Abstract
Objective
Skeletal muscle AMP-activated protein kinase (AMPK) is important for regulating glucose homeostasis, mitochondrial content and exercise capacity. R419 is a mitochondrial complex-I inhibitor that has recently been shown to acutely activate AMPK in myotubes. Our main objective was to examine whether R419 treatment improves insulin sensitivity and exercise capacity in obese insulin resistant mice and whether skeletal muscle AMPK was important for mediating potential effects.
Methods
Glucose homeostasis, insulin sensitivity, exercise capacity, and electron transport chain content/activity were examined in wildtype (WT) and AMPK β1β2 muscle-specific null (AMPK-MKO) mice fed a high-fat diet (HFD) with or without R419 supplementation.
Results
There was no change in weight gain, adiposity, glucose tolerance or insulin sensitivity between HFD-fed WT and AMPK-MKO mice. In both HFD-fed WT and AMPK-MKO mice, R419 enhanced insulin tolerance, insulin-stimulated glucose disposal, skeletal muscle 2-deoxyglucose uptake, Akt phosphorylation and glucose transporter 4 (GLUT4) content independently of alterations in body mass. In WT, but not AMPK-MKO mice, R419 improved treadmill running capacity. Treatment with R419 increased muscle electron transport chain content and activity in WT mice; effects which were blunted in AMPK-MKO mice.
Conclusions
Treatment of obese mice with R419 improved skeletal muscle insulin sensitivity through a mechanism that is independent of skeletal muscle AMPK. R419 also increases exercise capacity and improves mitochondrial function in obese WT mice; effects that are diminished in the absence of skeletal muscle AMPK. These findings suggest that R419 may be a promising therapy for improving whole-body glucose homeostasis and exercise capacity.
Keywords: Exercise-mimetic, Mitochondrial, Diabetes, Obesity, AMPK, Complex-I, R419
Abbreviations: 2-DG, 2-deoxyglucose; ACC, acetyl-CoA carboxylase; AICAR, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside; AMPK, AMP-activated protein kinase; AMPK-MKO, skeletal muscle-specific AMPK β1β2 floxed Cre-; AUC, area under the curve; COX, cytochrome c oxidase; CT, computed tomography; EDL, extensor digitorum longus; HFD, high-fat diet (45% kcal fat); GDR, glucose disposal rate; GIR, glucose infusion rate; GLUT4, glucose transporter 4; HGO, hepatic glucose output; OXPHOS, proteins involved in oxidative phosphorylation (electron transport chain); R419, N-(1-(4-cyanobenzyl) piperidin-4-yl)-6-(4-(4-methoxybenzoyl) piperidine-1-carbonyl; Tbp, TATA-binding protein; TA, tibialis anterior; WT, wildtype
Highlights
-
•
The beneficial effects of a novel complex I inhibitor, R419, were examined in skeletal muscle AMPK deficient obese mice.
-
•
R419 improves skeletal muscle insulin sensitivity in obese mice.
-
•
R419 improves exercise capacity in obese mice via an AMPK dependent pathway.
-
•
These findings suggest that R419 may have therapeutic importance in improving exercise capacity and skeletal muscle insulin sensitivity.
1. Introduction
The AMP-activated protein kinase (AMPK) is an αβγ heterotrimer that plays an important role in regulating whole-body glucose homeostasis and insulin sensitivity (for review see Ruderman et al., 2013 [1] and Steinberg & Jorgensen 2007 [2]). Over the last decade, many activators of AMPK have been shown to improve insulin sensitivity and glycemic control [3–7] through AMPK dependent [8–10] and independent [11–13] pathways. Of these AMPK activators, metformin, which is a mild complex-I inhibitor, has been extensively studied and found to exert its glucose lowering effects primarily by acting in the liver (for review see Foretz et al., 2014 [14]). This liver predominant effect of metformin is believed to be the result of high expression of the organic cation transporter 1 (OCT1) that is required for metformin uptake and thus limits the effectiveness of metformin to act in other tissues such as skeletal muscle [14]. Similarly, direct activators of AMPK like A769662 [15] and salicylate [16] activate AMPK through the β1 subunit which is prevalent in liver but has low expression in skeletal muscle [17,18]. While recent studies have identified new direct activators of AMPK that may be effective in muscle [19–21], their role in regulating skeletal muscle insulin sensitivity and muscle mitochondrial content is currently not known. As improvements in skeletal muscle insulin sensitivity are important for restoring glucose homeostasis, there is a need to develop and evaluate new activators of AMPK that may exert positive effects on skeletal muscle metabolism and insulin sensitivity.
In addition to improving insulin sensitivity, AMPK is also vital for controlling muscle mitochondrial content and exercise capacity (for review see Richter & Ruderman 2009 [22] and O'Neill et al., 2013 [23]). For example, mice with undetectable levels of AMPK activity due to genetic deletion of α or β subunits in skeletal muscle have a severely impaired capacity for treadmill running that is associated with alterations in mitochondrial content and/or function [24,25]. In contrast, the activation of AMPK through genetic manipulation or a wide variety of pharmacological agents has been shown to increase treadmill exercise performance in most studies [10,26–29]. These data indicate that activators of AMPK may also be able to increase muscle mitochondrial content and improve exercise performance.
R419 (N-(1-(4-cyanobenzyl)piperidin-4-yl)-6-(4-(4-methoxybenzoyl)piperidine-1-carbonyl)nicotinamide) is a recently described mitochondrial complex I inhibitor that acutely activates AMPK in a variety of cell systems including myotubes and hepatocytes [30]. R419 acutely increases glucose uptake and fatty acid oxidation in cultured myotubes via an AMPK dependent pathway. In contrast, R419 was shown to acutely inhibit glucose production in isolated hepatocytes via an AMPK-independent process. Metabolite tracer analysis of db/db mice treated with R419 suggested increased flux through both skeletal muscle glycolytic and fatty acid oxidative pathways and increases in glucose transporter 4 (GLUT4) promoter activity [30]. However, whether chronic R419 treatment improves glucose homeostasis and insulin sensitivity is not currently understood. Since the tissue specific uptake of R419 would not be limited by OCT1, as is the case with metformin, we hypothesized that R419 would improve glucose homeostasis through improvements in skeletal muscle insulin sensitivity and that this effect would require skeletal muscle AMPK. Furthermore, since activation of AMPK has been associated with muscle mitochondrial biogenesis, we also hypothesized that R419 would increase exercise capacity through an AMPK dependent process. We find that in obese mice fed a high-fat diet (HFD), R419 improves glucose tolerance and enhances insulin-stimulated glucose disposal into skeletal muscle in WT and AMPK-MKO mice while also enhancing exercise capacity only in WT controls.
