Abstract
IFN-γ release assays (IGRAs) are better indicators of Mycobacterium tuberculosis infection than the tuberculin skin test (TST) in Bacillus Calmette–Guérin (BCG)-vaccinated populations. However, IGRAs do not discriminate active and latent infections (LTBI) and no gold standard for LTBI diagnosis is available. Thus, since improved tests to diagnose M. tuberculosis infection are required, we assessed the efficacy of several M. tuberculosis latency antigens. BCG-vaccinated healthy donors (HD) and tuberculosis (TB) patients were recruited. QuantiFERON-TB Gold In-Tube, TST and clinical data were used to differentiate LTBI. IFN-γ production against CFP-10, ESAT-6, Rv2624c, Rv2626c and Rv2628 antigens was tested in peripheral blood mononuclear cells. LTBI subjects secreted significantly higher IFN-γ levels against Rv2626c than HD. Additionally, Rv2626c peptide pools to which only LTBI responded were identified, and their cumulative IFN-γ response improved LTBI discrimination. Interestingly, whole blood stimulation with Rv2626c allowed the discrimination between active and latent infections, since TB patients did not secrete IFN-γ against Rv2626c, in contrast to CFP-10 + ESAT-6 stimulation that induced IFN-γ response from both LTBI and TB patients. ROC analysis confirmed that Rv2626c discriminated LTBI from HD and TB patients. Therefore, since only LTBI recognizes specific epitopes from Rv2626c, this antigen could improve LTBI diagnosis, even in BCG-vaccinated people.
Keywords: Latent tuberculosis, Active tuberculosis, Diagnosis, IFN-gamma, Antigens
Highlights
-
•
Stimulation with Rv2626c M. tuberculosis antigen induced differential amounts of IFN-γ in LTBI individuals as compared to HD.
-
•
The cumulative response to specific Rv2626c-derived peptide pools improved the discrimination of LTBI individuals from HD.
-
•
PBMC or whole blood stimulation with Rv2626c differentiated latent from active infection (LTBI from TB patients).
1. Introduction
Tuberculosis (TB) continues to be one of the most prevalent infectious diseases worldwide, with 9 million new cases reported and 1.5 million deaths in 2013 (W.H.O., 2014). Furthermore, an estimated one third of the world population is latently infected with Mycobacterium tuberculosis (LTBI) and at risk of disease reactivation (Dye et al., 1999). The existence of such a huge reservoir of the bacteria denotes the need of a rapid diagnosis of TB infection for its early detection and control.
The tuberculin skin test (TST) has been long the most used tool for the diagnosis of M. tuberculosis infection (Vukmanovic-Stejic et al., 2006). However, TST specificity is limited since it employs antigens shared with environmental mycobacteria and Mycobacterium bovis Bacillus Calmette–Guérin (BCG) (Shingadia and Novelli, 2008). Furthermore, TST cannot differentiate active disease from LTBI, requiring additional tests to establish the diagnosis. The discovery of the diagnostic potential of the M. tuberculosis antigens ESAT-6 and CFP-10 led to the development of IFN-γ release assays (IGRAs) (Diel et al., 2011). These antigens are encoded in the region of deletion-1 locus present in M. tuberculosis, but absent in BCG and most environmental mycobacteria, making IGRAs more specific than TST (Diel et al., 2011, Ganguly et al., 2008). However, a deficiency of current IGRAs is that, like TST, they do not discriminate active from latent infection, a key problem in cases with uncertain clinical symptoms or smear negative sputum samples, like extra-pulmonary TB cases (Chegou et al., 2011). Though this is not the main objective of IGRAs, such discrimination would be desired, since it would bring greater specificity to the assay, providing an extra tool for fast diagnosis of active infection. In addition, there is as yet no gold standard for the diagnosis of LTBI. Besides, several vaccine studies are being currently performed employing antigens present in existing IGRAs, with many having demonstrated the potential of ESAT-6 to confer protection against M. tuberculosis (Davila et al., 2012, Cervantes-Villagrana et al., 2013, Reither et al., 2014, van Dissel et al., 2011). Therefore, if these vaccines prove to be successful and become commonly used, it might signify the end of ESAT-6 as a diagnostic marker, since immune response to this antigen would reflect both M. tuberculosis infection and vaccination, losing its present specificity. Finally, the performance of available IGRAs exhibits great variability among different groups of immunocompromised patients, being dependent upon the nature and extent of immunodeficiency (Sauzullo et al., 2015). Thus, the reasons described above support the search for better assays and/or new immunological biomarkers to diagnose M. tuberculosis infection, which might complement or improve current assays to diagnose both latent and active infections.
LTBI is thought to be associated with a dormancy state of the pathogen and several antigens up-regulated during these conditions have been analyzed as potential diagnostic markers (Schuck et al., 2009). We studied the response of healthy subjects, LTBI individuals and TB patients to several M. tuberculosis antigens. DosR regulon-encoded antigens Rv2624c, Rv2626c and Rv2628 were selected based on previous reports that suggested they were recognized by household contacts or TST+ individuals (Leyten et al., 2006, Chegou et al., 2012). Our results demonstrated that LTBI individuals recognized specific epitopes from Rv2626c that induced the secretion of significant amounts of IFN-γ, in sharp contrast to non-infected individuals. Moreover, our findings indicate that Rv2626c would be a useful antigen to discriminate LTBI individuals from both active TB patients and non-infected healthy subjects in a BCG-vaccinated population. Additional studies will validate the potential of Rv2626c to discriminate LTBI from TB patients and healthy donors in non-BCG-vaccinated populations.
