Abstract
Background
Validating the high-risk (HR) and ultra-high-risk (UHR) stages of bipolar disorder (BP) may help enable early intervention strategies.
Methods
We followed up with 44 offspring of parents with BP, subdividing into the HR and UHR categories. The offspring were aged 8–28 years and were free of any current DSM-IV diagnoses. Our multilevel, integrative approach encompassed gray matter (GM) volumes, brain network connectivity, neuropsychological performance, and clinical outcomes.
Findings
Compared with the healthy controls (HCs) (n = 33), the HR offspring (n = 26) showed GM volume reductions in the right orbitofrontal cortex. Compared with the HR offspring, the UHR offspring (n = 18) exhibited increased GM volumes in four regions. Both the HR and UHR offspring displayed abnormalities in the inferior occipital cortex regarding the measures of degree and centrality, reflecting the connections and roles of the region, respectively. In the UHR versus the HR offspring, the UHR offspring exhibited upwards-shifted small world topologies that reflect high clustering and efficiency in the brain networks. Compared with the HCs, the UHR offspring had significantly lower assortativity, which was suggestive of vulnerability. Finally, processing speed, visual–spatial, and general function were impaired in the UHR offspring but not in the HR offspring.
Interpretation
The abnormalities observed in the HR offspring appear to be inherited, whereas those associated with the UHR offspring represent stage-specific changes predisposing them to developing the disorder.
Keywords: Bipolar disorder, Affective disorder, Network analysis, High-risk design, Neuroimaging, Cognition, Ultra-high-risk
Highlights
-
•
Pathophysiological alterations were identified in the high-risk and ultra-high-risk stages of bipolar disorder (BP).
-
•
Deficits in processing speed and visual-spatial memory were observed in the ultra-high-risk stage of BP.
-
•
Abnormalities associated with the ultra-high-risk stage are suggestive of risk for the full development of BP.
-
•
Our data support that the underlying abnormalities of BP may become apparent long before the official onset.
-
•
Identifying the early development of BP opens the avenue for early intervention.
1. Introduction
Bipolar disorder (BP) is a major psychiatric disorder that is disabling and recurrent. It is highly heritable (more than 70%) (Tsuang and Faraone, 2000) and is characterized by hypo/manic episodes. Pioneering work on the trajectory of BP has identified early-risk syndromes that precede the official onset (Akiskal et al., 1985, Duffy et al., 2014, Mesman et al., 2013) and are proposed to represent early stages in the development of BP (Duffy et al., 2014). Identifying the early stages of BP is important for prevention and early intervention strategies, which have been shown to be successful in early psychosis and can avert or delay the transition from clinically ultra-high-risk conditions to a full psychotic disorder (McGorry et al., 2009). The early symptoms (and syndromes) that precede full-blown BP are usually non-specific during childhood (e.g., anxiety, sleep disturbance, and attention deficit hyperactivity disorder (ADHD) symptoms/signs) and then manifest as adjustment disorder during early adolescence and later as subthreshold depression and/or hypomania that falls short of the official criteria (Akiskal et al., 1985, Correll et al., 2014, Duffy et al., 2014, Egeland et al., 2000, Mesman et al., 2013). In a Canadian follow-up study (up to 16 years) of the offspring of parents with BP, the accumulated incidence of major mood disorders (depressive spectrum and bipolar disorders) was unfortunately high — 83.3% (Duffy et al., 2014). Another Dutch study of genetically high-risk offspring (12 years of follow-up) showed that 72% of the offspring developed a lifetime DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) axis I disorder (Mesman et al., 2013). Furthermore, in modeling the developmental stages of BP – from non-specific symptoms, followed by minor mood disorder, major depressive episodes, and finally hypo/manic episodes – once entering the model, these high-risk offspring progressed linearly through the stages without skipping any stage (Duffy et al., 2014), which suggests a progressive process and an urgent need for intervention. Recently, clinical staging models for BP that cut across the dichotomous category of the present classification systems have been proposed with attempts at better understanding the underlying pathophysiology and providing potential targets for early intervention (Frank et al., 2014, Scott et al., 2013). The clinical staging models hypothesize that there are stages (0, 1a and 1b) that precede full-blown BP. Stage 0 and stage 1a may together represent a phase of biological vulnerability (i.e., genetic risk) with no or mild, non-specific symptoms that can be subsumed into the HR stage (Scott et al., 2013), whereas stage 1b can be described as ultra-high-risk (UHR), manifesting subthreshold syndromes, alterations in cortical (and subcortical) volumes, and deficits in cognitive function (Frank et al., 2014).
Interrogating the underlying pathophysiology prior to the full-blown manifestations can help clarify some confounding effects. For example, extant neuroimaging research on established BP has suggested that the volume of gray matter (GM) decreases in the regions underpinning emotion processing and regulation and cognitive processes, such as the ventrolateral prefrontal cortex (vlPFC), orbitofrontal cortex (OFC) and amygdala (Phillips and Swartz, 2014). Nevertheless, these changes more likely reflect the net effect of inherited neuropathological vulnerability and a number of contributing factors including illness progression, symptoms (e.g., psychotic symptoms), comorbid conditions, and medications such as lithium that can normalize or increase GM volumes (Kalmar et al., 2009, Moore et al., 2000, Moorhead et al., 2007, Nugent et al., 2006, Strasser et al., 2005).
Brain network analysis has been increasingly adopted in neuroscience research. This method provides useful information about brain organization in terms of how spatially segregated brain regions are integrated globally via connecting fiber tracts (i.e., an anatomical network) to form an integrated system (for a comprehensive review, see (Bullmore and Sporns, 2009)). Accumulating evidence suggests that inter-regional integration is crucial for cognitive performance, particularly for effortful psychological tasks, such as working memory (Kitzbichler et al., 2011). Moreover, brain network analysis is particularly helpful for research on those psychiatric disorders that are conceptualized as dysconnectivity syndromes, such as schizophrenia and BP, disorders that may be caused by the failure of integrating spatially distributed brain regions to form a large-scale network (Catani and ffytche, 2005). Among measures of brain network topology, small-world properties are useful for describing anatomical connectivity networks that reflect a high clustering of functionally associated regions with short path length (high efficiency) (Bassett and Bullmore, 2006, Bullmore and Sporns, 2012). These properties were reported to be heritable in twin studies (Smit et al., 2008), related to cognitive performance (Micheloyannis et al., 2009), and significantly altered in schizophrenia (Bassett et al., 2008, Liu et al., 2008) (a disorder sharing many overlapping features with BP, including genetics). In contrast, assortativity, an index that can be used to measure the robustness to assaults (i.e., structurally abnormal regions) of a brain network (Newman, 2002), may potentially assist in capturing the vulnerability of the UHR stage of BP.
At the macroscopic level, cognitive deficits have been demonstrated to be an important aspect of full-blown BP that adversely affects the quality of life in people with the disorder (Wingo et al., 2009). A significant research gap is whether cognitive deficits are the consequences of the course of illness and its related factors, such as medications (e.g., valproate) and recurrent subthreshold syndromes (Martinez-Aran et al., 2004, Rosa et al., 2014, Xu et al., 2012), or are inherited, e.g., the deficits in visual–spatial memory (Ferrier et al., 2004) and working memory observed in the “unaffected” relatives of patients with BP (Kulkarni et al., 2010). Indeed, our previous study showed that patients with BP, following clinical remission from depression after six-week treatments, did not recover their processing speed and visual–spatial memory functioning (Xu et al., 2012). To fill the research gap, it is necessary to investigate whether cognitive deficits exist in genetically high-risk individuals at the very early stages before the onset of full-blown syndromes.
A multi-dimensional approach can inform complementary and mutually informative connections between different levels of descriptions across stages, thus yielding patterns of abnormalities (Phillips and Kupfer, 2013). Such an approach may assist in understanding how neural substrates affect cognitive function and behavioral phenotypes across stages.