2. Material and methods
2.1. Mouse experiments
Male AMPK β1β2 floxed muscle creatine kinase (MCK)-Cre- (WT) and AMPK β1β2 floxed MCK-Cre+ (AMPK-MKO) littermates were used in all experiments and have been described previously [25]. The McMaster University (Hamilton, Canada) Animal Ethics Committee approved all experimental protocols. To measure R419-mediated AMPK activation, WT and AMPK-MKO mice were fasted for 12 h and re-fed ad libitum with HFD (45 kcal% fat, D12451, Research Diets; New Brunswick, NJ) formulated with R419 at a dose of 100 mg/kg HFD (HFD + R419) or the same HFD without R419. After 3 h of re-feeding, blood glucose was measured by glucometer with a small nick of the tail vein. Mice were then anesthetized and tibialis anterior (TA) muscle was collected, snap frozen and kept at minus 80 °C for later analysis. To examine the chronic effects of R419 treatment, mice were maintained on a 12 h light/dark cycle and fed a HFD starting at 6–8 weeks of age for 12 weeks. After the first 6 weeks of HFD, mice were continued on HFD or given HFD + R419. Mice were allocated to different experiments in order to reduce stress from excessive testing and handling. Food intake was measured over a 72 h timeframe using the Oxymax Lab Animal Monitoring System (Columbus Instruments, OH).
2.2. Exercise capacity test
Mice were acclimated to the treadmill as described previously [17,25,31]. Exercise capacity test was completed once after 5 weeks of treatment. Mice began running at 8 m/min and treadmill speed was increased by 1 m/min every 2 min as previously described [17,25]. At exhaustion, time and speed were recorded and distance traversed calculated. Exhaustion was defined as the point at which instead of running on the treadmill, mice remained on the shockers that serve to encourage running for more than 10 s. Experimenters were unaware of the genotype/treatment of the mice while they were performing the experiment. Mice were quickly removed from the treadmill, blood was collected by facial bleed, and blood glucose recorded. Lactate Assay Kit was used to measure serum lactate, according to manufacturer instructions (Biovision), in the fed basal state and at exhaustion.
2.3. Metabolic studies
After 6 weeks of treatment, computed tomography (CT) was used to assess whole body adiposity and analyzed with the Amira Visage Imaging Software Program, as described previously [10]. Mice were fasted for 12 h in the evening and ∼100 μL of blood was collected by facial bleed in the morning. Blood glucose concentrations were recorded by glucometer. Serum analysis of insulin (Millipore) and non-esterified free fatty acids (NEFA) (Wako NEFA-HR) were completed, according to manufacturer instructions. For glucose (GTT, after 5 weeks of treatment) and insulin (ITT, after 6 weeks of treatment) tolerance tests, mice were fasted for 6 h and injected with d-glucose (1 g/kg) and human insulin (1 U/kg, NovoRapid), respectively. Blood glucose was monitored during a 2 h span. Blood glucose was measured by glucometer from a small nick of the tail vein. Hyperinsulinemic-euglycaemic clamps were performed after 12 weeks of the study as previously described [32,33]. The jugular vein was surgically cannulated and if mice had <10% loss in body weight change, they were clamped 5 days later. On the day of the clamp, mice were fasted for 6 h and basal D- [3-3H]-glucose (7.5 μCi/h, 0.12 ml/h in 0.9% saline) was infused for 1 h. This was followed by a constant infusion of insulin (10 mU/kg/min insulin in 0.9% saline) (Novorapid), containing D- [3-3H]-glucose (7.5 μCi/h, 0.12 ml/h in 0.9% saline). Blood glucose was titrated with infusion of 50% dextrose that was gradually increased until euglycaemia was reached, defined as blood glucose between 5.8 and 7.0 mmol/L for at least 30 min [32]. Additionally, glucose uptake in tissues was measured after intravenous injection of 2-[14C]-deoxy-glucose (2-[14C] DG) (10 μCi). Blood samples were taken at 10, 20, and 30 min. Mice were euthanized with an intravenous injection of ketamine-xylazine and sacrificed by exsanguination. Tissues were removed, snap frozen in liquid nitrogen, and stored at minus 80 °C for later analysis [10].
2.4. Analytical techniques
Extensor digitorum longus (EDL), TA and quadriceps muscles were powdered on dry ice and homogenized in cell lysis buffer using a Precellys 24 Homogenizer (Bertin Technologies; Paris, France). Lysates were collected from centrifuged homogenate (13 000 rpm). Tissue-specific glucose uptake (2-[14C] DG-Phosphate) was quantified by scintillation counting in which the unphosphorylated 2-[14C] DG fraction was subtracted from the total fraction of homogenate. Values were expressed to blood glucose and 2-[14C] DG infusion in the blood. Additionally, lysates were used to measure cytochrome c oxidase (COX) activity, as described previously [34]. Blood was collected for serum biochemistry measurements post-hyperinsulinemic euglycemic clamp, including NEFA and insulin (Iso-insulin ELISA kit (Mercodia)). Steele's equation for steady state conditions was used to determine rates of hepatic glucose output and glucose disposal in the basal and clamped state [35]. Lipid content in quadriceps muscle was measured by Bligh and Dyer procedure [36]. Briefly, lipids were extracted using chloroform and methanol. Lipids from chloroform phase were saponified and glycerol content measured with Glycerol Reagent (Sigma). Glycogen content was measured by incubating powdered tissue in 6N HCl at 80 °C and then neutralized with 6N NaOH. Glucose content was measured using a Glucose Assay (Sigma).
2.5. Western blotting
SDS-PAGE system was used to separate protein extracts. Immunoblotting was performed once proteins were transferred on nitrocellulose or polyvinylidene difluoride membranes. Membranes were first blocked for 1 h at room temperature with 5% BSA in 1xTBST (Tris buffered saline-Tween-20, 25 mM Tris·HCl (pH 7.5), 1 mM NaCl, and 0.1% Tween-20). Membranes were then incubated overnight at 4 °C with primary antibodies (from Cell Signaling, unless indicated) phosphorylated (p) acetyl-CoA carboxylase Ser79/212 (1:1000), ACC total (1:1000), pAMPK Thr172 (1:1000), AMPK total (1:1000), pAkt Ser473 (1:1000), pAkt Thr308 (1:1000), Akt (1:1000), GLUT4 (Millipore, 1:1000), OXPHOS (Complex I subunit NDUFB8, Complex II subunit CII-30, Complex III subunit Core 2 CIII-core2, Complex IV subunit I CIV-I, and ATP synthase subunit alpha CV-alpha) (MitoSciences, 1:5000), and GAPDH (Cell Signaling, 1:10 000) in TA and/or quadriceps muscle. Membranes were then washed (3 × 5 min) with TBST and incubated at room temperature for 1 h with corresponding secondary antibody. Membranes were washed (3 × 5 min) with TBST and developed with Clarity™ Western ECL Substrate (Biorad). Densitometry was performed using ImageJ software.