2. Methods
2.1. Study Subjects
BCG-vaccinated healthy adults lacking a history of TB (household contacts and healthcare workers) were recruited at Argentinean Referral Hospitals between January 2012 and August 2014. Among this group of individuals, diagnosis of LTBI was established using QuantiFERON-TB Gold In-Tube (QFT-GIT; Qiagen, USA; according to the manufacturer's directions) and TST (see below) tests. LTBI diagnosis was assigned to any subject with a positive QFT-GIT/TST and no clinical or radiological evidence of active TB. In the event of discordant QFT-GIT/TST results, individuals were assigned to the corresponding group on the basis of the QFT-GIT result. The group of healthy donors (HD) was comprised by adult individuals without TB disease (tested by chest X-rays and analysis of acid-fast bacilli in sputum) and with negative QFT-GIT/TST. HIV-uninfected patients with active TB were evaluated at Dr. F. Muñiz or Dr. E. Tornú Hospitals (Buenos Aires, Argentina). Diagnosis of TB disease was established based on clinical and radiological data together with culture-confirmation and the identification of acid-fast bacilli in sputum. Patients included in this study had received less than one week of anti-TB therapy. Information regarding demographic data and prior TB exposure was obtained at the time of recruitment. A total of 56 LTBI individuals, 56 TB patients and 60 HD participated in this study. All participants provided written informed consent for sample collection and subsequent analysis. The protocols conducted were approved by the Ethical Committee of the Dr. F. Muñiz and the Dr. E. Tornú Hospitals.
2.2. Study Inclusion and Exclusion Criteria for Individuals Participating in the Study
Inclusion criteria: a) adult (over 18 years old) men and women with active pulmonary TB and b) healthy volunteers with high level of exposure to M. tuberculosis (household contacts of TB patients and healthcare workers of National Referral Hospitals for TB). All recruited subjects were BCG-vaccinated. QFT-GIT and TST assays were used to determine the presence of LTBI among individuals without clinical or microbiological diagnosis of active TB. All TB patients included in the study had a positive culture for M. tuberculosis.
Exclusion criteria: a) HIV positive or positive serology to other viral or bacterial infections; b) patients with diabetes, cancer, autoimmune diseases or other conditions that may affect the immune system of the individual; c) pregnant women and d) children. Among the population of active TB patients we excluded: a) patients with multidrug-resistant tuberculosis (MDR-TB) infection and b) patients with more than seven consecutive days of anti-TB treatment. Individuals with indeterminate QFT-GIT results were also excluded from the study.
2.3. Tuberculin Skin Test (TST)
TST was administered by medical staff according to the Mantoux method (American Thoracic Society, CDC, 2000). Briefly, 2 tuberculin units (0.1 ml) of purified protein derivative (Instituto Malbrán, Buenos Aires, Argentina) were intradermally injected into the inner surface of the forearm. The skin induration was measured (in mm) after 48–72 h by trained personnel at one of the medical centers. A positive TST was defined as an induration size ≥ 10 mm, according to National Guidelines (Zerbini, 2013).
2.4. Antigens
Recombinant antigens (Rv2624c, Rv2626c, Rv2628, CFP-10, ESAT-6) were produced in Escherichia coli BL21 (DE3) pLysS and purified using a nickel nitrilotriacetic system (Ni-NTA Agarose, Qiagen), following the manufacturers' directions. After purity control, proteins were concentrated using Amicon (Millipore, USA) commercial columns and then Detoxi-Gel Endotoxin Removing Resin (Pierce, USA) was used to remove possible endotoxin traces. CFP-10 and ESAT-6-induced IFN-γ responses were compared to those elicited with the respective protein reference standard obtained from BEI Resources (NIAID, NIH; CFP-10 Recombinant Protein Reference Standard, NR-14869 and ESAT-6 Recombinant Protein Reference Standard, NR-14868). Cell lysate from the virulent M. tuberculosis H37Rv strain (Mtb-Ag) was prepared by probe sonication (BEI Resources, NIAID, NIH: M. tuberculosis, Strain H37Rv, Whole Cell Lysate, NR-14822).
2.5. Peptides
Overlapping synthetic peptides (13–15-mers, overlapped by 11 amino acids; 36 in total) spanning the sequence of Rv2626c were synthesized by Biomatik Corp. (Canada) using Fmoc chemistry. Peptide purity was > 80%, as assayed by HPLC, and the composition was verified by mass spectrometry. Lyophilized peptides were dissolved in dimethyl sulfoxide, aliquoted and stored at − 70 °C. For in vitro stimulation, peptides were arranged in pools composed of six peptides, obtaining six consecutive pools (A–E) covering the complete Rv2626c sequence.
2.6. Cell preparation and reagents
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Ficoll-Hypaque (GE Healthcare, USA) and cultured (1 × 106 cells/ml) with/without the different M. tuberculosis antigens (Rv2624c, Rv2626c, Rv2628, CFP-10, ESAT-6; 2.5 μg/ml) or Rv2626c peptide pools (each peptide at 5 μg/ml) with RPMI 1640 (Gibco, USA) supplemented with l-glutamine, penicillin/streptomycin and 10% human serum (Sigma-Aldrich, USA), during different times. Briefly, PBMCs were stimulated for five days and then cell free supernatants were collected to determine the levels of IFN-γ by ELISA (Human IFN-γ ELISA MAX™ Standard Kit, BioLegend, USA). In different experiments, cells were incubated with the antigens for four days and then harvested to determine IFN-γ expression by flow cytometry (see below).
2.7. Whole blood stimulation
Briefly, 0.5 ml of heparinized blood was treated with Rv2626c or CFP-10 + ESAT-6 (2.5 μg/ml) for 24 h at 37 °C. Afterwards, plates were centrifuged and plasma was recovered to determine IFN-γ production by ELISA.
