animals without Cre (tdRFPStopFlox) (C). Quantification of the percentage of RFP expressing α and β cells (D). n = 39 islets from three animals. Data are expressed as mean ± SEM.
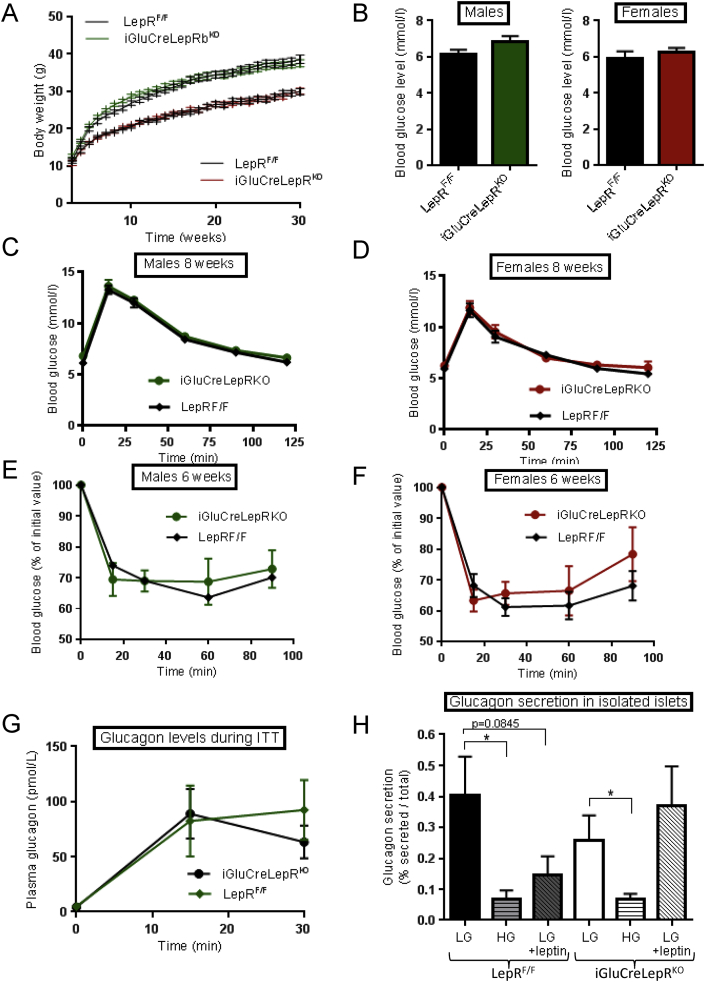
No differences in growth curves were apparent between iGluCreLepRbKO and LepRbF/F mice (Figure 7A), nor were differences in fasting blood glucose detected (Figure 7B). Intraperitoneal glucose (Figure 7C,D) and insulin (Figure 7E,F) tolerance were also identical between genotypes. Likewise, there were no apparent differences in glucagon release in response to hypoglycaemia in vivo (Figure 7G), nor in vitro in response to low or high glucose (Figure 7H). A tendency for leptin (10 nM) to impair glucagon release at low glucose was apparent in wild-type, but not iGluCreLepRbKO mice (Figure 7H).
Figure 7.
iGluCreLepRKOmice display normal glucose tolerance and fasting blood glucose levels. (A) Body weight followed for 30 weeks in male (green) and female (red) iGluCreLepRKO and LepRF/F mice (n = 12–19 mice per genotype). (B) Fasting blood glucose levels in 8 week old males and females (n = 6–11 mice per genotype). (C,D) Blood glucose concentration after IPGTT in male and female iGluCreLepRKO (square symbol) and LepRF/F (green triangle) mice. Blood glucose concentration after ITT in males: (E) iGluCreLepRKO, n = 8, LepRF/F, n = 7; and females: (F) iGluCreLepRKO, n = 8, LepRF/F, n = 10. Plasma glucagon levels during ITT, iGluCreLepRKO, n = 6, LepRF/F, n = 4, (G), and glucagon release from isolated islets in the presence of the indicated glucose concentrations: LG = 0.5 mM, HG = 10 mM glucose; n = 6 animals per genotype (H). When present, leptin was added to 10 nM. Data are expressed as the mean ± SEM and statistical comparison was through two-way ANOVA, *p < 0.05. Other details are provided in the Materials and Methods section.
