Abstract
Although injuries to the posterolateral corner of the knee were previously considered to be a rare condition, they have been shown to be present in almost 16% of all knee injuries and are responsible for sustained instability and failure of concomitant reconstructions if not properly recognized. Although also once considered to be the “dark side of the knee”, increased knowledge of the posterolateral corner anatomy and biomechanics has led to improved diagnostic ability with better understanding of physical and imaging examinations. The management of posterolateral corner injuries has also evolved and good outcomes have been reported after operative treatment following anatomical reconstruction principles.
Keywords: Knee injuries, Knee joint, Reconstructive surgical procedures/methods, Knee/anatomy & histology, Biomechanical phenomena
Resumo
Embora as lesões do canto posterolateral do joelho tenham sido previamente consideradas como uma condição rara, elas estão presentes em quase 16% de todas as lesões de joelho e são responsáveis pela instabilidade sustentada e falha das reconstruções concomitantes caso não tenham sido adequadamente reconhecidas. Embora tenha sido considerado como o “lado negro do joelho”, o maior conhecimento da anatomia e da biomecânica do canto posterolateral levou à melhoria da capacidade diagnóstica e à melhor compreensão do exame físico e de imagem. O manejo das lesões do canto posterolateral evoluiu e bons desfechos têm sido relatados após o tratamento cirúrgico que segue princípios da reconstrução anatômica.
Palavras-chave: Lesões do joelho, Articulação do joelho, Procedimentos de cirurgia reconstrutiva/métodos, Anatomia & histologia do joelho, Fenômeno biomecânico
Introduction
Posterolateral instability may cause significant functional limitations. Although previously considered rare, posterolateral corner (PLC) injuries have been increasingly recognized and account for approximately 16% of all knee ligament injuries,1 often presenting with concomitant anterior and posterior cruciate ligament injuries.2, 3, 4 Failure to detect these injuries has been shown to be an important cause of recurrent instability and failed cruciate ligament reconstructions.5, 6, 7, 8, 9, 10 In the past, treatment of lateral side instability has been challenging due to limited data on the anatomy and biomechanics of the PLC structures and under-reporting of clinical outcomes following non-operative and operative treatment. However, more recently, the anatomy and biomechanics have become well-defined and good outcomes have been reported after PLC operative treatment following anatomic reconstruction principles.11 The purpose of this article is to review the current state of knowledge regarding PLC injuries.
Anatomy and biomechanics
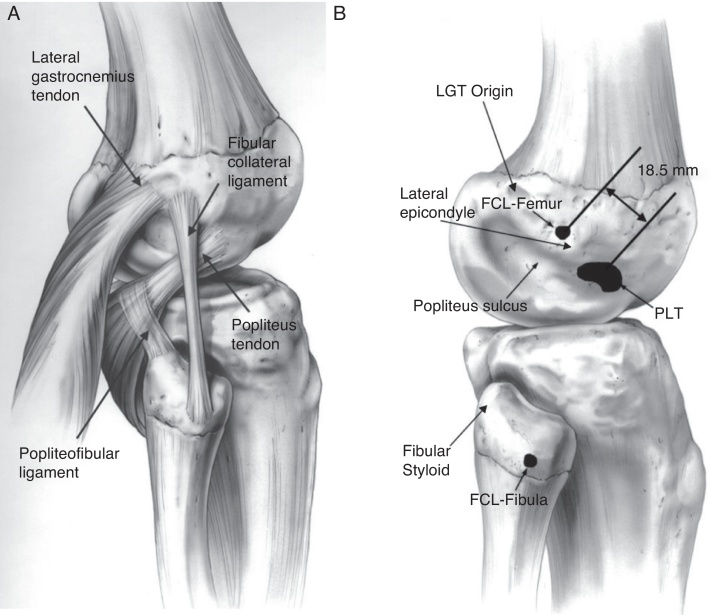
Appreciation of the complex anatomy and biomechanics of the PLC is critical for understanding the physical exam, imaging, and treatment of PLC injuries. The main structures that provide stability to the lateral aspect of the knee are the fibular collateral ligament (FCL), popliteus tendon, and popliteofibular ligament.8, 12, 13, 14, 15 (Fig. 1).
Fig. 1.
Anatomy of the posterolateral corner is represented (A) with the three main structures responsible for lateral side stability: popliteus tendon, popliteofibular ligament and fibular collateral ligament. The anatomical footprints of these structures are highlighted in (B) B. (Reprinted with permission from Am J Sports Med. 2003;31:854–860.).
The FCL is a ligamentous structure that originates from a depression located 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle.15 The distal insertion is located 28.4 mm distal to the tip of the fibula head.15 The FCL averages 7 cm in length and courses underneath the superficial layer of the iliotibial band. The FCL acts as the primary stabilizer to varus stress on the knee and helps stabilize against external rotation torque in lower degrees of flexion.16
The popliteus tendon runs obliquely from the posteromedial aspect of the tibia becoming more tendinous as it courses laterally. It inserts on a relatively broad area (59 mm2) on the anterior fifth of the popliteus sulcus, just posterior to the lateral femoral condyle articular cartilage surface.15 This insertion site is consistently anterior to the FCL insertion site by an average distance of 18.5 mm,15 demonstrating that an anatomic reconstruction is not achievable with a one femoral tunnel technique. The popliteus tendon runs underneath the FCL, through the femoral popliteus sulcus and becomes intra-articular on the posterior aspect of the lateral femoral condyle.