2.6. Statistical analysis
All results are expressed as mean ± standard error of the mean (SEM). Results were analyzed using a two-way ANOVA with Bonferroni post-hoc tests for group comparisons (unless otherwise denoted), using GraphPad Prism software. Repeated measures ANOVA was used to analyze body mass, GTT, ITT, and GIR. Significance was accepted at p ≤ 0.05.
3. Results
3.1. R419 acutely activates skeletal muscle AMPK but does not result in hypoglycemia
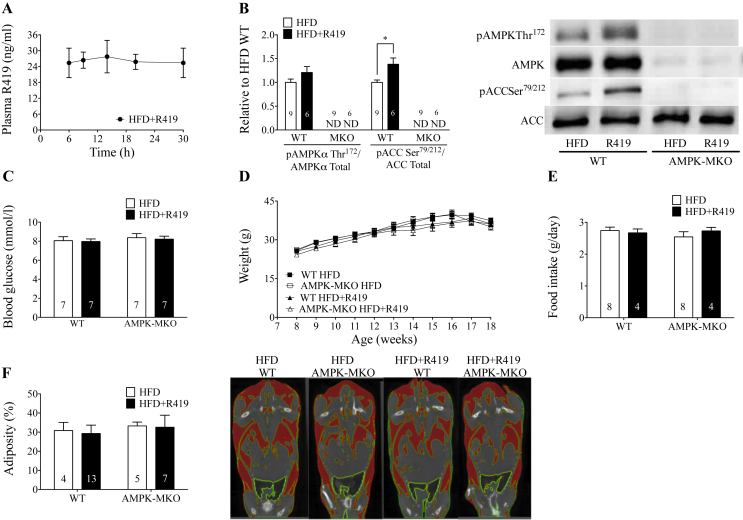
Treatment with R419 at 100 mg/kg HFD resulted in a plasma concentration of about 24 ng/mL and AUC of 600 ng*hr/mL over 24 h (Figure 1A), which is comparable to previous administration of R419 (5–10 mg/kg body weight) by oral gavage [30]. In WT, but not AMPK-MKO TA muscle, R419 resulted in a tendency for increased AMPK Thr172 phosphorylation and significant increases in ACC Ser79/212 phosphorylation compared to HFD alone (Figure 1B). It should be noted that ACC Ser79/212 phosphorylation is considered a more sensitive measure of cellular AMPK activity as it takes into account the significant role that allosteric and covalent regulation of AMPK has on its activity [37]. R419 treatment did not alter blood glucose in either WT or AMPK-MKO mice relative to HFD alone (Figure 1C).
Figure 1.
R419 acutely activates skeletal muscle AMPK but does not induce hypoglycemia. A lack of skeletal muscle AMPK or R419 treatment does not alter weight gain or adiposity in mice fed a HFD. (A) Plasma concentration of R419 when WT mice were given HFD + R419 at a dose of 100 mg/kg HFD (n = 5). (B) AMPK Thr172 relative to AMPKα (from separate gels) (p = 0.16) and ACC Ser79/212 relative to ACC (from separate gels) in TA muscle from WT and AMPK-MKO mice 3 h following HFD or HFD + R419 administration. ND, not detectable. (C) Blood glucose concentrations 3 h following HFD or HFD + R419 administration in WT and AMPK-MKO mice. (D) Body mass of HFD WT and AMPK-MKO mice treated with or without R419 (two-way repeated measures ANOVA). (E) Food intake of HFD WT and AMPK-MKO mice treated with or without R419. (F) Measured body percent adiposity with representative CT images. Highlighted red areas represent adipose region. Numbers within bars represent number of mice per group. Data are expressed as means ± SEM, ∗p < 0.05, for difference from HFD vs HFD + R419, as assessed by student's t-test (B).
3.2. A lack of skeletal muscle AMPK or treatment with R419 does not alter weight gain or adiposity in mice fed a HFD
Subsequent experiments were performed in WT and AMPK-MKO mice chronically treated with HFD + R419. A loss of skeletal muscle AMPK did not exacerbate the development of HFD-induced obesity or weight gain over time (Figure 1D). Treatment with HFD + R419 did not result in differences in weight gain (Figure 1D), food intake (Figure 1E) or adiposity (Figure 1F) between groups. There was also no effect of R419 on liver, adipose tissue, soleus, or EDL mass (Table 1). We were specifically interested in the effects of R419 in obesity, therefore, we did not test the effects of the compound in control chow-fed mice but the weight gain and adiposity obtained in these HFD-fed mice were very comparable to our recent publications using the same HFD and time course in which chow control mice were examined [33,38].
Table 1.
Analysis of tissue weights, blood glucose, serum lactate, muscle glycogen and triglyceride and serum non-esterified free fatty acids (NEFA).
| AMPK-WT |
AMPK-MKO |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFD |
HFD+R419 |
HFD |
HFD+R419 |
|||||||||
| Average | ±SEM | N | Average | ±SEM | N | Average | ±SEM | N | Average | ±SEM | N | |
| Liver | ||||||||||||
| Weight (mg) | 1257.9 | 57.3 | 14 | 1192.4 | 124.4 | 9 | 1172.2 | 60.3 | 8 | 1230.0 | 78.53 | 8 |
| Weight/gram bw (mg/g bw) | 32.5 | 0.9 | 14 | 32.8 | 2.2 | 9 | 34.9 | 2.5 | 8 | 35.6 | 1.9 | 8 |
| Epididymal adipose | ||||||||||||
| Weight (mg) | 1299.3 | 93.9 | 16 | 1224.1 | 104.4 | 12 | 1182.8 | 190.0 | 8 | 1173.4 | 119.9 | 9 |
| Weight/gram bw (mg/g bw) | 34.5 | 1.7 | 16 | 34.5 | 1.9 | 12 | 32.2 | 4.5 | 8 | 33.6 | 3.1 | 9 |
| Soleus (mg) | 11.4 | 0.4 | 13 | 11.7 | 0.3 | 7 | 11.6 | 0.5 | 8 | 10.9 | 0.5 | 7 |
| EDL (mg) | 11.0 | 0.5 | 9 | 11.6 | 0.5 | 7 | 10.9 | 0.9 | 8 | 11.2 | 0.4 | 7 |
| Glucose (mmol/l) | ||||||||||||
| Fed | 9.1 | 0.4 | 13 | 8.4† | 0.3 | 9 | 10.3§ | 0.6 | 11 | 9.2†§ | 0.5 | 6 |
| Post-Exercise | 11.6 | 0.6 | 10 | 9.5 | 0.5 | 8 | 12.5§§ | 1.2 | 10 | 10.3§§ | 0.4 | 6 |
| Lactate (mmol/l) | ||||||||||||
| Fed | 6.5 | 1.0 | 10 | 9.3 | 1.5 | 7 | 8.3 | 1.0 | 4 | 8.4 | 2.2 | 5 |
| Post-Exercise | 8.6 | 0.8 | 11 | 8.1 | 0.9 | 7 | 11.0§ | 1.3 | 7 | 11.1§ | 0.4 | 5 |
| Glycogen (mg/g tissue) | ||||||||||||
| Quadriceps | 137.3 | 6.4 | 16 | 141.3 | 9.8 | 8 | 124.9§ | 3.6 | 8 | 118.3§ | 10.3 | 8 |
| Triglycerides (μg/g tissue) | ||||||||||||
| Quadriceps | 323.9 | 29.0 | 11 | 371.8 | 107.5 | 5 | 271.1 | 54.4 | 6 | 412.8 | 57.7 | 4 |
| NEFA (mmol/l) | ||||||||||||
| Fasting (12 h) | 1.1 | 0.1 | 15 | 1.0 | 0.1 | 8 | 1.6§§§ | 0.1 | 6 | 1.4§§§ | 0.1 | 6 |
Data are expressed as means ± SEM, †p < 0.05, for difference from HFD control vs HFD + R419, and §p < 0.05, §§p < 0.01, §§§p < 0.001 for difference from WT vs AMPK-MKO, as determined by two-way ANOVA and Bonferroni post hoc test.