4. Discussion
4.1. Leptin receptor signalling in β cells
The studies described herein present the first examination to our knowledge of the impact of highly selective abrogation of leptin receptor function in pancreatic β cells using Ins1Cre-mediated deletion. We show firstly that levels of leptin receptor mRNA and of leptin signalling are vanishingly low in highly purified mouse islet cells and whole islets, respectively (Figure 1). These results corroborate work from Fujikawa, Coppari and colleagues [50] who showed, through the use of a leptin receptor promoter Cre crossed to Rosa26RFP reporter mice, that RFP expression (reflecting the activity of the LepR promoter) is essentially undetectable in the endocrine pancreas, both during development and in adult mice. In contrast, recombination was detected in a more poorly-defined cell population outside of the islet, possibly corresponding to duct cells or ductal precursors. Importantly, the latter cells are likely to contaminate islets after isolation, and, as such, may reflect the major pool of LepRb-expressing cells in these ex vivo preparations. Consequently, the use of Cre deleter strains active throughout the pancreas, notably Pdx1Cre, is expected to cause recombination and loss of LepRb from these contaminating (non-islet) cells. The loss of LepRb mRNA apparent in islets from the above mice in a previous report [24], therefore, seems likely to reflect deletion from the above population other than from β-cells per se. We note that the report by Covey et al. [23] using the Rip2Cre deleter strain, and therefore expected in the pancreas to delete only in β cells, does not include any data on LepRb mRNA levels in wild type versus null mouse islets. Only measures of DNA recombination, as performed in our own study, were provided.
Nevertheless, and consistent with earlier findings using more promiscuous Cres [23,24], we show that the levels of leptin receptors on β cells may be sufficient to restrict β cell function in vivo under some circumstances. Thus, we report mild glycaemic effects of LepRb deletion which appeared transiently in female mice (Figure 3A,B). Although challenged by the findings of Coppari and colleagues [50] (above) one possibility, not explored here, is that this might reflect a transiently higher level of expression of leptin receptors on β cells at this developmental stage in females. However, the alternative possibility that a very low level of LepR expression, perhaps on a minority of well-connected (“pacemaker” or “hub”) β cells [51], is sufficient to exert islet-wide consequences on insulin secretion in juvenile females, cannot be discounted.
We note that, whilst in one previous report [23] the effects of β cell-targeted LepRb deletion were examined using oral glucose tolerance tests (OGTTs), in a second paper [24], equally dramatic effects of deleting these receptors throughout the pancreas were observed using IPGTTs, where peak blood glucose was reduced from ∼22 to 16 (27%) and from >16 to ∼9.5 mM (40%) in 6 month old males and females, respectively. Our own experiments, using the more selective Ins1Cre driver line to delete LepRb, revealed a much more modest reduction in peak glucose during IPGTT, from ∼12 to 10 mM (16%) in 8 week old females, whereas no differences were observed between control and knockout male mice of the same age, or between 4 month old mice of either sex (Figure 3). The measurements in the present study are thus best compared to the latter report [24], using IPGTTs, and support our contention of a limited role for LepR in β cells beyond juvenile stages. The more marked effects of LepRb deletion described in earlier studies [23,24] would seem to be attributable, at least in large part, to the loss of leptin receptors at other sites, notably the brain, with consequent effects on food intake (and hence insulin sensitivity) [23] or potentially in other non-endocrine pancreatic cells [24]. Interestingly, Morioka et al. [24] did not observe changes in body weight after Pdx1Cre-mediated deletion, whilst markedly elevated insulin secretion and β cell mass were reported.
