Abstract
Background
The Woman's Condom is a new female condom that uses a dissolvable polyvinyl alcohol (PVA) capsule to simplify vaginal insertion. This preclinical study assessed the feasibility to incorporate an antiviral drug, UC781, into the Woman's Condom capsule, offering a unique drug delivery platform.
Study Design
UC781 capsules were fabricated using methods from the development of the Woman's Condom capsules as well as those used in vaginal film development. Capsules were characterized to evaluate physical/chemical attributes, Lactobacillus compatibility, in vitro safety and bioactivity, and condom compatibility.
Results
Two UC781 capsule platforms were assessed. Capsule masses (mg; mean ± SD) for platforms 1 and 2 were 116.50 ± 18.22 and 93.80 ± 8.49, respectively. Thicknesses were 0.0034 ± 0.0004 in and 0.0033 ± 0.0004 in. Disintegration times were 11 ± 3 sec and 5 ± 1 sec. Puncture strengths were 21.72 ± 3.30 N and 4.02 ± 0.83 N. Water content measured 6.98 ± 1.17 % and 7.04 ± 1.92 %. UC781 content was 0.59 ± 0.05 mg and 0.77 ± 0.11 mg. Both platforms retained in vitro bioactivity and were non-toxic to TZM-bl cells and Lactobacillus. Short-term storage of UC781 capsules with the Woman's Condom pouch did not decrease condom mechanical integrity.
Conclusions
UC781 was loaded into a polymeric capsule similar to that of the Woman's Condom product. This study highlights the potential use of the Woman's Condom as a platform for vaginal delivery of drugs relevant to sexual/reproductive health, including those for short or long-acting HIV prevention.
Keywords: Woman's Condom, contraception, UC781, HIV prevention, topical pre-exposure prophylaxis, microbicide
1. Introduction
PATH, with CONRAD and international research partners, used an iterative user-driven development process to design a female condom that is easy to use, comfortable, and provides good sensation for both partners. The resulting product, the Woman's Condom (Figure 1), is safe and has good performance in clinical studies across multiple countries [1-6]. The Woman's Condom has been preferred over other female condoms for its ease of use, appearance, and fit [2, 5]. In a multi-site trial comparing new female condoms to the FC2 female condom, the Woman's Condom showed performance similar to the FC2 female condom, with the same low rates of safety concerns [6].
Figure 1.
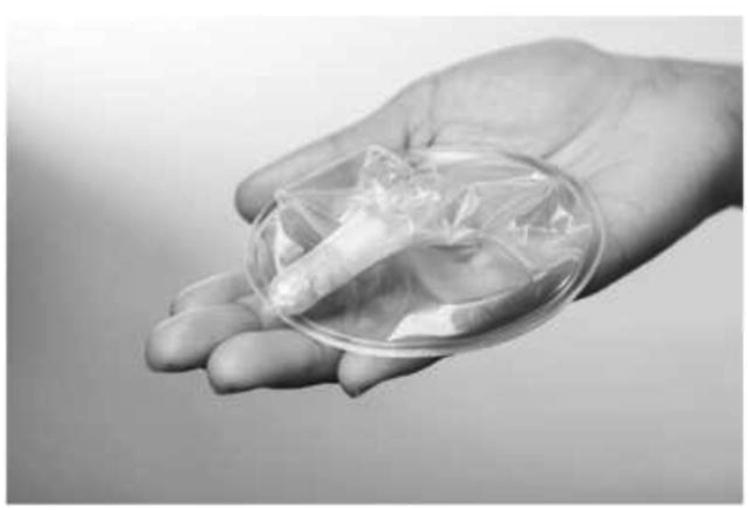
Woman's Condom. The polyvinyl alcohol (PVA) based capsule contains the condom pouch and is used for condom insertion. Once the condom is vaginally inserted, the capsule quickly dissolves, and the condom is ready for use. PATH/Patrick McKern.