2.8. Flow cytometry
After four days of PBMCs stimulation, GolgiStop® (BD Biosciences, USA) reagent was added for the last 5 h of culture. Cells were then harvested and stained for intracellular IFN-γ (eBioscience, USA) and surface CD4 (BioLegend) expression using Cytofix/Cytoperm™ fixation/permeabilization buffers following the manufacturer's instructions (BD Biosciences) (Supplementary appendix). Negative control samples were incubated with irrelevant isotype-matched antibodies in parallel with experimental samples. Samples were analyzed on a FACS ARIA II flow cytometer (BD Biosciences).
2.9. Statistics
Individuals with a similar pattern of IFN-γ cytokine secretion were grouped together as clusters and presented as a heat map; with heat distances computed using Euclidean distances as similarity measure, employing NetWalker 1.0 software.
The Mann–Whitney test was used to analyze differences between unpaired samples. Fisher's exact test was used for categorical variables. Receiver operating characteristic (ROC) curve analysis was conducted to analyze the predictive value of IFN-γ response to Rv2626c or CFP-10 + ESAT-6, calculating the area under the curve (AUC) and the 95% confidence interval (CI). Analyses were performed using GraphPad Prism 5 software. p < 0.05 was considered statistically significant.
3. Results
3.1. Evaluation of IFN-γ Responses to M. tuberculosis Antigens in BCG-Vaccinated People
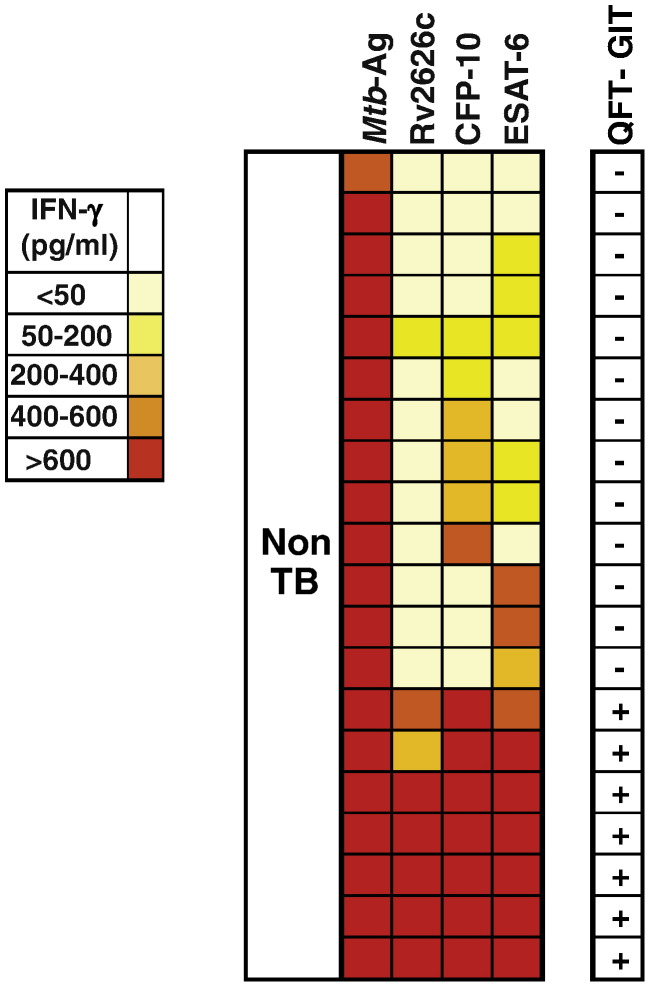
To assess the frequency of LTBI individuals among healthy BCG-vaccinated people highly exposed to M. tuberculosis, we analyzed the IFN-γ response to several dormancy (Rv2626c, Rv2628, Rv2624c) and specific early secretory (ESAT-6, CFP-10) antigens from the pathogen. Initially, IFN-γ production against the mentioned antigens or Mtb-Ag was evaluated in PBMCs from 20 healthy individuals. As shown by the heat map in Fig. 1, all subjects produced elevated amounts of IFN-γ against Mtb-Ag (> 600 pg/ml), as expected in BCG-vaccinated individuals. Interestingly, 7 out of 20 (35%) of the persons tested also responded simultaneously with high intensity to three antigens: Rv2626c, ESAT-6 and CFP-10. In fact, the three antigens induced > 600 pg/ml of IFN-γ in 5 out of the 7 subjects. The two exceptions were one individual in which Rv2626c and ESAT-6 induced 400–600 pg/ml and CFP-10 induced > 600 pg/ml of IFN-γ, and another person in which Rv2626c induced 200–400 pg/ml and CFP-10 and ESAT-6 induced > 600 pg/ml. In contrast to our data resulting from stimulation with Rv2626c, ESAT-6 and CFP-10 showing that 7 individuals simultaneously secreted high levels of IFN-γ to those antigens (all above 200 pg/ml), none of those subjects secreted IFN-γ against Rv2624c, and only 2 produced levels of IFN-γ above 200 pg/ml in response to Rv2628 stimulation (data not shown). Therefore, Fig. 1 represents the results obtained with antigens that displayed noticeable differences among individuals: Rv2626c, ESAT-6 and CFP-10.
Fig. 1.
Heat map for IFN-γ responses to Mtb-Ag, Rv2626c, CFP-10 and ESAT-6 M. tuberculosis antigens in subjects without active tuberculosis.
Heat map showing the IFN-γ responses to Mtb-Ag, Rv2626c, CFP-10 or ESAT-6 antigens as measured in cell free supernatants of peripheral blood mononuclear cells (PBMCs) from subjects without active tuberculosis (non-TB; n = 20). Colors represent pg/ml of IFN-γ produced in each individual, where high values are shown in red and low values in light yellow, as shown in the figure scale. Right column displays the result of QuantiFERON-TB Gold In-tube (QFT-GIT) test.
We then characterized the healthy population that secreted high IFN-γ levels to the mentioned three antigens with the QFT-GIT test. Fig. 1 shows that all the subjects that highly responded to Rv2626c, ESAT-6 and CFP-10 simultaneously were QFT-GIT+, which would be indicative of LTBI; while all those individuals that did not respond were QFT-GIT−.
