Abstract
Stress-induced abscisic acid (ABA) is mainly catabolized by ABA 8′-hydroxylase (ABA8ox), which also strictly regulates endogenous ABA levels. Although three members of the ABA8ox gene family are conserved in rice, it is not clear which stressors induce expression of these genes. Here, we found that OsABA8ox1 was induced by cold stress within 24 h and that OsABA8ox2 and OsABA8ox3 were not. In contrast, OsABA8ox2 and OsABA8ox3 were ABA-inducible, but OsABA8ox1 was not. OsABA8ox1, OsABA8ox2, and OsABA8ox3 restored germination of a cyp707a1/a2/a3 triple mutant of Arabidopsis to rates comparable to those of the wild type, indicating that OsABA8ox1, OsABA8ox2, and OsABA8ox3 function as ABA-catabolic genes in vivo. Transgenic rice lines overexpressing OsABA8ox1 showed decreased levels of ABA and increased seedling vigor at 15 °C. These results indicate that sustained low levels of ABA lead to increased seedling vigor during cold stress. On the other hand, excessively low endogenous ABA levels caused reduced drought and cold tolerance, although some of the transgenic rice lines expressing OsABA8ox1 at moderate levels did not show these harmful effects. Adequate regulation of endogenous ABA levels is thought to be crucial for maintaining seedling vigor under cold stress and for cold and drought tolerance in rice.
Rice is a globally important, staple crop. Environmental stressors such as low temperature, drought, heat, and high salinity affect rice growth and grain yield. In particular, cold stress causes early seedling growth retardation in rice cultivated in temperate areas1. Seedling vigor is mainly defined by rapid growth of shoots and roots in the early vegetative stage2,3. Generally, inhibition of seedling growth by exposure to low temperatures causes retardation of flowering, heading, and ripening. In addition, rice grain yields are reduced as a result of these delays, because plants are forced to mature under cold temperatures in late fall. Despite these adverse effects on crop yield, little is known about the physiological effects of low temperatures on seedling growth in rice.
Production of abscisic acid (ABA), a plant stress hormone, is increased by cold and drought and acts to help plants withstand these conditions4,5,6,7. Changes in ABA levels in response to drought stress control stomatal action: increased levels of ABA trigger stomatal closure, which reduces transpiration under drought conditions, and rehydration decreases ABA levels, leading to stomatal opening8. Under conditions of high humidity, an ABA-catabolic gene is specifically expressed in guard cells to promote transpiration in Arabidopsis thaliana9. Thus, ABA levels in the stomata regulate stomatal action in response to drought. However, the function of ABA with regard to cold stress remains unclear.
There are fewer data for ABA in rice than in Arabidopsis. An ABA-deficient mutant of Arabidopsis that exhibited severe growth retardation was found10, but no such mutant has been obtained yet in rice. Alternatively, several carotenoid (an ABA biosynthetic precursor molecule) deficient rice mutants showed strong cold tolerance, larger stomatal aperture, and earlier wilting than wild-type rice, at both the seedling and panicle development stages11. Furthermore, transgenic rice lines with anther-specific overexpression of a wheat ABA catabolic gene demonstrated reduced sterility caused by cold stress during the pollen developmental stage, when compared with each null-segregant line12. These findings suggest that high ABA levels negatively affect cold stress tolerance, in contrast to their positive effects in drought conditions. Therefore, rice seedlings might grow more vigorously if ABA levels are sustained at low levels under cold stress, although the physiological mechanisms promoting seedling vigor under cold stress could be different from those providing cold tolerance at the booting stage.
ABA 8′-hydroxylase (ABA8ox) oxidizes ABA to 8′-hydroxy-ABA, which is later spontaneously isomerized to phaseic acid (PA). PA is further reduced to dihydrophaseic acid (DPA) by an unknown reductase13,14,15. In Arabidopsis, the genes encoding ABA8ox (CYP707A1, CYP707A2, CYP707A3, and CYP707A4) have been identified and are known to possess catabolic enzyme activity16,17. In the case of ABA catabolism, the hydroxylation pathway that converts ABA to PA is predominant in higher plants18. Another ABA-inactivation pathway exists, in which ABA is conjugated with glucose to produce ABA glucosyl ester (ABA-GE)19. ABA-GE can then be catabolized back to ABA by β-glucosidase20. Although three ABA8ox genes are conserved in the rice genome (OsABA8ox1, OsABA8ox2, and OsABA8ox3), only OsABA8ox1 has been biochemically demonstrated to encode ABA 8′-hydroxylase21.
Here, we analyzed the expression of OsABA8ox1, OsABA8ox2, and OsABA8ox3 under several stress conditions, and we tested for compensation for these genes with a cyp707a1/cyp707a2/cyp707a3 triple mutant of Arabidopsis22. To analyze the relationships between ABA levels and seedling vigor under cold stress, we produced transgenic rice plants overexpressing OsABA8ox1. These transgenic plants had reduced ABA levels and improved seedling vigor under cold stress.
Results
Expression of OsABA8ox genes under stress conditions in rice
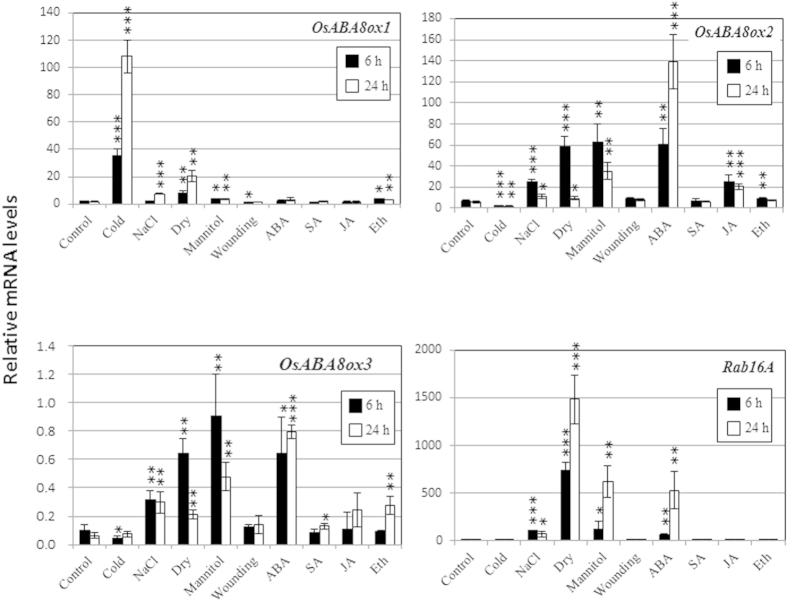
In rice, three OsABA8ox genes that encode ABA 8′-hydroxylases have been revealed by phylogenetic analysis23. Arabidopsis contains four genes for ABA 8′-hydroxylases (CYP707A1, CYP707A2, CYP707A3, and CYP707A4) that are induced by various stressors9,24,25. To examine stress induction of OsABA8ox genes in relation to diverse abiotic conditions, we analyzed expression of OsABA8ox1, OsABA8ox2, and OsABA8ox3 under cold (4 °C), dehydration, high salt (250 mM NaCl), osmotic stress (500 mM mannitol), and wounding, and following exposure to 100 μM ABA, 100 μM salicylic acid, 100 μM jasmonic acid, and 100 μM ethephon. qRT-PCR showed that expression of OsABA8ox1, but not of OsABA8ox2 or OsABA8ox3, was drastically increased by cold stress (Fig. 1). However, OsABA8ox2 and OsABA8ox3 were substantially induced by ABA, while OsABA8ox1 was not. All three genes were induced by high salinity, drought, and osmotic stress, but induction of OsABA8ox1 was much less than that of OsABA8ox2 or OsABA8ox3. Expression of the ABA-responsive gene Rab16A was similar to that of OsABA8ox2 and OsABA8ox3 (Fig. 1).
