Summary
Background
PA28γ was suggested to play a role in malignant progression. This paper aimed to investigate the association between PA28γ and the prognosis of oral squamous cell carcinoma (OSCC) in cohort studies.
Methods
The PA28γ expression level was assessed by immunohistochemistry in a total of 368 OSCC patients from three independent cohorts. The Cox proportional hazards regression model was used to determine multivariate hazard ratios for Overall Survival (OS). Model discrimination was measured using C Statistic. Additionally, OS was analyzed in Head Neck Squamous Cell Carcinoma (HNSCC) patients from The Cancer Genome Atlas (TCGA) data set. Functional analyses were conducted both in-vitro and in-vivo.
Findings
The median follow-up times of patients in the three studies were 60, 52, and 51 months. High expression of PA28γ was identified in tumors from 179 of 368 patients (48.6%). Compared with low expression, high expression of PA28γ was strongly associated with worse OS, with relative risks of 5.14 (95% CI, 2.51–10.5; P < 0.001), 2.82 (95% CI, 1.73–4.61; P < 0.001), and 3.85 (95% CI, 1.59–9.37; P = 0.003). PA28γ expression was also associated with disease-free survival in all three cohorts (P < 0.005). These findings are consistent with TCGA HNSCC data (P < 0.006). The prediction of all-cause mortality was significantly improved when PA28γ was added to the traditional clinical factors (Model 3, C statistic value: 0.78 VS 0.73, P = 0.016). In functional analyses, we found that PA28γ silencing dramatically inhibited the growth, proliferation and mobility of OSCC cells in vitro and reduced tumor growth and angiogenesis in tumor-bearing mice.
Interpretation
PA28γ overexpression is associated with adverse prognosis in patients with OSCC. The aberrant expression of PA28γ may contribute to the pathogenesis and progression of OSCC.
Research in context
OSCC is one of the most common HNSCC, which have a high lethally rate. However, few prognostic markers have been applied in the clinical practice. We found that PA28γ in OSCC tumor tissues were significantly high expression than those in normal tissues. As the results of the three cohorts from two independent research centers and from an additional validation cohort from a US population in the TCGA dataset, we demonstrate PA28γ is a good predictor of the risk of death in OSCC. Meanwhile, we demonstrate PA28γ have a potential role in OSCC tumorigenesis.
Keywords: PA28γ, OSCC, Prognosis, Carcinogenesis
Highlights
-
•
PA28γ protein over-expressed in a large subset of patients with OSCC
-
•
PA28γ was a prognostic factor in OSCC based on the results of three cohorts in China
-
•
The analysis of PA28γ mRNA abundance in a US/non-Chinese cohort from TCGA dataset consistent with Chinese-cohort study
-
•
PA28γ silencing could affect the tumor biological behavior of OSCC both in vitro and vivo.
1. Introduction
OSCC is one of the most common HNSCC, with an estimated 260,000 new cases and 120,000 deaths worldwide each year (Jemal et al., 2011). Despite recent advances in diagnosis and treatment, the 5-year survival rate of patients with OSCC is no more than 50% (Panzarella et al., 2014). Over the past two decades, numerous prognostic and predictive markers for clinical outcomes in OSCC have been proposed (Ratajczak-Wrona et al., 2013); however, few have been applied in clinical practice due to the non-reproducibility of the initial findings (Choi and Myers, 2008, Principe et al., 2013). To date, the classical clinic pathological parameters of tumor such as primary site, tumor stage, lymph nodal stage and clinical TNM stage remain the most significant factors to affect outcome of patients with OSCC. However, it is impossible to predict patients at a high risk of death mainly based on these parameters. Therefore, it is critical to identify novel and effective prognostic predictors and therapeutic targets for treating this common malignancy.
In eukaryotic cells, proteasomes play an essential role in intracellular proteolysis and are involved in the control of most biological processes through regulated degradation of key proteins. PA28 is a member of a unique family of proteasomal activators that has the ability to stimulate the proteolytic activity of the 20S core proteasome independent of ubiquitination and ATP (Li et al., 2007). Unlike PA28α and PA28β, PA28γ (also known as Ki antigen, 11Sγ, or REGγ) localizes in the nucleus and forms a homo-heptamer (Kloetzel and Ossendorp, 2004, Rechsteiner et al., 2000, Rivett and Hearn, 2004). PA28γ, regulated by MEKK3, B-RAF, caspase-3/7 and targeted by miR-7-5p, is a multifunctional protein that is involved in the degradation of important regulatory proteins, such as SRC-3, PTTG1 and cyclin-dependent kinase inhibitors p21/16/19 in an ubiquitin- and ATP-independent manner, and has been implicated in the regulation of cell cycle progression (Li et al., 2007, Araya et al., 2002, Chen et al., 2007, Ying et al., 2006, Shi et al., 2015). Moreover, PA28γ-deficient mice have been shown to exhibit growth retardation (Barton et al., 2004). Several targets of PA28γ have been identified in recent years, suggesting that it plays important roles in angiogenesis, hepatic lipid metabolism, infectious diseases and premature aging (Liu et al., 2014, Dong et al., 2013, Yan et al., 2014, Li et al., 2013). PA28γ is over-expressed in some cancer tissues, suggesting that this protein may also have a potential role in tumorigenesis (Wang et al., 2011, Roessler et al., 2006). Some studies found that PA28γ may facilitate the turnover of the tumor suppressor p53 by promoting murine double minute 2 (MDM2)-mediated p53 ubiquitination (He et al., 2012, Zhang and Zhang, 2008) and PA28γ could take part in the ATM-DBC1-SIRT1 axis induced p53-dependent apoptosis (Magni et al., 2014). Recently researchers found that mutant p53 (p53-R248Q) could up-regulate PA28γ in endometrial cancer (Wang et al., 2015), thus, there is an auto-regulatory feedback loop between p53 and PA28γ (Wan et al., 2014). Nevertheless, the mechanism by which PA28γ exerts its effects on tumor cells remains unclear.
In our previous study, we conducted a comprehensive proteomic analysis to identify candidate biomarkers in OSCC (Wang et al., 2008). Expression levels of 52 proteins in OSCC tumor tissues were significantly different from those in normal tissues (Wang et al., 2009). One of these proteins was PA28γ. The bioprocesses and interaction network analysis indicated that PA28γ might play an important role in malignant transformation. Given these findings, we hypothesized that PA28γ may be involved in malignant development and progression of OSCC and would have effect on the prognosis of this disease. To test this hypothesis, we first explored the protein expression profile of PA28γ and its relations with the outcome of OSCC patients in three independent cohorts from two centers. Then, we constructed models to predict death of patients with OSCC using PA28γ individually and jointly with other prognostic factors identified in these three cohorts. Finally, we investigated the effects of PA28γ on the biological behavior of OSCC cells both in vitro and in vivo.
2. Methods and patients
2.1. Patients
The Institutional Review Boards of the West China Hospital of Stomatology, Sichuan University and Guangdong Provincial Stomatological Hospital approved this study. The study was approved by the ethics committee both of the West China Hospital of Stomatology and the Guangdong Provincial Stomatological Hospital and was conducted in agreement with the Helsinki Declaration. Written informed consent was provided by all participants at baseline and during follow-up.
A total of 368 postoperative patients with primary OSCC tumors received regular follow-up. Follow-up visits entailed at least a medical history and clinical examination. In addition to scheduled visits, all patients could initiate visits if they were concerned that they had recurrence or a new primary tumor. The survival time of each patient was calculated from the day of surgery until the time of cancer-related death or the end of the follow-up period, death for other reasons led to censoring of data. The detailed information of three cohorts was described in Supplemental Patients and Methods.
An independent cohort of 460 patient specimens obtained between 1992 and 2013 in the TCGA database (http://tcga-data.nci.nih.gov/tcga/) was used as an external validation cohort to validate the prognostic value of PA28γ in patients with HNSCC (Table S1).