In view of the findings described above, we next investigated the response to M. tuberculosis antigens in a larger population of QFT-GIT− HD and QFT-GIT+ LTBI individuals (Table 1). We also stimulated all subjects' PBMCs simultaneously with the combination of antigens included in the QFT-GIT assay, i.e.,: CFP-10 + ESAT-6. As shown in Table 2, LTBI produced significantly higher IFN-γ levels against CFP-10, ESAT-6 and CFP-10 + ESAT-6 than HD. Interestingly, LTBI subjects also secreted significantly more elevated IFN-γ levels upon Rv2626c stimulation than HD (Table 2), suggesting that stimulation with Rv2626c might also be useful to discriminate between HD and LTBI. To confirm these findings we used flow cytometry, a more sensitive technique than ELISA (Supplementary Fig. 1). In agreement to the results obtained by ELISA, PBMCs from LTBI displayed significantly higher numbers of IFN-γ secreting CD4+ cells in response to Rv2626c as compared to HD (Supplementary Table 1). Regarding the other antigens tested, in both HD and LTBI, Rv2624c induced a very weak IFN-γ response (Table 2). Moreover, although LTBI subjects secreted low IFN-γ levels in response to Rv2628, they were not significantly different compared to those produced by HD (Table 2).
Table 1.
Characteristics of Individuals without active TB infection.
| Characteristics |
Individuals without active M. tuberculosis infection |
||
|---|---|---|---|
| (N = 116) | p value | ||
| QFT-GIT (−) | QFT-GIT (+) | ||
| 52% (N = 60) | 48% (N = 56) | ||
| Age (years) | 40.5 ± 11.9 | 42.0 ± 11.6 | 0.49 (a) |
| Sex | |||
| Male | 38% (N = 23) | 40% (N = 22) | 0.85 (b) |
| Female | 62% (N = 37) | 60% (N = 34) | |
| Country of birth | |||
| Argentina | 88% (N = 53) | 80% (N = 45) | 0.31 (b) |
| Other Latin American countries | 12% (N = 7) | 20% (N = 11) | |
| TST | |||
| Positive | 14% (N = 8) | 78% (N = 44) | < 0.001 (b) |
| Negative | 86% (N = 52) | 22% (N = 12) | |
Abbreviations: QuantiFERON-TB Gold In-tube, QFT-GIT; tuberculin skin test, TST.
(a) Age values are displayed as Mean ± SEM. p values were calculated by the Mann–Whitney U test for continuous variables; (b) p values were calculated by Fisher's exact test for categorical variables.
Table 2.
IFN-γ production against M. tuberculosis antigens by PBMCs from non-TB individuals.
| pg/ml of IFN-γ, Mean ± SEM (range, pg/ml) |
|||||||
|---|---|---|---|---|---|---|---|
| N | CFP-10 | ESAT-6 | CFP-10 + ESAT-6 | Rv2626c | Rv2624c | Rv2628 | |
| HD | 45 | 250 ± 52.8 (1–1102) |
196 ± 43.3 (2–895) |
248 ± 76.3 (8–578) |
69 ± 24.5 (1–476) |
36 ± 13.9 (1–104) |
33 ± 14.4 (1–150) |
| LTBI | 56 | 1452 ± 202.0 (1–4127) |
973 ± 156.2 (5–3958) |
1517 ± 210.0 (7–3888) |
549 ± 106.6 (14–2892) |
39 ± 24.9 (1–169) |
135 ± 60.2 (1–461) |
| p value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.84 | 0.15 | |
Peripheral blood mononuclear cells (PBMCs) from healthy donors (HD) and latently M. tuberculosis infected individuals (LTBI) were cultured with M. tuberculosis antigens CFP-10, ESAT-6, CFP-10 + ESAT-6, Rv2624c, Rv2626c or Rv2628 (2.5 μg/ml) for five days. Then, cell free supernatants were recovered and the production of IFN-γ was evaluated by ELISA. p values were calculated using the Mann–Whitney test for unpaired samples.
In order to evaluate the potential use of Rv2626c in discriminating LTBI vs. HD, we next performed a ROC analysis for the IFN-γ responses to this antigen (shown in Table 2), obtaining significant results for AUC analysis (AUC, 0.86; p < 0.001; 95% CI: 0.78–0.93). Together, these findings demonstrated that Rv2626c might allow discriminating LTBI individuals from HD.
3.2. Identification of Immunodominant Peptide Pools Derived from Rv2626c Antigen
Since a low percentage of HD showed a weak IFN-γ production against Rv2626c (Table 2), and an ideal diagnostic test requires the highest discrimination among groups under study, we next searched for the immunodominant regions of Rv2626c to which only LTBI responded. For this, we employed overlapping peptides arranged in pools composed of six peptides each, covering the entire Rv2626c protein. As can be observed in Table 3, pools B, D and F induced high IFN-γ levels, which significantly discriminated between LTBI and HD, while pools C and E also distinguished between both groups but with a weaker significance (Table 3). In contrast, although stimulation with pool A did induce the production of IFN-γ in LTBI subjects, no significant differences with HD were detected (Table 3). Therefore, pool A would belong to a less specific region of Rv2626c than pools B, D and F, the most specific and immunogenic ones, which could strongly differentiate the two groups of individuals.
Table 3.