Figure 1. qRT-PCR analysis of OsABA8ox1, OsABA8ox2, OsABA8ox3, and Rab16A under stress conditions and hormone treatments.
One-week-old seedlings of rice were used for these experiments. For drought treatment (Dry), seedlings were dehydrated on a paper towel. For hormone or osmotic stress treatment, plants were transferred to buffer containing 100 μM abscisic acid (ABA), 100 μM salicylic acid (SA), 100 μM jasmonic acid (JA), 100 μM ethephon (Eth), 250 mM NaCl, or 500 mM mannitol. For cold treatment, plants were transferred to and kept at 4 °C for 6 h or 24 h. For wounding, shoots were punctured by a needle and grown hydroponically in the buffer for 6 or 24 h. Gene expression values are means (±SD) of three biological replicates. Significant differences relative to controls were evaluated using Student’s t-test. *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001, respectively.
To elucidate the cold inducibility of OsABA8ox1, we investigated the 1.1–1.5 kb promoter regions of each OsABA8ox gene, and it was found to harbor the low temperature responsive elements LTRE1HVLT49 and LTREATLTI78, according to the PLACE database (Supplementary Fig. S1). In contrast, these elements were not found in the promoter regions of OsABA8ox2 or OsABA8ox3 (Supplementary Fig. S1). This suggests that the low temperature responsive elements provide the attribute of cold inducibility to the OsABA8ox1 gene.
Rescue of the cyp707a1/cyp707a2/cyp707a3 triple mutant phenotype of Arabidopsis by rice OsABA8ox genes
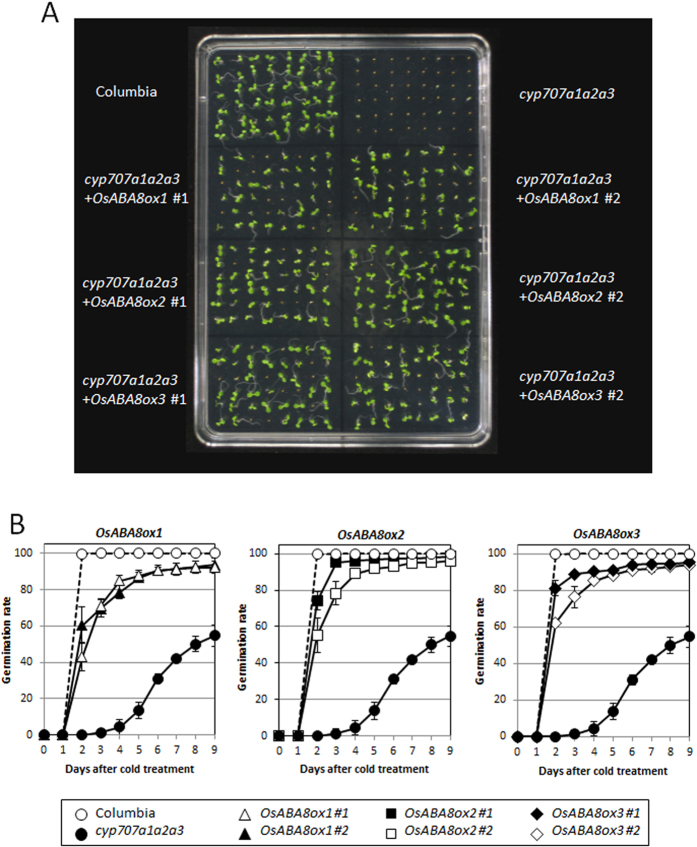
To verify whether rice OsABA8ox genes possess ABA 8′-hydroxylase function like the Arabidopsis cyp707a genes, we overexpressed OsABA8ox1, OsABA8ox2, or OsABA8ox3 in a cyp707a1/cyp707a2/cyp707a3 triple mutant (Cyp707a1a2a3) of Arabidopsis. Cyp707a1a2a3 is extremely difficult to germinate because of excessive ABA accumulation22. We assessed ABA 8′-hydroxylase function by comparing the germination rates of these transgenic lines with those of non-transgenic cyp707a1a2a3 and wild-type Arabidopsis. Germination of cyp707a1a2a3 was recovered by overexpression of OsABA8ox1, OsABA8ox2, or OsABA8ox3 (Fig. 2). These results indicate that rice OsABA8ox1, OsABA8ox2, and OsABA8ox3 can function as ABA 8′-hydroxylases in vivo.
Figure 2. Complementation by OsABA8ox1, OsABA8ox2, or OsABA8ox3 in the cyp707a1a2a3 triple mutant.
(A) Typical images of germination of wild-type (Columbia), triple mutant, and transgenic plants. (B) Germination rates of wild-type, cyp707a1a2a3, and transgenic plants after cold treatment. Values are means ± SD of four replicates.
OsABA8oxs expression and ABA content under various temperature conditions in rice
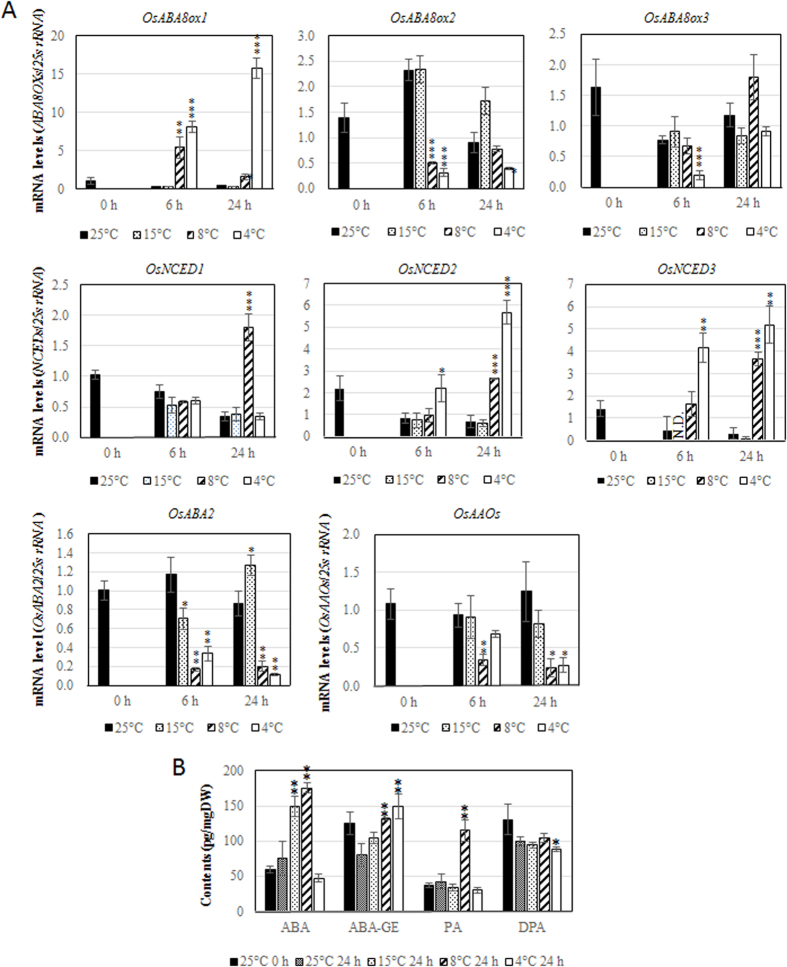
To investigate the effects of temperature on expression of OsABA8oxs, we cultivated seedlings at various temperatures and then isolated total RNA for qRT-PCR. OsABA8ox1 expression was strongly induced after cold stress at 4 °C or 8 °C; in contrast, the expression of OsABA8ox2 and OsABA8ox3 decreased (Fig. 3A). The 15 °C treatment did not induce expression of OsABA8ox1 (Fig. 3A). To comprehensively assess ABA metabolism under cold stress, we analyzed mRNA levels of ABA synthesis genes using qRT-PCR; the genes were differentially expressed according to temperature and time (Fig. 3A).