2.2. Laboratory experiments
All animal studies were approved by the Animal Care and Use Committee, State Key Laboratory of Oral Diseases, in compliance with the Guide for the U.S. Public Health Service's policy on humane care and use of laboratory animals (Kilkenny et al., 2010). Animals were housed within 12-h light/dark cycles and received food, standard rodent chow, and water ad libitum in compliance with the Association for Assessment and Accreditation of Laboratory Animal Care International guidelines. Other methods are detailed in Supplemental Patients and Methods including Cell Culture and siRNA transfections, western blot analysis, MTT, colony-formation, propidium iodide (PI) staining, flow cytometry, TUNEL, cell invasion, cell migration, in vivo tumor-formation assay, and immunohistochemistry.
2.3. Statistical analysis
Baseline characteristics among the patients were compared using the mixed linear model for continuous variables and the χ2 test or Fisher's Exact Test for categorical variables. OS and DFS were estimated using the Kaplan–Meier method, with a log-rank test in a univariate analysis. Multivariate survival analysis was done using the Cox proportional hazards model. Model discrimination was measured using C Statistic for survival analysis. Receiver operating characteristic (ROC) curve area was used in the prediction model.
Statistical analyses were performed using SAS software, version 9.3 (SAS Institute Inc., Cary, NC). Unless stated otherwise, two-sided P < 0.05 were considered significant. Details on data analysis are provided in the Supplementary.
3. Results
3.1. Patient and disease characteristics
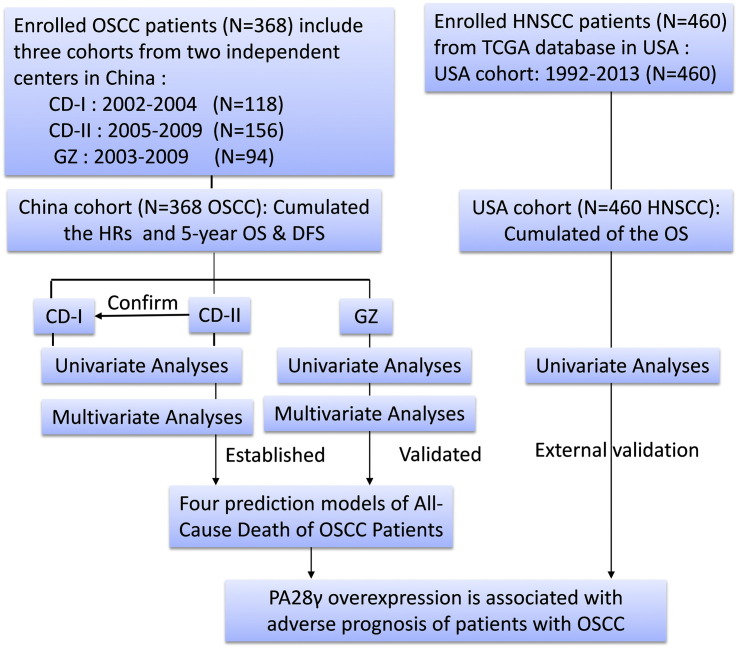
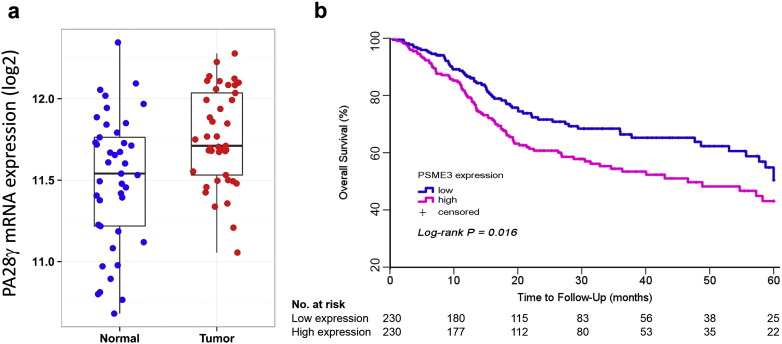
A total of 368 patients from three independent cohorts (118, 156, and 94 patients in CD-I cohort, CD-II cohort, and GZ cohort, respectively) were included in this study (Fig. 1). All patients were treated with curative intent. Some of these patients had been treated by radiotherapy and/or chemotherapy. The mean age and gender distribution were comparable across the three cohorts. The median durations of follow-up in the cohorts were 60, 52, and 51 months, respectively. IHC staining showed that PA28γ has a very clear nuclear positive in most tumor but not normal tissues (Fig. S1). High expression of PA28γ was observed on OSCC cell lines, and in 52.54%, 38.46%, and 60.64% of the patients in the CD-I cohort, CD-II cohort and GZ cohort, respectively (Table 1 and Fig. S2). This finding is consistent with the TCGA database analysis of PA28γ mRNA abundance in human primary oral cancers (Fig. S3a).
Fig. 1.
Study flow chart.
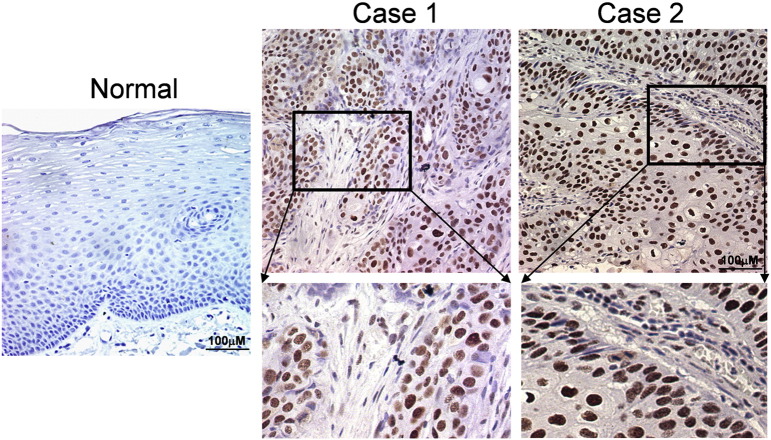
Fig. S1.
PA28γ staining pattern in both normal and tumor tissues. The staining of PA28γ in two selected tumor but not normal tissues has very clear nuclear positive, as shown by IHC assay.
Table 1.
Baseline characteristics of the patients with oral squamous cell carcinoma in three cohorts.