The Woman's Condom, manufactured by Dahua Medical Apparatus Company (Dahua, Shanghai, China), is approved by regulatory bodies in Europe, China, South Africa, Malawi, and Zambia and is currently available in limited markets in China and South Africa. Expansion of access to this new multipurpose prevention technology (MPT) for protection from sexually transmitted infections (STIs), including HIV, and unintended pregnancy is underway.
The Woman's Condom has several unique features. The thin, polyurethane pouch provides good sensation and comfort for both partners. The dissolving polyvinyl alcohol (PVA) capsule contains the condom pouch to facilitate handling and insertion. Once the capsule is inserted, it dissolves within 30-60 seconds and the pouch unfolds inside the vagina. Although female condoms are designed to protect from both unintended pregnancy and STIs, exploring the feasibility of using this dissolving capsule as a vaginal drug delivery platform is of interest.
Several formulations and delivery platforms are under investigation for vaginal administration of drugs to prevent HIV and other STIs. These include gels, vaginal rings, cervical barriers such as diaphragms, tablets, and polymeric films (Table 1). Multiple microbicide candidates have been formulated into vaginal films which provide discrete, easy to use, low-cost, and stable platforms for drug delivery [7-16]. Marketed vaginal films include the Vaginal Contraceptive Film (VCF), VCF Lubricating Film, and Vaginal Scented Film (Apothecus Pharmaceutical Corporation, New York, USA). One main polymer used in films, as well as in the polymer capsule of the Woman's Condom, is PVA. Additional examples of combination chemical and physical barriers for multipurpose prevention include Acidform gel and BufferGel, both used with a diaphragm [17-18], BufferGel Duet™ [19], and a microbicide releasing SILCS diaphragm [20].
Table 1. Vaginal Drug Delivery Methods.
| Type | Dosing Regimens | Advantages | Disadvantages |
|---|---|---|---|
| Gel | Coitally independent: Daily Coitally dependent: Prior to coitus; Before and after coitus |
Female controlled Familiar vaginal dosage form Provides lubrication Can be utilized for MPT Easy to manufacture |
Applicator is required (not discrete, product waste) Leakage/messiness Drug stability Frequency of application Adherence |
| Ring | Coitally independent: Every one to three months | Female controlled No applicator required Long-term drug exposure Adherence Can be utilized for MPT |
Unfamiliar vaginal dosage form Bulky/Uncomfortable Unnecessary exposure to drug after cessation of product use Placement Manufacturing |
| Film | Coitally independent: Daily; Every few days to ≤ once a week Coitally dependent: Prior to coitus; Before and after coitus |
Female controlled No applicator required Discrete Portable Easy to use Option for aqueous instable drug No leakage Can be utilized for MPT Easy to manufacture Cost |
Disintegration of product and dissolution of drug depends on local hydration Cervicovaginal distribution may be of concern Unfamiliar vaginal dosage form Placement |
| Tablet | Coitally independent: Daily; Every few days to ≤ once a week Coitally dependent: Prior to coitus; Before and after coitus |
Female controlled Discrete Option for aqueous instable drug No leakage Easy to manufacture Cost |
Applicator may be required Disintegration of product and dissolution of drug depends on local hydration Cervicovaginal distribution may be of concern |
| Female condom | Coitally dependent: During coitus | Female controlled No applicator required Can be combined with film, gel, or drug delivery Can be utilized for MPT |
Integrity of barrier could become compromised Placement Cost |
| Cervical barrier, diaphragm | Coitally dependent: During coitus | Female controlled No applicator required Can be combined with gel or drug delivery Can be utilized for MPT |
Integrity of barrier could become compromised Placement Cost |
UC781 (thiocarboxanilide, N-[4-chloro-3-(3-methyl-2-butenyloxy) phenyl]-2-methyl-3-furancarbothioamide) is a tight-binding non-nucleoside reverse transcriptase inhibitor (NNRTI) that has favorable anti-HIV-1 properties in vitro [21-24]. UC781 has been formulated within several dosage platforms including gel [25-27], vaginal ring [28], and vaginal film [12]. Prior to and during the time of our studies, UC781 was a leading microbicide candidate, which is a reason for its utilization. However, further pursuit of a UC781 microbicide has ceased. Although UC781 is no longer under development as a vaginal microbicide candidate, it is a model compound representative of many hydrophobic small molecule NNRTIs which are under development for HIV prevention in preclinical and clinical stages.
