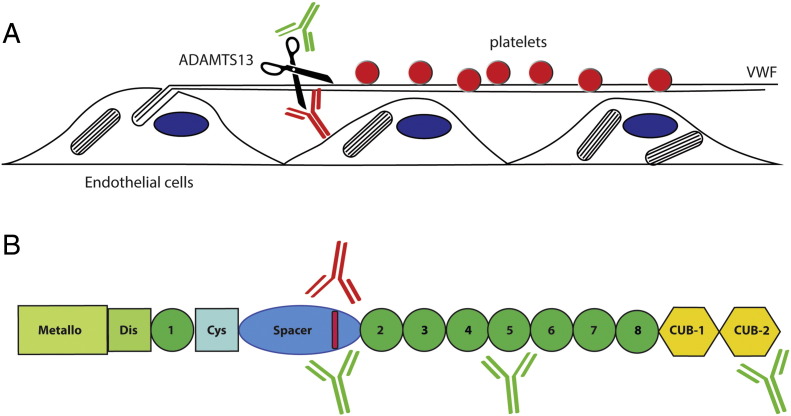
The study by Thomas and co-workers in this issue of EBioMedicine provides detailed characteristics on the pathogenic properties of antibodies that develop in patients with acquired thrombotic thrombocytopenic purpura (TTP) (Thomas et al., 2015). It is now well-established that TTP is due to dysregulation of primary hemostasis. A crucial initial step in this process involves von Willebrand factor-mediated adhesion of blood platelets to a damaged vessel wall. The platelet-adhesive properties of VWF are dependent on its ability to assemble into large polymers which are released from Weibel–Palade bodies, unique storage organelles present in endothelial cells (Valentijn et al., 2011). Upon their release, these polymers form extended parallel aligned strings that recruit platelets to sites of vascular perturbation. The VWF cleaving protease ADAMTS13 controls VWF multimer size by selectively cleaving a peptide bond in the A2 domain of VWF that becomes exposed under the influence of fluidic shear stress (Crawley et al., 2011). In the absence of ADAMTS13 processing of VWF polymers is incomplete, resulting in enhanced platelet adhesion at sites of vascular injury (Fig. 1A).
Fig. 1.
Pathogenicity of anti-ADAMTS13 antibodies. (A) Release of platelet recruiting von Willebrand factor polymers from endothelial cells. VWF polymers that are released from Weibel–Palade bodies assemble into ultra-large strings on the surface of endothelial cells. ADAMTS13 rapidly cleaves VWF strings thereby regulating adhesion of blood platelets. In the absence of ADAMTS13 adhesion of blood platelets to persisting VWF strings may promote microvascular thrombosis. Antibodies that develop in patients with acquired TTP may inhibit the processing activity of ADAMTS13 (indicated in red) or promote clearance of ADAMTS13 from the circulation (indicated in green). (B) Domain structure of ADAMTS13. Inhibitory antibodies that develop in patients with acquired TTP are directed towards an antigenic determinant in the spacer domain (indicated in red). Low-affinity non-inhibitory antibodies (indicated in green) may potentially also target this site. Non-inhibitory antibodies (indicated in green) that promote the clearance of ADAMTS13 may also bind to the TSP2-8 and CUB domains. The study by Thomas shows that clearance of ADAMTS13-Ig complexes contributes significantly to the pathogenesis of acquired TTP.
For reasons that are so far poorly understood, autoantibodies directed against ADAMTS13 can develop in previously healthy individuals. Like many other autoimmune disorders the highly polymorphic MHC class II locus has been implicated in the pathogenesis of TTP. The frequency of HLA-DR11 was significantly increased in patients with acquired TTP (Scully et al., 2010, Coppo et al., 2010). Feeding of antigen presenting cells with ADAMTS13 revealed that a CUB2 domain derived peptide is preferentially presented on HLA-DR11 (Sorvillo et al., 2013). Based on this it was hypothesized that microbial peptides with sequence overlap to this peptide may contribute to the onset of acquired TTP (Verbij et al., 2014). While our knowledge on the underlying cause for development of acquired TTP is still in its infancy, an increasing number of studies have described the binding characteristics of pathogenic antibodies in patients with acquired TTP (for review see Verbij et al., 2014).
In the current study Thomas and co-workers take this approach a step further by elegantly integrating functional and binding studies in a well-characterized cohort of patients with acquired TTP (Thomas et al., 2015). Their study confirms that in the majority of patients antibodies are present that are directed towards the spacer domain (Fig. 1B). In a subset of patients antibodies directed towards the TSP2-8 and CUB1-2 domain were identified. Antibodies exclusively targeting the carboxy-terminal TSP2-8 and CUB1-2 domains were observed in 3 out of 92 patients analyzed. These results are in excellent agreement with previous studies on the domain specificity of anti-ADAMTS13 antibodies (Pos et al., 2011, Zheng et al., 2010). A major asset of the current study is provided by combining mapping studies and linking these to the inhibitory properties of IgG purified from patient plasma. Their results show that inhibitory antibodies primarily target the spacer domain of ADAMTS13. Unexpectedly, in about half of the patients only weakly inhibitory antibodies were detected suggesting that functional inhibition of ADAMTS13 alone cannot account for the severe deficiency of ADAMTS13 in patients with acquired TTP.