Given the above considerations, this study applied a multi-dimensional approach to investigating neural correlates and cognitive function at the HR and UHR stages of BP. We began by proposing operational UHR criteria for BP and delineating the clinical characteristics and general functions for both the HR and UHR stages. We first searched for structural abnormalities in the GM that form the neural basis of the functional system by comparing HR offspring (stage 0 and stage 1a) with healthy controls – neuroanatomical endophenotypes – and further tested the validity of UHR (stage 1b) by comparing UHR with HR offspring as an attempt to dissect stage-specific (i.e., adaptive) from inherited alterations. Then, in a regional-level network connectivity analysis, we compared the degree and centrality of the prior identified abnormal regions (reflecting the connections and roles of a region, respectively, see Section 2), which could help elucidate whether volumetric changes in GM (i.e., GM reduction) were a consequence of the lack of connecting fiber bundles. We also quantitatively examined whether the significance (centrality) of the structurally abnormal regions would be affected within the brain networks. Next, a whole-brain network topology analysis was applied to encapsulate all the individual abnormalities that reflect the whole-brain network properties, including small-world and assortativity properties. Finally, at the cognitive function level, we delineated whether cognitive deficits predate the onset, and if so, at what stages; we then searched for their neural correlates.
We hypothesized that HR offspring versus healthy controls had decreased GM volumes in the regions underlying emotion processing and regulation. Compared with HR offspring, UHR offspring might exhibit decreased GM volumes in the regions (e.g., the OFC) related to emotion processing and regulation and in the regions (e.g., the parietal and occipital cortex) involved in the cognitive deficits of bipolar disorder (e.g., processing speed). For the brain network measures, HR and UHR offspring may display impairments in the degree and centrality of those abnormal regions identified by prior GM volume analyses when compared to healthy controls. At the whole-brain level, small-world properties in UHR offspring might be lower (worse) than those in HR offspring and healthy controls. Compared to healthy controls, UHR offspring might display lower values in assortativity, which is suggestive of vulnerability. Finally, we hypothesized that the deficits in processing speed and visual–spatial memory existed prior to the official onset of bipolar disorder (in the HR and/or UHR stages); there may be a correlation between the cognitive deficits and GM volumes in those structurally abnormal regions.
2. Methods
2.1. Participants
This study derived and extended from the project “The Recognition and Early Intervention of Prodromal Bipolar Disorders (REI-PBD)”, whose aims were to identify bipolar prodromal syndromes and to evaluate the effectiveness of exercise interventions for UHR offspring (ClinicalTrials.gov Identifier: NCT01863628). The participants were offspring of parents with BP (bipolar I or bipolar II disorder), and the parents referred their offspring to the project; they were recruited from March 2013 to January 2015. Parents with BP were initially identified from the Guangzhou Brain hospital (a tertiary national hospital) – the earliest psychiatric hospital in China – where they received psychiatric services and from population-based registers. The study was approved by the Institutional Review Board of the Guangzhou Brain Hospital. Written informed consent was obtained from all participants and their parents when appropriate (i.e., age < 18 years).
2.2. Procedures
Parents with BP with offspring aged 8–28 years lacking a diagnosis of any psychiatric disorder were interviewed; the diagnosis of proband BP was based on the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition (SCID-I/P) and all available medical information, including blood and brain-imaging tests and treatment history. The selected age range for the offspring is important for testing our hypotheses, given that i) the typical onset of BP is in adolescence or early adulthood, ii) some individuals with bipolar disorder can manifest symptoms as early as 8 years old, long before onset (Akiskal et al., 1985, Duffy et al., 2014, Mesman et al., 2013), and iii) the period is developmentally crucial for structural maturation (Paus et al., 2008). The offspring underwent in-depth and systematic assessments and were prospectively followed up at several time points (scheduled at weeks 1, 2, 4, 8, and 12 for the UHR offspring) during the first 3 months and then yearly (up to 2 years) or anytime there were changes in symptoms. In this process, we first applied a self-made, 74-item symptom checklist (which consisted of a constellation of symptoms/signs encompassing the commonly reported prodromal symptoms/signs and the defining symptoms/signs by the inclusion criteria (below)) to assess current symptoms and a modified retrospective instrument based on the Bipolar Prodrome Symptom Scale-Retrospective: Patient Version (BPSS-R-Pt) (Correll et al., 2007) to assess past symptoms. Second, the offspring aged < 18 years were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Aged Children: Present and Lifetime Version (K-SADS-PL) or the SCID-I/P for offspring > 18 years to exclude any psychiatric disorders. Third, the Hamilton Depression Rating Scale (HAM-D), the Young Mania Rating Scale (YMRS), and the Brief Psychiatric Rating Scale (BPRS) were implemented to assess the extent of depression, mania, and psychotic symptoms, respectively. Finally, the Global Assessment of Functioning Scale (GAF) was used to assess general function, and a family history of psychiatric disorders was confirmed using the Family Interview for Genetic Studies (FIGS). Of the 747 offspring with parents with BP screened by research psychiatrists, 554 were not eligible according to the inclusion and exclusion criteria (e.g., having a psychiatric diagnosis, in treatment, or too young/old), 130 declined further participation, and 19 offspring did not complete the MRI data collections. The resultant 44 offspring were included in this study, with 18 offspring defined as UHR by the operational criteria we tentatively proposed (below). Given that non-specific symptoms are not uncommonly endorsed by high-risk offspring and that they are usually transient and can be contingent, we subsumed stage 0 and stage 1a into the HR stage, which is in agreement with the clinical staging model for psychotic and severe mood disorders (Scott et al., 2013). Thirty-three age-matched healthy controls who were free of any psychiatric disorders and had no family history of psychiatric disorders were recruited by advertisement and word of mouth. They were systematically assessed in a similar fashion as the high-risk offspring.
2.3. Inclusion and Exclusion Criteria
The criteria for the UHR offspring were i) having at least one biological parent with bipolar disorder (bipolar I and bipolar II) and ii) manifesting at least one of the following defining syndromes: 1) two (or more) hypomania symptoms lasting at least 4 days but not meeting DSM-IV hypomanic episode criteria; 2) two (or more) major depressive symptoms lasting at least 1 week but falling short of a major depressive episode; 3) one (or more) of the following attenuated psychotic symptoms lasting at least 10 min for each manifestation and 2–7 manifestations per week for at least 3 months — ideas of reference, odd ideas, odd beliefs, unusual perceptual experiences, bizarre thoughts or speech, grandiosity, suspicious ideas, paranoid ideas, odd mannerisms, hallucinations, disorganized/catatonic behaviors; and 4) two (or more) of the hyperactivity and impulsivity symptoms/signs defined by the DSM-IV for attention deficit hyperactivity disorder (ADHD) that were observable by teachers, peers, and/or parents.
The construction of this UHR criteria referred to the clinical staging model for psychotic disorder and severe mood disorder recently proposed by Scott and colleagues (Scott et al., 2013), which is based on intervention data in early psychosis (McGorry et al., 2006) and on the studies of prodromal symptoms of bipolar disorder (Bechdolf et al., 2012). According to the clinical staging model (Scott et al., 2013), in the UHR stage, high-risk individuals manifest subthreshold syndromes that fall short of criteria for a psychiatric disorder. Thus, this study considered any established psychiatric disorders as exclusion criteria (below). Moreover, because commonly reported “prodromal” symptoms consist of manic and depressive symptoms and ADHD hyperactivity and impulsivity symptoms/signs (Bechdolf et al., 2012), these symptoms were included in the UHR criteria. Though attenuated psychotic symptoms were reported prior to bipolar disorder, they were less frequently presented compared to manic and depressive symptoms (Correll et al., 2014). As such, the UHR criteria required the participants to have at least one biological parent with bipolar disorder (Duffy, 2014b) to increase the specificity of the sub-syndromes in terms of progression into bipolar disorder. This requirement may be particularly helpful for the ADHD symptoms and attenuated psychotic symptoms that may lack specificity for predicting bipolar disorder.