The popliteofibular ligament is consistently present, originating from popliteus musculotendinous junction and inserting on the posteromedial aspect of the fibula head. Both the popliteus tendon and popliteofibular ligament contribute to external rotatory stability. The posterolateral complex and the posterior cruciate ligament (PCL) have a synergistic relationship, with the PCL acting as a secondary restraint preventing external rotation and the PLC helping in resisting posterior tibial translation, mostly in lower degrees of flexion.
Other structures are also found in the posterolateral corner of the knee. The long head of the biceps attachment is divided into a direct arm that attaches in the posterolateral aspect of the fibula head and an anterior arm that fans out superficial to the FCL, forming a bursa that must be accessed during an FCL reconstruction. The posterior most aspect of the posterolateral corner is composed of the lateral head of the gastrocnemius muscle, which attaches on the supracondylar ridge on the lateral femoral condyle. In addition, the gastrocnemius is an important landmark during a PLC surgical procedure because the area between the gastrocnemius muscle belly and the posterolateral capsule and soleus muscle must be dissected down to allow placement of retractors to protect the neurovascular bundle during tibial tunnel drilling. The iliotibial band is a thick fascial structure that runs superficial to the tensor fasciae lata muscle, immediately under the subcutaneous tissue, and covers all of the PLC femoral attachments. It originates on the anterior superior iliac spine and the external lip of the iliac crest and inserts on the lateral aspect of tibia at Gerdy's tubercle.
The common peroneal nerve originates from a bifurcation of the sciatic nerve in the distal thigh. The nerve runs distal, lying posterior to the long head of the biceps, and crossing around the lateral aspect of the fibula neck before dividing into superficial and deep peroneal nerves. The proximity of the nerve to the PLC structures makes identification and neurolysis of the nerve important aspects of the surgical technique.
The lateral side of the knee is inherently unstable due to the lack of conformity between the convex lateral femoral condyle and the convex tibial lateral plateau, coupled with higher mobility of the lateral meniscus.17 Additionally, the normal mechanical axis of the main population crosses the knee slightly medial to the neutral axis of the knee and, during the adductor moment, this axis becomes even more medial. The integrity of the PLC is of paramount importance to avoid the opening of the lateral side of the joint and overloading of the medial compartment.
The primary role of the PLC in preventing tibial anterior translation in a normal knee is minimal. However, in an ACL deficient knee, the medial meniscus and the PLC function as secondary stabilizers, with the PLC acting mostly in the early degrees of flexion. Posterior translation is mainly controlled by the PCL, but the PLC acts as a secondary restraint in early knee flexion. However, combined PLC and PCL injuries present with greatly increased posterior tibial translation compared to isolated PCL injuries. The FCL functions as the primary stabilizer to varus stress at all degrees of flexion. The highest load on the FCL occurs at 30° of flexion when secondary stabilizers contribute less. No varus gapping occurs in PLC injuries where the FCL remains intact. However, a FCL injury associated popliteus complex injury presents with increased varus gapping compared to an isolated FCL injury. Traditionally, the popliteus complex was understood to be the primary restrainer of the external rotation of the knee.18 However, recent studies have described that the FCL helps to control external rotation in the beginning of knee flexion (0–30°),5 while the popliteus complex controls external rotation at higher degrees of knee flexion. The PCL also contributes to external rotatory stability as a secondary restrainer when a PLC injury is presentmost effectively after 90° of flexion.
Evaluation
Clinical evaluation
An accurate assessment of PLC injuries is important since the failure to diagnose and treat PLC instability can lead to recurrent instability and failure of concomitant reconstruction procedures.6, 19 The PLC patient usually presents with a history of acute trauma related to motor vehicle accidents and sports injuries.20 Blunt trauma to the anteromedial aspect of the tibia with a posterolateral directed force, knee hyperextension, and external tibial rotation over a fixed foot are the most common injury mechanisms.21 In acute cases, pain over the joint line, ecchymosis, swelling, and inability to walk are the main complains. In chronic cases, instability with side-to-site activities and limited ability to resume sports activities are common complaints. Usually, PLC injuries are associated to ACL or PCL tears, with only 28% of all PLC injuries been an isolated tears.22
Regarding the knee physical exam, a detailed examination should be performed to assess range of motion, patellar instability, and extensor function and to look for possible concomitant injuries. Several special tests have been described for assessing posterolateral instability including the varus stress test, posterolateral drawer test, dial test, reverse pivot-shift test, and external rotation recurvatum test.