In this pre-clinical study, UC781 was added to polymeric capsules to illustrate the feasibility of incorporating a microbicide candidate into the Woman's Condom product. As a combined physical barrier and drug delivery platform, the Woman's Condom could provide an alternative method for delivery of drugs relevant for women's sexual and reproductive health, including long-acting HIV prevention drugs that could provide added protection for subsequent acts of sex if no preventative method is used.
2. Materials and Methods
2.1. Materials
UC781 was supplied by CONRAD. PVA, Polyethylene glycol (PEG) 400, PEG 600, glycerin, and propylene glycol were purchased from Spectrum (New Brunswick, NJ). PEG 4000 was supplied by the Dow Chemical Company (Midland, MI), and hydroxypropyl methylcellulose (HPMC K4M; Methocel®) was supplied by Colorcon (Harleysville, PA). Woman's Condom samples were provided by PATH (manufactured by Dahua). A MilliQ (Millipore; Milford, MA) water filtration system operating at 18.2 MΩcm was used for water.
2.2. Capsule Preparation
The first step of the capsule manufacturing process consisted of preparation of a polymeric semi-solid/melt. PVA was dissolved while in a 90°C water bath over one h. Remaining ingredients were combined utilizing overhead mixing. Mandrels, devices that allow for capsule formation, were coated with Cami 410 Food Processing Silicone Spray Lubricant to facilitate capsule peeling. Mandrels were dipped into the melt twice, at a depth of approximately two in (approximately five cm). Mandrels were dried for five min at ambient temperature, and then cured at 65°C in a convection oven for 60 min. Mandrels were cooled for 15 min at ambient temperature, and the procedure was repeated. Finally, dried capsules were peeled from the mandrels.
2.3. Capsule Formulation
Placebo and UC781 containing formulations were developed as described above. The multipolymeric platform was based on a UC781 vaginal film formulation [12]. UC781 capsules were prepared by dispersing 1% w/w micronized UC781 into polymer melts. Various concentrations of PVA were evaluated to develop a PVA platform that was compatible with UC781. The PVA platform will be referred to as platform 1, and the multipolymeric platform as platform 2.
2.4. Capsule Assessments
2.4.1. Appearance, Length, Thickness, and Mass
Capsule appearance was evaluated based on texture, color, uniformity, particulates/debris, bubbles, holes, tears, or unevenness. Capsules were cut to a length of 1.5 in (3.81 cm). The average capsule thickness (in) was measured using calipers. Capsule mass (mg) was measured using an analytical balance.
2.4.2. Disintegration Time
In vitro disintegration (sec) was performed using a 3/4 inch diameter Temper Foam® cylinder inserted into each capsule. The capsule, with the foam cylinder securely inserted, was submerged into 500 mL of water set to 37 ± 1.2°C. Disintegration time started at the moment that the sample was completely submerged and ended when the foam cylinder completely expanded, at which point the capsule is broken into pieces, i.e. disintegrated.
2.4.3. Mechanical Strength
Puncture strength was determined using a texture analyzer (TA.XT Plus with a TA 8A ball probe). Puncture strength was defined as the force (Newtons; N) required for the probe to break through the capsule or Woman's Condom pouch.
2.4.4. Water Content
Residual water content of capsules (water %) was evaluated using a Karl Fischer apparatus (Metrohm, 758 KFD Titrino).