The following conditions were excluded: DSM-IV-defined disorders; serious general medical illness; mental retardation; the prescription of psychoactive drugs or thyroxine; the inability to complete neuropsychological tests because of physical conditions; and drug or alcohol use.
2.4. Neuroimaging Data
T1-weighted and diffusion tensor images were acquired using a 3.0 Tesla scanner (Philips, Best, Netherlands). The parameters of the T1-weighted images and the processing of these images are reported in supplementary Appendix A. The parameters used to obtain the diffusion tensor images and the methods of constructing the anatomical network connectivity matrix are fully reported in Appendix B. Figure S1 describes a schematic of the brain network construction.
Graph metrics were computed in MATLAB with the Brain Connectivity Toolbox (www.brain-connectivity-toolbox.net). Two node-level network measurements were applied to investigate our research questions: i) degree of a region (node), indicating the number of tracts (edges) linking it to the rest of the network; and ii) the betweenness centrality of a region, measuring the fraction of the shortest paths between any pair of regions that pass through it, which is indicative of its structural or functional importance. The small-world property is thought to reflect an economical trade-off between maximizing adaptive values and minimizing wiring costs (i.e., high clustering and high efficiency) (Bassett and Bullmore, 2006, Bullmore and Sporns, 2012). Assortativity indicates that nodes of high degrees tend to connect with each other and can reflect the robustness to assaults to networks that suffer from the structural integrity of the network. The mathematic definitions of all the network properties are reported in Appendix C.
2.5. Neuropsychological Performance
We applied the MATRICS Consensus Cognitive Battery (MCCB), suggested by the International Society for Bipolar Disorder-Battery for Assessment of Neurocognition (ISBD-BANC), to assess neuropsychological performance. We emphasized processing speed and visual–spatial memory because our previous findings suggested that these functions would not recover after clinical remission (Xu et al., 2012).
2.6. Statistical Analysis
Analysis of variance (ANOVA) and Chi-square tests were applied for the demographic and clinical data. As in the T1-weighted image analysis, two planned comparisons – HR offspring versus healthy controls and UHR versus HR offspring – were conducted in the Statistical Parametric Mapping 8 software Package (SPM8; Wellcome Department of Cognitive Neurology, London, UK). A preliminary whole-brain analysis was performed using a statistical threshold of p < 0.001 and an extent threshold of k > 50. Then, regions of an a priori mask that met the threshold were analyzed with FWE-correction at the individual voxel level for the mask. The significance threshold was set at p < 0.05. The mask was drawn using the Wake Forest University Pick Atlas (Maldjian et al., 2003), which was based on previous research on bipolar disorder (Nugent et al., 2006) and UHR psychosis (Pantelis et al., 2003), including the superior prefrontal, orbital, cingulate, superior temporal cortex and cerebellum. Moreover, different types of neuroimaging data have suggested the involvement of the parietal and occipital cortex in bipolar disorder (Cerullo et al., 2014, Fears et al., 2015, James et al., 2011, Yuksel et al., 2015). Our previous study found that patients with bipolar disorder did not recover their visual–spatial memory and processing speed after clinical remission from depression, implying that the deficits in these two domains may be serving as potential neurocognitive endophenotypes (Xu et al., 2012). Furthermore, there is some evidence suggesting an association between GM volumes and processing speed in bipolar patients (Fears et al., 2015) and the role of the visual cortex (occipital region) in cognitive functioning (Cooke et al., 2015, Fears et al., 2015). Thus, these two regions were added to the mask. Age, gender, handedness, and total intracranial volume (the brain volumes had been demodulated during the processing of the T1-weighted images, Supplementary Appendix A) were included as covariates.
To reduce multiple statistical testing, multivariate analysis of variance (MANOVA) was first applied to compare the degree (and centrality) of the regions that were identified by prior comparisons of gray matter volumes between groups across the offspring and healthy control groups. Permutation tests were further applied to test the significantly different measures identified by the MANOVA analysis, which could be considered an attempt to control for type I error (Camargo et al., 2008). MANOVA was applied to compare neuropsychological performance among groups, with age, gender, and years of education as covariates. Mixed-effect regression models were applied by modeling membership (HR and UHR offspring) as a fixed factor and GM volumes as covariates to examine the relationships between the GM volumes and the cognitive deficits (beta, Wald X2, 95% confidence interval (CI) and p value were reported).
3. Results
3.1. Clinical Characteristics and General Function
The demographic and clinical characteristics are shown in Table 1. Of the 18 UHR offspring, 12 (66.7%) manifested manic sub-syndrome, nine (50.0%) manifested depressive sub-syndrome, five (27.8%) manifested hyperactivity and impulsivity symptoms/signs, and four manifested attenuated psychotic symptoms (22.2%). The age of the first symptom and the duration were on average 11.7 (SD = 5.4) years old and 4.4 (SD = 3.6) years, respectively. Three individuals developed full-blown BP identified after follow-up. Apart from the depressive, manic, and psychotic symptoms, general function measured by the GAF was significantly worse in the UHR offspring (n = 26) than in the HR offspring and healthy controls (n = 33) (p < 0.05, Bonferroni correction).
Table 1.
Demographic and clinical data in the offspring groups and healthy controls.
| HR offspring N = 26 |
UHR offspring N = 18 |
HC N = 33 |
F or X2 | p | Post hoca | |
|---|---|---|---|---|---|---|
| Age at assessment, years | 17.7 (5.4) | 16.2 (7.0) | 15.9 (4.4) | 0.841 | 0.435 | NA |
| Gender, female/male | 15/11 | 9/9 | 18/15 | 0.254 | 0.881 | NA |
| Right handedness, % | 96.2 | 94.4 | 100 | 1.663 | 0.435 | NA |
| Years of education | 10.1 (3.9) | 7.9 (4.5) | 10.1 (3.8) | 2.06 1 | 0.135 | NA |
| HAM-D | 0.8 (1.2) | 8.0 (11.8) | 0.3 (1.0) | 11.721 | < 0.001 | B > A; B > C |
| YMRS | 0 (0) | 4.4 (3.4) | 0.2 (0.9) | 22.724 | < 0.001 | B > A; B > C |
| BPRS | 18.2 (0.5) | 23.9 (8.4) | 18.4 (1.1) | 12.725 | < 0.001 | B > A; B > C |
| Global Assessment Scale | 94.9 (2.8) | 79.6 (14.7) | 93.5 (4.4) | 23.654 | < 0.001 | A > B; C > B |
| TONI-3 | 26.1 (8.7) | 26.3 (9.1) | 29.9 (8.0) | 1.818 | 0.170 | NA |
Abbreviations: HC, healthy controls; HR, high-risk; UHR, ultra-high-risk; NA, not applicable; HAM-D, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; BPRS, Brief Psychiatric Rating Scale; and TONI-3, Test of Nonverbal Intelligence, Third Edition.
Note: A = high-risk offspring; B = ultra-high-risk offspring.
The threshold for significance was p < 0.05 with the Bonferroni correction.