The varus stress test is performed by positioning the knee at both 30° of flexion and in full extension while applying a varus force through the patient's foot and ankle with one hand and stabilizing the knee at the proximal thigh using the other hand. The examiner should place his fingers at the joint line to grade joint line opening relative to the contralateral knee. A positive varus stress test with opening of the lateral compartment at 30° of knee flexion but not at full extension indicates an isolated complete tear of the FCL. If gapping is still present at full extension, concomitant cruciate injury is presumed.23, 24
The posterolateral drawer test is performed with the patient in supine position, the knee flexed at 90°, and the foot 15° externally rotated and stabilized by the examiner. A posterior directed force is applied against the tibia and a positive test consists of increased posterior translation and external rotation when compared to the contralateral side, indicating injury of FCL, popliteus tendon, and popliteofibular ligament.
With the patient in the supine position, the external rotation recurvatum test is performed by lifting the patient's leg by the great toe while stabilizing the distal thigh with the other hand. The amount of genu recurvatum produced by the maneuver should be compared to the uninjured side. Measurement of the heal heights using a ruler can objectively determine the amount of recurvatum. A negative test should be interpreted with caution due to the high incidence of false negative results.
The reverse pivot-shift test is performed by positioning the patient in supine position with the knee flexed to 90°. A valgus load and external rotation force is applied while the knee is slowly extended. If a PLC injury is present, the load will cause posterolateral subluxation of the tibial plateau and, when the knee reaches around 30° of flexion, the iliotibial band will cause to tibia to abruptly reduce. A positive reverse pivot-shift must always be compared to the uninjured side because it can be positive in 35% of normal knees.
Rotational stability can be evaluated using the dial test. The dial test is performed with the patient both in the prone and supine positions by stabilizing the patient's thigh and applying an external rotation force at the patient's ankle. The test is performed both at 30° of knee flexion and at 90° of knee flexion. If the patient presents with a PLC injury, a side-to-side difference of more than 10° of external rotation is expected at 30° of flexion. Because the PCL functions as a secondary stabilizer of external rotation, especially at higher degrees of flexion, a decrease in the external rotation should be seen in isolated PLC injuries at 90°. If the external rotation increases at 90°, a combined PLC and PCL injury is present.
In addition, gait must be assessed for varus thrust or hyperextension patterns, and the overall limb alignment must be evaluated because this could change the surgical plan for chronic injuries. Limb alignment and weightbearing axis should be evaluated using long-leg radiographs. A line is extended on the radiograph from the center of the femoral head to the center of the ankle mortise joint. The line should pass within the region of the eminences on the tibial plateau. If the patient is in varus alignment and has a chronic PLC tear, an opening wedge high tibial osteotomy with bone grafting is recommended to correct the alignment deformity prior to performing a PLC reconstruction procedure.
Finally, trauma related to isolated and combined PLC injuries endangers the posterior neurovascular bundle. A popliteal artery injury may be present in as many as 32% of knee dislocations,25 making assessment of distal pulses at the foot and ankle an important part of the initial evaluation. The peroneal nerve may also be injured, with 13% of all PLC injuries26 presenting symptoms that must be identified and documented. A detailed physical exam recording paresthesias or numbness over the dorsum of the foot and the first web space, muscle force grading for ankle dorsiflexion, foot eversion, and great toe extension must be performed.
Imaging
A routine x-ray workup with standing anteroposterior (AP), lateral, and axial views should be acquired to rule out the presence of fractures. A standing long-leg AP view should be obtained in chronic cases because the limb alignment should be corrected using an osteotomy prior to or at the same time of the reconstruction procedure. Additionally, varus and PCL stress X-rays can be used to obtain objective quantification of the amount of lateral compartment varus gapping and a combined PLC and PCL injury, respectively (Table 1).
Table 1.
Staging instability of the knee through stress x-rays for PLC and PCL injuries.
| Varus stress x-ray27 | <2.7 mm: normal knee or minor sprains 2.7 mm to 4 mm: complete FCL tear >4 mm: complete posterolateral injury |
| Kneeling PCL stress x-ray28 | <4 mm: Difference possible in normal patients or minor sprains 4 mm to 12 mm: found in isolated PCL injuries >12 mm: observed in patients with combined injuries of the PCL and PLC |
The magnetic resonance imaging (MRI) is another important tool for PLC management that allows identification of concurrent lesions such as meniscus tears, cartilage lesions, and occult fractures. It has been shown to have 90% sensitivity and specificity for IT band, biceps tendon, FCL, and popliteus tendon injury. The only PLC structure with lower diagnostic accuracy values was the popliteofibular ligament, with 68.8% sensitivity and 66.7% specificity.1, 29 However, for the optimal MRI diagnostic accuracy for PLC injuries, an imaging sequence using 2 mm slices in a coronal oblique plane following the obliquity of the popliteus tendon30 should be employed. Finally, bone bruise patterns can offer additional clues to the present injury, since these are found in 81% of all PLC injuries, usually on the anteromedial femoral condyle.22 Together, these imaging techniques are excellent tools to augment the diagnosis of PLC injury.
