Abstract
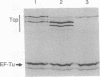
Salmonella typhimurium shows an attractant response to citrate and a repellent response to phenol, and a chemoreceptor mediating these responses has been identified and named Tcp (taxis to citrate and away from phenol). Tcp is one of the methyl-accepting chemotaxis proteins that have a molecular mass of approximately 60 kDa estimated by SDS/PAGE, and its methylation level is increased by citrate and decreased by phenol. Tcp also mediates an attractant response to metal-citrate complexes. The complete nucleotide sequence of the tcp coding region has been determined. The deduced amino acid sequence of Tcp, consisting of 547-amino acid residues, is homologous with that of the aspartate chemoreceptor of S. typhimurium. Thus, Tcp is another member of the bacterial transmembrane chemoreceptor family. Because citrate is a good carbon source for S. typhimurium but is not a carbon source for the closely related species Escherichia coli and because citrate utilization is used as a key diagnostic character to distinguish these species, it is reasonable to assume that Tcp is specific to S. typhimurium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswad D., Koshland D. E., Jr Isolation, characterization and complementation of Salmonella typhimurium chemotaxis mutants. J Mol Biol. 1975 Sep 15;97(2):225–235. doi: 10.1016/s0022-2836(75)80036-2. [DOI] [PubMed] [Google Scholar]
- Boyd A., Kendall K., Simon M. I. Structure of the serine chemoreceptor in Escherichia coli. Nature. 1983 Feb 17;301(5901):623–626. doi: 10.1038/301623a0. [DOI] [PubMed] [Google Scholar]
- Gardina P., Conway C., Kossman M., Manson M. Aspartate and maltose-binding protein interact with adjacent sites in the Tar chemotactic signal transducer of Escherichia coli. J Bacteriol. 1992 Mar;174(5):1528–1536. doi: 10.1128/jb.174.5.1528-1536.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. DNA sequence analysis suggests that expression of flagellar and chemotaxis genes in Escherichia coli and Salmonella typhimurium is controlled by an alternative sigma factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6422–6424. doi: 10.1073/pnas.84.18.6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imae Y., Oosawa K., Mizuno T., Kihara M., Macnab R. M. Phenol: a complex chemoeffector in bacterial chemotaxis. J Bacteriol. 1987 Jan;169(1):371–379. doi: 10.1128/jb.169.1.371-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Iijima T., Hasegawa T. Transport of tricarboxylic acids in Salmonella typhimurium. J Bacteriol. 1973 Jun;114(3):961–965. doi: 10.1128/jb.114.3.961-965.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Koshland D. E., Jr Response to a metal ion-citrate complex in bacterial sensing. J Bacteriol. 1979 Dec;140(3):798–804. doi: 10.1128/jb.140.3.798-804.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro N., Sato G., Yoshikawa M. Lack of chemotactic response to tricarboxylic acids by Escherichia coli carrying a plasmid determining citrate utilization. J Bacteriol. 1981 Oct;148(1):383–385. doi: 10.1128/jb.148.1.383-385.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Cameron M. Citrate transport in Salmonella typhimurium. Arch Biochem Biophys. 1978 Sep;190(1):270–280. doi: 10.1016/0003-9861(78)90276-x. [DOI] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Chemotaxis of Salmonella typhimurium toward citrate. J Bacteriol. 1979 Oct;140(1):297–300. doi: 10.1128/jb.140.1.297-300.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake K., Ohya Y., Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L., Imae Y. Role of threonine residue 154 in ligand recognition of the tar chemoreceptor in Escherichia coli. J Bacteriol. 1990 Jan;172(1):377–382. doi: 10.1128/jb.172.1.377-382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L., Mizuno T., Imae Y. Thermosensing properties of Escherichia coli tsr mutants defective in serine chemoreception. J Bacteriol. 1988 Oct;170(10):4769–4774. doi: 10.1128/jb.170.10.4769-4774.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. D., Blank V., Brade G., Higgins C. F. Peptide chemotaxis in E. coli involves the Tap signal transducer and the dipeptide permease. Nature. 1986 May 15;321(6067):253–256. doi: 10.1038/321253a0. [DOI] [PubMed] [Google Scholar]
- Milburn M. V., Privé G. G., Milligan D. L., Scott W. G., Yeh J., Jancarik J., Koshland D. E., Jr, Kim S. H. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science. 1991 Nov 29;254(5036):1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Mutoh N., Panasenko S. M., Imae Y. Acquisition of maltose chemotaxis in Salmonella typhimurium by the introduction of the Escherichia coli chemosensory transducer gene. J Bacteriol. 1986 Mar;165(3):890–895. doi: 10.1128/jb.165.3.890-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A. F., Koshland D. E., Jr Identification of the tip-encoded receptor in bacterial sensing. J Bacteriol. 1986 Jan;165(1):276–282. doi: 10.1128/jb.165.1.276-282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A. F., Koshland D. E., Jr Separation of signal transduction and adaptation functions of the aspartate receptor in bacterial sensing. Science. 1983 Jun 3;220(4601):1016–1020. doi: 10.1126/science.6302843. [DOI] [PubMed] [Google Scholar]
- Widenhorn K. A., Boos W., Somers J. M., Kay W. W. Cloning and properties of the Salmonella typhimurium tricarboxylate transport operon in Escherichia coli. J Bacteriol. 1988 Feb;170(2):883–888. doi: 10.1128/jb.170.2.883-888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Conley M. P., Kramer T. J., Berg H. C. Reconstitution of signaling in bacterial chemotaxis. J Bacteriol. 1987 May;169(5):1878–1885. doi: 10.1128/jb.169.5.1878-1885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff C., Parkinson J. S. Aspartate taxis mutants of the Escherichia coli tar chemoreceptor. J Bacteriol. 1988 Oct;170(10):4509–4515. doi: 10.1128/jb.170.10.4509-4515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Macnab R. M., Imae Y. Repellent response functions of the Trg and Tap chemoreceptors of Escherichia coli. J Bacteriol. 1990 Jan;172(1):383–388. doi: 10.1128/jb.172.1.383-388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]