2.4.5. Drug Content
UC781 was extracted from a capsule by dissolving in water and vortexing for five min. Acetonitrile (ACN) was added to precipitate polymer(s). Supernatant samples were analyzed for UC781 by high performance liquid chromatography (HPLC) on a Dionex HPLC system with ultraviolet detection at 275 nm. 75% ACN:25% Water was used as mobile phase at 1 ml/min for isocratic elution of UC781 on a Dionex C18 column (120Å, 4.6 × 150mm) with a particle size of 3μm maintained at 25°C. Retention time of UC781 was 6.0 min.
2.4.6. TZM-bl Cell-based Assay (anti-HIV-1 activity and cell viability)
The anti-HIV-1 activity and cell viability of the polymeric capsules were evaluated in a cell-based assay with a HeLa-derived TZM-bl indicator cell line, as previously described [29]. Identical but separate plates were set up to measure bioactivity and cell viability. Briefly, TZM-bl cells were added to a 96-well Packard ViewPlate, and allowed to adhere for 24 h at 37°C. Each capsule was dissolved in 2 mL of normal saline. Ten-fold serial dilutions up to 1:107 of the original 2 mL sample of the capsule were made. Cell viability and drug bioactivity were determined as previously described [30].
2.4.7. Lactobacillus Compatibility
The standard microbicide safety test was used, as previously described [31] to assess capsule-Lactobacillus compatibility. Strains tested were L. crispatus ATCC 33197, L. jensenii ATCC 25258, and L. jensenii LBP 28Ab, which are present within normal human vaginal fluid. Bacterial suspensions were prepared in N-(2-acetamido)-2-aminoethanesulfonic acid buffer. Each capsule was dissolved in the suspensions and incubated at 37°C for 30 min. Samples were taken at time zero and 30 min. Lactobacillus viability was determined by standard plate count and calculated as the log difference between the capsule plate count and the 30 min plate count for the experiment. If the calculated viability value is decreased by 10-fold or greater, then the viability of the specified bacterial strain has been compromised, and the capsule failed this assessment.
2.5. UC781 Capsule and Woman's Condom Pouch
Woman's Condom pouches were placed inside UC781 capsules of both platforms, sealed within moisture-resistant packages, and stored at 25°C/60% relative humidity (RH) and 40°C/75% RH. After two and four weeks of storage, puncture strength of the condom pouch was evaluated to determine if pouch mechanical strength was compromised.
2.6. Statistical Analyses
Capsule mass, thickness, disintegration time, puncture strength, water content, and UC781 content data values were reported as mean ± standard deviation. The UC781 capsule data were evaluated using the Student's t-test. The condom pouch data were analyzed using one-way analysis of variance (ANOVA). p values of ≤ 0.05 were considered statistically significant.
3. Results
3.1. Capsule Formulation Development
Two polymeric platforms were identified for incorporation of UC781. The flow diagram (Supplemental Figure 1) depicts the process by which the PVA-based platform was chosen. Viscosity issues or peeling difficulties caused the 10, 15, 18, and 20% PVA platforms to fail. The 12.5% PVA polymeric platform (Supplemental Table 1) was selected as platform 1 since it was compatible with every step of the process. Polyethylene glycol (PEG) 400 was utilized for UC781 dispersion. A multipolymeric platform (Supplemental Table 2) was evaluated for manufacturing compatibility, and posed no issues with mandrel dip-ability, curing, or peel-ability.
3.2. Capsule Assessments
UC781 was added at 1% w/w to both platforms, consistent with UC781 vaginal film research [12]. Platform 1 capsules were translucent and smooth, while platform 2 capsules were opaque, smooth, and more soft and flexible than those of platform 1. Both platforms yielded the characteristic yellow hue of UC781.
Capsule characterizations for platform 1 and platform 2 (Table 2) showed platform 1 to be significantly larger with increased disintegration time and puncture strength (p < 0.05). Despite being of greater mass, platform 1 contained significantly less drug than platform 2 (p < 0.05). In comparison, the mass of the Woman's Condom capsule was 119.11 ± 17.70 mg, thickness 0.0030 ± 0.0006 in, disintegration time 33 ± 10 sec, puncture strength 31.52 ± 4.57 N, and water content 2.53 ± 0.73%.