Classification and treatment rationale
Treatment of PLC injuries depends mostly on the injury grade, chronicity, and presence of associated injuries. Despite its subjectivity and a lack of relation to anatomic cutting studies, the Hughston classification31 is still very important for treatment guidance. A different classification system describing rotational instability was created by Fanelli et al.32 (Table 2).
Table 2.
| Hugston Scale for FCL instability31 (based only in the varus stress opening compared to the opposite side) | Grade I: 0–5 mm* Grade II: 5–10 mm* Grade III: >10 mm* |
| Fanelli Classification for PLC instability32 (location based, addresses rotational instability) |
Type A: mainly rotational instability (popliteus tendon and popliteofibular ligament tear) Type B: rotational instability with a mild varus stress grapping (popliteus tendon, FCL and popliteofibular ligament injury) 4 mm to 12 mm: found in isolated PCL injuries Type C: disruption of the PLC structures with a cruciate ligament injury, marked varus and external rotation instability |
Opening difference from the contralateral side.
Although non-surgical management of PLC injuries is not well documented in the literature, it seems to be effective in grades I and II isolated PLC acute injuries. The low symptomatology of low grade PLC injuries can make the evaluation of this small subgroup difficult. Good results for non-operative treatment of PLC grades I and II injuries were reported previously using an early mobilization protocol.33, 34 Minimal radiographic changes were found at 8 years follow-up. By contrast, grade III PLC injuries treated non-operatively had poor functional outcomes, persistent instability, and increased degenerative arthritic changes.33, 34 The rehabilitation protocol used by the authors for PLC conservative treatment consists of knee bracing with a knee immobilizer or brace locked in extension for 4–6 weeks. Weight bearing is usually allowed and progresses as tolerated. Active and passive range of motion exercises in the prone position are encouraged to prevent stiffness. Comparative stress x-rays after 6 weeks are recommended to assess for remaining laxity. After the initial healing period, sports-specific therapy is initiated and return to sport is allowed within 3–4 months if good balance, muscular strength, and muscular endurance are achieved.
Surgical treatment of PLC injuries is the treatment of choice for patients with isolated grade III PLC injuries, combined PLC injuries, and failed non-operative treatment. Acute surgical treatment (<3 weeks) results in improved outcomes14, 35, 36 and can avoid the necessity of an additional procedure for limb alignment correction that may be necessary in chronic cases.
Patients treated acutely may undergo repair or reconstruction procedures. Primary repairs of FCL and popliteus tendons avulsions, without midsubstance injury, may be performed within 2–3 weeks after the injury. After that point, the tissue becomes retracted and scars down, making it nearly impossible to reattach the injured structures to their native anatomic locations. However, midsubstance tears cannot be repaired regardless the time of the injury. Stannard et al.37 evaluated repair vs. reconstruction outcomes after PLC injuries and reported higher failure rates in the repair group (9% vs. 37%). The results were confirmed by a later study by Levy et al.38 with 6% failure for reconstructions versus 40% for repairs.
Several PLC reconstruction procedures have been described and can be classified as anatomic and non-anatomic according to the ligaments reconstructed and the positioning of the reconstruction tunnels. The Clancy procedure39 consists of a biceps tenodesis on the distal lateral femur to mimic the FCL. The technique recommends the placement of a screw and washer at a point anterior to the lateral epicondyle and re-routing the biceps tendon or a strip of the tendon above the screw. This creates an “isometric” construct to replace the FCL and reestablish varus stability. The Larson technique40 is performed by reconstructing the FCL with a vertical graft limb from the anterior aspect of the fibula head to the lateral femoral condyle, while adding an oblique graft limb from the posterior aspect of the fibula head to the femoral epicondyle. However, the rationale was still placing the femoral tunnel in an isometric, non-anatomic point. Modifications of the Larson technique were developed by Fanelli and Arciero and aim to achieve a more anatomical femoral FCL graft placement. Fanelli et al.32 uses a washer lock in the midpoint in between FCL and popliteus tendon and crosses the graft in a figure of eight. Arciero41 drills two holes on the femoral site to recreate the footprint of popliteus tendon and FCL. However, both techniques still use only one graft with two limbs to reconstruct three major PLC structures and fail to reproduce native anatomy.
The Stannard et al.42 reconstruction technique is a non-anatomic technique that reconstructs the FCL, popliteus tendon, and popliteofibular ligament. An anterior or posterior tibialis allograft with a minimum 24 mm length is used. After performing an exposure of the lateral knee, a tibia tunnel is drilled from anterior to posterior, exiting at the popliteus musculotendineous junction on the tibia. A second tunnel is created through the fibula head from anterolateral to posteromedial, exiting on the fibula styloid. A third fixation point for a screw and washer is created on the lateral femoral condyle, just anterior to where FCL and popliteus tendon cross each other at the theoretical isometric point on the femoral condyle. After all the tunnels are prepared, the graft is passed through the tibial tunnel from front to back and secured with an interference screw, exiting the posterior aspect of the tibia. The free limb of the graft is passed in the popliteus sulcus and looped around the femoral screw, re-routed through the fibular tunnel from posterior to anterior, before exiting through the anterior aspect of the fibular head and then back to the screw and washer. Although the three major structures are reconstructed in this technique, the reconstruction is non-anatomic since it does not place the reconstruction tunnels at the location of the native footprints.