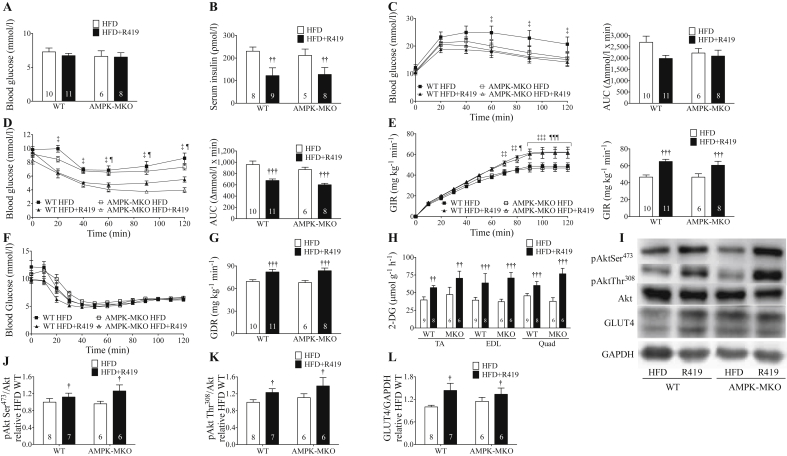
3.3. R419 improves insulin sensitivity independent of skeletal muscle AMPK
HFD AMPK-MKO mice had comparable fasting blood glucose and insulin levels compared to WT littermates irrespective of R419 treatment (Figure 2A,B). AMPK-MKO mice had higher fasting serum NEFA (Table 1). Glucose and insulin tolerance and corresponding areas under the curve (AUC) were also similar between AMPK-MKO and WT littermates fed a HFD (Figure 2C,D), indicating that a lack of AMPK does not promote the dysregulation of glucose homeostasis. Of note, the degree of glucose and insulin intolerance achieved in the HFD-fed mice of this study was comparable to many previous studies from our laboratory in which normal chow diet fed controls were also examined in parallel [33,38].
Figure 2.
R419 improves insulin sensitivity and insulin-stimulated glucose disposal in skeletal muscle. (A) 12 h fasting blood glucose. (B) 12 h fasting serum insulin. (C) Glucose tolerance test (GTT) and GTT AUC. (D) Insulin tolerance test (ITT) and ITT AUC. (E) Glucose infusion rate (GIR) curve and clamped GIR. (F) Blood glucose concentration curve during clamp. (G) Glucose disposal rate (GDR). (H) 2-[14C]DG uptake in TA, EDL, and quadriceps (quad) muscle in WT and AMPK-MKO mice treated with or without R419. (I) Representative western blots of pAkt Ser473, pAkt Ser308, total Akt, GLUT4, and GAPDH. (J) Protein expression of pAkt Ser473 relative to Akt (from separate gels). (K) Protein expression pAkt Thr308 relative to Akt (from separate gels). (L) Protein expression of GLUT4 relative to GAPDH. Numbers within bars represent number of mice per group. Data are expressed as means ± SEM, †p < 0.05, ††p < 0.01, †††p < 0.001 for difference from HFD control vs HFD + R419, as determined by two-way ANOVA and Bonferroni post hoc test; ‡p < 0.05, ‡‡p < 0.01, ‡‡‡p < 0.001 for difference between HFD WT and HFD + R419 WT, and ¶p < 0.05, ¶¶¶p < 0.001 for difference between HFD AMPK-MKO and HFD + R419 MKO, as determined by two-way repeated measures ANOVA and Bonferroni post hoc test (C,D,E,F).
Treatment of mice with R419 did not alter fasting blood glucose (Figure 2A) but it did significantly reduce fasting serum insulin in both WT and AMPK-MKO mice (Figure 2B). Consistent with these findings, R419 tended to improve glucose tolerance and significantly increased whole-body insulin tolerance in both WT and AMPK-MKO mice (Figure 2C,D). These data indicate that R419 improves whole-body insulin sensitivity in obese mice through a pathway independent of skeletal muscle AMPK.
To examine the tissues contributing to these improvements in insulin sensitivity, we conducted hyperinsulinemic-euglycaemic clamps. R419 treatment did not affect basal blood glucose or basal glucose turnover (Supplementary Table 1). Clamped serum glucose and insulin levels were comparable between groups (Supplementary Table 1). However, R419 treatment increased insulin-stimulated glucose infusion rates (GIR) in both WT and AMPK-MKO mice (Figure 2E,F). Consistent with an elevated GIR, the insulin-stimulated glucose disposal rate (GDR) was increased in both WT and AMPK-MKO mice treated with R419 (Figure 2G). Enhanced GDR with R419 treatment was accompanied by elevated 2-[14C]DG uptake at the completion of the clamp into all muscle types of both WT and AMPK-MKO mice (Figure 2H). There were no differences between groups in circulating NEFA (Supplementary Table 1), suggesting that adipose tissue insulin sensitivity was not altered with R419 treatment. During the clamp, R419 treatment had a modest effect on reducing hepatic glucose output (HGO) and tended to increase the percent (%) suppression of HGO in WT mice (Supplementary Table 1).