3.2. Voxel-Based Morphometry (VBM) Analysis
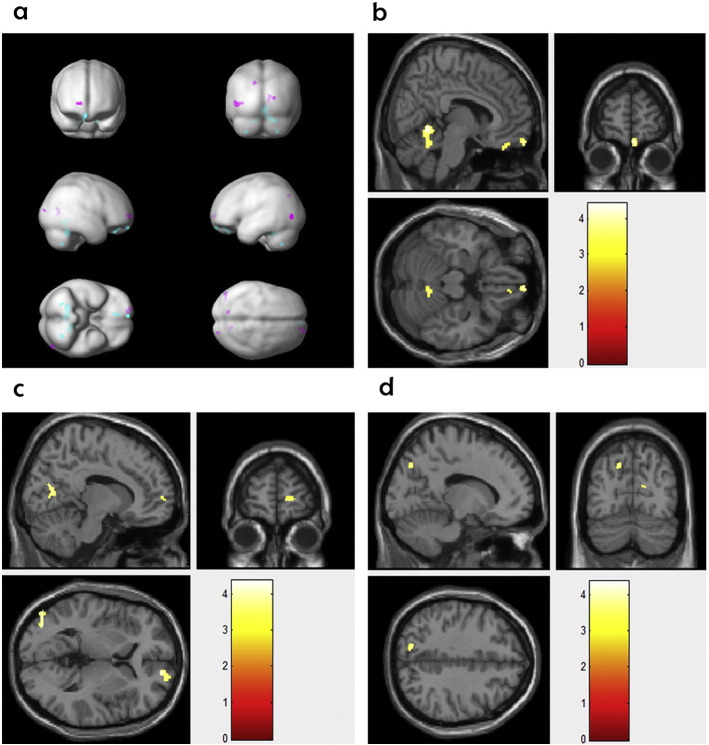
Compared with the healthy controls, the HR offspring showed significantly lower GM volumes in the right OFC and the right cerebellum. Compared to the HR offspring, the UHR offspring had significantly greater GM volumes in four regions, including the right superior frontal gyrus, posterior cingulate cortex, left parietal cortex, and the right middle and inferior occipital gyrus (Table 2 and Fig. 1, FWE-corrected p < 0.05).
Table 2.
Differences in gray matter volumes for high-risk offspring versus healthy controls and ultra-high-risk offspring.
| Regions | BA | Cluster sizea | MNI coordinatesb |
T tests | PFWE-correctedc | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| HR offspring versus HC | |||||||
| Right OFC | 11 | 90 | 6 | 62 | − 20 | 4.10 | 0.023 |
| Right cerebellum lobe | NA | 935 | 8 | − 54 | − 6 | 4.41 | 0.029 |
| UHR versus HR offspring | |||||||
| Right SFG | 10 | 118 | 15 | 59 | 3 | 3.89 | 0.023 |
| Right PCC | 31 | 118 | 15 | − 70 | 15 | 4.24 | 0.017 |
| Left middle and inferior OG | 31/30 | 187 | − 48 | − 79 | 0 | 4.19 | 0.033 |
| Left SPC | 7 | 59 | − 15 | − 73 | 39 | 3.78 | 0.047 |
Abbreviations: HR, high-risk; HC, healthy controls; BA, Brodmann's areas; OFC, orbitofrontal cortex; SFG, superior frontal gyrus; PCC, posterior cingulate cortex; OG, occipital gyrus; and SPC, superior parietal cortex.
Statistics at voxel-level set to a minimum uncorrected threshold of p < 0.001, k > 50 voxels.
Montreal Neurological institute coordinates in millimeters.
Family-wise error correction for multiple comparisons.
Fig. 1.
(a) Turquoise and pink represent the regions showing significant changes in gray matter volumes in the high-risk offspring versus healthy controls and in the high-risk versus ultra-high-risk offspring, respectively. (b) The regions in which the high-risk offspring had significantly lower gray matter volumes than the healthy controls. (c) and (d) The regions in which the ultra-high-risk offspring displayed significantly higher gray matter volumes than the high-risk offspring.
3.3. Regional-Level and Whole-Brain Network Analysis
For the abnormal regions that were identified in the prior VBM analysis, we examined the degree and centrality of these regions, which reflects the connection (of a region to the rest) and the importance of a region in the brain network, respectively. Among the five regions, the MANOVA analysis revealed that the degree of the left inferior occipital gyrus was significantly different across the three groups (F = 5.541, df = 2,68, p = 0.006). The centrality of this region was also significantly different across the three group (F = 3.22, df = 2,68, p = 0.047) (Figure S2). The permutation tests (the permutation distribution is shown in the supplementary materials) further revealed that both the HR and UHR offspring had significantly fewer degrees of this region than the healthy controls (p = 0.003 and p = 0.03, respectively), thus indicating fewer connections from this region to other regions. The centrality of the region was significantly lower in the HR offspring than in the healthy controls (p = 0.021), whereas the UHR offspring showed a similar trend, although it did not reach significance (p = 0.07).
A secondary analysis was conducted to examine the centrality of the anterior cingulate cortex (ACC) in the offspring, as abnormalities in this region have been reported in individuals who met the criteria of “at-risk mental state” (ARMS) (Lord et al., 2011). As shown in Table S1, both the HR and UHR offspring displayed significantly lower centrality in comparison to healthy controls (permutation tests, p < 0.05). The centrality of the ACC was significantly lower in the UHR offspring than that in the HR offspring (permutation test, p = 0.006).
In the global-level network analysis (Table 3), the UHR offspring displayed a significantly higher small-world property than the HR offspring (p = 0.030). As for assortativity, the UHR offspring displayed significantly lower values than the healthy controls (p = 0.020), which is suggestive of vulnerability, whereas the HR offspring did not display significant differences from the healthy controls (p = 0.189).
Table 3.
Network properties of neuropsychological performance in the high-risk offspring, ultra-high-risk offspring, and healthy controls.
| Groups |
Contrasts | Permutation testsa |
||||
|---|---|---|---|---|---|---|
| HR | UHR | HC | p value | |||
| Small-world property (SD) | 3.11 (0.30) | 3.38 (0.50) | 3.22 (0.44) | HR versus HC | 0.836 | |
| UHR versus HC | 0.875 | |||||
| HR versus UHR | 0.030 | |||||
| Assortativityb (median) | 0.03 (0.03) | 0.01 (0.02) | 0.05 (0.04) | HR versus HC | 0.190 | |
| UHR versus HC | 0.020 | |||||
| HR versus UHR | 0.828 | |||||
| MANOVA |
||||||
| F(1,50) | p value | |||||
| Processing speedc | 60.7 (12.3) | 51.7 (10.5) | 61.7 (8.7) | HR versus HC | 0.261 | 0.612 |
| UHR versus HC | 6.109 | 0.017 | ||||
| Visual–spatial memoryd | 25.0 (5.8) | 23.2 (5.6) | 27.0 (5.1) | HR versus HC | 0.087 | 0.769 |
| UHR versus HC | 4.669 | 0.036 | ||||
Abbreviations: HR, high-risk; UHR, ultra-high-risk; HC, healthy controls; SD, standard deviation; and MANOVA, Multivariate Analysis of Variance.
Notes:
Number of resamples = 5000; the distribution of mean differences is shown in the supplementary materials.
Shown as the mean (median); other data: mean (SD).
Measured by the Brief Assessment of Cognition in Schizophrenia: Symbol Coding.
Measured by the Brief Visuospatial Memory Test.
3.4. Cognitive Function and Correlation Analysis
As shown in Table 3, compared to the healthy controls, the MANOVA revealed that the UHR offspring displayed deficits in both processing speed and visual-working memory (F = 6.109, df = 1,50, p = 0.017; F = 4.669, df = 1,50, p = 0.036, respectively). The HR offspring were not significantly impaired in either processing speed or visual-working memory (F = 0.261, df = 1,59, p = 0.612; F = 0.087, p = 0.769, respectively). The mixed-effect regression models found that processing speed was significantly related to the GM volumes of the three regions, including the right posterior cingulate cortex (p = 0.005), right superior frontal gyrus (p = 0.011), and left superior parietal cortex (p = 0.027). There were no significant relationships between visual–spatial memory and the GM volumes of the regions (p > 0.05) (Table S2).
4. Discussion
This study provides preliminary evidence that some pathophysiological changes (i.e., GM volumes and brain network measures) may become apparent in certain early stages (i.e., the HR and UHR stages) preceding the official onset of bipolar disorder. Some results were unexpected. For example, instead of decreased GM volumes, the UHR versus HR offspring displayed increased GM volumes in some regions. UHR versus HR offspring showed an upward-shifted small-world property that reflects high clustering and short path lengths (discussed below).
At the macroscopic level, our data suggest that deficits in processing speed and visual–spatial memory may exist in the UHR stage but not in the HR stage. Moreover, the deficits in processing speed may be related to the GM volumes in the right superior frontal gyrus, posterior cingulate cortex, and the left superior parietal cortex.