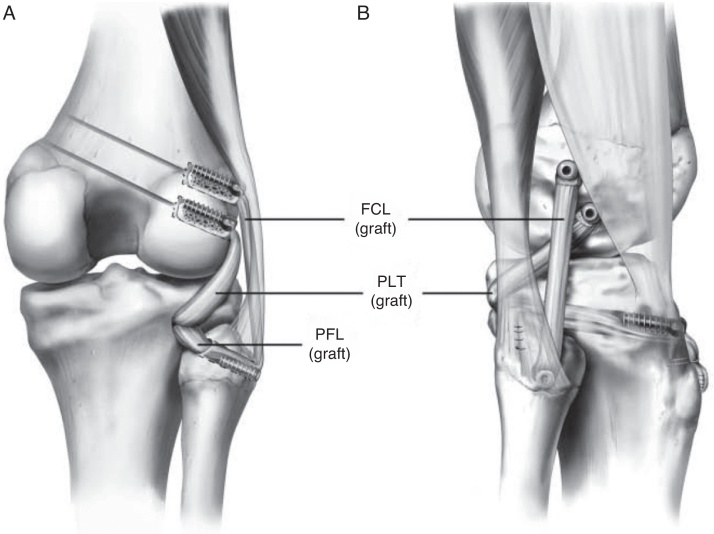
The authors’ preferred technique is an anatomical reconstruction for the PLC (Fig. 2), which has been biomechanically43 and clinically validated11 and shown to re-establish stability and clinical outcomes.44 A hockey stick incision extending from the femoral shaft and lateral femoral condyle to the area between Gerdy's tubercle and the fibula head is performed to develop a posterior-based skin flap. Next, dissection is carried down to the superficial layer of the IT band and the fascial layer of the biceps. Identification, isolation, and protection of the common peroneal are performed next. The nerve is usually found posterior to the long head of the biceps femoris muscle and a neurolysis is performed allowing safe assess to the posterior aspect of the knee. A small horizontal incision is created over the biceps bursa, exposing the FCL distal fibers and fibular attachment.
Fig. 2.
Anatomical reconstruction of the posterolateral corner with two free grafts reconstructing the three major structures, through two femoral tunnels, one tibial tunnel and one fibular tunnel. (Reprinted with permission from Am J Sports Med. 2010;38:1674–1680.).
Blunt dissection between the soleus and the lateral head of gastrocnemius muscle is carried out, allowing the identification of the musculotendinous junction of the popliteus and the popliteofibular insertion on the fibular head. A guide pin is passed from the FCL footprint on the lateral side of the fibula head to the posteromedial aspect of the fibula at the popliteofibular ligament's attachment. After proper position is confirmed, a retractor is placed and a 7 mm drill is used to ream the tunnel. Dissection of a flat area just distal to the Gerdy's tubercle is next performed to identify the tibial reconstruction tunnel entry point. A blunt obturator is placed into the fibula tunnel to serve as a palpable guide for the tibia tunnel placement. The tibial tunnel should be 1 cm medial and 1 cm proximal to fibular tunnel exit point. An aiming device is used to pass a guide pin from the flat spot entry point. After checking the tunnel position, a retractor is placed and the tunnel is created by overreaming the guide pin in an anterior to posterior direction with a 9 mm reamer.
A longitudinal opening in the IT band anterior to the lateral epicondyle is now performed in order to expose the femoral attachments for the FCL and popliteus tendon. Once the FCL attachment is identified, a guide pin is advanced across the femur in the anteromedial direction, avoiding the intercondylar notch. Identifying the popliteus tendon insertion is the next step. Previous anatomic studies showed the distance between these two attachments to be 18.5 mm.15 After the insertion area is identified, a second guide pin is placed across the femur. The distance between the two guide pins must be confirmed to be 18.5 mm. Finally, a 9 mm drill is used to ream to a depth of 25 mm for both reconstruction tunnels.
After all tunnels are reamed, the intra-articular procedure is performed and all concurrent ligament, meniscal, and cartilage pathology should be addressed. At the same time, the grafts may be prepared at the back table by an assistant. A split Achilles allograft is preferred, with the calcaneus bone block split in the middle. Two 9 mm of diameter and 25 mm long bone plugs are prepared and the distal aspect of the graft is tubularized with whipstitches to facilitate graft passage and traction.