Table 2. Characterization of UC781 Capsules (mean ± standard deviation).
| Assessmenta | Platform 1 | Platform 2 | p valueb |
|---|---|---|---|
| Mass (mg) | 116.50 ± 18.22 | 93.80 ± 8.49 | <0.01 |
| Thickness (in) | 0.0034 ± 0.0004 | 0.0033 ± 0.0004 | 0.90 |
| Disintegration (sec) | 11 ± 3 | 5 ± 1 | 0.04 |
| Puncture Strength (N) | 21.72 ± 3.30 | 4.02 ± 0.83 | <0.01 |
| Water Content (%) | 6.98 ± 1.17 | 7.04 ± 1.92 | 0.82 |
| UC781 Content (mg) | 0.59 ± 0.05 | 0.77 ± 0.11 | 0.04 |
| UC781 (% w/w) | 0.61 ± 0.02 | 0.91 ± 0.08 | <0.01 |
n = 16 for mass and thickness; n = 4 for all other assessments.
p value ≤ 0.05 was considered statistically significant (only the two drug containing platforms were statistically compared).
UC781 activity, as measured by the TZM-bl assay, for platforms 1 and 2 had EC50 values of 0.18 and 0.23 nM, respectively, and both platforms retained cell viability. Likewise, both UC781 platforms were compatible with the Lactobacilli – a < 10-fold change in bacterial growth occurred. The Woman's Condom capsules were also compatible with the bacterial flora (data not shown).
3.3. UC781 Capsule and Woman's Condom Pouch
For platforms 1 and 2, across time, storage condition, and the combination of time and storage condition, significant increases in pouch puncture strength were found (p < 0.01 and < 0.05, respectively). Significant differences in pouch puncture strength were also found when comparing the two platforms (p < 0.01). Overall, platform 2 increased the puncture strength of the condom pouch to a lesser extent. However, storage with both capsule platforms did not decrease the mechanical strength of the Woman's Condom (Figure 2).
Figure 2.

Mechanical Strength of the Woman's Condom. Woman's Condoms were stored in sealed packages with UC781 loaded capsules of both platforms. Storages conditions included 25°C/60% RH and 40°C/75% RH. Assessment time points were time zero (baseline, prior to storage), two weeks, and four weeks. Data are presented as average newtons ± SD. N = 3 condom pouches per data point.
4. Discussion
This proof-of-concept study illustrates the feasibility of incorporating a microbicide candidate, UC781, into a polymeric capsule of the Woman's Condom. Two capsule platforms were developed and underwent chemical and physical assessments for pharmaceutical product development. Capsule masses, disintegration times, and puncture strength values varied across platforms due to polymer and other excipient differences. Platform 1 and Woman's Condom capsules mainly contain PVA. Consequently, their masses were similar, as compared to the mass of platform 2 capsules. The shorter disintegration time for platform 1 and 2 capsules, in comparison to that of the Woman's Condom capsules, may result in decreased ease of administration. Platform 2 capsules possessed the least strength; they contained less PVA and several other excipients (glycerin, a plasticizer, and PEG 4000, which is a disintegrant). Also, presence of drug can relax the network of the polymer matrix, causing a decrease in puncture strength. A target puncture strength value has not been defined for capsules to be used with the Woman's Condom, but it is anticipated that a very weak or very strong capsule could have negative impacts on usage and effectiveness. Further formulation and manufacturing modifications are necessary to optimize in vitro disintegration time and mechanical strength.
Both UC781 capsule platforms contained similar water content which was greater than the Woman's Condom capsules. This difference was most likely due to the variation in formulation compositions between drug-loaded capsules and the Woman's Condom capsules. For example, both UC781 platforms contain low molecular weight PEGs, which are hygroscopic. Additionally, small molecule drug substance can relax the polymer matrix and allow for water to be more easily absorbed. These details also aid in explanation of puncture strength results. Water content can ultimately impact product shelf life. Neither UC781 platform reached the targeted drug level (Table 2), which was likely due to inadequate dispersion, polymer melt viscosity, and drug sedimentation. However, both platforms improved UC781 activity by ≥1 log10 likely through increasing solubility of the drug. To achieve the target drug level, formulation and/or manufacturing alterations should be explored.