Figure 3.
(A) Temperature-dependent expression of OsABA8oxs, OsNCEDs, OsABA2, and OsAAOs. (B) Endogenous ABA, ABA-GE, PA, and DPA content. Three-day-old rice seedlings grown hydroponically were transferred to and kept at 4 °C, 8 °C, 15 °C, and 25 °C for 24 h. Expression of ABA-related genes and endogenous ABA and catabolite contents are means ± SD of three biological replicates. Significant differences between each treatment and the 25 °C treatment were determined by Student’s t-tests. *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001, respectively.
ABA and its catabolites were analyzed by liquid chromatography (LC) tandem mass spectrometry (MS/MS). After 4 °C treatment for 24 h, ABA-GE increased, but ABA, PA, and DPA contents did not change substantially (Fig. 3B) despite significant induction of OsNCED2, OsNCED3, and OsABA8ox1 expression by this treatment (Fig. 3A), suggesting that most ABA-biosynthetic and catabolic activities were suppressed at 4 °C at the post-transcriptional levels. Contents of ABA, ABA-GE, and PA increased after treatment at 8 °C (Fig. 3B), consistent with the significant induction of ABA-biosynthetic genes and transient induction of OsABA8ox1 after 8 °C treatment (Fig. 3A). After 15 °C treatment, ABA content was increased but expression of OsNCEDs, thought to be the limiting step of ABA biosynthesis in plants, was not changed. However, expression of OsABA2 (the downstream gene of OsNCEDs) was increased (Fig. 3A). PA and DPA contents did not change after treatment at 15 °C, consistent with the non-induction of OsABA8ox1 at this temperature (Fig. 3B).
Growth, OsABA8ox1 expression, and ABA content under long-term cold stress
Because we found that OsABA8ox1 was not induced at 15 °C and that ABA content increased at this temperature (Fig. 3B), we measured shoot growth, ABA content, and expression changes of OsABA8ox1 and other ABA metabolic genes under long-term cold stress to confirm the effect of ABA accumulation.
Shoot growth of rice seedlings grown at 15 °C was substantially retarded compared to seedlings grown at 25 °C (Fig. 4A). ABA contents increased under cold treatment, reaching a maximum at 9 d (Fig. 4B). ABA-GE contents increased, and PA and DPA contents decreased, after 3 d of cold treatment; and DPA contents generally increased after cold treatment (Fig. 4B).
Figure 4. Growth analysis and ABA content under long-term cold stress.
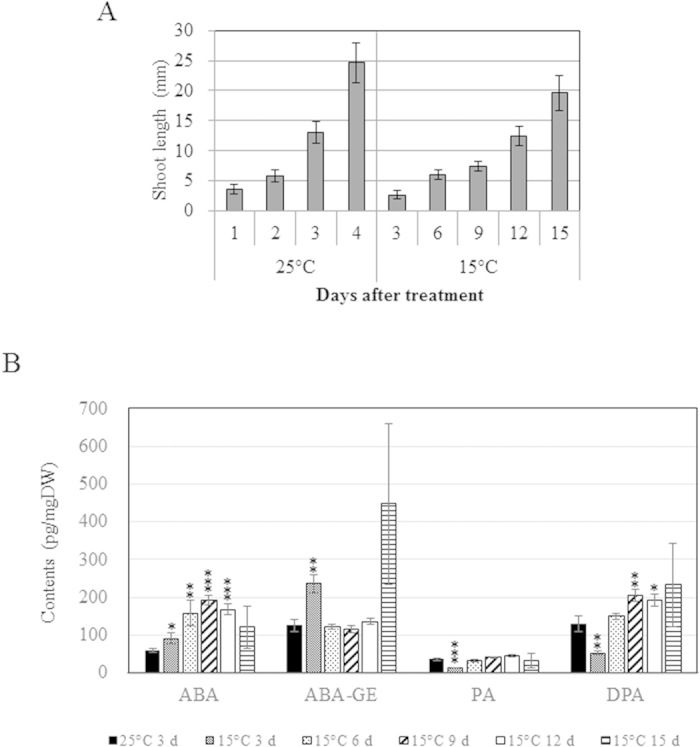
Rice seedlings after imbibition were transferred to and grown hydroponically at 15 °C for 15 d. (A) Shoot length in seedlings grown at 15 °C or 25 °C after imbibition. Values are means ± SD of nine biological replicates. (B) Endogenous ABA, ABA-GE, PA, and DPA contents. Values are means ± SD of three biological replicates. Significant differences between each treatment and the 25 °C treatment were determined by Student’s t-tests. *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001, respectively.
OsABA8ox1 expression increased and then declined during cold treatment (Supplementary Fig. S2). Expression of OsABA8ox2 and OsABA8ox3 after cold treatment was higher than that of control seedlings grown at 25 °C (Supplementary Fig. S2). Thus, only OsABA8ox1 tended to be suppressed at 15 °C. Expression of OsNCED1–3 was suppressed by cold stress, and expression of OsABA2 and OsAAOs was substantially induced after 3 d of cold treatment (Supplementary Fig. S2).