Graft fixation begins at the femoral tunnels. The two bone plugs are fixed with a 7 × 20 mm metallic interference screw. Next, the popliteus equivalent graft is passed through the popliteus hiatus exiting in the posterior aspect of the knee. The FCL graft is then passed distally over the popliteus graft and underneath the superficial layer of the IT band. A looped suture is used to guide the passage of the graft through the fibular head in a posteromedial direction, exiting in the back of the knee. The FCL reconstruction is tensioned with the knee at 20° flexion while applying a valgus reduction force in neutral tibial rotation. The graft is fixed with an absorbable 7 × 23 mm screw in the fibula head tunnel. The two free limbs of the grafts are passed through the tibia tunnel from posterior to anterior. The grafts should be tensioned once again using an alternating motion to remove any residual slack in the grafts. Finally, fixation is performed with a 9 × 23 mm absorbable screw with the knee in 60° of flexion, neutral tibial rotation, and tension applied on both grafts.
Post-operative rehabilitation
The postoperative rehabilitation protocol consists of 6 weeks of non-weight bearing while wearing an immobilizer brace in full extension at all times, except during range of motion exercises which are initiated on postoperative day one. Quadriceps sets and patellar mobilization should be started immediately. Hamstrings sets should be avoided in the first 6 weeks, to minimize the risk of the grafts stretching out. At the 6-week point, the patient can start weight bearing as tolerated and the immobilizer brace can be discontinued if the patient is able to perform a straight leg raise without a lag of extension. Biking exercises can be added as soon as 100° of knee flexion is achieved. Sports specific training is started at 4 months. Varus stress radiographs are obtained at 6 months post-operatively to assess for stability. Return to sports activities is delayed until a normal range of motion, strength, and stability is achieved (usually after 6 to 9 months).
Outcomes
The anatomic reconstruction technique has demonstrated the ability to reduce objective laxity on varus stress x-ray from 6.2 mm preoperatively to a 0.1 mm side-to-side difference at final follow-up. The Cincinnati and IKDC45 subjective outcomes scores increased significantly from 21.9 and 29.1, respectively, to 81.4 and 81.5.36
For chronic cases, the limb alignment must be assessed prior to a reconstruction surgery. Varus alignment stresses the PLC reconstruction grafts,46, 47 and needs to be corrected prior to any other surgical procedure. A high tibial medial opening wedge osteotomy was demonstrated to reduce laxity in PLC injured knees. In 38% of patients, the improvement in stability was enough that the patient did not need an additional PLC reconstruction surgery.48, 49
Conclusion
The posterolateral corner, previously known as the “dark side of the knee”, has been subject of innumerous studies lately. Improved understanding of PLC anatomy and biomechanics has led to improved diagnostics and development of surgical techniques that successfully restore knee stability.
Conflicts of interest
Dr LaPrade is aconsultant for Arthrex. The others authors declare no conflicts of interest.
Footnotes
Study conducted at the Steadman Philippon Research Institute, Vail, United States and Instituto Brasil de Tecnologias da Saúde, Rio de Janeiro, RJ, Brasil.
References
- 1.LaPrade R.F., Wentorf F.A., Fritts H., Gundry C., Hightower C.D. A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy. 2007;23(12):1341–1347. doi: 10.1016/j.arthro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Csintalan R., Ehsan A. Biomechanical and anatomical effects of an external rotational torque applied to the knee a cadaveric study. Am J Sports Med. 2006;34(10):1623–1629. doi: 10.1177/0363546506288013. [DOI] [PubMed] [Google Scholar]
- 3.Fanelli G., Orcutt D., Edson C. The multiple-ligament injured knee: evaluation, treatment and results. Arthroscopy. 2005;21(4):471–486. doi: 10.1016/j.arthro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Fanelli G., Edson C. Posterior cruciate ligament injuries in trauma patients: part II. Arthroscopy. 1995;11(5):526–529. doi: 10.1016/0749-8063(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 5.LaPrade R.F., Tso A., Wentorf F. Force measurements on the fibular collateral ligament, popliteofibular ligament, and popliteus tendon to applied loads. Am J Sports Med. 2004;32(7):1695–1701. doi: 10.1177/0363546503262694. [DOI] [PubMed] [Google Scholar]
- 6.LaPrade R.F., Muench C., Wentorf F., Lewis J.L. The effect of injury to the posterolateral structures of the knee on force in a posterior cruciate ligament graft: a biomechanical study. Am J Sports Med. 2002;30(2):233–238. doi: 10.1177/03635465020300021501. [DOI] [PubMed] [Google Scholar]