A vaginally applied product should not exert any harmful effects on the innate commensal microflora of the cervicovaginal environment. UC781 capsules were found to be safe toward the epithelial cell line, TZM-bl, and toward three stains of commensal Lactobacillus bacteria present in the normal human vagina. These observations provide evidence of the safety of a UC781 polymeric capsule.
Short-term compatibility of UC781 capsules with Woman's Condom pouches showed that the condom pouch did not deteriorate during this time frame for both platforms, as reflected by mechanical strength. This suggests that neither UC781 nor the excipients compromised the condom pouch mechanical property. However, a strengthening effect was observed. This finding is important to ensure that the addition of drug to the condom capsule does not increase the likelihood of condom breakage during storage or use. In future stability studies, longer term assessments of condom elongation, burst strength, and tensile strength would be needed in order to comply with international procurement requirements.
Further, efforts to develop alternative strategies for the prevention of pregnancy and STIs are urgently needed. Utilizing the polymeric capsule of the Woman's Condom as a drug delivery platform leverages the current technologies for drug-loaded films with an existing female-controlled HIV/pregnancy prevention method, thereby offering coital delivery of drugs for sexual and reproductive health. The methodologies presented can be translated for the formulation development of other hydrophobic small molecules that are to be vaginally delivered. This current proof-of-concept study suggests that incorporating a drug into a polymeric capsule is feasible and could offer a viable platform for future vaginal drug delivery research, including and beyond HIV prevention.
Supplementary Material
Implications.
We determined the proof-of-concept feasibility of incorporation of a HIV-preventative microbicide into the Woman's Condom capsule. This study highlights various in vitro physical and chemical evaluations as well as bioactivity and safety assessments necessary for vaginal product development related to female sexual and reproductive health.
Acknowledgments
We would like to thank Tiantian Gong and Sheila Grab for providing manufacturing and analytical support and Kevin Uranker for the TZM-bl cellular assessments (all of Magee-Womens Research Institute). We would like to thank Hrushikesh Agashe and Sheila Grab of Magee-Womens Research Institute and Maggie Kilbourne-Brook and Laura East of PATH for assistance in reviewing this manuscript. Funding for this research was made possible by the generous support of the American people through the United States Agency for International Development (USAID) under the terms of the HealthTech IV Cooperative Agreement #GPH-A-00-01-00005-00. We would also like to thank PATH for providing the Woman's Condom manufactured by Dahua Medical Apparatus Co., and CONRAD for providing UC781 drug substance for use in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coffey PS, Kilbourne-Brook M, Austin G, Seamans Y, Cohen J. Short-term acceptability of the PATH Woman's Condom among couples at three sites. Contraception. 2006;73(6):588–93. doi: 10.1016/j.contraception.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz JL, Barnhart K, Creinin MD, Poindexter A, Wheeless A, Kilbourne-Brook M, et al. Comparative crossover study of the PATH Woman's Condom and the FC Female Condom. Contraception. 2008;78(6):465–73. doi: 10.1016/j.contraception.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Zirong H, Junqing W, Coffey PS, Kilbourne-Brook M, Yufeng Z, Wang C, et al. Performance of the woman's condom among couples in Shanghai, China. Eur J Contracept Reprod Health Care. 2012;17(3):212–8. doi: 10.3109/13625187.2012.663016. [DOI] [PubMed] [Google Scholar]