IFN-γ production against overlapping peptides of Rv2626c by PBMCs from non-TB individuals.
| pg/ml of IFN-γ, Mean ± SEM |
||||||||
|---|---|---|---|---|---|---|---|---|
| N | Pool A | Pool B | Pool C | Pool D | Pool E | Pool F | Cumulative response to pools B, D and F | |
| HD | 33 | 7.6 ± 4.09 | 3.9 ± 1.6 | 4.1 ± 6.0 | 18.4 ± 18.4 | 4.9 ± 3.4 | 34.4 ± 20.0 | 55.3 ± 20.92 |
| LTBI | 40 | 216.6 ± 82.7 | 275.9 ± 93.1 | 119.2 ± 35.7 | 416.2 ± 120.3 | 48.7 ± 20.7 | 517.1 ± 119.3 | 1177.0 ± 253.1 |
| p value | 0.09 | < 0.001 | 0.03 | < 0.001 | 0.02 | < 0.001 | < 0.001 | |
Peripheral blood mononuclear cells (PBMCs) from healthy donors (HD) and latently M. tuberculosis infected individuals (LTBI) were cultured with six peptide pools (A–F) derived from Rv2626c (5 μg/ml of each peptide) for five days and then IFN-γ production was evaluated in cell free supernatants by ELISA. The cumulative response to pools B, D and F was calculated for each individual as the sum of the secretion of IFN-γ in response to each of the three pools. p values were obtained though the Mann–Whitney test for unpaired samples.
We next analyzed the difference in the levels of IFN-γ secreted by LTBI and HD in response to Rv2626c antigen or each of the most specific peptide pools. We observed that upon stimulation with Rv2626c, LTBI subjects showed an eight times greater response than HD (Table 2), whereas with the pools, the response was 70 (pool B), 23 (pool D) or 15 (pool F) times greater in LTBI than in HD (Table 3), clearly improving the discrimination between HD and LTBI individuals. Since each LTBI subject recognized the individual peptide pools with different intensity, we then calculated the cumulative IFN-γ response to the most specific and immunogenic pools (B, D and F) to capture the overall immune response to the tested peptide pools for each person. As shown in Table 3, our results showed a significantly higher cumulative IFN-γ response to these pools in the group of LTBI individuals than in HD. Thus, our findings indicate that LTBI individuals recognized epitopes from Rv2626c antigen and showed a strong cumulative IFN-γ response, in clear contrast to HD that display weak or no response to those peptides.
3.3. IFN-γ Responses to M. tuberculosis Specific Antigens in Healthy Donors, LTBI Individuals and TB Patients
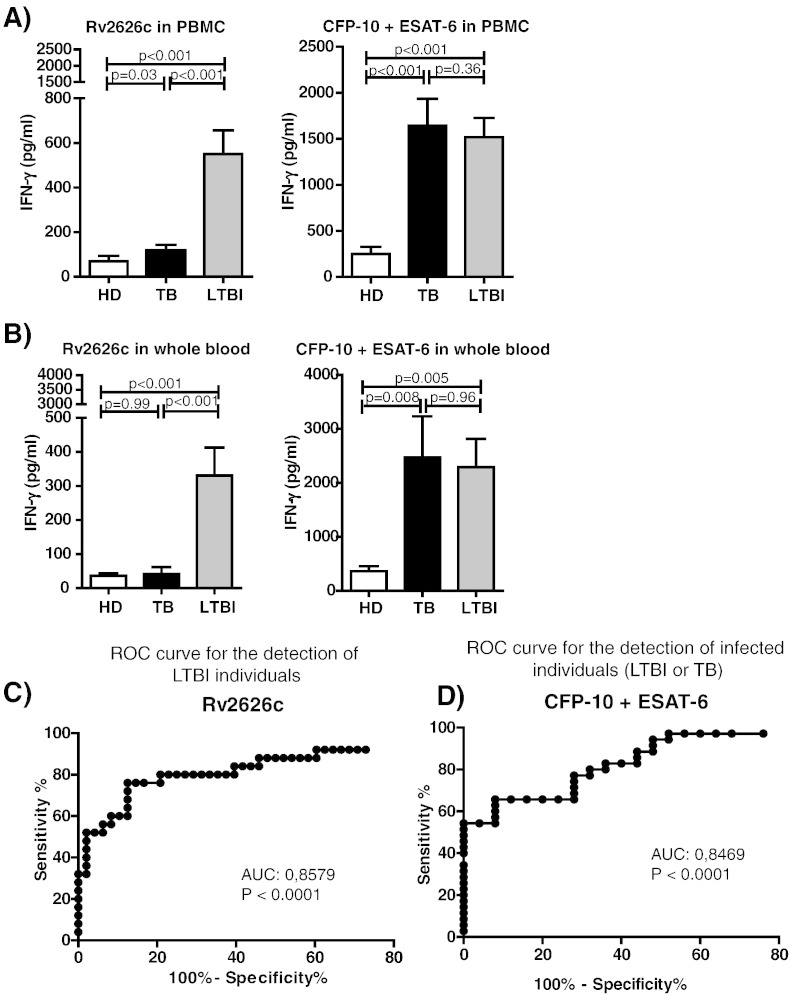
Since we classified our population using QFT-GIT assay, and considering that this test uses whole blood for more practical and faster testing, we also analyzed the response to Rv2626c stimulating whole blood. Given that both current IGRAs discriminate M. tuberculosis infection (active or latent) from non-infected individuals, we also examined the response of active TB patients (Table 4). We first investigated the response of PBMCs from TB patients, LTBI and HD to Rv2626c and CFP-10 + ESAT-6 (Fig. 2A). We found that, as expected, both active and latently infected individuals (TB patients and LTBI) secreted significantly higher IFN-γ amounts in response to CFP-10 + ESAT-6 as compared to HD. Interestingly, we observed that stimulation with Rv2626c allowed the discrimination between active and latent infections (Fig. 2A) since TB patients did not secrete IFN-γ against this antigen (Fig. 2A), in sharp contrast to the results obtained with CFP-10 + ESAT-6. When we performed our experiments in whole blood, we obtained similar results to those observed in PBMCs: LTBI subjects secreted significantly higher amounts of IFN-γ in response to Rv2626c than HD. In addition, like in PBMCs (Fig. 2A), we observed that TB patients did not produce IFN-γ against Rv2626c (Fig. 2B), in sharp contrast to the results obtained with CFP-10 + ESAT-6 (Fig. 2B). Furthermore, the ROC analysis for IFN-γ responses to Rv2626c in whole blood reinforced the potential of Rv2626c antigen for discriminating LTBI individuals from non-LTBI individuals (patients with active TB or HD; Fig. 2C; AUC, 0.86; p < 0.001; 95% CI: 0.78–0.93). Additionally, by using the data obtained in whole blood and the Youden index (Youden, 1950, Perkins and Schisterman, 2006), we were able to establish an optimal cut off point of > 63.35 pg/ml of IFN-γ, with 78.26% sensitivity and 89.36% specificity. For comparison, Fig. 2D also shows the ROC curve obtained after analyzing the IFN-γ response to CFP-10 + ESAT-6 for the discrimination of M. tuberculosis infected individuals (LTBI + TB patients) versus HD. In conclusion, our findings show that the response to Rv2626c might be useful to differentiate between HD and LTBI, similar to ESAT-6 and CFP-10, antigens employed in IGRAs like QFT-GIT. However, in contrast to QFT-GIT assay, stimulation with Rv2626c would also allow the discrimination between active and latent infections. Therefore, our results indicate that Rv2626c might be useful not only to diagnose LTBI, but also, to differentiate latent from active M. tuberculosis infection.