- 7.LaPrade R.F., Resig S., Wentorf F., Lewis J.L. The effects of grade III posterolateral knee complex injuries on anterior cruciate ligament graft force. A biomechanical analysis. Am J Sports Med. 1999;27(4):469–475. doi: 10.1177/03635465990270041101. [DOI] [PubMed] [Google Scholar]
- 8.Harner C.D.C., Mauro C.S.C., Lesniak B.P., Romanowski J.R. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Surgical technique. J Bone Joint Surg Am. 2008;91(2):257–270. doi: 10.2106/JBJS.I.00500. [DOI] [PubMed] [Google Scholar]
- 9.Harner C., Höher J., Vogrin T. The effects of a popliteus muscle load on in situ forces in the posterior cruciate ligament and on knee kinematics a human cadaveric study. Am J Sports Med. 1998;26(5):669–673. doi: 10.1177/03635465980260051201. [DOI] [PubMed] [Google Scholar]
- 10.Noyes F., Barber-Westin S. Posterior cruciate ligament revision reconstruction, part 1: causes of surgical failure in 52 consecutive operations. Am J Sports Med. 2005;33(5):646–654. doi: 10.1177/0363546504271210. [DOI] [PubMed] [Google Scholar]
- 11.LaPrade R.F., Johansen S., Wentorf F.A., Engebretsen L., Esterberg J.L., Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405–1414. doi: 10.1177/0363546503262687. [DOI] [PubMed] [Google Scholar]
- 12.Seebacher J., Inglis A. The structure of the posterolateral aspect of the knee. J Bone Joint Surg Am. 1982;64(4):536–541. [PubMed] [Google Scholar]
- 13.Watanabe Y., Moriya H., Takahashi K. Functional anatomy of the posterolateral structures of the knee. Arthroscopy. 1993;9:57–62. doi: 10.1016/s0749-8063(05)80344-5. [DOI] [PubMed] [Google Scholar]
- 14.Veltri D., Deng X., Torzilli P., Maynard M., Warren R. The role of the popliteofibular ligament in stability of the human knee a biomechanical study. Am J Sports Med. 1996;24(1):19–27. doi: 10.1177/036354659602400105. [DOI] [PubMed] [Google Scholar]
- 15.LaPrade R.F., Ly T.V., Wentorf F.A., Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854–860. doi: 10.1177/03635465030310062101. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez A.R., Sugalski M.T., LaPrade R.F. Anatomy and biomechanics of the lateral side of the knee. Sports Med Arthrosc Rev. 2006;14(1):2–11. doi: 10.1097/00132585-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Laprade R.F., Wentorf F.A., Olson E.J., Carlson C.S. An in vivo injury model of posterolateral knee instability. Am J Sports Med. 2006;34(8):1313–1321. doi: 10.1177/0363546506286785. [DOI] [PubMed] [Google Scholar]
- 18.Laprade R.F., Wozniczka J.K., Stellmaker M.P., Wijdicks C.A. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543–549. doi: 10.1177/0363546509349493. [DOI] [PubMed] [Google Scholar]
- 19.Wentorf F.A., LaPrade R.F., Lewis J.L., Resig S. The influence of the integrity of posterolateral structures on tibiofemoral orientation when an anterior cruciate ligament graft is tensioned. Am J Sports Med. 2002;30(6):796–799. doi: 10.1177/03635465020300060701. [DOI] [PubMed] [Google Scholar]
- 20.Covey D. Injuries of the posterolateral corner of the knee. J Bone Joint Surg Am. 2001;83(1):106–118. doi: 10.2106/00004623-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Fornalski S., McGarry M. Biomechanical and anatomical assessment after knee hyperextension injury. Am J Sports Med. 2008;36(1):80–84. doi: 10.1177/0363546507308189. [DOI] [PubMed] [Google Scholar]
- 22.Geeslin A.G., Laprade R.F. Location of bone bruises and other osseous injuries associated with acute grade III isolated and combined posterolateral knee injuries. Am J Sports Med. 2010;38(12):2502–2508. doi: 10.1177/0363546510376232. [DOI] [PubMed] [Google Scholar]
- 23.Gollehon D., Torzilli P., Warren R. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg Am. 1987;69(2):233–242. [PubMed] [Google Scholar]
- 24.Grood E., Stowers S., Noyes F. Limits of movement in the human knee. J Bone Joint Surg Am. 1988;70(1):88–97. [PubMed] [Google Scholar]
- 25.Green N., Allen B. Vascular injuries associated with dislocation of the knee. J Bone Joint Surg Am. 1977;59(2):236–239. [PubMed] [Google Scholar]
- 26.LaPrade R.F., Terry G. Injuries to the posterolateral aspect of the knee. Am J Sports Med. 1997;25(4):434–438. doi: 10.1177/036354659702500403. [DOI] [PubMed] [Google Scholar]
- 27.LaPrade R., Heikes C. The reproducibility and repeatability of varus stress radiographs in the assessment of isolated fibular collateral ligament and grade-III posterolateral knee. J Bone Joint Surg Am. 2008;90(10):2069–2076. doi: 10.2106/JBJS.G.00979. [DOI] [PubMed] [Google Scholar]