A potential concern with the present studies is that deletion may not have occurred throughout the β cell complement, as we have assumed [27]. Whilst LepRb mRNA levels were too low to quantitate accurately either in wild type or Ins1CreLepRKO islets (Figure 1A–C), we believe this possibility to be unlikely. Firstly, we observed clear recombination at the genomic level in isolated islets (Figure 2B). Secondly, Ins1Cre leads to highly efficient recombination at LoxP sites in other targets including in LKB1/STK11, AMPKα1 and α2 alleles [27], at the Rosa26-eYFP locus [28], and in mice carrying a single halorhodopsin allele downstream of a LoxP-STOP-LoxP cassette (D.J.H. and G.A.R. results not shown). In the two latter models, recombination is observed in 97.8% and 94.7% of β cells, respectively.
Further supporting a limited role of LepRb on β cells are studies by Chua and colleagues [52] demonstrating an absence of metabolic phenotype in mice deleted selectively for LepR in the periphery. In light of the above observations, our own data in Ins1CreLepRKO animals reporting only a weak metabolic phenotype are not entirely unexpected.
Interestingly, the inhibitory actions of leptin observed in the present study appear to be independent of glucose-induced increases in intracellular Ca2+, since the latter showed only a non-significant tendency to be impaired in Ins1CreLepRKO mice (Figure 5).
4.2. Leptin receptor signalling in α cells
Using the less efficient PPG.Cre [33], earlier studies [32] have reported that elimination of LepRb from a subset of α cells exerted no effect on glucose tolerance. However, and consistent with findings from Tuduri et al. [48], we observed a tendency for leptin to inhibit glucagon secretion in vitro (Figure 7G), an effect which was abolished in iGluCreLepRbKO mouse islets. Similarly, a tendency was observed towards a more rapid recovery towards normal glycaemia after insulin injection in female KO mice (Figure 7F). Thus, at least under the conditions examined here, leptin receptor signalling in α cells has no more than a marginal role at most in the control of glucagon secretion during hypoglycaemia.
4.3. Leptin receptors in GLP-1 neurons
In the present study, we failed to observe differences in weight gain between wild-type and iGluCreLepRbKO mice. Whilst the simplest interpretation of these data would be that GLP-1-expressing neurones in the NTS are not involved in the control of food intake, we note that Scott and colleagues [53] recently observed that disruption of LepRb signalling in this brain region using a less selective Phox2B deleter strain led to hyperphagia but not weight gain. This was attributed to an increase in energy expenditure in null mice. Whether deletion of LepRb using the iGluCre transgene similarly affected food intake is a possibility which will require testing in individually-housed mice in the future.
4.4. Leptin receptors in other proglucagon-expressing cells
Intestinal L-cells, which synthesise and secrete GLP-1, are also an important site of proglucagon expression, and consequently recombination in iGluCre mice [37,38]. Although not measured directly in the present studies, changes in GLP-1 output from these cells would seem to be unlikely in iGluCreLepRKO mice given their unaltered body weight gain (Figure 7A) and glucose tolerance (Figure 7C,D), as well as the low expression of leptin receptor mRNA in FACS-purified mouse L-cells (FR and FMG, data not shown).
5. Conclusions
Using a well-defined and efficient Cre deleter strain we demonstrate that leptin signalling in β cells plays a physiologically relevant, albeit very limited role, to control insulin secretion in vivo. Similarly, leptin has a relatively minor, if any, role in the control of glucagon secretion from pancreatic α cells. No direct evidence was obtained in the present study for a role for leptin receptors in GLP-1-expressing cells in the gut or brain.
