Abstract
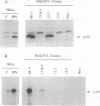
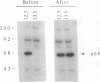
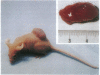
RNA-dependent protein kinase is a M(r) 68,000 protein in human cells (p68 kinase) or a M(r) 65,000 protein in murine cells (p65 kinase). p65/p68 is a serine/threonine kinase induced by interferon treatment and generally activated by double-stranded RNAs. Once activated, the known function of this kinase is inhibition of protein synthesis through phosphorylation of the eukaryotic initiation factor 2. Here we have investigated the potential for tumorigenicity in mice of murine NIH 3T3 clones expressing human p68 kinase, either the wild-type or a mutant inactive kinase with a single amino acid substitution in the invariant lysine-296 in the catalytic domain II. Expression of the mutant p68 kinase was correlated with a malignant transformation phenotype, giving rise to the production of large tumors of at least 1 cm in diameter within 7-12 days in all inoculated mice. In contrast, no tumor growth was observed for several weeks in mice inoculated with NIH 3T3 cell clones expressing either the wild-type recombinant p68 kinase or only the endogenous p65 kinase, the murine analogue of the p68 kinase. These results suggest that functional p65/p68 kinase (recently called PKR), by a still undefined mechanism, may also act as a tumor suppressor. Consequently, one of the pathways by which interferon inhibits tumor growth might be through its capacity to induce the enhanced expression of this kinase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Gresser I., Hovanessian A. G., Bandu M. T., Blanchard B., Blangy D. Specific high-affinity binding of 125I-labeled mouse interferon to interfevon resistant embryonal carcinoma cells in vitro. Virology. 1981 Oct 30;114(2):585–588. doi: 10.1016/0042-6822(81)90241-5. [DOI] [PubMed] [Google Scholar]
- Bischoff J. R., Samuel C. E. Mechanism of interferon action. Activation of the human P1/eIF-2 alpha protein kinase by individual reovirus s-class mRNAs: s1 mRNA is a potent activator relative to s4 mRNA. Virology. 1989 Sep;172(1):106–115. doi: 10.1016/0042-6822(89)90112-8. [DOI] [PubMed] [Google Scholar]
- Black T. L., Safer B., Hovanessian A., Katze M. G. The cellular 68,000-Mr protein kinase is highly autophosphorylated and activated yet significantly degraded during poliovirus infection: implications for translational regulation. J Virol. 1989 May;63(5):2244–2251. doi: 10.1128/jvi.63.5.2244-2251.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffet-Janvresse C., Vannier J. P., Laurent A. G., Robert N., Hovanessian A. G. Enhanced level of double-stranded RNA-dependent protein kinase in peripheral blood mononuclear cells of patients with viral infections. J Interferon Res. 1986 Apr;6(2):85–96. doi: 10.1089/jir.1986.6.85. [DOI] [PubMed] [Google Scholar]
- Chong K. L., Feng L., Schappert K., Meurs E., Donahue T. F., Friesen J. D., Hovanessian A. G., Williams B. R. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J. 1992 Apr;11(4):1553–1562. doi: 10.1002/j.1460-2075.1992.tb05200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever T. E., Feng L., Wek R. C., Cigan A. M., Donahue T. F., Hinnebusch A. G. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992 Feb 7;68(3):585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Dubois M. F., Galabru J., Lebon P., Safer B., Hovanessian A. G. Reduced activity of the interferon-induced double-stranded RNA-dependent protein kinase during a heat shock stress. J Biol Chem. 1989 Jul 25;264(21):12165–12171. [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Feng G. S., Chong K., Kumar A., Williams B. R. Identification of double-stranded RNA-binding domains in the interferon-induced double-stranded RNA-activated p68 kinase. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5447–5451. doi: 10.1073/pnas.89.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galabru J., Hovanessian A. G. Two interferon-induced proteins are involved in the protein kinase complex dependent on double-stranded RNA. Cell. 1985 Dec;43(3 Pt 2):685–694. doi: 10.1016/0092-8674(85)90241-7. [DOI] [PubMed] [Google Scholar]