| Characteristic | CD-I cohort (N = 118) |
CD-II cohort (N = 156) |
GZ cohort (N = 94) |
P value⁎ |
|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | ||
| Age–yr (mean ± sd) | 58.97 ± 13.67 | 58.76 ± 10.62 | 60.39 ± 12.35 | 0.563 |
| < 60 yr | 55 (46.61) | 76 (48.72) | 42 (44.68) | 0.821 |
| ≥ 60 yr | 63 (53.39) | 80 (51.28) | 52 (55.32) | |
| Sex | ||||
| Male | 81 (68.64) | 112 (71.79) | 55 (58.51) | 0.089 |
| Female | 37 (31.36) | 44 (28.21) | 39 (41.49) | |
| Smoking | ||||
| Never | 65 (55.08) | 76 (48.72) | 50 (53.19) | 0.556 |
| Ever | 53 (44.92) | 80 (51.28) | 44 (46.81) | |
| Drinking | ||||
| Never | 62 (52.54) | 76 (48.72) | 55 (58.51) | 0.324 |
| Ever | 56 (47.46) | 80 (51.28) | 39 (41.49) | |
| Differentiation | ||||
| High | 75 (63.56) | 104 (66.67) | 72 (76.60) | 0.144 |
| Moderate | 37 (31.36) | 40 (25.64) | 20 (21.28) | |
| Low | 6 (5.08) | 12 (7.69) | 2 (2.13) | |
| Primary site | ||||
| Ventral tongue/floor of mouth | 50 (42.37) | 71 (45.51) | 54 (57.45) | < 0.001 |
| Buccal mucosa | 18 (15.25) | 27 (17.31) | 10 (10.64) | |
| Gingiva | 15 (12.71) | 23 (14.74) | 25 (26.60) | |
| Othersa | 35 (29.66) | 35 (22.44) | 5 (5.32) | |
| Tumor stage | ||||
| T1 | 36 (30.51) | 34 (21.79) | 19 (20.21) | < 0.001 |
| T2 | 61 (51.69) | 48 (30.77) | 48 (51.06) | |
| T3 | 15 (12.71) | 53 (33.97) | 12 (12.77) | |
| T4 | 6 (5.08) | 21 (13.46) | 15 (15.96) | |
| Nodal stage | ||||
| N0 | 82 (69.49) | 108 (69.23) | 71 (75.53) | 0.522 |
| N1–N3 | 36 (30.51) | 48 (30.77) | 23 (24.47) | |
| Clinical TNM stage | ||||
| I | 28 (23.73) | 29 (18.59) | 15 (15.93) | 0.009 |
| II | 45 (38.14) | 37 (23.72) | 38 (40.43) | |
| III | 24 (20.34) | 57 (36.54) | 20 (21.28) | |
| IV | 21 (17.80) | 33 (21.15) | 21 (22.34) | |
| Surgery type | ||||
| Local | 49 (41.53) | 55 (35.26) | 18 (19.15) | < 0.001 |
| Unilateral neck | 62 (52.54) | 82 (52.56) | 65 (69.15) | |
| Bilateral neck | 6 (5.08) | 4 (2.56) | 6 (5.32) | |
| Other | 1 (0.85) | 15 (9.62) | 5 (5.32) | |
| Radiotherapy | ||||
| Yes | 9 (7.63) | 28 (17.95) | 15 (15.96) | 0.044 |
| No | 109 (92.37) | 128 (82.05) | 79 (84.04) | |
| Chemotherapy | ||||
| Yes | 65 (55.08) | 84 (53.85) | 42 (44.68) | 0.262 |
| No | 53 (44.92) | 72 (46.15) | 52 (55.32) | |
| Radiotherapy or chemotherapy | ||||
| Yes | 67 (56.78) | 93 (59.62) | 49 (52.13) | 0.512 |
| No | 51 (43.22) | 63 (40.38) | 45 (47.87) | |
| PA28γ | ||||
| Low expression | 56 (47.46) | 96 (61.54) | 37 (39.36) | 0.002 |
| High expression | 62 (52.54) | 60 (38.46) | 57 (60.64) |
Abbreviations: CD, Chengdu; GZ, Guangzhou.
P value of comparison between studies was generated using mixed linear model for continuous variables and chi-square test or Fisher's exact test for categorical variables.
Others included hard palate, mandibular and lip mucosa.
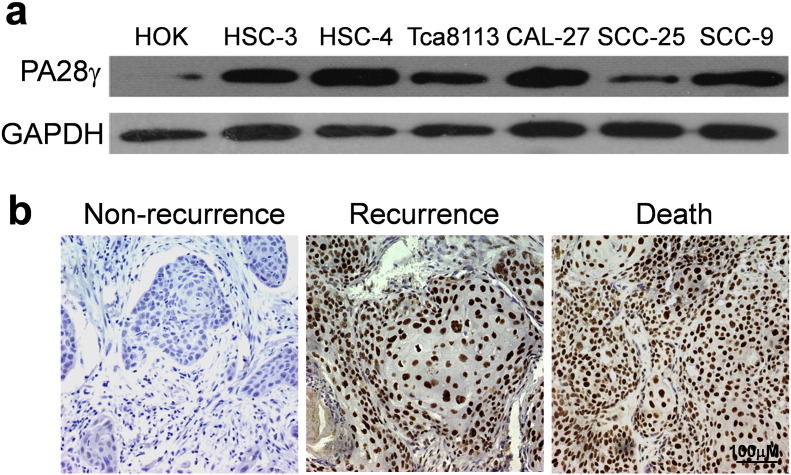
Fig. S2.
Expression of PA28γ related to carcinogenesis and prognosis in OSCC cells and human tissues. (a) Validation of PA28γ over-expression in six OSCC cells compared with normal oral keratinocyte by Western blot as described in material and methods. (b) Validation the role of PA28γ expression in OSCC prognosis in 118 clinical OSCC samples. IHC staining showed those cases with poorer prognosis exhibited stronger staining. Left: a moderate-differentiated OSCC sample with disease free survival. Middle: a well-differentiated OSCC sample with regional neck recurrence within 2 years postoperation. Right: a well-differentiated OSCC sample with cancer-related death with 4 year's postoperation. The scale bar represents 100 μm.
Fig. S3.
TCGA database analysis of HNSCC patients with high and low expression of PA28γ. (a) TCGA database analysis of PA28γ mRNA abundance in human primary oral cancers compared with human normal oral tissue. (b). Kaplan–Meier plot of estimated 5-year Overall Survival time distributions according to PA28γ mRNA expression.
3.2. Univariate and multivariate analyses of PA28γ expression and its predictive value
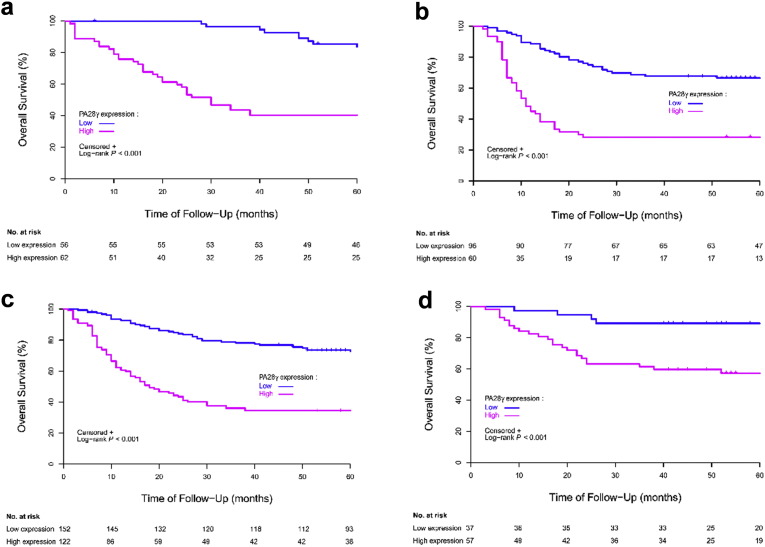
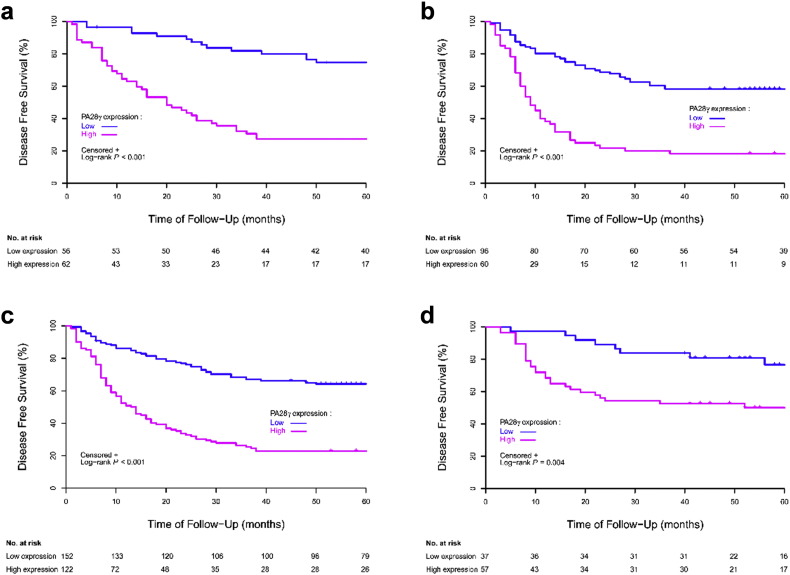
In all three cohorts, the results of univariate analysis showed that OS at five years was associated with PA28γ expression (Table 2). For the CD-I cohort, estimated 5-year OS values for patients in the low and high PA28γ expression groups were 84% (95% CI, 0.71–0.91) and 40% (95% CI, 0.28–0.52; Fig. 2a), respectively. For the CD-II cohort, the estimated 5-year OS values for patients in the low and high PA28γ expression groups were 67% (95% CI, 0.56–0.57) and 28% (95% CI, 0.18–0.40; Fig. 2b), respectively. For the joint CD-I and CD-II cohorts, the estimated 5-year OS for patients also showed that the risk increased associated with positive staining (P < 0.001; Fig. 2c). For the GD validation cohort, the estimated 5-year OS values for patients in the low and high PA28γ expression groups were 89% (95% CI, 0.74–0.96) and 57% (95% CI, 0.43–0.69; Fig. 2d), respectively. Consistent with an external validation cohort of 460 HNSCC patients from the TCGA database analysis in the US population, high PA28γ mRNA was associated with poor survival (P = 0.016, Fig. S3b). Furthermore, the association between PA28γ high expression with lower rates of 5-year Disease-Free Survival (DFS) was also statistically significant in those three independent cohorts (P < 0.001; CD-II: P < 0.001; joint CD-I and -II cohorts: P < 0.001; GD: P = 0.004; Table S2; Fig. S4). Several conventional prognostic factors, including lower cell differentiation, positive nodal stage, higher tumor stage, higher clinical TNM stage and radiotherapy or chemotherapy, were associated with a significantly increased risk of death. History of smoking and alcohol consumption were significantly related with survival (P < 0.005). Some of these factors were included in the multivariable Cox proportional-hazards model and fixed.