4.1. Changes in GM Volumes
In terms of structural abnormalities, the HR offspring showed a volumetric reduction in the right OFC and the right cerebellum. The OFC, the most inferior and ventral part of the prefrontal cortex, has been found to be involved in set shifting and reversal learning (Rygula et al., 2010), encoding reward values in reward processing (Grabenhorst and Rolls, 2011), decision-making processes (Bechara et al., 2000), and emotion processing (Liu et al., 2012), which may underlie certain characteristics of BP, such as mood lability, mood dysregulation, and reward sensitivity (Phillips and Swartz, 2014). Decreased OFC volumes have been reported in pediatric and adult BP patients (James et al., 2011, Stanfield et al., 2009), but a reduction in this region has also been reported to relate to depressive symptoms (Nery et al., 2009). By minimizing the effects of symptoms, this study indicates that the decreased volumes in the two regions may represent genetic susceptibility. In addition, the connections of the OFC with other regions might not be impaired in the HR offspring in terms of the degree of this region within the brain networks. Orbitofrontal reduction has also been observed in those individuals with prodromal symptoms of psychosis who were subsequently predisposed to psychosis (mainly schizophrenia and BP with psychotic features) (Pantelis et al., 2003). In the UHR versus HR offspring, there were increased GM volumes in several regions (most of which were located in the parietal and occipital cortex), suggestive of stage-specific changes that may predispose subjects to the full development of BP. This finding is in agreement with research on UHR psychosis, which shows that volumetric changes in prefrontal regions are related to genetic susceptibility, whereas volumetric changes in the parietal and temporal regions are related to the transition stage into psychotic disorder (the stage in which attenuated psychotic symptoms are manifested) (Lawrie et al., 1999, Pantelis et al., 2003). This finding also coincides with morphometric studies reporting that the structural abnormalities in unaffected relatives of BP proband were relatively restricted to the prefrontal cortex, whereas the widespread volumetric changes identified in pediatric BP extended to the temporal, occipital and parietal cortexes and the amygdala, some of which were associated with symptoms (Chang et al., 2005, Frazier et al., 2005, Hajek et al., 2013, Matsuo et al., 2012, Nery et al., 2009). The increased GM volumes observed in the UHR offspring are unlikely to be protective factors because the selected age range for the offspring represents the most at-risk time for the onset of full-blown BP. Moreover, the UHR offspring manifested varying sub-syndromes, and some of them received a diagnosis of full-blown BP during follow-up. Furthermore, we found that there was an inverse relationship between the GM volumes from these regions and cognitive function. During normative brain maturation from childhood to post-adolescence, gray matter reductions are found in these regions, including in the superior frontal, parietal and occipital regions. Such gray matter reductions are associated with increased brain growth (Sowell et al., 2001). As such, the observation of increased GM volumes in the UHR offspring might indicate disruptions in the process of brain maturation that may predispose an individual to developing BP. To test this speculation, we conducted a complementary analysis to examine differences in the relationship of age to GM volumes in the four regions between the HR and UHR offspring. Our speculation is supported by the results of this analysis, which showed that there were differential relationships between age and GM volumes across the HR and UHR offspring groups (supplementary Figure S7).
In addition, the increased volumes in UHR offspring may relate to the phenomenon of “allostasis,” which refers to the body's reactions to repeated adverse physical or psychosocial conditions that cause stress (McEwen, 2000). Allostasis is fundamentally crucial for adaptation, the maintenance of homeostasis and survival in the short term, which might manifest as increased GM changes. However, over longer intervals, prolonged reactions/over-compensations may lead to neurotoxicity effects; such neurotoxicity effects could manifest as brain structure atrophy (Drevets et al., 1997, Kasai et al., 2003, Moorhead et al., 2007), as reported in individuals at UHR for psychosis over a period of 12 months or longer (Pantelis et al., 2003).
4.2. Brain Network Properties
At the finer-grain level, we observed that both the HR and UHR offspring displayed significantly fewer degrees of the inferior occipital region. This region is not critical for emotion processing and regulation, dysfunction of which may underlie the clinical characteristics of BP (Phillips and Swartz, 2014), e.g., mood instability and manic symptoms. Rather, the occipital cortex plays a key role in sensory function and visual–spatial memory in particular (Cooke et al., 2015, Fears et al., 2015). We performed an exploratory analysis (Pearson correlation) showing that the degree of the inferior occipital cortex was positively associated with processing speed and visual–spatial memory in the healthy controls (p < 0.05). The finding of fewer degrees of the inferior occipital regions is consistent with the observation of deficits in processing speed and visual–spatial memory in the UHR offspring. This observation seems to support the notion that abnormalities of the occipital region may be related to the impairments of cognitive function in BP (Fears et al., 2015, Lyoo et al., 2006). Additionally, it is possible that the involvement of the inferior occipital region reflects the high vulnerability to developing severe forms of bipolar disorder because the abnormalities of this site are relatively common in psychotic bipolar disorder, schizoaffective disorder, (Ivleva et al., 2013), and poor-outcome BP (Bonne et al., 1996). One study on schizophrenia found significantly changed degrees in some regions, including the inferior occipital cortex (Bassett et al., 2008). Moreover, the ARMS criteria were defined by Yung et al. using the UHR criteria for the onset of psychotic disorders (Yung et al., 2005). Lord et al. found that the centrality of this site was associated with the severity of psychotic symptoms in ARMS individuals (Lord et al., 2011). Using the present UHR criteria (which were more specific to BP), this study found decreased centrality in the ACC in UHR versus HR offspring, implying that the centrality of the ACC may be related to the severity of symptoms that are not limited to psychotic symptoms.
Small-world properties, which are characterized by high clustering and short path lengths (high efficiency), support rapid synchronization and information transfer across spatially separated regions (Bullmore and Sporns, 2012). The development of a small-world topology is a dynamic process, evolving from early brain development (< 2 years) (Fan et al., 2011) and demonstrating more long-distance connections during late childhood and adolescence (Chen et al., 2013). Long-distance connections facilitate information processing across regions (high efficiency) and lead to shorter path lengths between spatially separated regions (i.e., the path length is shorter compared to when those regions were connected via a number of shorter path-connecting nodes in between). Research has shown that brain networks increase efficiency by configuring more long-distance connections while performing effortful cognitive tasks (Kitzbichler et al., 2011). Thus, the increased incidence of small-world topology in UHR offspring may be tentatively interpreted as a compensation for impaired cognitive function by configuring more long-distance connections in the brain network. However, these long-distances connections, which likely integrate “hub” regions, are expensive in terms of the wiring and metabolic costs. They are subject to selective attack in the networks during pathophysiological processes (Bullmore and Sporns, 2012). In what may be a trade-off between adaptive value and wiring cost, psychiatric disorders such as schizophrenia have been reported to be associated with abnormal shifts in small-world properties (Bassett et al., 2008, Bullmore and Sporns, 2012, Liu et al., 2008). Alternatively, synaptic pruning (which results in the reduction of fiber connections and gray matter density) is known to occur during the normative development of the brain from childhood through adolescence (Penzes et al., 2011); thus, the upwards-shifted small world topology in the UHR offspring may be due to disruptions in synaptic pruning that can lead to brain networks of higher clustering and efficiency. Whole-brain network properties help to uncover dysfunction in an entire system comprised of interconnected regions, providing more comprehensive information than just a focus on individual regions (Bullmore and Sporns, 2009). Assortativity was able to capture the clinically defined UHR stage, which is a critical stage prior to official onset that includes varying sub-syndromes.