Effects of overexpression of OsABA8ox1 on rice seedling vigor under cold conditions
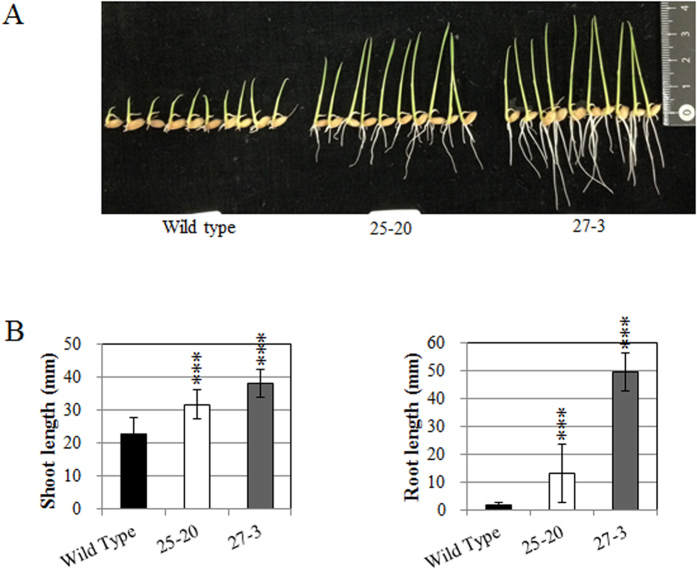
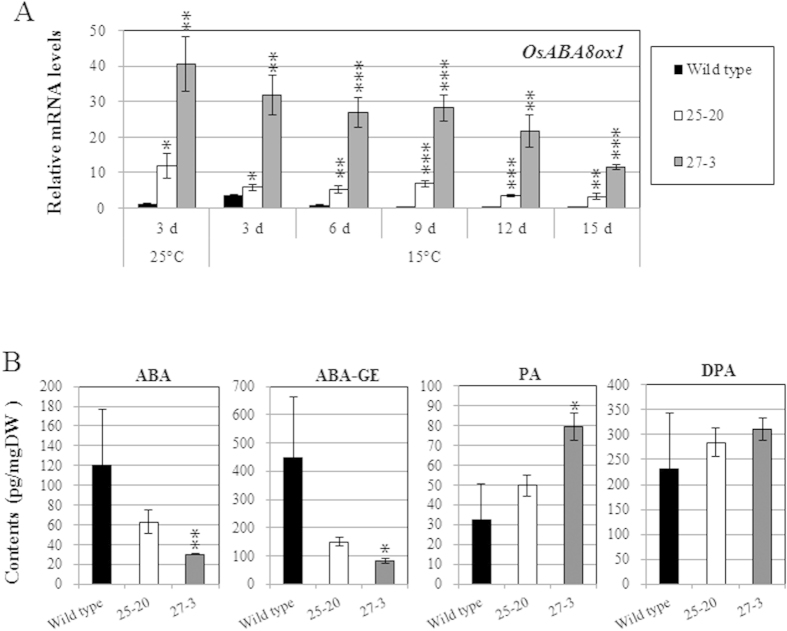
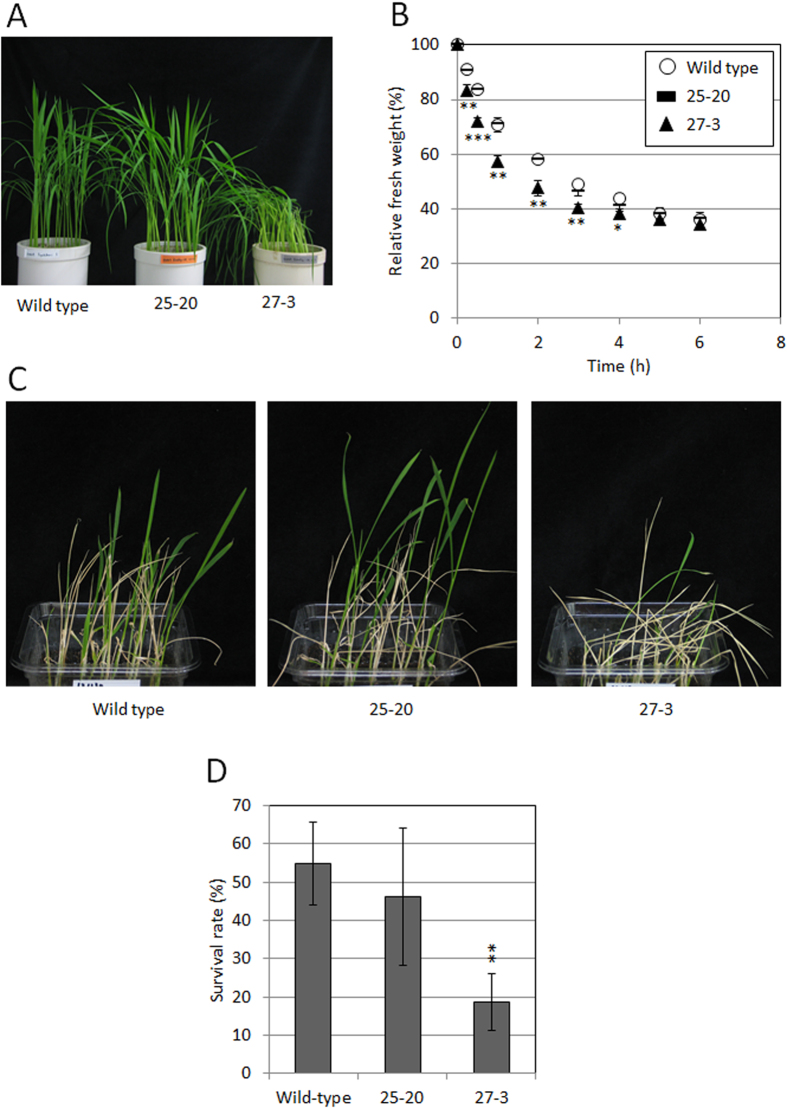
To analyze the relationships between endogenous ABA levels and seedling vigor under cold conditions, we generated transgenic rice plants overexpressing OsABA8ox1 and compared the vigor of transgenic lines E0082::ABA8ox1 25–20 (25–20) and E0082::ABA8ox1 27–3 (27–3) with that of wild-type rice (Toyohikari) at 15 °C. Shoots and roots of 25–20 and 27–3 were longer than those of the wild-type seedlings (Fig. 5A,B), and qRT-PCR showed that expression of OsABA8ox1 was higher in 25–20 and 27–3 (Fig. 6A). ABA and ABA-GE levels were lower in transgenic than in wild-type seedlings, and PA contents were higher in 27–3 than in wild type (Fig. 6B). We also compared seedling vigor and expression of OsABA8ox1 in two other transgenic lines (E0082::ABA8ox1 11–22 and E0082::ABA8ox1 49–38) with that of wild type and observed results similar to those for 25–20 and 27–3 (Supplementary Fig. S3).
Figure 5. Growth of transgenic rice seedlings overexpressing OsABA8ox1 and wild-type seedlings (control) at cold temperatures.
(A) Typical seedlings of Toyohikari (wild type) and transgenic lines overexpressing OsABA8ox1 at 15 °C. (B) Shoot and root length of seedlings grown at 15 °C for 15 days. Values are means ± SD of 27 biological replicates. Significant differences between transgenic and wild-type seedlings were determined by Student’s t-tests. *** represent P < 0.001.
Figure 6. Expression of OsABA8ox1 and ABA content in wild-type rice seedlings and transgenic rice seedlings overexpressing OsABA8ox1.
(A) qRT-PCR analysis of OsABA8ox1 in wild-type and transgenic seedlings (25–20: E0082::ABA8ox1 25–20, 27–3: E0082::ABA8ox1 27–3) grown at 25 °C and 15 °C. (B) Endogenous ABA, ABA-GE, PA, and DPA contents in wild-type and transgenic seedlings (25–20, 27–3) grown at 25 °C and 15 °C. OsABA8ox1 gene expression and endogenous Values are means ± SD of three biological replicates. Significant differences between transgenic and wild-type seedlings were determined by Student’s t-tests. *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001, respectively.
Effects of overexpression of OsABA8ox1 on drought and cold stress tolerance
Drought and cold stress tolerance of 25–20 and 27–3 were compared with those of wild-type rice (Toyohikari). The relative fresh weight of leaves was found to be substantially lower in 27–3 than in wild-type plants and 25–20, 15 min to 4 h after harvest (Fig. 7A,B). Thus, drought tolerance of 27–3 was shown to be lower than that of wild-type plants or 25–20. The survival rate of seedlings of 27–3 after cold treatment (4 °C, 5 d) was substantially lower than that of seedlings of in wild-type or 25–20 (Fig. 7C,D). Therefore, cold stress tolerance at the seedling stage of 27–3 was lower than that of wild-type plants or 25–20.