Table 2.
Univariate analyses of selected characteristics with survival among patients with oral squamous cell carcinoma.
| Characteristic | CD-I cohort (N = 118) |
CD-II cohort (N = 156) |
GZ cohort (N = 94) |
|||
|---|---|---|---|---|---|---|
| OS at five years |
P value⁎ |
OS at five years |
P value⁎ |
OS at five years |
P Value⁎ |
|
| (95% CI) | (95% CI) | (95% CI) | ||||
| Age | ||||||
| < 60 yr | 0.65 (0.51–0.76) | 0.510 | 0.47 (0.36–0.58) | 0.139 | 0.81 (0.66–0.90) | 0.057 |
| ≥ 60 yr | 0.56 (0.43–0.68) | 0.56 (0.45–0.66) | 0.61 (0.46–0.73) | |||
| Sex | ||||||
| Male | 0.58 (0.46–0.67) | 0.327 | 0.54 (0.45–0.63) | 0.422 | 0.67 (0.52–0.78) | 0.352 |
| Female | 0.68 (0.50–0.80) | 0.45 (0.30–0.59) | 0.74 (0.58–0.85) | |||
| Smoking history | ||||||
| Never | 0.75 (0.63–0.84) | < 0.001 | 0.58 (0.46–0.68) | 0.108 | 0.75 (0.60–0.85) | 0.097 |
| Ever | 0.43 (0.30–0.56) | 0.46 (0.35–0.57) | 0.64 (0.48–0.76) | |||
| Drinking history | ||||||
| Never | 0.69 (0.56–0.79) | 0.037 | 0.65 (0.54–0.75) | < 0.001 | 0.78 (0.65–0.87) | 0.027 |
| Ever | 0.52 (0.38–0.64) | 0.39 (0.28–0.49) | 0.58 (0.41–0.72) | |||
| Cell differentiation | ||||||
| High | 0.70 (0.58–0.79) | 0.003 | 0.58 (0.48–0.66) | 0.020 | 0.75 (0.63–0.83) | 0.042 |
| Moderate or Low | 0.44 (0.29–0.58) | 0.40 (0.27–0.53) | 0.55 (0.32–0.72) | |||
| Primary site | ||||||
| Ventral tongue/floor of mouth | 0.59 (0.44–0.72) | 0.814 | 0.56 (0.44–0.67) | 0.477 | 0.72 (0.58–0.82) | 0.265 |
| Buccal mucosa | 0.67 (0.40–0.83) | 0.44 (0.25–0.62) | 0.86 (0.33–0.98) | |||
| Gingiva | 0.67 (0.38–0.85) | 0.43 (0.23–0.62) | 0.50 (0.25–0.71) | |||
| Othersa | 0.57 (0.39–0.71) | 0.54 (0.37–0.69) | 0.60 (0.13–0.88) | |||
| Tumor stage | ||||||
| T1 or T2 | 0.66 (0.55–0.74) | 0.003 | 0.56 (0.45–0.66) | 0.183 | 0.76 (0.64–0.85) | 0.076 |
| T3 or T4 | 0.33 (0.15–0.53) | 0.47 (0.36–0.58) | 0.53 (0.32–0.71) | |||
| Nodal stage | ||||||
| N0 | 0.72 (0.60–0.80) | < 0.001 | 0.61 (0.51–0.70) | < 0.001 | 0.78 (0.67–0.86) | < 0.001 |
| N1-N3 | 0.36 (0.21–0.51) | 0.31 (0.19–0.44) | 0.43 (0.23–0.62) | |||
| Clinical TNM stage | ||||||
| I or II | 0.76 (0.65–0.85) | < 0.001 | 0.61 (0.48–0.71) | 0.024 | 0.79 (0.66–0.88) | 0.041 |
| III or IV | 0.36 (0.22–0.49) | 0.46 (0.35–0.55) | 0.57 (0.40–0.71) | |||
| Surgical method | ||||||
| Local | 0.61 (0.46–0.73) | 0.878 | 0.60 (0.46–0.72) | 0.116 | 0.78 (0.51–0.91) | 0.318 |
| Unilateral or bilateral or other | 0.60 (0.48–0.71) | 0.48 (0.38–0.57) | 0.68 (0.56–0.77) | |||
| Radiotherapy | ||||||
| Yes | 0.78 (0.36–0.94) | 0.347 | 0.43 (0.25–0.60) | 0.218 | 1.00 (1.00–1.00) | 0.008 |
| No | 0.59 (0.48–0.68) | 0.54 (0.45–0.62) | 0.64 (0.52–0.74) | |||
| Chemotherapy | ||||||
| Yes | 0.74 (0.61–0.83) | 0.002 | 0.57 (0.46–0.67) | 0.204 | 0.75 (0.58–0.86) | 0.147 |
| No | 0.44 (0.31–0.57) | 0.46 (0.34–0.57) | 0.65 (0.51–0.77) | |||
| Radiotherapy or chemotherapy | ||||||
| Yes | 0.75 (0.62–0.83) | < 0.001 | 0.58 (0.47–0.67) | 0.097 | 0.78 (0.63–0.88) | 0.015 |
| No | 0.42 (0.28–0.55) | 0.43 (0.31–0.55) | 0.60 (0.44–0.73) | |||
| PA28γ | ||||||
| Low expression | 0.84 (0.71–0.91) | < 0.001 | 0.67 (0.56–0.75) | < 0.001 | 0.89 (0.74–0.96) | < 0.001 |
| High expression | 0.40 (0.28–0.52) | 0.28 (0.18–0.40) | 0.57 (0.43–0.69) | |||
Abbreviations: OS, Overall Survival; CD, Chengdu; GZ, Guangzhou.
P value was determined using the log-rank test.
Others included hard palate, mandibular and lip mucosa.
Fig. 2.
Overall Survival (OS) of OSCC patients with high and low expression of PA28γ in Three cohorts defined by the Kaplan–Meier survival curves. (a) Overall Survival in CD-I cohort. (b) Overall Survival in CD-II cohort. (c) Overall Survival in CD-I and -II cohorts. (d) Overall Survival in GZ cohort.
Fig. S4.
Disease-Free Survival (DFS) of OSCC patients with high and low expression of PA28γ in Three cohorts defined by the Kaplan–Meier survival curves. (a) DFS in CD-I cohort. (b) DFS in CD-II cohort. (c) DFS in CD-I and -II cohorts. (d) DFS in GZ Cohort.