4.3. Neuropsychological Performance and the Neural Correlates
At the cognitive level, our finding is that processing speed and visual–spatial memory may not be impaired at the HR stage; however, as the disease progresses further into the UHR stage, deficits in these domains can become apparent. Although emerging before official onset, genetically high-risk individuals who manifest no or mild, non-specific symptoms may have intact cognitive functioning in these domains. Our finding is at odds with previous findings that suggested cognitive deficits were inherited abnormalities in bipolar disorder. These studies examined neuropsychological performance in the “unaffected” relatives of individuals with BP (Drysdale et al., 2013, Kulkarni et al., 2010). Thus, the discrepancy probably lies in the definition of “unaffected”. By applying the clinical staging model, this study allowed the further subdivision of the unaffected status into the HR and UHR stages. In the HR stage, the impact of subthreshold symptoms on neuropsychological performance could be minimized. Indeed, previous research has shown that cognitive deficits are not characteristic of genetically high-risk, unaffected offspring (i.e., those with intact visual information processing) (Duffy et al., 2009) or of individuals later diagnosed with BP (both high and low academic performance was associated with risk for bipolar disorder) (MacCabe et al., 2010) but were apparent in those high-risk individuals who later developed psychotic affective disorder and schizophrenia (Cannon et al., 2008). Moreover, we observed a negative correlation between processing speed performance and the GM volumes of the regions in which the UHR offspring displayed abnormalities, including the right posterior cingulate cortex, superior frontal gyrus, and left superior parietal cortex. This result resonates with the observation that UHR offspring exhibited increased GM volumes and deficits in processing speed. The GM volumes in the parietal cortex have been reported to be negatively correlated with executive function in bipolar patients (Haldane et al., 2008). However, a recent multi-generational family study of bipolar disorder by Fears et al. reported that the GM volume of the posterior cingulate cortex was positively correlated with processing speed in bipolar patients, although the correlation did not survive the FDR-correction (Fears et al., 2015). They also found a positive correlation between the GM volumes in both the post cingulate and superior parietal cortex and visual–spatial memory. This observation is not supported by our study. Although the etiology of the GM change is unknown, factors such as age and affected status may contribute to the discrepancy.
4.4. Limitations
The following limitations must be noted for the interpretation of these results. First, the follow-up in this study was relatively short (up to 2.5 years), and only 17% of the UHR offspring have been confirmed to develop bipolar disorder by follow-up. Additionally, the sample size was relatively small. These findings need to be replicated in large, independent samples. Moreover, given that certain psychiatric disorders (e.g., substance use disorder and major depression) can precede the onset of bipolar disorder (Duffy et al., 2014) and this study excluded DSM-IV diagnosable disorders, the findings of this study may not be generalizable to other populations in which psychiatric disorders are comorbid with or precede the sub-syndromes specified in the UHR criteria (e.g., subthreshold depression). The UHR criteria for bipolar disorder need to be refined to better represent the full spectrum of symptoms. Furthermore, the manic sub-syndrome duration requirement (at least 4 days) may be conservative. Offspring with manic sub-syndromes may already be in a relatively “late” prodromal stage of bipolar disorder. The definition of bipolar disorder not otherwise specified (bipolar NOS) by DSM-IV is vague. Applying a duration of at least 4 days, the Course and Outcome of Bipolar Youth (COBY) study found that youths with bipolar NOS had a high rate of conversion into bipolar I or bipolar II disorder (29% in two years and 38% in four years) (Birmaher et al., 2006, Birmaher et al., 2009). Finally, the etiology of bipolar disorder may be heterogeneous (Duffy, 2014b). The current clinical staging model may be biased towards severe cases of bipolar disorder, in which a proportion of the UHR offspring displayed attenuated psychotic symptoms (the symptoms were partly overlapping with the UHR criteria for psychosis despite genetic susceptibility for BP). Thus, the findings of this study may not be generalizable to other high-risk populations.
4.5. Conclusions
Our data suggest that the underlying abnormalities of BP may become apparent long before the official onset of BP. Specifically, the decreased GM volumes in the OFC observed in the HR offspring may serve as neuroanatomical endophenotypes. In this stage, genetically high-risk offspring may not yet have cognitive deficits in processing speed and visual memory. In the next stage, UHR offspring may display increased GM volumes in the right posterior cingulate cortex, superior frontal gyrus, and left superior parietal cortex, which may partly explain the cognitive deficits found in UHR offspring. Although some network properties, such as small-world property, were significantly different between the HR and UHR offspring, the nature of these changes are currently hard to interpret, and these findings need to be replicated. We tentatively speculate that some changes may be related to compensatory reactions, which may be worthy of future investigation.
Clinical staging models are scientifically and clinically important, and they emphasize progression from a very early stage prior to the sub-syndromes. At this stage, common confounding effects (including symptoms, illness duration and medications) are minimized, and the possibility of investigating the underlying pathophysiology is maximized. More importantly, identifying abnormalities prior to full-blown onset opens up the possibility of putting into practice early intervention (i.e., when cognitive deficits are observed in the UHR stage) or even primary intervention strategies (i.e., when intact cognitive function but disrupted brain connections are observed in the HR stage), of particular importance because BP is currently considered irremediable and becomes progressively worse. A range of early interventions that show preliminary evidence of effectiveness, including sleep hygiene, nutraceuticals (e.g., omega-3 fatty acids), mindfulness, emotion regulation strategies, and anti-inflammatory agents (Duffy, 2014a), might be justified for offspring at familiar risk and those in the UHR stage in particular.
Contributors
TMCL and GX conceptualized the project. KL and GX prepared the original protocol with later input from KFS and TMCL. TMCL and KFS supervised the project's overall undertaking. KL, HW, TL, WL, KC, XC, BL, and LZ implemented the project. KL and NMW managed the data and performed the data analysis. KL, NMW, WL, and XC constructed the figures. KL drafted the manuscript. GX, KFS, and TMCL provided critical comments on the final version of the manuscript.
Declaration of Interests
We declare no competing interests.
Acknowledgments
The project was funded by the National Natural Science Foundation of China (NSFC, 81471375); the Key Medical discipline of Guangzhou, China (GBH2014-ZD04); the Science and Technology Planning Project of Guangdong Province, China (2011B031800154); and a Research Grant Council Humanities and Social Sciences Prestigious Fellowship (HKU703-HSS-13).
Role of the Funding Source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report. All authors had access to the data, and all authors agreed to submit the paper for publication.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.06.027.
Contributor Information
Guiyun Xu, Email: xuguiyun2908@hotmail.com.
Tatia M.C. Lee, Email: tmclee@hku.hk.
Appendix A. Supplementary Data
Supplementary material.