To investigate the intracellular mechanisms mediating enhanced insulin-stimulated 2-[14C]DG uptake into muscle, we assessed Akt phosphorylation and GLUT4 expression (Figure 2I). Improvements in skeletal muscle insulin-stimulated glucose disposal with R419 treatment were associated with enhanced phosphorylation of Akt Thr308 and Ser473 (Figure 2J,K). As AMPK may also increase insulin-stimulated glucose uptake by increasing the transcription of GLUT4 through phosphorylation of histone deacetylases (HDACs) [39], we examined skeletal muscle GLUT4 expression. R419 treatment increased skeletal muscle GLUT4 protein expression in both WT and AMPK-MKO mice (Figure 2L). These data indicate that R419 likely increases glucose disposal in skeletal muscle by increasing both insulin sensitivity and GLUT4 expression via pathways that are independent of skeletal muscle AMPK.
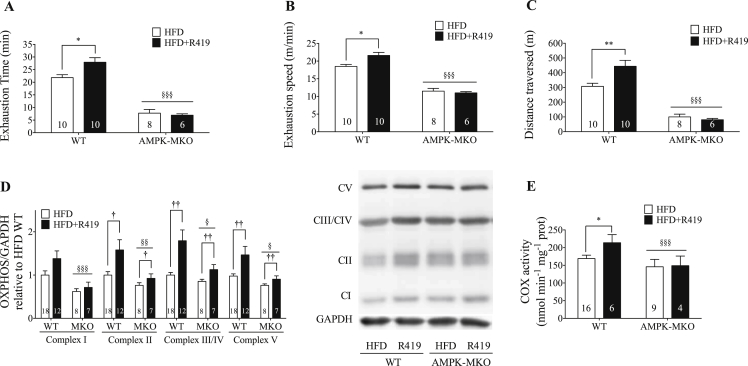
3.4. R419 improves exercise capacity via an AMPK dependent pathway
In agreement with our previous findings in chow-fed mice [25], time and speed at exhaustion were significantly impaired in AMPK-MKO compared to WT mice (Figure 3A,B). Despite obtaining a much lower maximal running speed, AMPK-MKO mice had elevated serum lactates and displayed significantly elevated blood glucose levels, consistent with their inability to stimulate muscle glucose uptake during treadmill running (Table 1), as previously reported [25]. R419 treatment increased time to exhaustion, speed at exhaustion and distance covered in WT but not AMPK-MKO mice (Figure 3A,B,C).
Figure 3.
R419 improves exercise capacity via an AMPK dependent pathway involving increased content and activity of the electron transport chain. (A) Time to exhaustion. Significant interaction between treatment and genotype (p = 0.03). (B) Speed at exhaustion. Significant interaction between treatment and genotype (p = 0.02). (C) Distance traversed. Significant interaction between treatment and genotype (p = 0.02). (D) OXPHOS complex expression in quadriceps muscle. Representative image of OXPHOS blot from the same membrane. (E) COX Activity. Data are expressed as means ± SEM, †p < 0.05, ††p < 0.01 for difference from HFD control vs HFD + R419; *p < 0.05 for difference from HFD control post hoc within genotype; §p < 0.05, §§p < 0.01, §§§p < 0.001 for difference from WT vs AMPK-MKO, as determined by two-way ANOVA and Bonferroni post hoc test.
To examine the potential mechanism mediating these effects, we first assessed muscle glycogen and triglycerides and found that R419 did not affect these parameters (Table 1). In HFD-fed mice, mitochondrial complex proteins were reduced in AMPK-MKO mice compared to WT (Figure 3D). R419 increased the protein content of Complex II, III/IV, and V of the respiratory chain in WT mice. These effects of R419 were blunted in AMPK-MKO mice (Figure 3D). There was also a tendency for increased protein expression of Complex I. R419 elevated COX activity in quadriceps (quad) muscle of WT but not AMPK-MKO mice. Collectively, these data suggest that R419 increases mitochondrial biogenesis (Figure 3E).
4. Discussion
R419 has recently been shown to acutely activate AMPK in a variety of cell systems [30] but its effects on whole-body glucose homeostasis, insulin sensitivity and exercise capacity are not known. We demonstrate that chronic R419 treatment lowered fasting insulin, improved glucose tolerance and insulin-stimulated glucose disposal into skeletal muscle, independently of alterations in adiposity in both WT and AMPK-MKO mice. Increases in skeletal muscle 2-DG uptake were associated with elevated Akt phosphorylation and increased GLUT4 expression. In addition, R419 treatment increased exercise capacity and electron transport chain content and activity in WT, but not AMPK-MKO mice. These data indicate that in the context of HFD-induced obesity, R419 may be a promising therapy for improving exercise capacity and glucose homeostasis.
Despite the well-documented role of AMPK activators improving insulin sensitivity, most studies have found that mice having reductions in skeletal muscle AMPK activity fed a control chow [25,34,40] or obesity-promoting HFD diet [40,41] have normal skeletal muscle insulin sensitivity compared to wildtype littermates; although it should be noted that some studies have detected modest reductions in muscle insulin sensitivity [17,42,43]. While previous studies have investigated the effects of an obesity-inducing HFD on a genetic background of lower muscle AMPK activity, it is possible that small amounts of residual AMPK activity may have been sufficient to maintain skeletal muscle insulin sensitivity in these previous reports [40,41]. Thus it was unknown whether AMPK-MKO mice might be more susceptible to HFD-induced obesity and insulin resistance. We found that body mass and adiposity were comparable between genotypes. Insulin tolerance was also unchanged between genotypes as were rates of glucose infusion and glucose disposal during hyperinsulinemic-euglycemic clamps. In contrast to our previous studies in young [25] and aged [34] AMPK-MKO mice which had normal glucose tolerance, we found that there was a tendency for AMPK-MKO mice to have improved glucose tolerance and increased muscle GLUT4 expression when fed HFD. We also found that the large reduction in muscle mitochondrial content we have previously observed in chow-fed AMPK-MKO mice [25] was largely attenuated (so that there were minimal genotype differences) when mice were fed HFD. These data are consistent with findings that HFD-induced mitochondrial biogenesis occurs via a pathway involving calcium/calmodulin-dependent protein kinase [44]. Collectively, these data indicate that a lack of skeletal muscle AMPK does not enhance the development of HFD-induced obesity or insulin resistance and suggest that future studies investigating the induction of compensatory pathways in AMPK-MKO mice in response to HFD are warranted.