Based on this observation the authors suggest that enhanced antibody-mediated clearance provides a major pathogenic mechanism in acquired TTP. This observation is corroborated by the reduced levels of circulating ADAMTS13 antigen at first presentation in patients with non-inhibitory antibodies which appears to be a major determinant of disease severity.
The current study strongly positions antibody-mediated clearance of ADAMTS13 as an important pathogenic mechanism for acquired TTP. Data included in this study show that residual levels of ADAMTS13 antigen at presentation are related to clinical outcome. Circulating ADAMTS13-Ig complexes have been detected in plasma samples of patients with acquired TTP (Ferrari et al., 2014). As yet our knowledge on the size, composition and clearance-rate of ADAMTS13-Ig complexes is insufficient. Given their importance for the pathogenesis of acquired TTP more detailed knowledge on the mechanism of clearance of ADAMTS13-Ig complexes is urgently needed. This may help to further improve current therapeutic approaches for treatment of patients with acquired TTP.
Disclosure
The authors declared no conflicts of interest.
References
- Coppo P., Busson M., Veyradier A., Wynckel A., Poullin P., Azoulay E. HLA-DRB1*11: a strong risk factor for acquired severe ADAMTS13 deficiency-related idiopathic thrombotic thrombocytopenic purpura in Caucasians. J. Thromb. Haemost. 2010;8(4):856–859. doi: 10.1111/j.1538-7836.2010.03772.x. (April) [DOI] [PubMed] [Google Scholar]
- Crawley J.T., de Groot R., Xiang Y., Luken B.M., Lane D.A. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. 2011;118(12):3212–3221. doi: 10.1182/blood-2011-02-306597. (September 22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Palavra K., Gruber B., Kremer Hovinga J.A., Knobl P., Caron C. Persistence of circulating ADAMTS13-specific immune complexes in patients with acquired thrombotic thrombocytopenic purpura. Haematologica. 2014;99(4):779–787. doi: 10.3324/haematol.2013.094151. (April) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pos W., Sorvillo N., Fijnheer R., Feys H.B., Kaijen P.H., Vidarsson G. Residues Arg568 and Phe592 contribute to an antigenic surface for anti-ADAMTS13 antibodies in the spacer domain. Haematologica. 2011;96(11):1670–1677. doi: 10.3324/haematol.2010.036327. (November) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully M., Brown J., Patel R., Mcdonald V., Brown C.J., Machin S. Human leukocyte antigen association in idiopathic thrombotic thrombocytopenic purpura: evidence for an immunogenetic link. J. Thromb. Haemost. 2010;8(2):257–262. doi: 10.1111/j.1538-7836.2009.03692.x. (February) [DOI] [PubMed] [Google Scholar]
- Sorvillo N., van Haren S.D., Kaijen P.H., ten B A., Fijnheer R., Meijer A.B. Preferential HLA-DRB1*11-dependent presentation of CUB2-derived peptides by ADAMTS13-pulsed dendritic cells. Blood. 2013;121(17):3502–3510. doi: 10.1182/blood-2012-09-456780. (April 25) [DOI] [PubMed] [Google Scholar]
- Thomas M.R., de Groot R., Scully M.A., Crawley J.T.B. Pathogenicity of Anti-ADAMTS13 Autoantibodies in Acquired Thrombotic Thrombocytopenic Purpura. EBio. Med. 2015;2:788–789. doi: 10.1016/j.ebiom.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn K.M., Sadler J.E., Valentijn J.A., Voorberg J., Eikenboom J. Functional architecture of Weibel–Palade bodies. Blood. 2011;117(19):5033–5043. doi: 10.1182/blood-2010-09-267492. (May 12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbij F.C., Fijnheer R., Voorberg J., Sorvillo N. Acquired TTP: ADAMTS13 meets the immune system. Blood Rev. 2014;28(6):227–234. doi: 10.1016/j.blre.2014.07.004. (November) [DOI] [PubMed] [Google Scholar]
- Zheng X.L., Wu H.M., Shang D., Falls E., Skipwith C.G., Cataland S.R. Multiple domains of ADAMTS13 are targeted by autoantibodies against ADAMTS13 in patients with acquired idiopathic thrombotic thrombocytopenic purpura. Haematologica. 2010;95(9):1555–1562. doi: 10.3324/haematol.2009.019299. (September) [DOI] [PMC free article] [PubMed] [Google Scholar]