References
- Akiskal H.S., Downs J., Jordan P., Watson S., Daugherty D., Pruitt D.B. Affective disorders in referred children and younger siblings of manic-depressives. Mode of onset and prospective course. Arch. Gen. Psychiatry. 1985;42:996–1003. doi: 10.1001/archpsyc.1985.01790330076009. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E., Verchinski B.A., Mattay V.S., Weinberger D.R., Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J. Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechdolf A., Ratheesh A., Wood S.J., Tecic T., Conus P., Nelson B., Cotton S.M., Chanen A.M., Amminger G.P., Ruhrmann S., Schultze-Lutter F., Klosterkotter J., Fusar Poli P., Yung A.R., Berk M., McGorry P.D. Rationale and first results of developing at-risk (prodromal) criteria for bipolar disorder. Curr. Pharm. Des. 2012;18:358–375. doi: 10.2174/138161212799316226. [DOI] [PubMed] [Google Scholar]
- Birmaher B., Axelson D., Strober M., Gill M.K., Valeri S., Chiappetta L., Ryan N., Leonard H., Hunt J., Iyengar S., Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Arch. Gen. Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B., Axelson D., Goldstein B., Strober M., Gill M.K., Hunt J., Houck P., Ha W., Iyengar S., Kim E., Yen S., Hower H., Esposito-Smythers C., Goldstein T., Ryan N., Keller M. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am. J. Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne O., Krausz Y., Gorfine M., Karger H., Gelfin Y., Shapira B., Chisin R., Lerer B. Cerebral hypoperfusion in medication resistant, depressed patients assessed by Tc99m HMPAO SPECT. J. Affect. Disord. 1996;41:163–171. doi: 10.1016/s0165-0327(96)00058-4. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Camargo A., Azuaje F., Wang H., Zheng H. Permutation — based statistical tests for multiple hypotheses. Source Code Biol. Med. 2008;3:15. doi: 10.1186/1751-0473-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon T.D., Cadenhead K., Cornblatt B., Woods S.W., Addington J., Walker E., Seidman L.J., Perkins D., Tsuang M., McGlashan T., Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Ffytche D.H. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Cerullo M.A., Eliassen J.C., Smith C.T., Fleck D.E., Nelson E.B., Strawn J.R., Lamy M., DelBello M.P., Adler C.M., Strakowski S.M. Bipolar I disorder and major depressive disorder show similar brain activation during depression. Bipolar Disord. 2014;16:703–712. doi: 10.1111/bdi.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K., Karchemskiy A., Barnea-Goraly N., Garrett A., Simeonova D.I., Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Chen Z., Liu M., Gross D.W., Beaulieu C. Graph theoretical analysis of developmental patterns of the white matter network. Front. Hum. Neurosci. 2013;7:716. doi: 10.3389/fnhum.2013.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke S.F., Komorowski R.W., Kaplan E.S., Gavornik J.P., Bear M.F. Visual recognition memory, manifested as long-term habituation, requires synaptic plasticity in V1. Nat. Neurosci. 2015;18:262–271. doi: 10.1038/nn.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll C.U., Penzner J.B., Frederickson A.M., Richter J.J., Auther A.M., Smith C.W., Kane J.M., Cornblatt B.A. Differentiation in the preonset phases of schizophrenia and mood disorders: evidence in support of a bipolar mania prodrome. Schizophr. Bull. 2007;33:703–714. doi: 10.1093/schbul/sbm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll C.U., Hauser M., Penzner J.B., Auther A.M., Kafantaris V., Saito E., Olvet D., Carrion R.E., Birmaher B., Chang K.D., DelBello M.P., Singh M.K., Pavuluri M., Cornblatt B.A. Type and duration of subsyndromal symptoms in youth with bipolar I disorder prior to their first manic episode. Bipolar Disord. 2014;16:478–492. doi: 10.1111/bdi.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Simpson J.R., Jr., Todd R.D., Reich T., Vannier M., Raichle M.E. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drysdale E., Knight H.M., McIntosh A.M., Blackwood D.H. Cognitive endophenotypes in a family with bipolar disorder with a risk locus on chromosome 4. Bipolar Disord. 2013;15:215–222. doi: 10.1111/bdi.12040. [DOI] [PubMed] [Google Scholar]
- Duffy A. Interventions for youth at risk of bipolar disorder. Curr. Treat. Options Psychiatry. 2014:37–47. [Google Scholar]
- Duffy A. Toward a comprehensive clinical staging model for bipolar disorder: integrating the evidence. Can. J. Psychiatry. 2014;59:659–666. doi: 10.1177/070674371405901208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy A., Hajek T., Alda M., Grof P., Milin R., MacQueen G. Neurocognitive functioning in the early stages of bipolar disorder: visual backward masking performance in high risk subjects. Eur. Arch. Psychiatry Clin. Neurosci. 2009;259:263–269. doi: 10.1007/s00406-008-0862-3. [DOI] [PubMed] [Google Scholar]
- Duffy A., Horrocks J., Doucette S., Keown-Stoneman C., McCloskey S., Grof P. The developmental trajectory of bipolar disorder. Br. J. Psychiatry. 2014;204:122–128. doi: 10.1192/bjp.bp.113.126706. [DOI] [PubMed] [Google Scholar]
- Egeland J.A., Hostetter A.M., Pauls D.L., Sussex J.N. Prodromal symptoms before onset of manic-depressive disorder suggested by first hospital admission histories. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:1245–1252. doi: 10.1097/00004583-200010000-00011. [DOI] [PubMed] [Google Scholar]
- Fan Y., Shi F., Smith J.K., Lin W., Gilmore J.H., Shen D. Brain anatomical networks in early human brain development. Neuroimage. 2011;54:1862–1871. doi: 10.1016/j.neuroimage.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears S.C., Schur R., Sjouwerman R., Service S.K., Araya C., Araya X., Bejarano J., Knowles E., Gomez-Makhinson J., Lopez M.C., Aldana I., Teshiba T.M., Abaryan Z., Al-Sharif N.B., Navarro L., Tishler T.A., Altshuler L., Bartzokis G., Escobar J.I., Glahn D.C., Thompson P.M., Lopez-Jaramillo C., Macaya G., Molina J., Reus V.I., Sabatti C., Cantor R.M., Freimer N.B., Bearden C.E. Brain structure–function associations in multi-generational families genetically enriched for bipolar disorder. Brain. 2015;138:2087–2102. doi: 10.1093/brain/awv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier I.N., Chowdhury R., Thompson J.M., Watson S., Young A.H. Neurocognitive function in unaffected first-degree relatives of patients with bipolar disorder: a preliminary report. Bipolar Disord. 2004;6:319–322. doi: 10.1111/j.1399-5618.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- Frank E., Nimgaonkar V.L., Phillips M.L., Kupfer D.J. All the world's a (clinical) stage: rethinking bipolar disorder from a longitudinal perspective. Mol. Psychiatry. 2014;20:23–31. doi: 10.1038/mp.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier J.A., Breeze J.L., Makris N., Giuliano A.S., Herbert M.R., Seidman L., Biederman J., Hodge S.M., Dieterich M.E., Gerstein E.D., Kennedy D.N., Rauch S.L., Cohen B.M., Caviness V.S. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disord. 2005;7:555–569. doi: 10.1111/j.1399-5618.2005.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn. Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hajek T., Cullis J., Novak T., Kopecek M., Blagdon R., Propper L., Stopkova P., Duffy A., Hoschl C., Uher R., Paus T., Young L.T., Alda M. Brain structural signature of familial predisposition for bipolar disorder: replicable evidence for involvement of the right inferior frontal gyrus. Biol. Psychiatry. 2013;73:144–152. doi: 10.1016/j.biopsych.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane M., Cunningham G., Androutsos C., Frangou S. Structural brain correlates of response inhibition in Bipolar Disorder I. J. Psychopharmacol. 2008;22:138–143. doi: 10.1177/0269881107082955. [DOI] [PubMed] [Google Scholar]