Acknowledgements
Funded by grants to GAR from the Wellcome Trust (Programme 081958/Z/07/Z; Senior Investigator Award WT098424AIA), the MRC (UK; Project GO401641; Programme MR/J0003042/1), and Diabetes UK (BDA 11/0004210), and a grant to ST and GAR from the MRC (MR/J013293/1). DJH thanks Diabetes UK for an RD Lawrence Fellowship (BDA 12/0004431). The work leading to this publication also received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n° 155005 (IMIDIA), resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies' in kind contribution. We thank Streamson Chua (Colombia University) for the provision of LepRbF/F mice and J. Ferrer (Imperial College London, U.K.) and Bernard Thorens (University of Lausanne, Switzerland) for providing Ins1Cre mice. Work by AEA, FR and FMG was supported by an EFSD/Boehringer Ingelheim programme grant. We are grateful for discussion to Drs Timothy Pullen and Aida Martinez-Sanchez (Imperial College).
Conflict of interest
None declared.
References
- 1.Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. Epub 2013 Dec 1011. [DOI] [PubMed] [Google Scholar]
- 2.Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. Epub 2013 Mar 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger R.H., Cherrington A.D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. Journal of Clinical Investigation. 2012;122:4–12. doi: 10.1172/JCI60016. Epub 62012 Jan 60013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutter G.A., Parton L.E. The beta-cell in type 2 diabetes and in obesity. Frontiers of Hormone Research. 2008;36:118–134. doi: 10.1159/000115360. [DOI] [PubMed] [Google Scholar]
- 5.Ahima R.S., Flier J.S. Leptin. Annual Review of Physiology. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 6.Montague C.T., Farooqi I.S., Whitehead J.P., Soos M.A., Rau H., Wareham N.J. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 7.Wabitsch M., Funcke J.B., Lennerz B., Kuhnle-Krahl U., Lahr G., Debatin K.M. Biologically inactive leptin and early-onset extreme obesity. New England Journal of Medicine. 2015;372:48–54. doi: 10.1056/NEJMoa1406653. [DOI] [PubMed] [Google Scholar]
- 8.Clement K., Vaisse C., Lahlou N., Cabrol S., Pelloux V., Cassuto D. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 10.Lee G.H., Proenca R., Montez J.M., Carroll K.M., Darvishzadeh J.G., Lee J.I. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 11.Fei H., Okano H.J., Li C., Lee G.H., Zhao C., Darnell R. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seufert J., Kieffer T.J., Leech C.A., Holz G.G., Moritz W., Ricordi C. Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. Journal of Clinical Endocrinology and Metabolism. 1999;84:670–676. doi: 10.1210/jcem.84.2.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkarni R.N., Wang Z.L., Wang R.M., Hurley J.D., Smith D.M., Ghatei M.A. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. Journal of Clinical Investigation. 1997;100:2729–2736. doi: 10.1172/JCI119818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poitout V., Rouault C., Guerre-Millo M., Briaud I., Reach G. Inhibition of insulin secretion by leptin in normal rodent islets of Langerhans. Endocrinology. 1998;139:822–826. doi: 10.1210/endo.139.3.5812. [DOI] [PubMed] [Google Scholar]
- 15.Poitout V., Rouault C., Guerre-Millo M., Reach G. Does leptin regulate insulin secretion? Diabetes & Metabolism. 1998;24:321–326. [PubMed] [Google Scholar]
- 16.Kieffer T.J., Heller R.S., Leech C.A., Holz G.G., Habener J.F. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes. 1997;46:1087–1093. doi: 10.2337/diab.46.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehmann H.C., Peiser C., Bode H.P., Stamm M., Staats P., Hedetoft C. Leptin: a potent inhibitor of insulin secretion. Peptides. 1997;18:1267–1273. doi: 10.1016/s0196-9781(97)00135-6. [DOI] [PubMed] [Google Scholar]
- 18.Emilsson V., Liu Y.L., Cawthorne M.A., Morton N.M., Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes. 1997;46:313–316. doi: 10.2337/diab.46.2.313. [DOI] [PubMed] [Google Scholar]