Results of the predictive analysis are provided in Table 3. Strong PA28γ expression was independently associated with significantly reduced OSCC patients' OS in the CD-I cohort (HR, 5.14; 95% CI, 2.51–10.5; P < 0.001) after accounting for smoking history, drinking history, cell differentiation, tumor stage, nodal stage and radiotherapy or chemotherapy; this was later confirmed in the CD-II cohort (HR, 2.82; 95% CI, 1.73–4.61; P < 0.001). There is evidence that patients with PA28γ high expression had worse DFS in the CD-I and CD-II cohorts, with hazard ratios of 3.82 (95% CI, 2.12–6.88; P < 0.001) and 2.96 (95% CI, 1.87–4.69; P < 0.001), respectively (Table S3), after accounting for the same factors as in the OS analysis. Furthermore, in the joint CD-I and CD-II cohorts and in the validation GZ cohort, the analysis results showed similar patterns (P < 0.001; Table 3; Table S3).
Table 3.
Multivariate analyses of survival among patients with oral squamous cell carcinoma.
| Characteristic | CD-I cohort (N = 118) |
CD-II cohort 2 (N = 156) |
CD cohort combined (N = 274) |
GZ cohort (N = 94) |
||||
|---|---|---|---|---|---|---|---|---|
| OS at five years |
OS at five years |
OS at five years |
OS at five years |
|||||
| HR |
P Value⁎ |
HR |
P Value⁎ |
HR |
P Value⁎ |
HR |
P Value⁎ |
|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||
| Smoking history | ||||||||
| Never | Reference | 0.072 | Reference | 0.979 | Reference | 0.180 | Reference | 0.349 |
| Ever | 1.87 (0.65–3.68) | 1.01 (0.59–1.72) | 1.32 (0.88–1.98) | 1.45 (0.67–3.13) | ||||
| Drinking history | ||||||||
| Never | Reference | 0.717 | Reference | 0.023 | Reference | 0.065 | Reference | 0.103 |
| Ever | 1.14 (0.57–2.28) | 1.86 (1.09–3.16) | 1.47 (0.98–2.22) | 1.89 (0.88–4.07) | ||||
| Cell differentiation | ||||||||
| High | Reference | 0.036 | Reference | 0.078 | Reference | 0.006 | Reference | 0.014 |
| Moderate or Low | 1.98 (1.05–3.75) | 1.55 (0.95–2.50) | 1.70 (1.16–2.48) | 2.84 (1.23–6.52) | ||||
| Tumor stage | ||||||||
| T1 or T2 | Reference | 0.040 | Reference | 0.399 | Reference | 0.011 | Reference | 0.991 |
| T3 or T4 | 2.20 (1.04–4.69) | 1.23 (0.76–1.97) | 1.65 (1.12–2.44) | 1.01 (0.46–2.19) | ||||
| Nodal stage | ||||||||
| N0 | Reference | 0.316 | Reference | 0.012 | Reference | 0.016 | Reference | 0.016 |
| N1-N3 | 1.40 (0.72–2.72) | 1.89 (1.15–3.10) | 1.62 (1.09–2.40) | 2.61 (1.20–5.69) | ||||
| Radiotherapy or chemotherapy | ||||||||
| Yes | Reference | 0.004 | Reference | 0.141 | Reference | 0.001 | Reference | 0.051 |
| No | 2.47 (1.34–4.53) | 1.44 (0.89–2.35) | 1.82 (1.26–2.61) | 2.17 (1.00–4.69) | ||||
| PA28γ | ||||||||
| Low expression | Reference | < 0.001 | Reference | < 0.001 | Reference | < 0.001 | Reference | < 0.001 |
| High expression | 5.14 (2.51–10.5) | 2.82 (1.73–4.61) | 3.02 (1.05–4.43) | 6.39 (2.12–19.3) | ||||
Abbreviations: OS, Overall Survival; CD, Chengdu; GZ, Guangzhou; HR, Hazard Ratio.
P value was determined using Cox proportional-hazards model.
3.3. Prediction models for all-cause death of OSCC patients
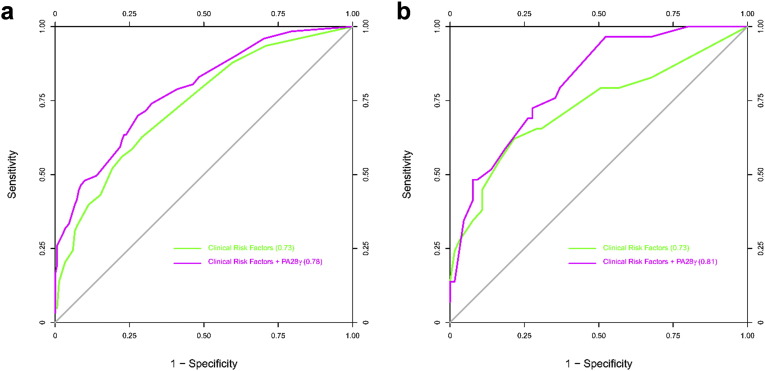
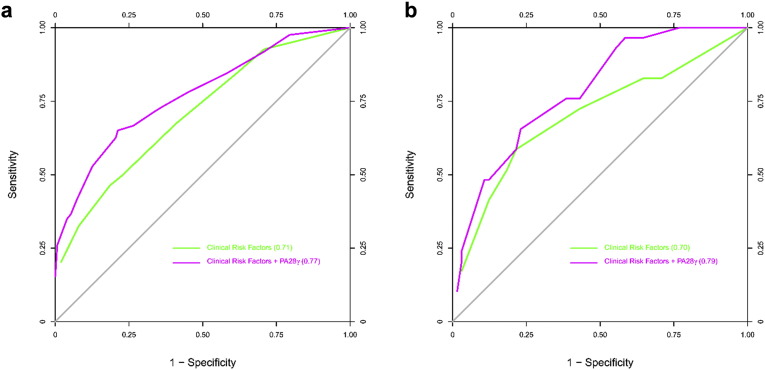
Multivariable models were constructed for the prediction of all-cause death in OSCC patients by using the combined CD cohort as the discovery cohort, and the GD cohort as a validation cohort. We assessed model discrimination using the C statistic for predictive value and compared the difference between basic models and models including PA28γ expression (Table S4). For the discovery of CD cohort, in Model 1, among the basic risk factors, the C statistic value of PA28γ was highest. The C statistic increased significantly when PA28γ was combined with those conventional risk factors in Models 2 to 4. In Models 3 and 4, when PA28γ was added, the C statistic was larger than 0.75, above which the prediction model is considered relatively good. Similar results were found in the validation GZ cohort. ROC curves were constructed for the mode Models 3 and 4, in which the area under the ROC curve indicates the C statistic (Fig. 3, Fig. S5).
Fig. 3.
ROC curves for all-cause death of OSCC patients with or without PA28γ expression in three cohorts of two independent centers. (a) ROC curve for Model 3 in CD-I and -II Cohorts. (b) ROC curve for Model 3 in GZ cohort.
Fig. S5.
ROC curves for all-cause death of OSCC patients with or without PA28γ expression in three cohorts of two independent centers. (a) ROC curve for Model 4 in CD-I and -II cohorts. (b) ROC curve for Model 4 in GZ cohort.