Table 4.
Characteristics of patients with active TB infection.
| Characteristics |
Tuberculosis patients (TB) |
|---|---|
| (N = 56) | |
| Age (years) (a) | 34.5 ± 13.4 |
| Sex (b) | |
| Male | 86% (N = 48) |
| Female | 14% (N = 8) |
| Country of birth | |
| Argentina | 53% (N = 30) |
| Other Latin American countries | 47% (N = 26) |
| TST | |
| Positive | 98% (N = 55) |
| Negative | 2% (N = 1) |
| AFB in sputum smear | |
| Positive | 84% (N = 47) |
| Negative | 16% (N = 9) |
| Radiological lesions (c) | |
| Severe | 80% (N = 45) |
| Moderate | 20% (N = 11) |
| Mild | 0% (N = 0) |
(a) Age values are displayed as Mean ± SEM. (b) Categorical data are expressed as percentages (number). (c) Radiological lesions: mild, patients with a single lobe involved and without visible cavities; moderate, patients presenting unilateral involvement of two or more lobes with cavities, if present, reaching a total diameter no greater than 4 cm; severe, bilateral disease with massive affectation and multiple cavities. Abbreviations: AFB, acid-fast bacilli.
Fig. 2.
IFN-γ production against M. tuberculosis antigens in PBMCs and whole blood from non-TB individuals and TB patients.
(A) Peripheral blood mononuclear cells (PBMCs) from healthy donors (HD; N = 45), patients with tuberculosis (TB; N = 56) and latently M. tuberculosis infected individuals (LTBI; N = 56) were cultured with Rv2626c or CFP-10 + ESAT-6 for 5 days. Then, cell free supernatants were recovered and IFN-γ production was evaluated by ELISA. (B) Whole blood from HD (N = 28), TB patients (N = 20) and LTBI individuals (N = 25) was cultured with Rv2626c or CFP-10 + ESAT-6 for 24 h. Afterwards, plasma samples were collected and IFN-γ production was evaluated by ELISA. (A–B) Bars represent the Mean ± SEM. Mann–Whitney test for unpaired samples. (C–D) ROC curve analyses for evaluation of the predictive value of whole blood IFN-γ levels produced in response to (C) Rv2626c or (D) CFP-10 + ESAT-6, for differentiating (C) LTBI individuals from non-LTBI individuals (HD or TB), or (D) infected individuals (LTBI or active TB) from HD.
Abbreviations: ROC, receiver operating characteristic; AUC, area under the ROC curve.
4. Discussion
A main health obstacle to control TB is that latent bacilli may remain viable for decades and reactivate later to cause active TB. Thus, there is a need to identify LTBI individuals and treat them to eliminate the risk of developing TB. Since there is no diagnostic gold standard for LTBI and several reports have demonstrated the potential use of antigens employed in current IGRAs as protective vaccines, it urges to find new M. tuberculosis antigens to detect latently infected individuals (Davila et al., 2012, Cervantes-Villagrana et al., 2013, Reither et al., 2014, van Dissel et al., 2011, Sester et al., 2011).
Currently, both TST and IGRAs are used for LTBI diagnosis worldwide. Still, in BCG-vaccinated populations IGRAs may be preferable to TST since they are more specific for M. tuberculosis infection (Pai et al., 2014). In fact, in our study 13% of the individuals assessed had discordant results between TST and IGRA tests (Supplementary Fig. 2). To develop T cell-based assays that discriminate active TB and LTBI, improving the current available tests, it is necessary to identify new host markers expressed in response to M. tuberculosis antigens that are uniquely recognized by LTBI individuals (Chegou et al., 2012). Thus, present IGRAs might be complemented by using antigens to which patients with active TB do not respond.