- 4.Coffey PS, Kilbourne-Brook M, Junqing W, Yufeng Z, Hongxin Z, Bin W, et al. Initial reactions to the Woman's Condom by potential user groups in Shanghai, China. J Fam Plann Reprod Health Care. 2013;39(2):111–20. doi: 10.1136/jfprhc-2011-100211. [DOI] [PubMed] [Google Scholar]
- 5.Joanis C, Beksinska M, Hart C, Tweedy K, Linda J, Smit J. Three new female condoms: which do South-African women prefer? Contraception. 2011;83(3):248–54. doi: 10.1016/j.contraception.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Beksinska ME, Piaggio G, Smit JA, Wu J, Zhang Y, Pienaar J, et al. Performance and safety of the second-generation female condom (FC2) versus the Woman's Condom, the VA worn-of-women, and the Cupid female condoms: a randomised controlled non-inferiority crossover trial. Lancet Glob Health. 2013;1(3):e146–52. doi: 10.1016/S2214-109X(13)70054-8. [DOI] [PubMed] [Google Scholar]
- 7.Yoo JW, Dharmala K, Lee CH. The physicodynamic properties of mucoadhesive polymeric films developed as female controlled drug delivery system. Int J Pharm. 2006;309(1-2):139–45. doi: 10.1016/j.ijpharm.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Neurath AR, Strick N, Li YY, Debnath AK. Cellulose acetate phthalate, a common pharmaceutical excipient, inactivates HIV-1 and blocks the coreceptor binding site on the virus envelope glycoprotein gp120. BMC Infect Dis. 2001;1:17. doi: 10.1186/1471-2334-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neurath AR, Strick N, Li YY. Water dispersible microbicidal cellulose acetate phthalate film. BMC Infect Dis. 2003;3:27. doi: 10.1186/1471-2334-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trifonova RT, Pasicznyk JM, Fichorova RN. Biocompatibility of solid- dosage forms of anti-human immunodeficiency virus type 1 microbicides with the human cervicovaginal mucosa modeled ex vivo. Antimicrob Agents Chemother. 2006;50(12):4005–10. doi: 10.1128/AAC.00588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee A, Bhowmik B, Awasthi D. Prolong Release Bioadhesive Vaginal Film of Anti-HIV Drug (Zidovudine): Formulation and In-Vitro Evaluation. International Journal of Pharmaceutical Sciences and Research. 2010;1(3):28–37. [Google Scholar]
- 12.Ferguson L, Dezzutti C, Moncla B, Parniak M, Wang L, Friend D, et al. Microbicides. Vol. 2010. Pittsburgh, PA: 2010. UC781 polymeric thin films - optimization and stability assessment. [Google Scholar]
- 13.Akil A, Parniak MA, Dezzuitti CS, Moncla BJ, Cost MR, Li M, et al. Development and Characterization of a Vaginal Film Containing Dapivirine, a Non- nucleoside Reverse Transcriptase Inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res. 2011;1(3):209–222. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ham AS, Rohan LC, Boczar A, Yang L, K WB, Buckheit RW., Jr Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm Res. 2012;29(7):1897–907. doi: 10.1007/s11095-012-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sassi AB, Cost MR, Cole AL, Cole AM, Patton DL, Gupta P, et al. Formulation development of retrocyclin 1 analog RC-101 as an anti-HIV vaginal microbicide product. Antimicrob Agents Chemother. 2011;55(5):2282–9. doi: 10.1128/AAC.01190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole AM, Patton DL, Rohan LC, Cole AL, Cosgrove-Sweeney Y, Rogers NA, et al. The formulated microbicide RC-101 was safe and antivirally active following intravaginal application in pigtailed macaques. PLoS One. 2010;5(11):e15111. doi: 10.1371/journal.pone.0015111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams DL, Newman DR, Ballagh SA, Creinin MD, Barnhart K, Weiner DH, et al. Phase I safety trial of two vaginal microbicide gels (Acidform or BufferGel) used with a diaphragm compared to KY jelly used with a diaphragm. Sex Transm Dis. 2007;34(12):977–84. doi: 10.1097/olq.0b013e31813347e9. [DOI] [PubMed] [Google Scholar]