Figure 7. Drought and cold stress tolerance of 25–20 and 27–3.
(A) Typical plants of Toyohikari (wild type) and transgenic lines overexpressing OsABA8ox1 were grown in a greenhouse at 25 °C/20 °C. (B) Drought stress tolerance of Toyohikari (wild type) and transgenic lines overexpressing OsABA8ox1. The weight of harvested leaves was measured at intervals of 15 min, 30 min, or 1 h. Relative fresh weight was calculated to estimate water loss; values are means ± SD of three biological replicates. Significant differences relative to the wild type were determined by Student’s t-tests. *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001 respectively. (C) Typical images of wild-type, 25–20, and 27–3, 2 weeks after cold treatment (4 °C, 5 d). (D) Survival rates of wild-type, 25–20, and 27–3, 2 weeks after cold treatment (4 °C, 5 d). The experiments were repeated four times. Significant differences relative to the wild type were determined by Student’s t-tests. **, P < 0.01.
Microarray analysis
Genes with differential expression between transgenic rice seedlings (E0082::ABA8ox1 27–3) and wild-type seedlings under cold stress (15 °C) were selected (Fig. 8 and Supplementary Table SII). The genes for transcription factors, including putative genes for ABA-responsive element binding factors, were induced lower in 27–3 by cold stress than that in wild type. Expression of PP2C genes and water-stress related genes, including dehydrin RAB and LEA protein genes, were lower in 27–3 than in wild type at both 25 °C and 15 °C. Heat shock-related genes, including HSP genes, were induced to a lesser extent by cold stress in 27–3 than in wild type. In contrast, peroxidase genes were expressed to a greater extent in 27–3 than in wild type at both 25 °C and 15 °C.
Figure 8. Heatmap of differentially expressed genes in wild-type seedlings and transgenic seedlings overexpressing OsABA8ox1 (27–3).
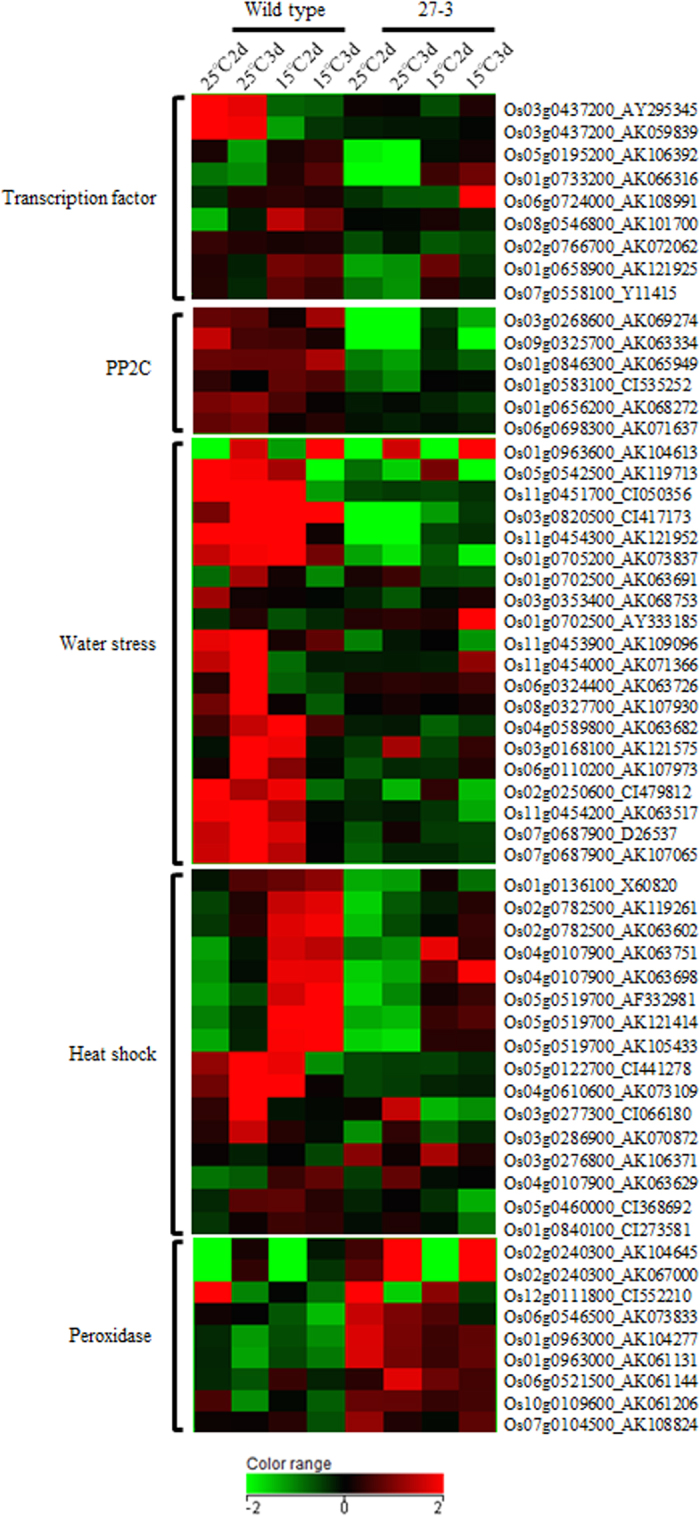
Genes were selected based on fold changes (>2) between wild-type and transgenic seedlings grown at 15 °C or 25 °C. Then, genes that were reported in the database (RiceXPro, http://ricexpro.dna.affrc.go.jp/) and in the literature as ABA responsive were further selected (Supplementary Table SII). Results are shown in the heatmap. Genes are categorized by function (left). Locus and Accession Nos. are shown on the right.
Discussion
Stress-dependent expression of OsABA8ox1, OsABA8ox2, and OsABA8ox3
In this study, we showed that expression of OsABA8ox1 was induced by cold temperatures, while expression of OsABA8ox2 and OsABA8ox3 was not. In contrast, expression of OsABA8ox2 and OsABA8ox3 was induced by ABA, but OsABA8ox1 was not. In addition, expression of OsABA8ox2 and OsABA8ox3 was induced at much higher levels than that of OsABA8ox1 by drought, high salinity, and osmotic stress (Fig. 1). These results suggest that OsABA8ox1 is a cold-inducible gene, whereas OsABA8ox2 and OsABA8ox3 are ABA-inducible genes with expression similarly to that of the ABA-responsive gene Rab16A. The differential inducibility among the OsABA8ox homologs may be unique to rice, because the Arabidopsis CYP707A1, CYP707A2, CYP707A3, and CYP707A4 genes are all induced by ABA, drought, high salinity, and osmotic stress17. Expression of these Arabidopsis CYP707A genes also increases in cauline leaves at 0 °C26. Thus, all ABA8ox homologs in Arabidopsis are induced by the same stressors. However, we demonstrated that the inducibility of OsABA8ox1 was different from that of OsABA8ox2 or OsABA8ox3 in rice, suggesting that the evolutionary origin of OsABA8ox1 may be different from that of OsABA8ox2 and OsABA8ox3. Phylogenetic analyses classify rice OsABA8ox2 and OsABA8ox3 into extremely close clades and place OsABA8ox1 in a different group23. In addition, OsABA8ox1 consists of 4 exons, whereas OsABA8ox2 and OsABA8ox3 consist of 9 and 8 exons, respectively. These findings suggest that OsABA8ox1 evolved in a manner distinct from OsABA8ox2 and OsABA8ox3 in rice.