Obese mice treated with R419 had lower fasting serum insulin levels, irrespective of genotype. Notable improvements were also observed in glucose and insulin tolerance with R419 treatment. Hyperinsulinemic-euglycaemic clamps revealed that improved insulin sensitivity was mediated by enhanced insulin-stimulated glucose disposal into skeletal muscle and that this effect was independent of skeletal muscle AMPK. In addition to enhancing insulin-stimulated skeletal muscle glucose uptake in a skeletal muscle AMPK independent manner, R419 modestly reduced insulin-stimulated hepatic glucose output; however, it should be noted that the insulin-simulated % suppression of HGO was not significantly different between treatments. Given the modest effect on HGO (and lack of a significant increase in the % suppression), these data suggest, that, in contrast to metformin that primarily elicits its insulin sensitizing effects by acting in the liver [33,45], R419 primarily elicits its glucose lowering/insulin sensitizing effects by enhancing skeletal muscle glucose uptake.
In order to assess the potential mechanisms contributing to the improved insulin-stimulated glucose disposal/2-DG uptake into skeletal muscle following R419 treatment, we assessed activating phosphorylation of Akt and total GLUT4 expression. We found that R419 treated mice had greater Akt phosphorylation and increased GLUT4 expression compared to HFD-fed mice. The overexpression of GLUT4 in skeletal muscle enhances insulin-stimulated glucose uptake and reduces fasting insulin in HFD-fed mice [46]. Enhanced GLUT4 expression may also alter hepatic glucose metabolism/insulin sensitivity, thus explaining the potentially modest changes observed in liver insulin sensitivity [47]. This suggests that increased glucose disposal in skeletal muscle via R419 may be mediated in part through increases in GLUT4 expression via AMPK-independent pathways. These findings are consistent with reports that GLUT4 expression is also increased following exercise via AMPK-independent pathways [48,49]. Future studies investigating the AMPK-independent mechanisms controlling GLUT4 transcription are warranted.
Exercise training is associated with the induction of mitochondrial biogenesis and enhanced exercise performance (for review see Richter & Ruderman 2009 [22] and O'Neill et al., 2011 [23]). R419 improved treadmill running capacity in WT mice by over 30%, while having no effect in AMPK-MKO mice. This increase in treadmill running capacity is comparable to other studies in mice with endurance exercise training [50,51]. A limitation of our study was that we defined fatigue/exhaustion as the point at which instead of running on the treadmill, mice remained on the shockers, which serve to encourage running, for more than 10 s. Future studies measuring biochemical measures of exhaustion ([52], e.g. muscle and liver glycogen, lactate) with and without R419 and/or muscle AMPK deficiency will be important to establish genuine fatigue and to confirm the effects were not due to changes in motivation or altered sensitivity to the electrical shocking system.
A feature of studies in which AMPK has been activated using either genetic gain of function [53] or pharmacological agents such as AICAR [54] is that muscle glycogen contents are elevated; which could be a primary factor contributing to the enhanced exercise performance [42]. With R419 treatment, there was no change in muscle glycogen content. Instead, improvements in treadmill running capacity in WT mice were associated with increased protein expression of subunits of the electron transport chain and COX activity. These observations that R419, a complex-I inhibitor, can enhance mitochondrial content and function are consistent with previous findings indicating that siRNA mediated complex-1 inhibition in C. elegans induces mitohormesis (an increase in mitochondrial biogenesis and efficiency) [55]. Interestingly, these effects were blunted in AMPK-MKO mice suggesting that R419 primarily elicits its effects on mitochondrial function through a pathway involving AMPK. Future studies investigating the downstream substrates mediating R419 effects on mitochondrial function are warranted.
In summary, chronic treatment with R419 leads to substantial improvements in glucose homeostasis, effects that are primarily mediated through enhanced skeletal muscle insulin sensitivity and are independent of skeletal muscle AMPK. In addition, chronic treatment of obese mice with R419 elicits improvements in exercise capacity and skeletal muscle electron transport chain content/activity in WT mice, effects which are blunted in the absence of AMPK. These data indicate that R419 mimics many of the effects of chronic exercise training in skeletal muscle and suggest that R419 may be of therapeutic importance for improving exercise capacity and skeletal muscle insulin sensitivity in obesity.
Author contributions
KM, YH and GRS conception and design of research; KM, ALB, JSL, RJF, THW, BKS, YJ, WL, TMK performed experiments; KM, ALB, JSL, THW, BKS analyzed data; KM, YH and GRS interpreted results of experiments; KM and GRS prepared figures; KM, YH and GRS drafted manuscript; KM, BEK, YH, YJ and GRS edited and revised manuscript; KM, ALB, JSL, RJF, THW, BKS, YJ, WL, TMK, BEK, YH and GRS approved final version of manuscript. KM and GRS are responsible for the integrity of the work as a whole.
Funding
These studies were supported by research grants from Rigel Pharmaceuticals, Inc. (K.04521.00.RES.FND) and the Canadian Institutes of Health Research (RGPIN-371870-2009) (GRS). GRS is a Canada Research Chair in Metabolism and Obesity and the J Bruce Duncan Chair in Metabolic Diseases. BEK was supported by grants from the Australian Research Council (DP130104548) and the National Health and Medical Research Council (1028254, 1078752) and in part by the Victorian Government's Operational Infrastructure Support Program.
Acknowledgments
We would like to acknowledge Drs. Donald G. Payan, Vadim Markovtsov, Kristen A. Baltgalvis for useful discussions and exchange of information and Dr. Simon J. Shaw for compound synthesis. We thank R. Rhem from the McMaster Centre for Translational Imaging for completing the computed tomography scans.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2015.06.002
Conflict of interest
YJ, WL, TMK and YH are employees of Rigel Pharmaceuticals, Inc. No other potential conflicts of interest relevant to this article were reported.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Hyperinsulinemic-euglycemic clamp serum analytes and parameters. Data are expressed as means ± SEM, §§p < 0.01, for difference from WT vs AMPK-MKO, ‡p < 0.05 for difference between HFD WT and HFD + R419 WT, as determined by two-way ANOVA and Bonferroni post hoc test.