3.4. Validation the functional role of PA28γ both in vitro and in vivo
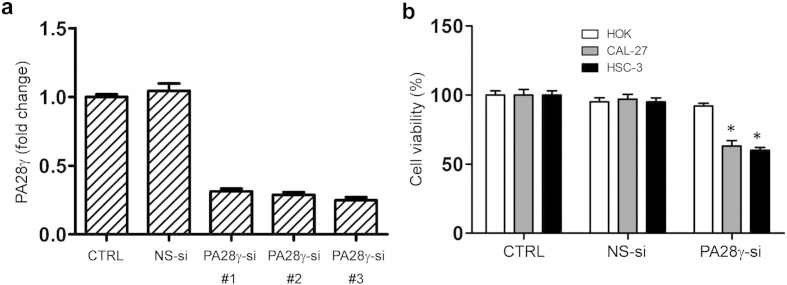
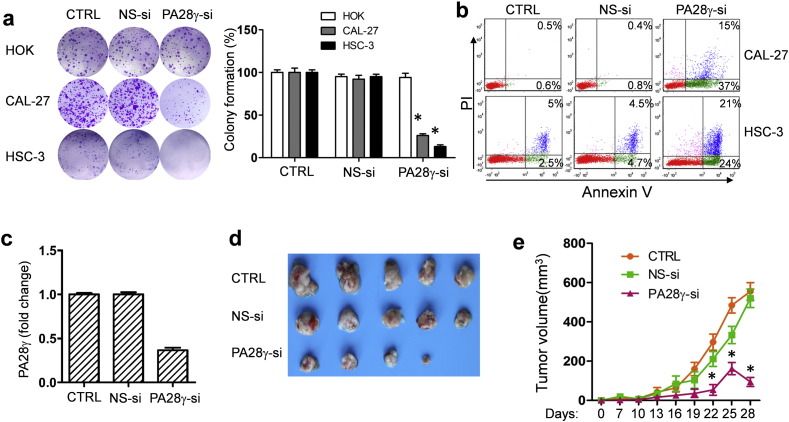
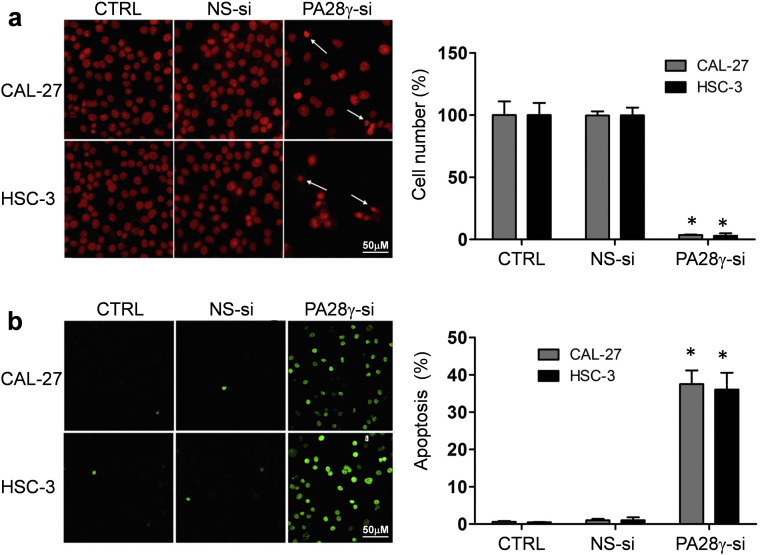
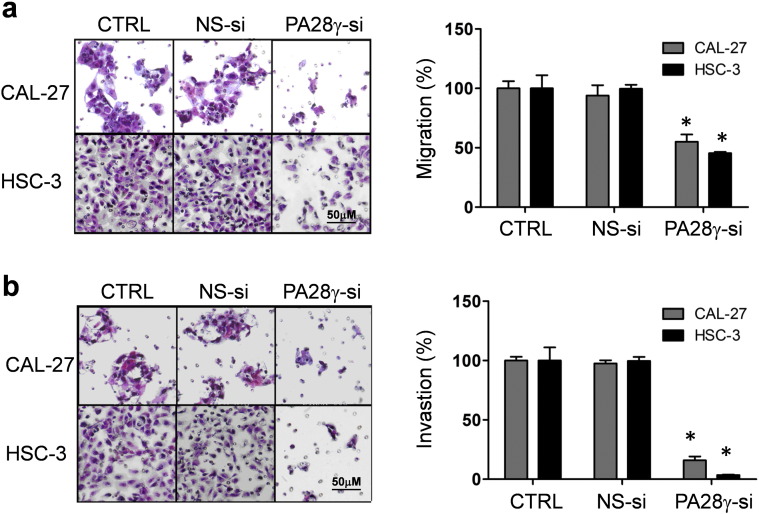
On the basis of the clinical findings described above, we evaluated the effect of PA28γ on OSCC cell lines and xenograft models. We hypothesized that PA28γ might act as a tumor promoter. If so, PA28γ silencing should reverse some of the early processes of tumorigenesis. PA28γ silencing in both OSCC cell lines caused decreased cell viability and colony growth (Fig. S6, Fig. 4a). However, this silencing had no such effect on HOK cells. A significant induction of apoptosis was observed after treatment of cells with PA28γ-specific siRNA (Fig. 4b). Similar results were also observed in the TUNEL assay (Fig. S7). These results suggest that PA28γ silencing can inhibit cell proliferation via induction of apoptosis in OSCC cells. Moreover, our data suggested that PA28γ silencing could inhibit the migration and invasion of OSCC cells in vitro (Fig. S8).
Fig. S6.
PA28γ silencing induced growth inhibition of OSCC cells. (a) siRNA-003 worked the best among the three strains of siRNA against PA28γ gene expression, illustrated by qPCR, 48 h. (b) MTT assay, 48 h. PA28γ silencing inhibited the survival of OSCC cells (HSC-3, CAL-27) but not in immortalized oral keratinocytes (HOK). The percentages of surviving cells for each of two OSCC cell lines relative to controls (defined as 100% survival). All data are represented as the mean ± SD from three independent experiments (*P < 0.05).
Fig. 4.
Functional role of PA28γ on the tumor growth. (a) Colony formation assay. PA28γ silencing led to colony formation inhibition in two OSCC cell lines (HSC-3 and CAL-27), after 10 days transfection with siRNA. All data are presented as the mean ± SD of three independent experiments (*P < 0.05). (b) PA28γ silencing induced the apoptosis in OSCC cells, determined by Flow Cytometry assay, after 48 h transfection with siRNA. All data are presented as the mean ± SD of three independent experiments (*P < 0.05). (c) Chemically-modified PA28γ-si could inhibit PA28γ gene expression in HSC-3, illustrated by qPCR, 48 h. All data are presented as the mean ± SD of three independent experiments (*P < 0.05) (d ande) The tumor volumes (mean ± SD) of PA28γ-si group were significantly smaller compared to the other two groups, 30 days after injecting transfected cells. (n = 5, *P < 0.05).
Fig. S7.
PA28γ silencing induced apoptosis of OSCC cells. (a) PA28γ silencing induced the apoptosis in OSCC (HSC-3, CAL-27) cells, illustrated by propidium iodide (PI) assay, 48 h. Cells undergoing apoptosis and nuclear fragmentation are indicated by arrows. The scale bar represents 50 μm. All data are represented as the mean ± SD from three independent experiments (*P < 0.05). (b) PA28γ silencing induced the apoptosis in OSCC (HSC-3, CAL-27) cells, as shown by TUNEL assay, 36 h. For each coverslip, ≧ 500 cells were counted. The scale bar represents 50 μm. All data are represented as the mean ± SD from three independent experiments (*P < 0.05).
Fig. S8.
PA28γ silencing decreased the migration and invasion of OSCC cells. (a) Cell migration was decreased by silencing PA28γ in OSCC cell lines (CAL-27, HSC-3), as shown by transwell migration assay, 24 h. Cells that migrated to the bottom of the chamber were counted in five fields under 20 × magnification. The scale bar represents 50 μm. Error bars mean ± SD (*P < 0.05). (b) Cell invasion was decreased by silencing PA28γ in OSCC cell lines, as shown by invasion assay, 30 h (right panels, scale bars represent 50 μm). Cells that invaded to the bottom of the chamber were counted in five fields under 20 × magnification. Error bars mean ± SD (*P < 0.05).
