Highlights
-
•
Ovarian metastases can occur after hysterectomy for cervical adenocarcinoma.
-
•
Cervical adenocarcinoma and ovarian metastases showed common genetic profiles.
-
•
Most likely mechanism is trans-tubal spread of neoplastic cells via ovarian stroma.
Keywords: Cervical adenocarcinoma, Ovarian metastases, Whole genome copy number analysis
Ovarian metastases occurring long after hysterectomy for cervical adenocarcinoma are a rare but known risk (Ronnett et al., 2008). It is often difficult to distinguish these from primary mucinous ovarian carcinomas (Ronnett et al., 2008). Here we present a case where three methods (p16 immunohistochemistry, human papillomavirus (HPV) DNA detected by polymerase chain reaction (PCR) and whole genomic copy number analysis) were used to confirm that the ovarian tumour was cervical in origin and we discuss the possible pathogenesis of this process.
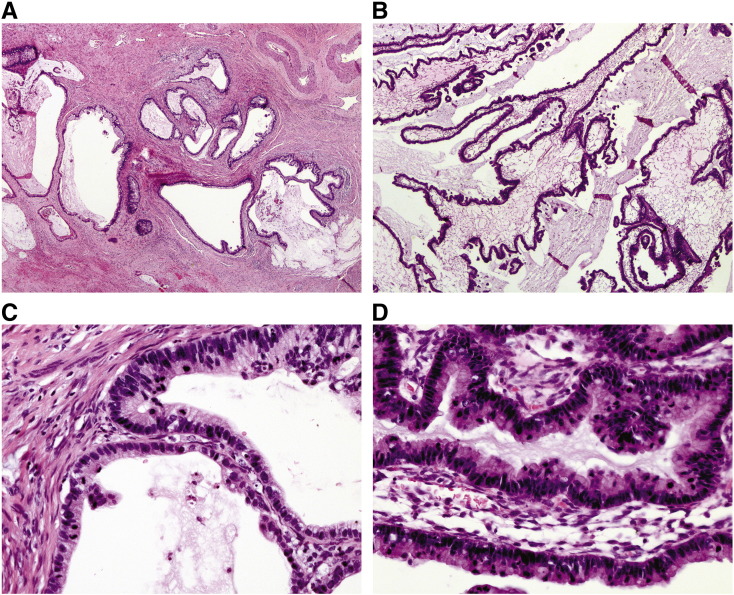
The patient was a 42-year-old, para 7, premenopausal woman, with no medical or surgical history. She presented with a Pap smear diagnosis of adenocarcinoma in situ (AIS) and proceeded to have a large loop excision of the transformation zone (LLETZ) followed by a simple hysterectomy. One month later, an upper vaginectomy, parametrectomy and bilateral pelvic and common iliac lymphadenectomy were performed. The pathology of the LLETZ showed AIS involving the margins. The simple hysterectomy showed in addition to AIS, invasive adenocarcinoma (FIGO stage 1B1) with a depth of invasion of 5 mm and a horizontal width of < 8 mm (a more accurate measurement was not possible because of tissue loss from the previous LLETZ). The cervical tumour consisted of glands and small cysts containing mucin and lined by atypical columnar epithelium (Fig. 1A and C). There was no lymphovascular space invasion (LVSI) and the margins on both the AIS and invasive carcinoma were clear. The upper vaginectomy, parametrectomy and bilateral pelvic and common iliac lymphadenectomy were clear.
Fig. 1.
Panels on the left (A, C) are of invasive cervical adenocarcinoma: A. H&E × 20 and C. H&E × 200. Panels on the right (B, D) are of ovarian metastases: B. H&E × 20 and D. H&E × 200.
A bilateral salpingo-oophorectomy (BSO) was performed for pelvic masses observed on computerised tomography (CT) 24 months after the simple hysterectomy. The right and left ovaries were replaced by solid and cystic tumours measuring 160 × 130 × 40 mm and 110 × 100 × 60 mm. The ovarian tumours showed analogous features to the cervical cancer, except the cystic component was much more pronounced (Fig. 1B and D). At 30 months post-hysterectomy, a CT showed ascites and omental deposits and a positron emission tomography (PET) scan revealed increased uptake in the abdomen, right sacrum and mid thoracic spine. She was initially treated with cisplatin and radiation followed by carboplatin and paclitaxel then pegylated liposomal doxorubicin, however, the patient died of progressive disease five years after the diagnosis of her cervical disease.