References
- 1.Ruderman N.B., Carling D., Prentki M., Cacicedo J.M. AMPK, insulin resistance, and the metabolic syndrome. The Journal of Clinical Investigation. 2013;123(7):2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberg G.R., Jorgensen S.B. The AMP-activated protein kinase: role in regulation of skeletal muscle metabolism and insulin sensitivity. Mini-Reviews in Medicinal Chemistry. 2007;7(5):519–526. doi: 10.2174/138955707780619662. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khalili L., Forsgren M., Kannisto K., Zierath J.R., Lönnqvist F., Krook A. Enhanced insulin-stimulated glycogen synthesis in response to insulin, metformin or rosiglitazone is associated with increased mRNA expression of GLUT4 and peroxisomal proliferator activator receptor gamma co-activator 1. Diabetologia. 2005;48(6):1173–1179. doi: 10.1007/s00125-005-1741-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y.-C., Lee S.-D., Ho L.-T., Kuo C.-H. The effects of Altitude training on the AMPK-related glucose transport pathway in the red skeletal muscle of both Lean and obese Zucker rats. High Altitude Medicine & Biology. 2011;12(4):371–378. doi: 10.1089/ham.2010.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes A.P., Duarte F.V., Nunes P., Hubbard B.P., Teodoro J.S., Varela A.T. Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochimica et Biophysica Acta – Molecular Basis of Disease. 2012;1822(2):185–195. doi: 10.1016/j.bbadis.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iglesias M.A., Ye J.M., Frangioudakis G., Saha A.K., Tomas E., Ruderman N.B. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51(10):2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 7.Kumar N., Dey C.S. Metformin enhances insulin signalling in insulin-dependent and-independent pathways in insulin resistant muscle cells. British Journal of Pharmacology. 2002;137(3):329–336. doi: 10.1038/sj.bjp.0704878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kjøbsted R., Treebak J.T., Fentz J., Lantier L., Viollet B., Birk J.B. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes. 2015;64(6):2042–2055. doi: 10.2337/db14-1402. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen J.M., Treebak J.T., Schjerling P., Goodyear L., Wojtaszewski J.F.P. Two weeks of metformin treatment induces AMPK-dependent enhancement of insulin-stimulated glucose uptake in mouse soleus muscle. American Journal of Physiology. Endocrinology and Metabolism. 2014;306(10):E1099–E1109. doi: 10.1152/ajpendo.00417.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watt M.J., Dzamko N., Thomas W.G., Rose-John S., Ernst M., Carling D. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nature Medicine. 2006;12(5):541–548. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z., Wang X., He Y., Qi L., Yu L., Xue B. The full capacity of AICAR to reduce obesity-induced inflammation and insulin resistance requires Myeloid SIRT1. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turban S., Stretton C., Drouin O., Green C.J., Watson M.L., Gray A. Defining the contribution of AMP-activated protein kinase (AMPK) and protein kinase C (PKC) in regulation of glucose uptake by metformin in skeletal muscle cells. Journal of Biological Chemistry. 2012;287(24):20088–20099. doi: 10.1074/jbc.M111.330746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee-Young R.S., Bonner J.S., Mayes W.H., Iwueke I., Barrick B.A., Hasenour C.M. AMP-activated protein kinase (AMPK)α2 plays a role in determining the cellular fate of glucose in insulin-resistant mouse skeletal muscle. Diabetologia. 2013;56(3):608–617. doi: 10.1007/s00125-012-2787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: from mechanisms of action to therapies. Cell Metabolism. 2014;20(6):953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Scott J.W., van Denderen B.J.W., Jorgensen S.B., Honeyman J.E., Steinberg G.R., Oakhill J.S. Thienopyridone drugs are selective activators of AMP-activated protein kinase β1-containing complexes. Chemistry and Biology. 2008;15(11):1220–1230. doi: 10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Hawley S.A., Fullerton M.D., Ross F.A., Schertzer J.D., Chevtzoff C., Walker K.J. The Ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336(6083):918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinberg G.R., O'Neill H.M., Dzamko N.L., Galic S., Naim T., Koopman R. Whole body deletion of AMP-activated protein kinase {beta}2 reduces muscle AMPK activity and exercise capacity. The Journal of Biological Chemistry. 2010;285(48):37198–37209. doi: 10.1074/jbc.M110.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wojtaszewski J.F.P., Birk J.B., Frøsig C., Holten M., Pilegaard H., Dela F. 5′ AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. Journal of Physiology. 2005;564(2):563–573. doi: 10.1113/jphysiol.2005.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter R.W., Foretz M., Bultot L., Fullerton M.D., Deak M., Ross F.A. Mechanism of action of compound-13: an α1-selective small molecule activator of AMPK. Chemistry and Biology. 2014;21(7):866–879. doi: 10.1016/j.chembiol.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen T.E., Ross F.A., Kleinert M., Sylow L., Knudsen J.R., Gowans G.J. PT-1 selectively activates AMPK-γ1 complexes in mouse skeletal muscle, but activates all three γ subunit complexes in cultured human cells by inhibiting the respiratory chain. Biochemical Journal. 2015;467(3):461–472. doi: 10.1042/BJ20141142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai Y.-C., Kviklyte S., Vertommen D., Lantier L., Foretz M., Viollet B. A small-molecule benzimidazole derivative that potently activates AMPK to increase glucose transport in skeletal muscle: comparison with effects of contraction and other AMPK activators. The Biochemical Journal. 2014;460(3):363–375. doi: 10.1042/BJ20131673. [DOI] [PubMed] [Google Scholar]
- 22.Richter E.A., Ruderman N.B. AMPK and the biochemistry of exercise: implications for human health and disease. The Biochemical Journal. 2009;418(2):261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neill H.M., Holloway G.P., Steinberg G.R. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity. Molecular and Cellular Endocrinology. 2013;366(2):135–151. doi: 10.1016/j.mce.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Lantier L., Fentz J., Mounier R., Leclerc J., Treebak J.T., Pehmøller C. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB Journal. 2014;28(7):3211–3224. doi: 10.1096/fj.14-250449. [DOI] [PubMed] [Google Scholar]
- 25.O'Neill H.M., Maarbjerg S.J., Crane J.D., Jeppesen J., Jorgensen S.B., Schertzer J.D. AMP-activated protein kinase (AMPK) 1 2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proceedings of the National Academy of Sciences. 2011;108(38):16092–16097. doi: 10.1073/pnas.1105062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes B.R., Glund S., Long Y.C., Hjälm G., Andersson L., Zierath J.R. 5’-AMP-activated protein kinase regulates skeletal muscle glycogen content and ergogenics. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2005;19(7):773–779. doi: 10.1096/fj.04-3221com. [DOI] [PubMed] [Google Scholar]