- 18.Behets FM, Turner AN, Van Damme K, Rabenja NL, Ravelomanana N, Swezey TA, et al. Vaginal microbicide and diaphragm use for sexually transmitted infection prevention: a randomized acceptability and feasibility study among high-risk women in Madagascar. Sex Transm Dis. 2008;35(9):818–26. doi: 10.1097/OLQ.0b013e318175d8ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballagh SA, Brache V, Mauck C, Callahan MM, Cochon L, Wheeless A, et al. A Phase I study of the functional performance, safety and acceptability of the BufferGel Duet. Contraception. 2008;77(2):130–7. doi: 10.1016/j.contraception.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Major I, Lowry D, Malcolm K, Woolfson D, Cohen J, Labarre P, et al. Development of a microbicide-releasing diaphragm as an HIV prevention strategy. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:1089–92. doi: 10.1109/IEMBS.2010.5627333. [DOI] [PubMed] [Google Scholar]
- 21.Balzarini J, Pelemans H, Aquaro S, Perno CF, Witvrouw M, Schols D, et al. Highly favorable antiviral activity and resistance profile of the novel thiocarboxanilide pentenyloxy ether derivatives UC-781 and UC-82 as inhibitors of human immunodeficiency virus type 1 replication. Mol Pharmacol. 1996;50(2):394–401. [PubMed] [Google Scholar]
- 22.Buckheit RW, Jr, Snow MJ, Fliakas-Boltz V, Kinjerski TL, Russell JD, Pallansch LA, et al. Highly potent oxathiin carboxanilide derivatives with efficacy against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus isolates. Antimicrob Agents Chemother. 1997;41(4):831–7. doi: 10.1128/aac.41.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnard J, Borkow G, Parniak MA. The thiocarboxanilide nonnucleoside UC781 is a tight-binding inhibitor of HIV-1 reverse transcriptase. Biochemistry. 1997;36(25):7786–92. doi: 10.1021/bi970140u. [DOI] [PubMed] [Google Scholar]
- 24.Borkow G, Arion D, Wainberg MA, Parniak MA. The thiocarboxanilide nonnucleoside inhibitor UC781 restores antiviral activity of 3′-azido-3′-deoxythymidine (AZT) against AZT-resistant human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1999;43(2):259–63. doi: 10.1128/aac.43.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz JL, Kovalevsky G, Lai JJ, Ballagh SA, McCormick T, Douville K, et al. A randomized six-day safety study of an antiretroviral microbicide candidate UC781, a non-nucleoside reverse transcriptase inhibitor. Sex Transm Dis. 2008;35(4):414–9. doi: 10.1097/OLQ.0b013e318162c4d8. [DOI] [PubMed] [Google Scholar]
- 26.Ventuneac A, Carballo-Dieguez A, McGowan I, Dennis R, Adler A, Khanukhova E, et al. Acceptability of UC781 gel as a rectal microbicide among HIV-uninfected women and men. AIDS Behav. 2010;14(3):618–28. doi: 10.1007/s10461-009-9611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anton PA, Saunders T, Elliott J, Khanukhova E, Dennis R, Adler A, et al. First Phase 1 Double-Blind, Placebo-Controlled, Randomized Rectal Microbicide Trial Using UC781 Gel with a Novel Index of Ex Vivo Efficacy. PLoS One. 2011;6(9):e23243. doi: 10.1371/journal.pone.0023243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark MR, Johnson TJ, McCabe RT, Clark JT, Tuitupou A, Elgendy H, et al. A hot-melt extruded intravaginal ring for the sustained delivery of the antiretroviral microbicide UC781. J Pharm Sci. 2011 doi: 10.1002/jps.22781. [DOI] [PubMed] [Google Scholar]
- 29.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dezzutti CS, Rohan LC, Wang L, Uranker K, Shetler C, Cost M, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. J Antimicrob Chemother. 2012;67(9):2139–42. doi: 10.1093/jac/dks173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moncla BJ, Pryke K, Rohan LC, Yang H. Testing of viscous anti-HIV microbicides using Lactobacillus. J Microbiol Methods. 2012;88(2):292–6. doi: 10.1016/j.mimet.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