Immunohistochemical expression was identical in the cervical and ovarian adenocarcinomas. Strong confluent nuclear p16 expression was seen. Cytokeratin 7 was diffusely positive and cytokeratin 20, oestrogen and progesterone receptor and CDX2 were negative. Polymerase chain reaction (PCR) analysis for HPV DNA genotype using the RHA kit HPV SPF10-LiPA25, version 1 (Labo Bio-medical Products BV, Rijswijk, The Netherlands) showed HPV18 in both tumours. Whole genomic copy number analysis was performed as previously described (Ashton et al., 2012). The copy number variation profile of the ovarian adenocarcinoma showed the same gains and losses observed in the cervical adenocarcinoma; however, there were a number of additional gains and losses across the genome, specifically chromosomes 3 (gain) and 4, 11, 16 and 20 (loss) in the ovarian adenocarcinoma (Fig. 2A and B). There was a copy number gain of TERC in the ovarian adenocarcinoma but no gain in c-MYC was observed. The same gains and losses are strong evidence of clonal origin. The additional changes are expected in a metastasis due to tumour progression.
Fig. 2.
Genetic profile of cervical adenocarcinoma (A) and genetic profile of ovarian metastases (B).
The chromosomes are labelled 1-Y. The chromosomal gains and losses are red and blue regions, respectively.
The histological similarities between the cervical and ovarian carcinomas are evidence of a common origin. The presence of AIS in the cervix supports that the cervical carcinoma is primary and the bilaterality and absence of benign or borderline mucinous tumour in the ovaries supports that the ovarian carcinoma is metastatic. The positive p16 and the presence of HPV 18 DNA in both sites argue that the adenocarcinomas are HPV-related. The similarities of the genomic profiles are strong evidence of clonality. The additional changes in the ovarian adenocarcinoma are in keeping with metastasis. All of the evidence points to a primary cervical adenocarcinoma which has metastasised to the ovaries.
The phenomenon that ovarian metastases may occur following cervical adenocarcinoma is well described, but unusual (Ronnett et al., 2008). In the largest study to date, Ronnett et al. (2008) described 29 cases of endocervical adenocarcinoma with ovarian metastases. Fifteen had a clinical history of cervical disease (9 AIS, 3 micro-invasive, 3 invasive), with an age range of 27–49 years (mean 37) (Ronnett et al., 2008). The presentation of metastatic cancer following identification of cervical adenocarcinoma ranged from 2 months to 7 years (Ronnett et al., 2008). As discussed by Ronnett et al. (2008), ovarian metastases can occur over a variable period of time and in premenopausal women without invasive disease (Ronnett et al., 2008).
Mechanisms to explain ovarian metastases with cervical adenocarcinoma that have been postulated are LVSI, ascending spread of HPV infection and transtubal spread of neoplastic cells (Ronnett et al., 2008, Reichert, 2005, Chang et al., 2009). Metastasis via lymphatics is virtually excluded by the absence of a direct lymphatic link between the cervix and ovaries, the small size of the primary tumour and the absence of LVSI. Furthermore, this hypothesis does not explain those cases in the literature of metastases occurring with AIS where there is no invasion (Ronnett et al., 2008).
In the HPV hypothesis, it has been proposed that the HPV is oncogenic and ascends from the cervix through the fallopian tube and into the ovary of premenopausal women at the time of ovulation (Reichert, 2005). This differs from transtubal spread which involves the passage of neoplastic cells from the cervix to the ovaries. Both these hypotheses would result in p16 positive, HPV DNA-containing tumours as seen in our case. They can be distinguished by genetic testing to determine whether the cervical and ovarian tumours are one or two separate primaries. In our case, the ovarian metastases and cervical tumour suggested clonal origin, excluding two separate primary HPV-related cancers.