- Ivleva E.I., Bidesi A.S., Keshavan M.S., Pearlson G.D., Meda S.A., Dodig D., Moates A.F., Lu H., Francis A.N., Tandon N., Schretlen D.J., Sweeney J.A., Clementz B.A., Tamminga C.A. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am. J. Psychiatry. 2013;170:1285–1296. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A., Hough M., James S., Burge L., Winmill L., Nijhawan S., Matthews P.M., Zarei M. Structural brain and neuropsychometric changes associated with pediatric bipolar disorder with psychosis. Bipolar Disord. 2011;13:16–27. doi: 10.1111/j.1399-5618.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- Kalmar J.H., Wang F., Spencer L., Edmiston E., Lacadie C.M., Martin A., Constable R.T., Duncan J.S., Staib L.H., Papademetris X., Blumberg H.P. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J. Int. Neuropsychol. Soc. 2009;15:476–481. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K., Shenton M.E., Salisbury D.F., Hirayasu Y., Lee C.U., Ciszewski A.A., Yurgelun-Todd D., Kikinis R., Jolesz F.A., McCarley R.W. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am. J. Psychiatry. 2003;160:156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzbichler M.G., Henson R.N., Smith M.L., Nathan P.J., Bullmore E.T. Cognitive effort drives workspace configuration of human brain functional networks. J. Neurosci. 2011;31:8259–8270. doi: 10.1523/JNEUROSCI.0440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S., Jain S., Janardhan Reddy Y.C., Kumar K.J., Kandavel T. Impairment of verbal learning and memory and executive function in unaffected siblings of probands with bipolar disorder. Bipolar Disord. 2010;12:647–656. doi: 10.1111/j.1399-5618.2010.00857.x. [DOI] [PubMed] [Google Scholar]
- Lawrie S.M., Whalley H., Kestelman J.N., Abukmeil S.S., Byrne M., Hodges A., Rimmington J.E., Best J.J., Owens D.G., Johnstone E.C. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353:30–33. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liang M., Zhou Y., He Y., Hao Y., Song M., Yu C., Liu H., Liu Z., Jiang T. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Liu J., Blond B.N., van Dyck L.I., Spencer L., Wang F., Blumberg H.P. Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disord. 2012;14:432–441. doi: 10.1111/j.1399-5618.2012.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord L.D., Allen P., Expert P., Howes O., Lambiotte R., McGuire P., Bose S.K., Hyde S., Turkheimer F.E. Characterization of the anterior cingulate's role in the at-risk mental state using graph theory. Neuroimage. 2011;56:1531–1539. doi: 10.1016/j.neuroimage.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Lyoo I.K., Sung Y.H., Dager S.R., Friedman S.D., Lee J.Y., Kim S.J., Kim N., Dunner D.L., Renshaw P.F. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- MacCabe J.H., Lambe M.P., Cnattingius S., Sham P.C., David A.S., Reichenberg A., Murray R.M., Hultman C.M. Excellent school performance at age 16 and risk of adult bipolar disorder: national cohort study. Br. J. Psychiatry. 2010;196:109–115. doi: 10.1192/bjp.bp.108.060368. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A., Vieta E., Reinares M., Colom F., Torrent C., Sanchez-Moreno J., Benabarre A., Goikolea J.M., Comes M., Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am. J. Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Kopecek M., Nicoletti M.A., Hatch J.P., Watanabe Y., Nery F.G., Zunta-Soares G., Soares J.C. New structural brain imaging endophenotype in bipolar disorder. Mol. Psychiatry. 2012;17:412–420. doi: 10.1038/mp.2011.3. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McGorry P.D., Hickie I.B., Yung A.R., Pantelis C., Jackson H.J. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust. N. Z. J. Psychiatry. 2006;40:616–622. doi: 10.1080/j.1440-1614.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- McGorry P.D., Nelson B., Amminger G.P., Bechdolf A., Francey S.M., Berger G., Riecher-Rossler A., Klosterkotter J., Ruhrmann S., Schultze-Lutter F., Nordentoft M., Hickie I., McGuire P., Berk M., Chen E.Y., Keshavan M.S., Yung A.R. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J. Clin. Psychiatry. 2009;70:1206–1212. doi: 10.4088/JCP.08r04472. [DOI] [PubMed] [Google Scholar]
- Mesman E., Nolen W.A., Reichart C.G., Wals M., Hillegers M.H. The Dutch bipolar offspring study: 12-year follow-up. Am. J. Psychiatry. 2013;170:542–549. doi: 10.1176/appi.ajp.2012.12030401. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S., Vourkas M., Tsirka V., Karakonstantaki E., Kanatsouli K., Stam C.J. The influence of ageing on complex brain networks: a graph theoretical analysis. Hum. Brain Mapp. 2009;30:200–208. doi: 10.1002/hbm.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G.J., Bebchuk J.M., Wilds I.B., Chen G., Manji H.K. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Moorhead T.W., McKirdy J., Sussmann J.E., Hall J., Lawrie S.M., Johnstone E.C., McIntosh A.M. Progressive gray matter loss in patients with bipolar disorder. Biol. Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Nery F.G., Chen H.H., Hatch J.P., Nicoletti M.A., Brambilla P., Sassi R.B., Mallinger A.G., Keshavan M.S., Soares J.C. Orbitofrontal cortex gray matter volumes in bipolar disorder patients: a region-of-interest MRI study. Bipolar Disord. 2009;11:145–153. doi: 10.1111/j.1399-5618.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- Newman M.E. Assortative mixing in networks. Phys. Rev. Lett. 2002;89:208701. doi: 10.1103/PhysRevLett.89.208701. [DOI] [PubMed] [Google Scholar]
- Nugent A.C., Milham M.P., Bain E.E., Mah L., Cannon D.M., Marrett S., Zarate C.A., Pine D.S., Price J.L., Drevets W.C. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30:485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Pantelis C., Velakoulis D., McGorry P.D., Wood S.J., Suckling J., Phillips L.J., Yung A.R., Bullmore E.T., Brewer W., Soulsby B., Desmond P., McGuire P.K. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P., Cahill M.E., Jones K.A., VanLeeuwen J.E., Woolfrey K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Kupfer D.J. Bipolar disorder diagnosis: challenges and future directions. Lancet. 2013;381:1663–1671. doi: 10.1016/S0140-6736(13)60989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Swartz H.A. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am. J. Psychiatry. 2014;171:829–843. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa A.R., Magalhaes P.V., Czepielewski L., Sulzbach M.V., Goi P.D., Vieta E., Gama C.S., Kapczinski F. Clinical staging in bipolar disorder: focus on cognition and functioning. J. Clin. Psychiatry. 2014;75:e450–e456. doi: 10.4088/JCP.13m08625. [DOI] [PubMed] [Google Scholar]
- Rygula R., Walker S.C., Clarke H.F., Robbins T.W., Roberts A.C. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J. Neurosci. 2010;30:14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Leboyer M., Hickie I., Berk M., Kapczinski F., Frank E., Kupfer D., McGorry P. Clinical staging in psychiatry: a cross-cutting model of diagnosis with heuristic and practical value. Br. J. Psychiatry. 2013;202:243–245. doi: 10.1192/bjp.bp.112.110858. [DOI] [PubMed] [Google Scholar]
- Smit D.J., Stam C.J., Posthuma D., Boomsma D.I., de Geus E.J. Heritability of “small-world” networks in the brain: a graph theoretical analysis of resting-state EEG functional connectivity. Hum. Brain Mapp. 2008;29:1368–1378. doi: 10.1002/hbm.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Tessner K.D., Toga A.W. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J. Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield A.C., Moorhead T.W., Job D.E., McKirdy J., Sussmann J.E., Hall J., Giles S., Johnstone E.C., Lawrie S.M., McIntosh A.M. Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disord. 2009;11:135–144. doi: 10.1111/j.1399-5618.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- Strasser H.C., Lilyestrom J., Ashby E.R., Honeycutt N.A., Schretlen D.J., Pulver A.E., Hopkins R.O., Depaulo J.R., Potash J.B., Schweizer B., Yates K.O., Kurian E., Barta P.E., Pearlson G.D. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol. Psychiatry. 2005;57:633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Tsuang M.T., Faraone S.V. The genetic epidemiology of bipolar disorder. In: Marneros A., Angst J., editors. Bipolar Disorders: 100 Years After Manic-Depressive Insanity. Kluwer Academic; Zurich, Switzerland: 2000. [Google Scholar]
- Wingo A.P., Harvey P.D., Baldessarini R.J. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disord. 2009;11:113–125. doi: 10.1111/j.1399-5618.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- Xu G., Lin K., Rao D., Dang Y., Ouyang H., Guo Y., Ma J., Chen J. Neuropsychological performance in bipolar I, bipolar II and unipolar depression patients: a longitudinal, naturalistic study. J. Affect. Disord. 2012;136:328–339. doi: 10.1016/j.jad.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Yuksel C., Du F., Ravichandran C., Goldbach J.R., Thida T., Lin P., Dora B., Gelda J., O'Connor L., Sehovic S., Gruber S., Ongur D., Cohen B.M. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Mol. Psychiatry. 2015 doi: 10.1038/mp.2015.13. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Yung A.R., Yuen H.P., McGorry P.D., Phillips L.J., Kelly D., Dell'Olio M., Francey S.M., Cosgrave E.M., Killackey E., Stanford C., Godfrey K., Buckby J. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust. N. Z. J. Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.