- 27.Rockl K.S.C., Hirshman M.F., Brandauer J., Fujii N., Witters L.A., Goodyear L.J. Skeletal muscle adaptation to exercise training - AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56(8):2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- 28.Winder W.W., Holmes B.F., Rubink D.S., Jensen E.B., Chen M., Holloszy J.O. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. Journal of Applied Physiology (Bethesda, Md.: 1985) 2000;88(6):2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 29.Zong H., Ren J.M., Young L.H., Pypaert M., Mu J., Birnbaum M.J. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(25):15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins Y., Sun T.Q., Markovtsov V., Foretz M., Li W., Nguyen H. AMPK activation through mitochondrial regulation results in increased substrate oxidation and improved metabolic parameters in models of diabetes. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0081870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzamko N., Schertzer J.D., Ryall J.G., Steel R., Macaulay S.L., Wee S. AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. The Journal of Physiology. 2008;586(Pt 23):5819–5831. doi: 10.1113/jphysiol.2008.159814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galic S., Fullerton M.D., Schertzer J.D., Sikkema S., Marcinko K., Walkley C.R. Hematopoietic AMPK1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. Journal of Clinical Investigation. 2011;121(12):4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fullerton M.D., Galic S., Marcinko K., Sikkema S., Pulinilkunnil T., Chen Z.-P. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nature Medicine. 2013;19(12):1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bujak A.L., Blümer R.M.E., Marcinko K., Fullerton M.D., Kemp B.E., Steinberg G.R. Reduced skeletal muscle AMPK and mitochondrial markers do not promote age-induced insulin resistance. Journal of Applied Physiology (Bethesda, Md.: 1985) 2014;117(2):171–179. doi: 10.1152/japplphysiol.01101.2013. (May) [DOI] [PubMed] [Google Scholar]
- 35.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 36.Bligh E.G., Dyer W.J. A rapid Method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 37.Gowans G.J., Hawley S.A., Ross F.A., Hardie D.G. AMP is a true physiological regulator of amp-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metabolism. 2013;18(4):556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jørgensen S.B., Nielsen J.N., Birk J.B., Olsen G.S., Viollet B., Andreelli F. The α2-5′ AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53(12):3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- 39.McGee S.L., Van Denderen B.J.W., Howlett K.F., Mollica J., Schertzer J.D., Kemp B.E. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57(4):860–867. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- 40.Beck Jørgensen S., O'Neill H.M., Hewitt K., Kemp B.E., Steinberg G.R. Reduced AMP-activated protein kinase activity in mouse skeletal muscle does not exacerbate the development of insulin resistance with obesity. Diabetologia. 2009;52(11):2395–2404. doi: 10.1007/s00125-009-1483-8. [DOI] [PubMed] [Google Scholar]
- 41.Frøsig C., Jensen T.E., Jeppesen J., Pehmøller C., Treebak J.T., Maarbjerg S.J. AMPK and insulin action–responses to ageing and high fat diet. PloS One. 2013;8(5):e62338. doi: 10.1371/journal.pone.0062338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodyear L.J. The exercise pill–too good to be true? The New England Journal of Medicine. 2008;359(17):1842–1844. doi: 10.1056/NEJMcibr0806723. [DOI] [PubMed] [Google Scholar]
- 43.Viollet B., Andreelli F., Jørgensen S.B., Perrin C., Geloen A., Flamez D. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. The Journal of Clinical Investigation. 2003;111(1):91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain S.S., Paglialunga S., Vigna C., Ludzki A., Herbst E. a, Lally J.S. High-fat diet-induced mitochondrial biogenesis is regulated by mitochondrial derived reactive oxygen species activation of CaMKII. Diabetes. 2014;63(6):1–15. doi: 10.2337/db13-0816. [DOI] [PubMed] [Google Scholar]
- 45.Shu Y., Sheardown S.A., Brown C., Owen R.P., Zhang S., Castro R.A. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. The Journal of Clinical Investigation. 2007;117(5):1422–1431. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsao T.S., Burcelin R., Katz E.B., Huang L., Charron M.J. Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle. Diabetes. 1996;45(1):28–36. doi: 10.2337/diab.45.1.28. [DOI] [PubMed] [Google Scholar]
- 47.Ranalletta M., Du X.Q., Seki Y., Glenn A.S., Kruse M., Fiallo A. Hepatic response to restoration of GLUT4 in skeletal muscle of GLUT4 null mice. American Journal of Physiology. Endocrinology and Metabolism. 2007;293(5):E1178–E1187. doi: 10.1152/ajpendo.00628.2006. [DOI] [PubMed] [Google Scholar]
- 48.Holmes B.F., Lang D.B., Birnbaum M.J., Mu J., Dohm G.L. AMP kinase is not required for the GLUT4 response to exercise and denervation in skeletal muscle. American Journal of Physiology. Endocrinology and Metabolism. 2004;287(4):E739–E743. doi: 10.1152/ajpendo.00080.2004. [DOI] [PubMed] [Google Scholar]
- 49.Murgia M., Jensen T.E., Cusinato M., Garcia M., Richter E.A., Schiaffino S. Multiple signalling pathways redundantly control glucose transporter GLUT4 gene transcription in skeletal muscle. The Journal of Physiology. 2009;587(Pt 17):4319–4327. doi: 10.1113/jphysiol.2009.174888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jordy A.B., Kraakman M.J., Gardner T., Estevez E., Kammoun H.L., Weir J.M. Analysis of the liver lipidome reveals insights into the protective effect of exercise on high-fat diet-induced hepatosteatosis in mice. American Journal of Physiology – Endocrinology and Metabolism. 2015;308(9):E778–E791. doi: 10.1152/ajpendo.00547.2014. [DOI] [PubMed] [Google Scholar]
- 51.Lund J., Hafstad A.D., Boardman N.T., Rossvoll L., Rolim N.P., Ahmed M.S. Exercise training promotes cardioprotection through oxygen-sparing action in high fat-fed mice. American Journal of Physiology – Heart and Circulatory Physiology. 2015;308(8):H823–H829. doi: 10.1152/ajpheart.00734.2014. [DOI] [PubMed] [Google Scholar]
- 52.Booth F.W., Laye M.J., Spangenburg E.E. Gold standards for scientists who are conducting animal-based exercise studies. Journal of Applied Physiology. 2010;108:219–221. doi: 10.1152/japplphysiol.00125.2009. [DOI] [PubMed] [Google Scholar]
- 53.Barré L., Richardson C., Hirshman M.F., Brozinick J., Fiering S., Kemp B.E. Genetic model for the chronic activation of skeletal muscle AMP-activated protein kinase leads to glycogen accumulation. American Journal of Physiology. Endocrinology and Metabolism. 2007;292(3):E802–E811. doi: 10.1152/ajpendo.00369.2006. [DOI] [PubMed] [Google Scholar]
- 54.Aschenbach W.G., Hirshman M.F., Fujii N., Sakamoto K., Howlett K.F., Goodyear L.J. Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes. 2002;51(3):567–573. doi: 10.2337/diabetes.51.3.567. [DOI] [PubMed] [Google Scholar]
- 55.Owusu-Ansah E., Song W., Perrimon N. XMuscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell. 2013;155(3) doi: 10.1016/j.cell.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hyperinsulinemic-euglycemic clamp serum analytes and parameters. Data are expressed as means ± SEM, §§p < 0.01, for difference from WT vs AMPK-MKO, ‡p < 0.05 for difference between HFD WT and HFD + R419 WT, as determined by two-way ANOVA and Bonferroni post hoc test.