As noted in Fig. 1, OsABA8ox1 is thought to play a pivotal role in responses to cold stress in rice. However, OsABA8ox2 and OsABA8ox3 function under different stress conditions (e.g., drought, ABA, high salinity, and osmotic stress). Since OsABA8ox3 is induced within 3 to 6 h of seed imbibition27, OsABA8ox3 might help promote seed germination by catabolizing ABA after imbibition.
In vivo ABA catabolic ability of OsABA8ox1, OsABA8ox2, and OsABA8ox3
Overexpression of OsABA8ox1, OsABA8ox2, or OsABA8ox3 resulted in the recovery of defective germination in the Arabidopsis cyp707a1a2a3 triple mutant, in which excess accumulation of ABA occurs in the seeds (Fig. 2). This indicates that the gene products of rice OsABA8ox1, OsABA8ox2, and OsABA8ox3 can catabolize ABA in vivo. However, the germination rates of all transgenic lines failed to reach the same level as that of wild-type plants, indicating that the three Arabidopsis cyp707a genes might function cooperatively. Although ABA levels of cyp707a1 or cyp707a2 single mutants of Arabidopsis are similar to those of wild-type plants, ABA levels of the cyp707a1/cyp707a2 double mutant increase 5- to 10-fold relative to those of the cyp707a1 or cyp707a2 single mutants24. Therefore, even though seed germination of cyp707a1a2a3 was rescued by overexpression of each single OsABA8ox gene, multiple genes might be required to completely recover the same germination rate as the wild-type plant. Alternatively, the rice OsABA8ox genes may not function the same as Arabidopsis ABA8ox genes in vivo.
Effects of lowered endogenous ABA levels on seedling vigor under cold stress
Comparisons of seedling vigor at 15 °C between wild-type seedlings and 25–20 and 27–3 transgenic seedlings revealed that overexpression of OsABA8ox1 increased seedling vigor under cold conditions (Fig. 5A,B). The expression level of OsABA8ox1 in 27–3 was even higher than that in 25–20 (Fig. 6A), and, as predicted, the endogenous ABA level in 27–3 was lower than that in 25–20 (Fig. 6B), and seedling vigor at 15 °C was superior in 27–3. Hence, overexpression of OsABA8ox1 resulted in lower levels of endogenous ABA, which in turn led to increased seedling vigor under cold conditions. These results suggest that growth of rice seedlings is inhibited by increased ABA levels during cold conditions, and that this inhibition can be blocked by lowering ABA levels. Research to date indicates that inhibition of carotenoid biosynthesis contributed to cold stress tolerance in the vegetative stage in rice11, and that overexpression of anther-specific wheat ABA8ox reduced seed sterility caused by exposure to cold stress12. Recently, we demonstrated that severe ABA inhibition of rice seedling growth was completely blocked by salicylic acid, which showed strong antagonistic effects towards ABA28. We found that expression of many stress-related genes was lowered or was not induced by cold stress, while peroxidase genes had higher expression in transgenic rice seedlings overexpressing OsABA8ox1 than in wild-type seedlings (Fig. 8 and Supplementary Table SII). Plant peroxidases can induce cell wall loosening and growth by elongation and cross-linking of cell wall components29. Seedling vigor might have been improved by release of ABA suppression against peroxidase genes in transgenic seedlings overexpressing OsABA80x1. Thus, reducing ABA levels or its effects might be a key mechanism for conferring tolerance to cold conditions to rice.
Although we succeeded in increasing seedling vigor under cold conditions, transgenic rice plants overexpressing OsABA8ox1 showed a few disadvantageous characteristics. First, drought tolerance of 27–3 was substantially lower than that of wild-type and 25–20 (Fig. 7A,B), which we suggest was caused by the extremely low endogenous ABA levels. One report demonstrated that tobacco plants overexpressing three ABA8ox genes from the common bean (Phaseolus vulgaris L.) had wilty phenotypes and broadly opened stomata compared to non-transgenic tobacco, and that these phenotypes were the result of reduced endogenous ABA levels30. ABA helps stomata to close and thus to reduce transpiration under drought conditions, and ABA degradation promotes stomatal opening8. Here, 27–3 probably wilted because constitutive OsABA8ox1 overexpression decreased endogenous ABA levels excessively, forcing stomata to remain open. Several ABA-deficient lines in Arabidopsis also show an extreme wilty phenotype under normal conditions10. In contrast, 25–20 showed growth that was similar to wild-type plants although endogenous ABA was reduced. These differences between 25–20 and 27–3 are thought to be caused by the differences in endogenous ABA levels. The ABA content in 27–3 was 30 pg/mg dry weight (DW), which was substantially lower than that of 25–20 (60 pg/mg DW) (Fig. 6B). Thus, the wilty phenotype of 27–3 might be caused by the excessively reduced ABA levels. Measuring the width of stomatal aperture might help to determine this threshold. Secondly, the survival rate after cold treatment (4 °C) in 27–3 was inferior to that of wild-type or 25–20 (Fig. 7C,D). This result was inconsistent with the increased cold tolerance of the carotenoid-deficient mutant11 and might be a result of excessive degradation of endogenous ABA. We found that the expression of many stress-related genes was lowered or not induced by cold stress in 27–3 compared to wild type (Fig. 8 and Supplementary Table SII), which could affect the survival rate of 27–3 after cold treatment (4 °C). In contrast, 25–20 showed almost the same survival rate as the wild-type strain (Fig. 7C,D). Therefore, excessive ABA degradation might actually reduce cold tolerance. Third, the seed filling rate of 27–3 was lower than that of wild-type or 25–20 under normal conditions (Supplementary Fig. S4), and seed filling rate was similar in 25–20 and wild type. This suggests that mild OsABA8ox1 overexpression has little effect on rice seed ripening, whereas excessive OsABA8ox1 expression results in increased seed sterility. Taken together, our findings suggest that adequate regulation of endogenous ABA levels in rice can lead to improved seedling vigor under cold temperatures without lowering cold and drought tolerance.
Conclusion
The findings presented here provide important knowledge toward understanding the relationships between endogenous ABA levels and seedling vigor under cold conditions in rice. By using transgenic rice plants overexpressing OsABA8ox1, we demonstrated that sustained low levels of endogenous ABA led to increased seedling vigor under cold conditions. On the other hand, excessively low endogenous ABA levels reduced drought and cold tolerance. We propose that adequate regulation of endogenous ABA levels in rice is crucial both for seedling vigor under cold conditions and for cold and drought tolerance.