- Galabru J., Hovanessian A. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J Biol Chem. 1987 Nov 15;262(32):15538–15544. [PubMed] [Google Scholar]
- Galabru J., Katze M. G., Robert N., Hovanessian A. G. The binding of double-stranded RNA and adenovirus VAI RNA to the interferon-induced protein kinase. Eur J Biochem. 1989 Jan 2;178(3):581–589. doi: 10.1111/j.1432-1033.1989.tb14485.x. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Holmes S. L., Mehra L. L. Interferon action against reovirus: activation of interferon-induced protein kinase in mouse L929 cells upon reovirus infection. Virology. 1982 Jul 30;120(2):495–499. doi: 10.1016/0042-6822(82)90051-4. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hershey J. W. Protein phosphorylation controls translation rates. J Biol Chem. 1989 Dec 15;264(35):20823–20826. [PubMed] [Google Scholar]
- Hovanessian A. G. Double-stranded RNA dependent protein kinase (s) in rabbit reticulocyte lysates analogous to kinase from interferon-treated cells. Biochimie. 1980;62(11-12):775–778. doi: 10.1016/s0300-9084(80)80132-5. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Galabru J., Meurs E., Buffet-Janvresse C., Svab J., Robert N. Rapid decrease in the levels of the double-stranded RNA-dependent protein kinase during virus infections. Virology. 1987 Jul;159(1):126–136. doi: 10.1016/0042-6822(87)90355-2. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Galabru J., Rivière Y., Montagnier L. Efficiency of poly(A).poly(U) as an adjuvant. Immunol Today. 1988 Jun;9(6):161–162. doi: 10.1016/0167-5699(88)91288-1. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Galabru J. The double-stranded RNA-dependent protein kinase is also activated by heparin. Eur J Biochem. 1987 Sep 15;167(3):467–473. doi: 10.1111/j.1432-1033.1987.tb13360.x. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G. Interferon-induced and double-stranded RNA-activated enzymes: a specific protein kinase and 2',5'-oligoadenylate synthetases. J Interferon Res. 1991 Aug;11(4):199–205. doi: 10.1089/jir.1991.11.199. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G. The double stranded RNA-activated protein kinase induced by interferon: dsRNA-PK. J Interferon Res. 1989 Dec;9(6):641–647. doi: 10.1089/jir.1989.9.641. [DOI] [PubMed] [Google Scholar]
- Katze M. G., DeCorato D., Safer B., Galabru J., Hovanessian A. G. Adenovirus VAI RNA complexes with the 68 000 Mr protein kinase to regulate its autophosphorylation and activity. EMBO J. 1987 Mar;6(3):689–697. doi: 10.1002/j.1460-2075.1987.tb04809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G. The war against the interferon-induced dsRNA-activated protein kinase: can viruses win? J Interferon Res. 1992 Aug;12(4):241–248. doi: 10.1089/jir.1992.12.241. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Wambach M., Wong M. L., Garfinkel M., Meurs E., Chong K., Williams B. R., Hovanessian A. G., Barber G. N. Functional expression and RNA binding analysis of the interferon-induced, double-stranded RNA-activated, 68,000-Mr protein kinase in a cell-free system. Mol Cell Biol. 1991 Nov;11(11):5497–5505. doi: 10.1128/mcb.11.11.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koromilas A. E., Roy S., Barber G. N., Katze M. G., Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992 Sep 18;257(5077):1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- Krust B., Galabru J., Hovanessian A. G. Further characterization of the protein kinase activity mediated by interferon in mouse and human cells. J Biol Chem. 1984 Jul 10;259(13):8494–8498. [PubMed] [Google Scholar]
- Krust B., Rivière Y., Hovanessian A. G. p67K kinase in different tissues and plasma of control and interferon-treated mice. Virology. 1982 Jul 15;120(1):240–246. doi: 10.1016/0042-6822(82)90022-8. [DOI] [PubMed] [Google Scholar]