- 19.Wang M.Y., Koyama K., Shimabukuro M., Mangelsdorf D., Newgard C.B., Unger R.H. Overexpression of leptin receptors in pancreatic islets of Zucker diabetic fatty rats restores GLUT-2, glucokinase, and glucose-stimulated insulin secretion. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:11921–11926. doi: 10.1073/pnas.95.20.11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclercq-Meyer V., Considine R.V., Sener A., Malaisse W.J. Do leptin receptors play a functional role in the endocrine pancreas? Biochemical and Biophysical Research. 1996;229:794–798. doi: 10.1006/bbrc.1996.1882. [DOI] [PubMed] [Google Scholar]
- 21.Park S.H., Ryu S.Y., Yu W.J., Han Y.E., Ji Y.S., Oh K. Leptin promotes K(ATP) channel trafficking by AMPK signaling in pancreatic beta-cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12673–12678. doi: 10.1073/pnas.1216351110. Epub 1216352013 Jul 1216351115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutter G.A., Pullen T.J., Hodson D.J., Martinez-Sanchez A. Pancreatic b-cell identity, glucose sensing and the control of insulin secretion. Biochemical Journal. 2015;466:202. doi: 10.1042/BJ20141384. [DOI] [PubMed] [Google Scholar]
- 23.Covey S.D., Wideman R.D., McDonald C., Unniappan S., Huynh F., Asadi A. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metabolism. 2006;4:291–302. doi: 10.1016/j.cmet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Morioka T., Asilmaz E., Hu J., Dishinger J.F., Kurpad A.J., Elias C.F. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. Journal of Clinical Investigation. 2007;117:2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wicksteed B., Brissova M., Yan W., Opland D.M., Plank J.L., Reinert R.B. Conditional gene targeting in mouse pancreatic ss-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59:3090–3098. doi: 10.2337/db10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niswender K.D., Magnuson M.A. Obesity and the beta cell: lessons from leptin. Journal of Clinical Investigation. 2007;117:2753–2756. doi: 10.1172/JCI33528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kone M., Pullen T.J., Sun G., Ibberson M., Martinez-Sanchez A., Sayers S. LKB1 and AMPK differentially regulate pancreatic beta-cell identity. FASEB Journal. 2014;28:4972–4985. doi: 10.1096/fj.14-257667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorens B., Tarussio D., Maestro M.A., Rovira M., Heikkila E., Ferrer J. Ins1 knock-in mice for beta cell-specific gene recombination. Diabetologia. 2014;11:11. doi: 10.1007/s00125-014-3468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamarina N.A., Roe M.W., Philipson L. Characterization of mice expressing Ins1 gene promoter driven CreERT recombinase for conditional gene deletion in pancreatic beta-cells. Islets. 2014;6 doi: 10.4161/isl.27685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gannon M., Shiota C., Postic C., Wright C.V., Magnuson M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis. 2000;26:139–142. doi: 10.1002/(sici)1526-968x(200002)26:2<139::aid-gene12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Brouwers B., de Faudeur G., Osipovich A.B., Goyvaerts L., Lemaire K., Boesmans L. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metabolism. 2014;20:979–990. doi: 10.1016/j.cmet.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuduri E., Denroche H.C., Kara J.A., Asadi A., Fox J.K., Kieffer T.J. Partial ablation of leptin signaling in mouse pancreatic alpha-cells does not alter either glucose or lipid homeostasis. American Journal of Physiology. Endocrinology and Metabolism. 2014;306:E748–E755. doi: 10.1152/ajpendo.00681.2013. Epub 02014 Jan 00628. [DOI] [PubMed] [Google Scholar]
- 33.Herrera P.L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 34.Sun G., da Silva Xavier G., Gorman T., Priest C., Solomou A., Hodson D.J. LKB1 and AMPKalpha1 are required in pancreatic alpha cells for the normal regulation of glucagon secretion and responses to hypoglycemia. Molecular Metabolism. 2015;4(4):277–286. doi: 10.1016/j.molmet.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilcox C.L., Terry N.A., Walp E.R., Lee R.A., May C.L. Pancreatic alpha-cell specific deletion of mouse Arx leads to alpha-cell identity loss. PLoS One. 2013;8:e66214. doi: 10.1371/journal.pone.0066214. Print 0062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J., Herrera P.L., Carreira C., Bonnavion R., Seigne C., Calender A. Alpha cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology. 2010;138:1954–1965. doi: 10.1053/j.gastro.2010.01.046. Epub 2010 Feb 1952. [DOI] [PubMed] [Google Scholar]