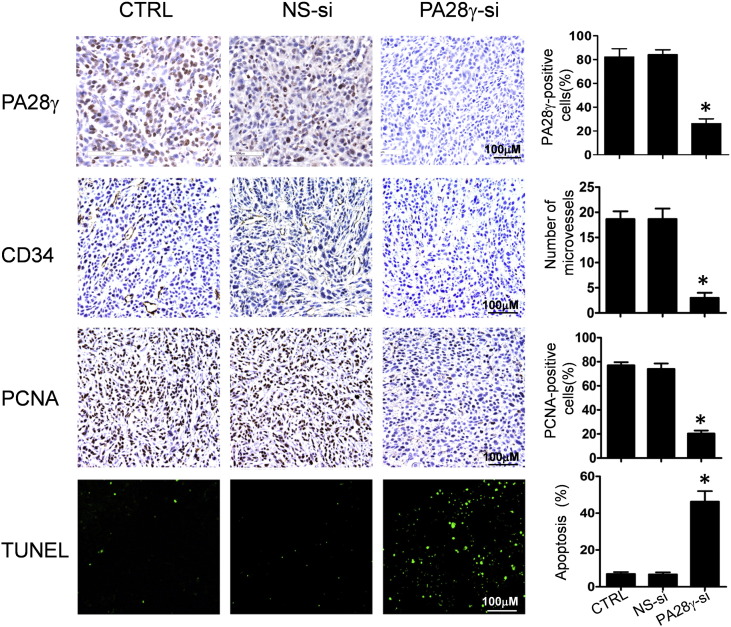
We further investigated whether PA28γ silence could inhibit tumor growth in vivo. OSCC cells treated with PA28γ siRNA modified with 2ʹ OMe and 3ʹ Chol (PA28γ-si group) which have a high effective interference (Fig. 4c), scramble siRNA (NS-si group) or PBS (CTRL group) were transplanted subcutaneously on the right back of BALB/c nude mice. Tumor growth in the PA28γ-si group was much slower than the other two groups. There was a 40–50% reduction in the average tumor volume in the PA28γ-si group (Fig. 4d, e). To investigate the potential mechanisms underlying the effects of PA28γ silencing in vivo, we examined tumor cell proliferation, microvessel density (MVD) Rechsteiner and Hill, 2005, and tumor cell apoptosis. As shown in Fig. S9, dramatic reductions in PCNA expression and tumor angiogenesis and significant increases in TUNEL-positive nuclei were found in the tumors in the PA28γ-si group compared with those in the other two groups.
Fig. S9.
Functional role of PA28γ on tumor growth. PA28γ-si could inhibit PA28γ expression in an OSCC xenografts model; angiogenesis in tumors was detected by CD34 staining. The average number of microvessels per vascular hot spot (mean ± SD) was significantly lower in PA28γ-si group tissues compared with those in the two control groups; Percentages of PCNA-positive nuclei (mean ± SD) in PA28γ-si group were significantly lower than those in the two control groups; Apoptosis was assessed by TUNEL assay. The scale bar represents 100 μm. (*P < 0.05).
4. Discussion
In the current study, our results showed that the proteasomal activator PA28γ is a prognostic biomarker in OSCC with higher expression levels correlating with worse outcomes compared with normal tissues. We studied three cohorts from China and found consistent results supporting a pronounced effect of PA28γ as a prognostic biomarker with a total of 368 patients, which was confirmed by an external validation cohort of 460 HNSCC patient specimens from the TCGA database. The corresponding role of this gene in regulating tumorigenesis and metastasis was also been evaluated.
PA28γ is a member of the PA28 protein family, which has been shown to bind specifically to 20S proteasomes and stimulate the hydrolysis of peptides (Rechsteiner et al., 2000). The PA28γ–20S proteasome pathway plays a very important role in cellular processes. Two recent studies have indicated a role for PA28γ in the regulation of the cell cycle and cell proliferation (Barton et al., 2004, Li et al., 2009). Some cellular targets of PA28γ related to the regulation of cell apoptosis have also been identified (He et al., 2012, Liu et al., 2010). PA28γ is overexpressed in some types of cancers and has been linked with multiple cancer-related pathways (He et al., 2012). Our results indicated that PA28γ may be a tumor promoter gene that can contribute to the development of a more aggressive form of oral carcinoma. Its expression negatively correlates with patient survival. An auto-regulatory feedback loop has recently been reported between p53 and PA28γ, while a p53 mutation, which is the most comprehensive genomic characterization of HNSCC (Cancer Genome Atlas N, 2015), could enhance PA28γ transcription in some cancer cells (Ali et al., 2013). Therefore, the prognostic significance of PA28γ may be driven by p53 mutation; this mechanism warrants further investigation.
We explored the PA28γ mRNA (gene name: PSME3) expression level in the head and neck cancer group from the TCGA database. Very few genomic changes (2 cases of mutation and 1 case of amplification in 279 head and neck squamous cell carcinoma cases) were observed, suggesting that the PA28γ contribution to oral cancer may not be through genomic events, but more likely through some local condition change events. This hypothesis is under further study in our laboratory. To date, there has been no well-established predictive model for all-cause death of patients with OSCC, or even head and neck cancer. In this study, we generated a series of basic models that included four conventional risk factors and one candidate biomarker, PA28γ. The integrated discrimination improvement was estimated when PA28γ was incorporated into different combinations of established risk factors in Models 2, 3, and 4. The incorporation of PA28γ with established risk factors improved the risk prediction for death, as shown by a substantial increase in the C Statistic. We also used an alternative model by replacement of tumor and nodal stage with clinical TNM stage in Model 3, as the latter is a more commonly used clinical prognosis factor. Thus, the role of each factor independently and in combination in predicting all-cause death could be determined. Most importantly, these models highlight the prognostic value of PA28γ by itself or in combination with conventional predictors. Our data were notable in that the replacement of multiple clinical factors with a simplified alternative clinical factor yields consistent results that would be extended to the clinical setting.
Furthermore, to evaluate the molecular basis for the clinical association described above, we investigated the biologic role of PA28γ in cancer cell lines and OSCC xenograft models. Our study provided the first biological evidence for the role of PA28γ in tumor growth and metastasis. Beyond its significance in metastasis-related outcome prediction, our data also showed that PA28γ silencing significantly suppressed tumor angiogenesis. Further studies are in progress in our laboratory.
The clinically significant role of PA28γ expression as a surrogate marker in OSCC is clearly established in this study. However, our model does have some limitations. Some reports have described prognosis according to a primary sub-site, which differs in HNSCC (Chung et al., 2014), the main primary sites of patients with OSCC in our cohorts were ventral tongue or floor of mouth. Although there is no difference between sub-sites in our cohorts, we could not examine survival outcomes based on PA28γ status and primary site, given the limited number of subset cases. Therefore, our data show that the use of PA28γ as a prognostic biomarker in OSCC requires more investigation before broad application in the clinical setting.
5. Conclusion
Overall, we found that PA28γ is a good predictor of the risk of death in OSCC and adds additional information to well-established prognostic factors, which were derived from OS and DFS analyses as well as the eventual four statistic models in those cohorts. Meanwhile, the functional studies in vitro and in vivo in a mouse xenograft model also validated the cellular effects of PA28γ in OSCC. However, the molecular mechanisms responsible for its function are still unclear. The identification of the targets and action model of PA28γ in OSCC needs to be further delineated in our further study.
The following are the supplementary data related to this article.
Baseline characteristics of the patients with head and neck.
Univariate analyses of selected characteristics with survival among patients.
Multivariate analyses of survival among patients with oral squamous cell carcinoma.
Prediction of all-cause death of patients with oral squamous cell carcinoma in discovery and validation cohorts, by PA28γ and clinical factors.
Contributors
Q. C, Z. W directed this study, coordinated the research. Q. C, Z. W, and J. L wrote the final manuscript. J. L, X. F, C. S and X. Z have contributed to the design of the experiments and performed research. M. Z, L. L, G. Liao, N. G, Y. L, X. L and L. Y have contributed to provide the study materials or patients. J. L, L. X, X. X, H. X, R. W, D. Z, H. D, Y. Z, P. D, Y. W, Z. W, N. J, G. Luo and L. J have contributed to the collection and assembly of data. J. L, Z. W, X. Z, H. X and T. L. have contributed to data analysis and interpretation. All authors reviewed and revised the paper.
Conflict of interests
The authors declare no potential conflicts of interest.
Funding
This project was supported by the National Natural Science Foundations of China, ISTCPC and the Open Foundation of State Key Laboratory of Oral Diseases.