Here, we describe an immune-stimulatory function of the 16-kDa conserved protein coded by the M. tuberculosis open reading frame Rv2626c, one of the most abundant proteins under low-oxygen conditions in M. tuberculosis lysates (Rosenkrands et al., 2002). Variable results using Rv2628 and Rv2626c latency antigens were reported by others, although the disparities could be related to differences in stimulation schemes, antigen concentrations, ethnic backgrounds, and BCG vaccination history (Schuck et al., 2009, Chegou et al., 2012, Lin et al., 2007, Black et al., 2009, Riano et al., 2012, Hozumi et al., 2013, Mensah et al., 2014). Bashir et al. reported that Rv2626c induced higher IFN-γ levels in TB patients than in HD, but LTBI individuals were not analyzed in this study (Bashir et al., 2010). Leyten et al. demonstrated that TST+ individuals strongly recognized more latency antigens (e.g.,: Rv2628 and Rv2626c) as compared to cured patients or patients undergoing anti-TB treatment (Leyten et al., 2006). The study by Goletti et al. evaluated T-cell IFN-γ responses to M. tuberculosis latency antigens in a mainly European population composed by subjects at different stages of TB infection, concluding that remote LTBI individuals showed significantly higher IFN-γ responses to Rv2628 antigen than recent LTBI subjects, active TB and controls (Goletti et al., 2010). However, we studied a very different population: 20% of QFT-GIT+ and 12% of QFT-GIT− subjects were from other Latin American countries (Table 1), while the rest were Argentinean. In addition, the genetic mixture of Argentinean people is comprised by 79.9% European, 15.8% Native American, and 4.3% African contributions, which would imply that there was a fairly diverse genetic background in the population studied (Corach et al., 2010, Avena et al., 2006).
Since some HD subjects produced low levels of IFN-γ against Rv2626c (Fig. 2), we searched for Rv2626c immunodominant epitopes to which only LTBI individuals responded. None of the mentioned studies that tested latency antigens investigated the immunodominance of peptide pools to discriminate LTBI individuals (Schuck et al., 2009, Leyten et al., 2006, Chegou et al., 2012, Lin et al., 2007, Black et al., 2009, Riano et al., 2012, Hozumi et al., 2013, Mensah et al., 2014, Bashir et al., 2010, Goletti et al., 2010). We observed that pools C, B, D, E and F all allowed improving the discernment between LTBI and HD (Table 3), but pools B, D and F resulted the most specific and immunogenic ones. These findings reinforced our data showing that the response to Rv2626c might allow discriminating between LTBI and HD. Additionally, a significantly higher cumulative IFN-γ response to the immunogenic peptide pools B, D and F was detected in LTBI individuals as compared to HD (Table 3). Together, we demonstrated that specific immunogenic peptide pools from Rv2626c might improve the discrimination of LTBI individuals from HD in a BCG-vaccinated population. Interestingly, when we analyzed the response to Rv2626c both in PBMCs and in whole blood from LTBI, HD and TB patients, we observed that only LTBI individuals secreted significant amounts of IFN-γ against this antigen (Fig. 2A and B), indicating that the use of Rv2626c or its immunogenic peptides might comprise a new tool to differentiate LTBI individuals from both HD and patients with active disease. Thus, the use of Rv2626c in combination with ESAT-6 and CFP-10 could improve current kits to allow the differential diagnosis of LTBI and active TB subjects. Additionally, Rv2626c or its immunogenic peptide pools might be candidates to replace potential vaccine antigens present in current IGRAs. In conclusion, the vast genetic mixture of Argentinean people makes the use of Rv2626c or its immunogenic peptide pools promising to discriminate individuals with active and latent M. tuberculosis infections in BCG-vaccinated people. However, future studies should determine whether Rv2626c might be also useful discriminating LTBI from TB patients and healthy donors in non-BCG-vaccinated populations.
Contributors
VEG and HEC designed the study. MG, RMM and DJP were in charge of patient recruitment, diagnosis and sample collection. DP, AIR, and REH were responsible for processing samples and performing ELISA and flow cytometry analysis. JP, NLT, NOA and AR contributed with standard laboratory work. DP, VP, JI, HEC and VEG did the data management and analysis. DP and VEG wrote the manuscript. MMG and JI provided expert advice. All authors contributed to data gathering and interpretation, and revision of the report.
Declaration of Interests
DP, AIR, VP, MMG, JI, HEC and VEG have a patent application for the use of the peptide pools for the LTBI detection (patent application number No. EP14306329.5).
Acknowledgments
This investigation received financial support from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PAE-PID-00127, PAE-PICT-207-02332 and PICT-0240 to VEG); University of Buenos Aires (20020100100221 and 20020130100236BA to VEG; 20020130100720BA to HEC), and Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET; PIP 2012–2014 to VEG. and HEC). The ANPCyT, the University of Buenos Aires, and CONICET had no role in study design, data gathering, analysis, and interpretation, or writing of the report. VEG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
We thank Dr. Peter Barnes for the insightful discussions.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.05.026.
Appendix A. Supplementary Data
Supplementary material.