- 28.Jackman T., Laprade R.F., Pontinen T., Lender P.A. Intraobserver and interobserver reliability of the kneeling technique of stress radiography for the evaluation of posterior knee laxity. Am J Sports Med. 2008;36(8):1571–1576. doi: 10.1177/0363546508315897. [DOI] [PubMed] [Google Scholar]
- 29.LaPrade R.F., Bollom T.S., Wentorf F.A., Wills N.J., Meister K. Mechanical properties of the posterolateral structures of the knee. Am J Knee Surg. 2005;33(9):1386–1391. doi: 10.1177/0363546504274143. [DOI] [PubMed] [Google Scholar]
- 30.LaPrade R., Wentorf F. Diagnosis and treatment of posterolateral knee injuries. Clin Orthop Relat Res. 2002;(402):110–121. doi: 10.1097/00003086-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Hughston J.C., Andrews J.R., Cross M.J., Moschi A. Classification of knee ligament instabilities Part II. The lateral compartment. J Bone Joint Surg Am. 1976;58(2):173–179. [PubMed] [Google Scholar]
- 32.Fanelli G., Stannard J.P., Stuart M.J., MacDonald P.B., Marx R.G., Whelan D.B. Management of complex knee ligament injuries. J Bone Joint Surg Am. 2010;60(12):2234–2246. [PubMed] [Google Scholar]
- 33.Kannus P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med. 1989;17(1):83–88. doi: 10.1177/036354658901700114. [DOI] [PubMed] [Google Scholar]
- 34.Krukhaug Y., Mølster A., Rodt A., Strand T. Lateral ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 1998;6(1):21–25. doi: 10.1007/s001670050067. [DOI] [PubMed] [Google Scholar]
- 35.Clancy W., Jr., Sutherland T. Combined posterior cruciate ligament injuries. Clin Sports Med. 1994;13(3):629–647. [PubMed] [Google Scholar]
- 36.Geeslin A.G., LaPrade R.F. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries. J Bone Joint Surg Am. 2011;93(18):1672–1683. doi: 10.2106/JBJS.J.01639. [DOI] [PubMed] [Google Scholar]
- 37.Stannard J.P., Brown S.L., Farris R.C., McGwin G., Volgas D.A. The posterolateral corner of the knee: repair versus reconstruction. Am J Sports Med. 2005;33(6):881–888. doi: 10.1177/0363546504271208. [DOI] [PubMed] [Google Scholar]
- 38.Levy B.A., Dajani K.A., Morgan J.A., Shah J.P., Dahm D.L., Stuart M.J. Repair versus reconstruction of the fibular collateral ligament and posterolateral corner in the multiligament-injured knee. Am J Knee Surg. 2010;38(4):804–809. doi: 10.1177/0363546509352459. [DOI] [PubMed] [Google Scholar]
- 39.Clancy W., Chapman M. Repair and reconstruction of the posterior cruciate ligament. Oper Orthop. 1988;3:1651–1665. [Google Scholar]
- 40.Larson R. Isometry of the lateral collateral and popliteofibular ligaments and techniques for reconstruction using a free semitendinosus tendon graft. Oper Techn Sports Med. 2001;9(2):84–90. [Google Scholar]
- 41.Arciero R.R. Anatomic posterolateral corner knee reconstruction. Arthroscopy. 2005;21(9):1147. doi: 10.1016/j.arthro.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Stannard J.P., Brown S.L., Robinson J.T.G.M., Jr., Volgas D.A. Reconstruction of the posterolateral corner of the knee. Arthroscopy. 2005;21(9):1051–1059. doi: 10.1016/j.arthro.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy M., Camarda L., Wijdicks C.A., Johansen S., Engebretsen L., Laprade R.F. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674–1681. doi: 10.1177/0363546510361220. [DOI] [PubMed] [Google Scholar]
- 44.LaPrade R.F., Johansen S., Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2011;93(Suppl 1):10–20. doi: 10.2106/JBJS.J.01243. [DOI] [PubMed] [Google Scholar]
- 45.Metsavaht L., Leporace G., Riberto M., de Mello Sposito M.M., Batista L.A. Translation and cross-cultural adaptation of the Brazilian version of the International Knee Documentation Committee Subjective Knee Form: validity and reproducibility. Am J Sports Med. 2010;38(9):1894–1899. doi: 10.1177/0363546510365314. [DOI] [PubMed] [Google Scholar]
- 46.Noyes F. High tibial osteotomy and ligament reconstruction for varus angulated anterior cruciate ligament-deficient knees. Am J Sports Med. 2000;28(3):282–296. doi: 10.1177/03635465000280030201. [DOI] [PubMed] [Google Scholar]
- 47.LaPrade R., Hamilton C., Engebretsen L. Treatment or acute and chronic combined anterior cruciate ligament and posterolateral knee ligament injuries. Sports Med Arthrosc Rev. 1997;5(2):91–99. [Google Scholar]
- 48.Arthur A., LaPrade R.F., Agel J. Proximal tibial opening wedge osteotomy as the initial treatment for chronic posterolateral corner deficiency in the varus knee: a prospective clinical study. Am J Sports Med. 2007;35(11):1844–1850. doi: 10.1177/0363546507304717. [DOI] [PubMed] [Google Scholar]
- 49.Laprade R.F., Engebretsen L., Johansen S., Wentorf F.A., Kurtenbach C. The effect of a proximal tibial medial opening wedge osteotomy on posterolateral knee instability: a biomechanical study. Am J Sports Med. 2008;36(5):956–960. doi: 10.1177/0363546507312380. [DOI] [PubMed] [Google Scholar]