- 37.Parker H.E., Adriaenssens A., Rogers G., Richards P., Koepsell H., Reimann F. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55:2445–2455. doi: 10.1007/s00125-012-2585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zac-Varghese S., Trapp S., Richards P., Sayers S., Sun G., Bloom S.R. The Peutz-Jeghers kinase LKB1 suppresses polyp growth from intestinal cells of a proglucagon-expressing lineage in mice. Disease Models & Mechanisms. 2014;7:1275–1286. doi: 10.1242/dmm.014720. Epub 012014 Sep 014724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anini Y., Brubaker P.L. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 40.Hisadome K., Reimann F., Gribble F.M., Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes. 2010;59:1890–1898. doi: 10.2337/db10-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMinn J.E., Liu S.M., Dragatsis I., Dietrich P., Ludwig T., Eiden S. An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mammalian Genome. 2004;15:677–685. doi: 10.1007/s00335-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 42.Sun G., Tarasov A.I., McGinty J.A., French P.M., McDonald A., Leclerc I. LKB1 deletion with the RIP2.Cre transgene modifies pancreatic beta-cell morphology and enhances insulin secretion in vivo. American Journal of Physiology, Endocrinology and Metabolism. 2010;298:E1261–E1273. doi: 10.1152/ajpendo.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravier M.A., Rutter G.A. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes. 2005;54:1789–1797. doi: 10.2337/diabetes.54.6.1789. [DOI] [PubMed] [Google Scholar]
- 44.Hodson D.J., Mitchell R.K., Bellomo E.A., Sun G., Vinet L., Meda P. Lipotoxicity disrupts incretin-regulated human beta cell connectivity. Journal of Clinical Investigation. 2013;123:4182–4194. doi: 10.1172/JCI68459. Epub 62013 Sep 68459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F.M. Glucose sensing in L cells: a primary cell study. Cell Metabolism. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chera S., Baronnier D., Ghila L., Cigliola V., Jensen J.N., Gu G. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature. 2014;514:503–507. doi: 10.1038/nature13633. Epub 12014 Aug 13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuduri E., Bruin J.E., Denroche H.C., Fox J.K., Johnson J.D., Kieffer T.J. Impaired Ca(2+) signaling in beta-cells lacking leptin receptors by Cre-loxP recombination. PLoS One. 2013;8:e71075. doi: 10.1371/journal.pone.0071075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuduri E., Marroqui L., Soriano S., Ropero A.B., Batista T.M., Piquer S. Inhibitory effects of leptin on pancreatic alpha-cell function. Diabetes. 2009;58:1616–1624. doi: 10.2337/db08-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luche H., Weber O., Nageswara Rao T., Blum C., Fehling H.J. Faithful activation of an extra-bright red fluorescent protein in “knock-in” Cre-reporter mice ideally suited for lineage tracing studies. European Journal of Immunology. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- 50.Fujikawa T., Berglund E.D., Patel V.R., Ramadori G., Vianna C.R., Vong L. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metabolism. 2013;18:431–444. doi: 10.1016/j.cmet.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rutter G.A., Hodson D.J. Beta cell connectivity in pancreatic islets: a type 2 diabetes target? Cell Mol Life Sci. 2015;72:453–467. doi: 10.1007/s00018-014-1755-4. Epub 02014 Oct 00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo K., McMinn J.E., Ludwig T., Yu Y.H., Yang G., Chen L. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148:3987–3997. doi: 10.1210/en.2007-0261. Epub 2007 May 3910. [DOI] [PubMed] [Google Scholar]
- 53.Scott M.M., Williams K.W., Rossi J., Lee C.E., Elmquist J.K. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. Journal of Clinical Investigation. 2011;121:2413–2421. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]