Methods
Plant materials, growth conditions, and treatments
Seeds of rice (Toyohikari) were washed with sterilized water and soaked in water for 2 d at 28 °C in the dark. After soaking, germinated seeds were grown under hydroponic conditions as follows. Nine seeds of Toyohikari were placed on a plastic grid (approximately 35 × 35 mm), which was floated in a plastic cup holding 100 mL distilled water. Seeds were grown hydroponically in water containing 2.5 mM MES-KOH (pH 5.8) in a growth chamber (12 h light: 12 h dark; 25 °C) for 1 week, and 1-week-old seedlings were used for the subsequent experiments. For drought treatment, seedlings were dehydrated on a paper towel. For hormone and osmotic stress treatment, seedlings were transferred to buffer containing either 100 μM ABA, 100 μM salicylic acid, 100 μM jasmonic acid, 100 μM ethephon, 250 mM NaCl, or 500 mM mannitol. For cold treatment, seedlings were transferred to and kept at 4 °C for 6 or 24 h. For wounding, shoots were punctured by a needle. Wounded seedlings were grown hydroponically in the buffer for 6 or 24 h. To investigate the effects of temperature, germinated seeds were hydroponically grown for 3 d as described above and then were transferred to and kept at 4 °C, 8 °C, 15 °C, and 25 °C for 6 or 24 h.
qRT-PCR
Shoots were harvested from stress-treated seedlings for qRT-PCR. The shoots were frozen with liquid nitrogen and applied to a Multi-beads Shocker (Yasui-Kikai, Osaka) cell disruptor at 1500 rpm for 10 s. Total RNA was isolated using an RNeasy plant mini kit (Qiagen, Venlo), according to the manufacturer’s manual, then treated with DNase I (Takara, Tokyo) to prevent contamination by genomic DNA. cDNA was then synthesized from 500 ng cleaned total RNA using PrimeScript RT Master Mix (Takara). qRT-PCR was performed using TaqMan with the Universal Probe Library (Roche, Basel, Switzerland). Primer sets used in qRT-PCR are provided in Supplementary Table SI. For normalization of data, 25S rRNA was used as an internal standard31.
ABA measurements
ABA and its catabolites were analyzed by liquid chromatography (LC) tandem mass spectrometry (MS/MS). Deuterium-labeled d6-ABA purchased from Icon Services (Summit) and deuterium-labeled d3-PA, d3-DPA, d5-ABA-GE, d3-neoPA, and d4-7′OH-ABA32 were used as internal standards. The tissue was frozen in liquid nitrogen and stored at −80 °C. Lyophilized plant materials were ground in 2 ml of 80% acetonitrile containing 1% acetic acid, extraction and purification of ABA and its catabolites were performed as described previously33. The hormone and metabolites were quantified by LC (Agilent 1200 UHPLC; Agilent Technologies) and a triple quadrupole mass spectrometer (Agilent 6410; Agilent Technologies). The LC conditions were the same as described previously33. The retention times of the compounds were 11.4 min (DPA and d3-DPA), 18.3 min (PA and d3-PA), 13.6 min (ABA-GE and d5-ABA-GE), 21.3 min (7′OH-ABA and d4-7′OH-ABA), 23.8 min (neoPA and d3-neoPA), 27.1 min (d6-ABA and ABA). MS/MS conditions were as follows: collision energy (eV) = 8.0 (ABA and d6-ABA), 10.0 (PA and d3-PA, neoPA, and d3-neoPA, ABA-GE and d5-ABA-GE), 12.0 (7′OH-ABA and d4-7′OH-ABA), 16.0 (DPA, d3-DPA) and fragmentor (V) = 140 (ABA and d6-ABA, DPA and d3-DPA), 130 (PA and d3-PA, neoPA, and d3-neoPA), 160 (ABA-GE and d5-ABA-GE), 110 (7′OH-ABA and d4-7′OH-ABA). MS/MS transitions (m/z) were: 263/153 (ABA), 269/159 (d6-ABA), 279/139 (PA), 282/142 (d3-PA), 281/171 (DPA), 284/174 (d3-DPA), 425/263 (ABA-GE), 430/268 (d5-ABA-GE), 279/217 (7′OH-ABA), 283/221 (d4-7′OH-ABA), 279/205 (neoPA), and 282/208 (d3-neoPA).
Introduction of OsABA8ox1, OsABA8ox2, or OsABA8ox3 into cyp707a1/cyp707a2/cyp707a3 triple mutant of Arabidopsis
OsABA8ox1, OsABA8ox2, or OsABA8ox3 cDNA fragments were amplified by PCR from synthesized total cDNA with the following primers: OsABA8ox1, ABA8ox1_fw and ABA8ox1_rv; OsABA8ox2, ABA8ox2_fw and ABA8ox2_rv; OsABA8ox3, ABA8ox3_fw and ABA8ox3_rv (Supplementary Table SI). These fragments were cloned into pENTR/D-TOPO vectors (Life Technologies, Rockville). After nucleotide sequences were checked, the cloned cDNAs were transferred to the binary vector pEarleyGate101 via the Gateway LR Clonase Enzyme mix (Life Technologies)34. The resulting plasmids were introduced into Agrobacterium strain GV3101, which was used to transform the cyp707a1a2a3 triple mutant22 by the floral-dipping method35. Transgenic plants were obtained by selection with Basta. T2-independent transgenic lines were analyzed.
Germination analysis of Arabidopsis plants
The in vivo function of rice OsABA8ox genes was estimated by quantifying the germination rate of transgenic Arabidopsis lines. Two hundred seeds were sown on 0.8% agar plates containing one-half Murashige and Skoog salts and 2.5 mM MES-KOH (pH 5.8). After 2 d of cold treatment at 4 °C, the plates were placed at 22 °C under continuous light for 9 d. Germination was scored daily for radicle emergence. The experiments were repeated four times.
Production of transgenic rice plants overexpressing OsABA8ox1
The 1416-bp OsABA8ox1 cDNA fragment was amplified from synthesized total cDNA with the primers ABA8ox1_fw2 and ABA8ox1_rv2 (Supplementary Table SI). The amplified cDNA fragment was cloned into a pSTARA-R4 vector (Funakoshi, Tokyo). The fragment was inserted downstream of the E0082 promoter36 that drives expression in green tissues. Transformation of wild-type rice (Toyohikari) and selection of transgenic rice were performed as described previously37.
Growth analysis of rice seedlings under long-term cold stress
Seeds of rice (Toyohikari) were washed with sterilized water and soaked in water for 2 d at 28 °C in the dark. After soaking, nine seeds each of transgenic rice plants overexpressing OsABA8ox1 and of wild-type plants were placed on a plastic grid (approximately 35 × 35 mm), which was floated in a plastic cup holding 100 mL distilled water. Plants were grown in the growth chamber (12 h light: 12 h dark; 15 °C) for 15 d.
Evaluation of drought and cold stress tolerance
For drought stress treatment, plants were grown in soil in a greenhouse (14 h light, 25 °C; 10 h dark, 20 °C) for 1 month, and then the leaves were harvested. The weight of harvested leaves was measured at intervals of 15 min, 30 min, or 1 h. Relative fresh weight was calculated to estimate water loss. For cold stress treatment, 20 plants each of the wild type and transgenic plants were pre-grown in soil in a greenhouse (14 h light, 25 °C; 10 h dark, 20 °C) for 10 d and then transferred to a growth chamber (12 h light, 4 °C; 12 h dark, 4 °C). After the stress treatment for 5 d, plants were returned to the greenhouse and grown for an additional 2 weeks. The numbers of plants that survived and continued to grow were counted. The experiments were repeated four times.
Microarray analysis
Shoot samples were collected from seedlings grown at 15 °C or 25 °C. Total RNA was extracted as described above (qRT-PCR). The integrity of each RNA sample was examined using lab-on-a-chip technology with the RNA 6000 Nano LabChip kit and a 2100 Bioanalyzer (Agilent Technologies, Santa Clara). Microarrays were performed using a Low-Input Quick Amp Labeling Kit (Agilent Technologies) and a rice 4 × 44 K custom oligo DNA microarray (Agilent Technologies) according to the manufacturer’s instructions. Hybridization microarray slides were scanned with a Microarray Scanner and the resulting images were analyzed using Feature Extraction software (Agilent Technologies), applying standard normalization procedures. The analyses were performed using three biological replicates. Heat maps were generated using GeneSpring 12.5 software.