Transtubal spread of neoplastic cells is possibly the only explanation that can account for all of the findings in our patient. To explain the normal appearance of the ovaries at hysterectomy, we propose two mechanisms. The metastases may have been so small that they were not seen at surgery or the pathogenesis is analogous to the same hypothesis that Sampson proposed 100 years ago to explain ovarian endometriosis (Scurry et al., 2001). In this proposition, transtubal retrograde spread of neoplastic endocervical cells gains access to the ovarian stroma via a ruptured corpus luteum, analogous to endometrial tissue from retrograde menstruation similarly gaining access to the ovary. This mechanism would explain why the ovaries appear normal at surgery, as neoplastic endocervical cells are buried beneath the healed ovarian cortex. If this hypothesis is true, women who develop ovarian metastases from a cervical adenocarcinoma should be premenopausal at the time their cervical cancer began and should not have had prior tubal ligation. Our case satisfies these conditions. In the literature, menopausal and tubal ligation status are usually not recorded, but we cannot find a single case where a patient was stated to be post-menopausal (or was of an age where post-menopausal status was not in doubt) or who had had a previous tubal ligation.
Our study raises the question of ovarian preservation in premenopausal women with adenocarcinoma of the cervix being treated by hysterectomy. Approximately 5% of women with cervical adenocarcinoma are at risk of ovarian metastases (Shimada et al., 2006) and in about half the cases, the metastases will occur post-hysterectomy (Ronnett et al., 2008). The difficulty is that there are no clinicopathological features that predict which women will develop ovarian metastases. In particular, normal appearing ovaries at surgery are not a safeguard against potentially lethal metastases.
In summary, we describe late ovarian metastases in a woman with a small cervical adenocarcinoma treated by hysterectomy. The common genomic profiles suggest that the ovarian metastases were due to spread of cervical adenocarcinoma. We hypothesise that trans-tubal spread of neoplastic cells implanted into the ovarian stroma via a ruptured corpus luteum.
Conflicts of interest
None.
References
- Ashton K.A., Scurry J., Rutherford J., Otton G., Scott R.J., Bowden N.A. Nodular prurigo of the vulva. Pathology. 2012;44(6):565–567. doi: 10.1097/PAT.0b013e328354e006. [DOI] [PubMed] [Google Scholar]
- Chang M.C., Nevadunsky N.S., Viswanathan A.N., Crum C.P., Feltmate C.M. Endocervical adenocarcinoma in situ with ovarian metastases: a unique variant with potential for long-term survival. Int J Gynecol Pathol. 2009;29(1):88–92. doi: 10.1097/PGP.0b013e3181acefbf. [DOI] [PubMed] [Google Scholar]
- Reichert R.A. Synchronous and metachronous endocervical and ovarian neoplasms: a different interpretation of HPV data. Am J Surg Pathol. 2005;29:1686–1687. doi: 10.1097/01.pas.0000183569.71269.01. [DOI] [PubMed] [Google Scholar]
- Ronnett B.M., Yemelyanova A.V., Vang R., Gilks C.B., Miller D., Gravitt P.E., Kurman R.J. Endocervical adenocarcinomas with ovarian metastases: analysis of 29 cases with emphasis on minimally invasive cervical tumours and the ability of the metastases to simulate primary ovarian neoplasms. Am J Surg Pathol. 2008;32(12):1835–1853. doi: 10.1097/PAS.0b013e3181758831. [DOI] [PubMed] [Google Scholar]
- Scurry J., Whitehead J., Healey M. Classification of ovarian endometriotic cysts. Int J Gynecol Pathol. 2001;20:147–154. doi: 10.1097/00004347-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Shimada M., Kigawa J., Nishimura R., Yamaguchi S., Kuzuya K., Nakanishi T., Suzuki M., Kita T., Iwasaka T., Terakawa N. Ovarian metastasis in carcinoma of the uterine cervix. Gynecol Oncol. 2006;101(2):234–237. doi: 10.1016/j.ygyno.2005.10.004. [DOI] [PubMed] [Google Scholar]