Acknowledgments
This project was supported by grants from the National Natural Science Foundations of China (No. 81321002, 81472533, 81472524, 81272954, 81102060, 81270040, 81302371), ISTCPC (2012DFA31370) and the Open Foundation of State Key Laboratory of Oral Diseases (SKLOD201501). The funding agency has no role in the actual experimental design, analysis, or writing of this manuscript.
Contributor Information
Xin Zeng, Email: zengxin22@163.com.
Zhi Wang, Email: wangzhi0506@vip.163.com.
Qianming Chen, Email: qmchen@scu.edu.cn.
References
- Ali A., Wang Z., Fu J. Differential regulation of the REGgamma-proteasome pathway by p53/TGF-beta signalling and mutant p53 in cancer cells. Nat. Commun. 2013;4:2667. doi: 10.1038/ncomms3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R., Takahashi R., Nomura Y. Yeast two-hybrid screening using constitutive-active caspase-7 as bait in the identification of PA28gamma as an effector caspase substrate. Cell Death Differ. 2002;9(3):322–328. doi: 10.1038/sj.cdd.4400949. [DOI] [PubMed] [Google Scholar]
- Barton L.F., Runnels H.A., Schell T.D. Immune defects in 28-kDa proteasome activator gamma-deficient mice. J. Immunol. 2004;172(6):3948–3954. doi: 10.4049/jimmunol.172.6.3948. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas N Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Barton L.F., Chi Y., Clurman B.E., Roberts J.M. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol. Cell. 2007;26(6):843–852. doi: 10.1016/j.molcel.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Myers J.N. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J. Dent. Res. 2008;87(1):14–32. doi: 10.1177/154405910808700104. [DOI] [PubMed] [Google Scholar]
- Chung C.H., Zhang Q., Kong C.S. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J. Clin. Oncol. 2014;32(35):3930–3938. doi: 10.1200/JCO.2013.54.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S., Jia C., Zhang S. The REGgamma proteasome regulates hepatic lipid metabolism through inhibition of autophagy. Cell Metab. 2013;18(3):380–391. doi: 10.1016/j.cmet.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Cui L., Zeng Y. REGgamma is associated with multiple oncogenic pathways in human cancers. BMC Cancer. 2012;12:75. doi: 10.1186/1471-2407-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel P.M., Ossendorp F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr. Opin. Immunol. 2004;16(1):76–81. doi: 10.1016/j.coi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Li X., Amazit L., Long W., Lonard D.M., Monaco J.J., O'Malley B.W. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol. Cell. 2007;26(6):831–842. doi: 10.1016/j.molcel.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Li J., Tian T., Wang X., Li F., Ren G. Expression and clinical significance of REGγ in gastric cancer tissue and variously differentiated gastric cancer cell lines. Clin. Oncol. Cancer Res. 2009;6(3):208–213. [Google Scholar]
- Li L., Zhao D., Wei H. REGgamma deficiency promotes premature aging via the casein kinase 1 pathway. Proc. Natl. Acad. Sci. U. S. A. 2013;110(27):11005–11010. doi: 10.1073/pnas.1308497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yu G., Zhao Y. REGgamma modulates p53 activity by regulating its cellular localization. J. Cell Sci. 2010;123(Pt 23):4076–4084. doi: 10.1242/jcs.067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Lai L., Zuo Q. PKA turnover by the REGgamma-proteasome modulates FoxO1 cellular activity and VEGF-induced angiogenesis. J. Mol. Cell. Cardiol. 2014;72:28–38. doi: 10.1016/j.yjmcc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni M., Ruscica V., Buscemi G. Chk2 and REGgamma-dependent DBC1 regulation in DNA damage induced apoptosis. Nucleic Acids Res. 2014;42(21):13150–13160. doi: 10.1093/nar/gku1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzarella V., Pizzo G., Calvino F., Compilato D., Colella G., Campisi G. Diagnostic delay in oral squamous cell carcinoma: the role of cognitive and psychological variables. Int. J. Oral Sci. 2014;6(1):39–45. doi: 10.1038/ijos.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Principe S., Hui A.B., Bruce J., Sinha A., Liu F.F., Kislinger T. Tumor-derived exosomes and microvesicles in head and neck cancer: implications for tumor biology and biomarker discovery. Proteomics. 2013;13(10-11):1608–1623. doi: 10.1002/pmic.201200533. [DOI] [PubMed] [Google Scholar]
- Ratajczak-Wrona W., Jablonska E., Antonowicz B., Dziemianczyk D., Grabowska S.Z. Levels of biological markers of nitric oxide in serum of patients with squamous cell carcinoma of the oral cavity. Int. J. Oral Sci. 2013;5(3):141–145. doi: 10.1038/ijos.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M., Hill C.P. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005;15(1):27–33. doi: 10.1016/j.tcb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M., Realini C., Ustrell V. The proteasome activator 11S REG (PA28) and class I antigen presentation. Biochem. J. 2000;345(Pt 1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Rivett A.J., Hearn A.R. Proteasome function in antigen presentation: immunoproteasome complexes, peptide production, and interactions with viral proteins. Curr. Protein Pept. Sci. 2004;5(3):153–161. doi: 10.2174/1389203043379774. [DOI] [PubMed] [Google Scholar]
- Roessler M., Rollinger W., Mantovani-Endl L. Identification of PSME3 as a novel serum tumor marker for colorectal cancer by combining two-dimensional polyacrylamide gel electrophoresis with a strictly mass spectrometry-based approach for data analysis. Mol. Cell. Proteomics. 2006;5(11):2092–2101. doi: 10.1074/mcp.M600118-MCP200. [DOI] [PubMed] [Google Scholar]
- Shi Y., Luo X., Li P. miR-7-5p suppresses cell proliferation and induces apoptosis of breast cancer cells mainly by targeting REGgamma. Cancer Lett. 2015;358(1):27–36. doi: 10.1016/j.canlet.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Wan Z.X., Yuan D.M., Zhuo Y.M. The proteasome activator PA28gamma, a negative regulator of p53, is transcriptionally up-regulated by p53. Int. J. Mol. Sci. 2014;15(2):2573–2584. doi: 10.3390/ijms15022573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Jiang L., Huang C. Comparative proteomics approach to screening of potential diagnostic and therapeutic targets for oral squamous cell carcinoma. Mol. Cell. Proteomics. 2008;7(9):1639–1650. doi: 10.1074/mcp.M700520-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Feng X., Liu X. Involvement of potential pathways in malignant transformation from oral leukoplakia to oral squamous cell carcinoma revealed by proteomic analysis. BMC Genomics. 2009;10:383. doi: 10.1186/1471-2164-10-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tu S., Tan J. REG gamma: a potential marker in breast cancer and effect on cell cycle and proliferation of breast cancer cell. Med. Oncol. 2011;28(1):31–41. doi: 10.1007/s12032-010-9546-8. [DOI] [PubMed] [Google Scholar]
- Wang H., Bao W., Jiang F. Mutant p53 (p53-R248Q) functions as an oncogene in promoting endometrial cancer by up-regulating REGgamma. Cancer Lett. 2015;360(2):269–279. doi: 10.1016/j.canlet.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Yan Q., Sharma-Kuinkel B.K., Deshmukh H. Dusp3 and Psme3 are associated with murine susceptibility to Staphylococcus aureus infection and human sepsis. PLoS Pathog. 2014;10(6):e1004149. doi: 10.1371/journal.ppat.1004149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H., Furuya F., Zhao L. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone beta receptor inhibits mitotic progression. J. Clin. Invest. 2006;116(11):2972–2984. doi: 10.1172/JCI28598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhang R. Proteasome activator PA28 gamma regulates p53 by enhancing its MDM2-mediated degradation. EMBO J. 2008;27(6):852–864. doi: 10.1038/emboj.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of the patients with head and neck.
Univariate analyses of selected characteristics with survival among patients.
Multivariate analyses of survival among patients with oral squamous cell carcinoma.
Prediction of all-cause death of patients with oral squamous cell carcinoma in discovery and validation cohorts, by PA28γ and clinical factors.