Statistics
For all statistical analyses, group data were averaged and standard deviations were calculated. Student’s t-tests were used to compare the appropriate experimental and control group means; P values <0.05 were considered statistically significant.
Additional Information
Accession numbers: OsABA8ox1:Os02g0703600; OsABA8ox2:Os08g0472800; OsABA8ox3:CI523426; Rab16A:Os11g0454300; 25S rRNA:AK119809; OsNCED1:CI049010; OsNCED2:Os12g0617400; OsNCED3:Os07g0154100; OsABA2:Os03g0810800; OsAAOs:Os03g0790900/Os10g0138100.
How to cite this article: Mega, R. et al. Sustained low abscisic acid levels increase seedling vigor under cold stress in rice (Oryza sativa L.). Sci. Rep. 5, 13819; doi: 10.1038/srep13819 (2015).
Supplementary Material
Acknowledgments
We would like to thank Noriko Goto for technical assistance. This work was supported by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry of Japan.
Footnotes
Author Contributions R.M. and A.M. contributed equally to this work. R.M. and A.M. conducted the characterization of rice samples. A.E. and E.N. performed investigation for Arabidopsis samples. E.S. and S.M. conducted the generation of transgenic rice plants. M.S., Y.K. and S.R.A. conducted ABA measurements. Y.S., R.M. and A.M. wrote the manuscript. Y.S. contributed to the study design and edited the manuscript.
References
- Redoña E. D. & Mackill D. J. Genetic variation for seedling vigor traits in rice. Crop Sci. 36, 285–90 (1996). [DOI] [PubMed] [Google Scholar]
- Williams J. F. & Peterson M. L. Relations between alpha-amylase activity at and growth of rice seedlings. Crop Sci. 13, 612–615 (1973). [Google Scholar]
- Sasahara T., Ikarashi K. & Kambayashi M. Genetic variations in embryo and endosperm weights, seedling growth parameters and alpha-amylase activity of the germinated grains in rice (Oryza sativa L.). Jpn J. Breed. 36, 248–261 (1986). [Google Scholar]
- Thomashow M. F. PLANT COLD ACCLIMATION: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. Annual Reviews 50, 571–599 (1999). [DOI] [PubMed] [Google Scholar]
- Shinozaki K. & Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 3, 217–223 (2000). [PubMed] [Google Scholar]
- Shinozaki K., Yamaguchi-Shinozaki K. & Seki M. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 6, 410–417 (2003). [DOI] [PubMed] [Google Scholar]
- Chinnusamy V., Schumaker K. & Zhu J. Molecular genetic perspectives on cross‐talk and specificity in abiotic stress signalling in plants. J. Exp. Bot. 55, 225–236 (2004). [DOI] [PubMed] [Google Scholar]
- Beardsell M. F. & Cohen D. Relationships between leaf water status, abscisic acid levels, and stomatal resistance in maize and sorghum. Plant Physiol. 56, 207–212 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M. et al. High humidity induces abscisic acid 8′-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant Physiol. 149, 825–834 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W. H. et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14, 2723–2743 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H. et al. Carotenoid deficiency impairs ABA and IAA biosynthesis and differentially affects drought and cold tolerance in rice. Plant Mol. Biol. 83, 475–488 (2013). [DOI] [PubMed] [Google Scholar]
- Ji X. et al. Control of ABA catabolism and ABA homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol. 156, 647–662 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard D. F. & Walton D. C. Abscisic acid metabolism by a cell-free preparation from Echinocystis lobata liquid endoserum. Plant Physiol. 58, 790–795 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krochko J. E., Abrams G. D., Loewen M. K., Abrams S. R. & Cutler A. J. (+)-Abscisic acid 8′-hydroxylase is a cytochrome P450 monooxygenase. Plant Physiol. 118, 849–860 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler A. J. & Krochko J. E. Formation and breakdown of ABA. Trends Plant Sci. 4, 472–478 (1999). [DOI] [PubMed] [Google Scholar]
- Kushiro T. et al. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J. 23, 1647–1656 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S. et al. Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol. 134, 1439–1449 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E. & Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 56, 165–185 (2005). [DOI] [PubMed] [Google Scholar]
- Xu Z. J., Nakajima M., Suzuki Y. & Yamaguchi I. Cloning and characterization of the abscisic acid-specific glucosyltransferase gene from adzuki bean seedlings. Plant Physiol. 129, 1285–1295 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H. et al. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126, 1109–1120 (2006). [DOI] [PubMed] [Google Scholar]
- Saika H. et al. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8′-hydroxylase in rice. Plant Cell Physiol. 48, 287–298 (2007). [DOI] [PubMed] [Google Scholar]
- Okamoto M. et al. Genome-wide analysis of endogenous abscisic acid-mediated transcription in dry and imbibed seeds of Arabidopsis using tiling arrays. Plant J. 62, 39–51 (2010). [DOI] [PubMed] [Google Scholar]
- Vallabhaneni R. & Wurtzel E. T. From epoxycarotenoids to ABA: The role of ABA 8′-hydroxylases in drought-stressed maize roots. Arch. Biochem. Biophys. 504, 112–117 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M. et al. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 141, 97–107 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T. et al. CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J. 46, 171–182 (2006). [DOI] [PubMed] [Google Scholar]
- Baron K. N., Schroeder D. F. & Stasolla C. Transcriptional response of abscisic acid (ABA) metabolism and transport to cold and heat stress applied at the reproductive stage of development in Arabidopsis thaliana. Plant Sci. 188–189, 48–59 (2012). [DOI] [PubMed] [Google Scholar]
- Zhu G., Ye N. & Zhang J. Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol. 50, 644–651 (2009). [DOI] [PubMed] [Google Scholar]
- Meguro A. & Sato Y. Salicylic acid antagonizes abscisic acid inhibition of shoot growth and cell cycle progression in rice. Sci. Rep. 4, 4555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passardi F., Penel C. & Dunand C. Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci. 9, 534–540 (2004). [DOI] [PubMed] [Google Scholar]
- Yang S. H. & Zeevaart J. A. D. Expression of ABA 8′-hydroxylases in relation to leaf water relations and seed development in bean. Plant J. 47, 675–686 (2006). [DOI] [PubMed] [Google Scholar]
- Jain M., Nijhawan A., Tyagi A. K. & Khurana J. P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 345, 646–651 (2006). [DOI] [PubMed] [Google Scholar]
- Priest D. M. et al. Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J. 46, 492–502 (2006). [DOI] [PubMed] [Google Scholar]
- Zheng C. et al. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J. Exp. Bot. 10.1093/jxb/eru519 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley K. W. et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45, 616–629 (2006). [DOI] [PubMed] [Google Scholar]
- Desfeux C., Clough S. J. & Bent A. F. Female reproductive tissues are the primary target of Agrobacterium-mediated transformation by the Arabidopsis floral-dip method. Plant Physiol. 123, 895–904 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki H. & Ohshima M. inventors; Inc Admin Agency Naro, assignee. Functional plant, promoter to be used for producing the functional plant and method of using the same. PCT/JP2002/002817. 2002 March 22.
- Sato Y., Masuta Y., Saito K., Murayama S. & Ozawa K. Enhanced chilling tolerance at the booting stage in rice by transgenic overexpression of the ascorbate peroxidase gene, OsAPXa. Plant Cell Rep. 30, 399–406 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.