- Laurent A. G., Krust B., Galabru J., Svab J., Hovanessian A. G. Monoclonal antibodies to an interferon-induced Mr 68,000 protein and their use for the detection of double-stranded RNA-dependent protein kinase in human cells. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4341–4345. doi: 10.1073/pnas.82.13.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Montine K. S., Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990 Jun 7;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Levin D., London I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marié I., Svab J., Robert N., Galabru J., Hovanessian A. G. Differential expression and distinct structure of 69- and 100-kDa forms of 2-5A synthetase in human cells treated with interferon. J Biol Chem. 1990 Oct 25;265(30):18601–18607. [PubMed] [Google Scholar]
- McCormack S. J., Thomis D. C., Samuel C. E. Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2 alpha protein kinase. Virology. 1992 May;188(1):47–56. doi: 10.1016/0042-6822(92)90733-6. [DOI] [PubMed] [Google Scholar]
- Meurs E. F., Watanabe Y., Kadereit S., Barber G. N., Katze M. G., Chong K., Williams B. R., Hovanessian A. G. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J Virol. 1992 Oct;66(10):5805–5814. doi: 10.1128/jvi.66.10.5805-5814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs E., Chong K., Galabru J., Thomas N. S., Kerr I. M., Williams B. R., Hovanessian A. G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990 Jul 27;62(2):379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Inhibition of protein synthesis in reovirus-infected HeLa cells with elevated levels of interferon-induced protein kinase activity. J Biol Chem. 1982 Dec 25;257(24):14593–14596. [PubMed] [Google Scholar]
- Patel R. C., Sen G. C. Identification of the double-stranded RNA-binding domain of the human interferon-inducible protein kinase. J Biol Chem. 1992 Apr 15;267(11):7671–7676. [PubMed] [Google Scholar]
- Rice A. P., Kerr I. M. Interferon-mediated, double-stranded RNA-dependent protein kinase is inhibited in extracts from vaccinia virus-infected cells. J Virol. 1984 Apr;50(1):229–236. doi: 10.1128/jvi.50.1.229-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière Y., Hovanessian A. G. Antitumoral action of interferon and poly(A)poly(U) on HeLa xenografts in the nude mouse. Ann Immunol (Paris) 1984 May-Jun;135C(3):333–343. doi: 10.1016/s0769-2625(84)80963-0. [DOI] [PubMed] [Google Scholar]
- Rivière Y., Hovanessian A. G. Direct action of interferon and inducers of interferon on tumor cells in athymic nude mice. Cancer Res. 1983 Oct;43(10):4596–4599. [PubMed] [Google Scholar]
- Roy S., Agy M., Hovanessian A. G., Sonenberg N., Katze M. G. The integrity of the stem structure of human immunodeficiency virus type 1 Tat-responsive sequence of RNA is required for interaction with the interferon-induced 68,000-Mr protein kinase. J Virol. 1991 Feb;65(2):632–640. doi: 10.1128/jvi.65.2.632-640.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Katze M. G., Parkin N. T., Edery I., Hovanessian A. G., Sonenberg N. Control of the interferon-induced 68-kilodalton protein kinase by the HIV-1 tat gene product. Science. 1990 Mar 9;247(4947):1216–1219. doi: 10.1126/science.2180064. [DOI] [PubMed] [Google Scholar]
- Safer B. 2B or not 2B: regulation of the catalytic utilization of eIF-2. Cell. 1983 May;33(1):7–8. doi: 10.1016/0092-8674(83)90326-4. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Duncan R., Knutson G. S., Hershey J. W. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eIF-2 alpha in interferon-treated, reovirus-infected mouse L929 fibroblasts in vitro and in vivo. J Biol Chem. 1984 Nov 10;259(21):13451–13457. [PubMed] [Google Scholar]
- SenGupta D. N., Silverman R. H. Activation of interferon-regulated, dsRNA-dependent enzymes by human immunodeficiency virus-1 leader RNA. Nucleic Acids Res. 1989 Feb 11;17(3):969–978. doi: 10.1093/nar/17.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Reynolds R. K., Luciw P. A., Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Tumor suppressor genes. Science. 1991 Nov 22;254(5035):1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Hovanessian A. G. Interferon enhances 2-5A synthetase in embryonal carcinoma cells. Nature. 1979 Nov 1;282(5734):74–76. doi: 10.1038/282074a0. [DOI] [PubMed] [Google Scholar]