References
- American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC) Targeted tuberculin testing and treatment of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 2000;161(4 Pt 2):S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2014. Global Tuberculosis Report. Geneva. [Google Scholar]
- Avena S.A., Goicoechea A.S., Rey J., Dugoujon J.M., Dejean C.B., Carnese F.R. Gene mixture in a population sample from Buenos Aires City. Med. (B Aires) 2006;66(2):113–118. [PubMed] [Google Scholar]
- Bashir N., Kounsar F., Mukhopadhyay S., Hasnain S.E. Mycobacterium tuberculosis conserved hypothetical protein rRv2626c modulates macrophage effector functions. Immunology. 2010;130(1):34–45. doi: 10.1111/j.1365-2567.2009.03196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black G.F., Thiel B.A., Ota M.O. Immunogenicity of novel DosR regulon-encoded candidate antigens of Mycobacterium tuberculosis in three high-burden populations in Africa. Clin. Vaccine Immunol. 2009;16(8):1203–1212. doi: 10.1128/CVI.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Villagrana A.R., Hernandez-Pando R., Biragyn A. Prime-boost BCG vaccination with DNA vaccines based in beta-defensin-2 and mycobacterial antigens ESAT6 or Ag85B improve protection in a tuberculosis experimental model. Vaccine. 2013;31(4):676–684. doi: 10.1016/j.vaccine.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chegou N.N., Hoek K.G., Kriel M., Warren R.M., Victor T.C., Walzl G. Tuberculosis assays: past, present and future. Expert Rev. Anti-Infect. Ther. 2011;9(4):457–469. doi: 10.1586/eri.11.23. [DOI] [PubMed] [Google Scholar]
- Chegou N.N., Black G.F., Loxton A.G. Potential of novel Mycobacterium tuberculosis infection phase-dependent antigens in the diagnosis of TB disease in a high burden setting. BMC Infect. Dis. 2012;12:10. doi: 10.1186/1471-2334-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corach D., Lao O., Bobillo C. Inferring continental ancestry of Argentineans from autosomal, Y-chromosomal and mitochondrial DNA. Ann. Hum. Genet. 2010;74(1):65–76. doi: 10.1111/j.1469-1809.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- Davila J., McNamara L.A., Yang Z. Comparison of the predicted population coverage of tuberculosis vaccine candidates Ag85B-ESAT-6, Ag85B-TB10.4, and Mtb72f via a bioinformatics approach. PLoS One. 2012;7(7):e40882. doi: 10.1371/journal.pone.0040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel R., Goletti D., Ferrara G. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur. Respir. J. 2011;37(1):88–99. doi: 10.1183/09031936.00115110. [DOI] [PubMed] [Google Scholar]
- Dye C., Scheele S., Dolin P., Pathania V., Raviglione M.C. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282(7):677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- Ganguly N., Siddiqui I., Sharma P. Role of M. tuberculosis RD-1 region encoded secretory proteins in protective response and virulence. Tuberculosis (Edinb.) 2008;88(6):510–517. doi: 10.1016/j.tube.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Goletti D., Butera O., Vanini V. Response to Rv2628 latency antigen associates with cured tuberculosis and remote infection. Eur. Respir. J. 2010;36(1):135–142. doi: 10.1183/09031936.00140009. [DOI] [PubMed] [Google Scholar]
- Hozumi H., Tsujimura K., Yamamura Y. Immunogenicity of dormancy-related antigens in individuals infected with Mycobacterium tuberculosis in Japan. Int. J. Tuberc. Lung Dis. 2013;17(6):818–824. doi: 10.5588/ijtld.12.0695. [DOI] [PubMed] [Google Scholar]
- Leyten E.M., Lin M.Y., Franken K.L. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes Infect. 2006;8(8):2052–2060. doi: 10.1016/j.micinf.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Lin M.Y., Geluk A., Smith S.G. Lack of immune responses to Mycobacterium tuberculosis DosR regulon proteins following Mycobacterium bovis BCG vaccination. Infect. Immun. 2007;75(7):3523–3530. doi: 10.1128/IAI.01999-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah G.I., Addo K.K., Tetteh J.A. Cytokine response to selected MTB antigens in Ghanaian TB patients, before and at 2 weeks of anti-TB therapy is characterized by high expression of IFN-gamma and Granzyme B and inter-individual variation. BMC Infect. Dis. 2014;14:495. doi: 10.1186/1471-2334-14-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai M., Denkinger C.M., Kik S.V. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin. Microbiol. Rev. 2014;27(1):3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N.J., Schisterman E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006;163(7):670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reither K., Katsoulis L., Beattie T. Safety and immunogenicity of H1/IC31(R), an adjuvanted TB subunit vaccine, in HIV-infected adults with CD4 + lymphocyte counts greater than 350 cells/mm3: a phase II, multi-centre, double-blind, randomized, placebo-controlled trial. PloS One. 2014;9(12):e114602. doi: 10.1371/journal.pone.0114602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riano F., Arroyo L., Paris S. T cell responses to DosR and Rpf proteins in actively and latently infected individuals from Colombia. Tuberculosis. 2012;92(2):148–159. doi: 10.1016/j.tube.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Rosenkrands I., Slayden R.A., Crawford J., Aagaard C., Barry C.E., III, Andersen P. Hypoxic response of Mycobacterium tuberculosis studied by metabolic labeling and proteome analysis of cellular and extracellular proteins. J. Bacteriol. 2002;184(13):3485–3491. doi: 10.1128/JB.184.13.3485-3491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauzullo I., Vullo V., Mastroianni C.M. Detecting latent tuberculosis in compromised patients. Curr Opin Infect Dis. 2015;28(3):275–282. doi: 10.1097/QCO.0000000000000158. [DOI] [PubMed] [Google Scholar]
- Schuck S.D., Mueller H., Kunitz F. Identification of T-cell antigens specific for latent Mycobacterium tuberculosis infection. PLoS One. 2009;4(5):e5590. doi: 10.1371/journal.pone.0005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sester M., Sotgiu G., Lange C. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur. Respir. J. 2011;37(1):100–111. doi: 10.1183/09031936.00114810. [DOI] [PubMed] [Google Scholar]
- Shingadia D., Novelli V. The tuberculin skin test: a hundred, not out? Arch. Dis. Child. 2008;93(3):189–190. doi: 10.1136/adc.2007.129585. [DOI] [PubMed] [Google Scholar]
- van Dissel J.T., Soonawala D., Joosten S.A. Ag85B-ESAT-6 adjuvanted with IC31(R) promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in volunteers with previous BCG vaccination or tuberculosis infection. Vaccine. 2011;29(11):2100–2109. doi: 10.1016/j.vaccine.2010.12.135. [DOI] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M., Reed J.R., Lacy K.E., Rustin M.H., Akbar A.N. Mantoux test as a model for a secondary immune response in humans. Immunol. Lett. 2006;107(2):93–101. doi: 10.1016/j.imlet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Zerbini E.V. Instituto Nacional de Enfermedades Respiratorias Dr. Emilio Coni; Santa Fe, Argentina: 2013. Programa Nacional de Control de la Tuberculosis: Normas Técnicas. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.