Abstract
Myeloproliferative neoplasms (MPNs) are characterized by the clonal expansion of one or more myeloid cell lineage. In most cases, proliferation of the malignant clone is ascribed to defined genetic alterations. MPNs are also associated with aberrant expression and activity of multiple cytokines; however, the mechanisms by which these cytokines contribute to disease pathogenesis are poorly understood. Here, we reveal a non-redundant role for steady-state IL-33 in supporting dysregulated myelopoiesis in a murine model of MPN. Genetic ablation of the IL-33 signaling pathway was sufficient and necessary to restore normal hematopoiesis and abrogate MPN-like disease in animals lacking the inositol phosphatase SHIP. Stromal cell–derived IL-33 stimulated the secretion of cytokines and growth factors by myeloid and non-hematopoietic cells of the BM, resulting in myeloproliferation in SHIP-deficient animals. Additionally, in the transgenic JAK2V617F model, the onset of MPN was delayed in animals lacking IL-33 in radio-resistant cells. In human BM, we detected increased numbers of IL-33–expressing cells, specifically in biopsies from MPN patients. Exogenous IL-33 promoted cytokine production and colony formation by primary CD34+ MPN stem/progenitor cells from patients. Moreover, IL-33 improved the survival of JAK2V617F-positive cell lines. Together, these data indicate a central role for IL-33 signaling in the pathogenesis of MPNs.
Introduction
Myeloproliferative neoplasms (MPNs) comprise a heterogeneous group of malignant clonal hematopoietic diseases including, among others, BCR-ABL1–negative polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), as well as BCR-ABL1–positive chronic myelogenous leukemia (CML). While the BCR-ABL1 fusion protein results in a constitutively activated tyrosine kinase activity in CML, BCR-ABL1–negative MPNs often harbor mutations in the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. Both types of genetic alterations lead to abnormal proliferation of myeloid cells in the absence of overt signs of morphological dysplasia (1).
Although the role of cytokines and growth factors in normal hematopoiesis is widely recognized, their precise contribution to MPN pathogenesis is still unclear. In vitro, cells from MPN patients are characterized by an intrinsic independence and/or hypersensitivity to growth-factor stimulation and cytokine stimulation (2, 3). In CML cells, the BCR-ABL1 protein stimulates constant autocrine production of IL-3, IL-6, G-CSF, and TNF (3–5). The long-term outcome of CML patients has substantially improved with the use of the tyrosine kinase inhibitor imatinib, yet its efficacy may be hampered by the action of cytokines (6). The JAK2V617F mutation is often found in BCR-ABL1–negative MPNs and similarly results in constitutively activated kinase activity, mimicking persistent signaling via multiple cytokine pathways. While JAK inhibitors have been successful in treating JAK2-mutated MPNs, elimination of the mutant clone and resistance may pose an important problem, and patients with WT JAK2 MPNs are not amenable to this therapy (7). The abnormal expression and activity of a number of proinflammatory cytokines are associated with BCR-ABL1–negative MPNs (8), possibly initiating clonal evolution (9) or promoting progression to myelofibrosis (10) and resistance against JAK2 inhibitors (11). Thus, cytokines and growth factors support the aberrant hematopoiesis associated with MPNs and provide mechanisms for drug resistance.
While several studies suggest that concomitant inflammation supports MPN pathogenesis and development, it remains elusive whether inflammation precedes and may thus initiate the myeloproliferation. Indeed, previous inflammatory conditions have been linked to myeloid malignancies (12). Moreover, recent epidemiologic studies have reported an association between MPNs and either certain autoimmune diseases (13) or antecedent chronic inflammatory conditions of infectious origin (14). Microbe-sensing receptors — and TLRs in particular — may initiate such inflammation, and monocytes from MPN patients may display an increased responsiveness to TLR ligands, leading to excessive cytokine production (15). Therefore, and although strong causative evidence is currently lacking, microbial infections driving sustained inflammation may potentially trigger MPNs.
IL-33 is a recently identified member of the IL-1 family of cytokines that binds to a heterodimeric receptor complex consisting of ST2 (IL-1RL1) and IL-1 receptor accessory protein. IL-33 signaling is then further transduced via recruitment of the adapters MyD88 and IL-1 receptor–associated kinase 4 (IRAK4), which are shared with TLRs. IL-33 is predominantly expressed by endothelial and epithelial cells (16, 17), and it has primarily been implicated in the induction of type-2 immune responses by binding to ST2, which is expressed on a variety of cells such as Th2 CD4+ T cells, mast cells, and basophils (16). While IL-33 has recently emerged as a cytokine with pleiotropic functions in diverse types of inflammatory processes — including inflammatory bowel disease (18) and virus-induced inflammation (19, 20) — its function in tumorigenesis is poorly characterized.
Here, we used a mutant allele of the inositol polyphosphate-5-phosphatase D (Inpp5d, which encodes SHIP, an SRC homology 2 domain–containing inositol-5-phosphatase) called styx to investigate the mechanisms underlying spontaneous development of MPN-like disease. Since microbial cues can be sufficient to drive inflammatory disease in genetically susceptible strains (21, 22), we assessed the suppressive effect of a genetic blockade of selected inflammatory pathways in the pathogenesis of MPN-like disease in Inpp5dstyx/styx mice (herein referred to as styx mice). We identified a critical role for MyD88 and IRAK4 in determining the phenotype. Our data indicate that microbial-derived signals are dispensable, whereas the IL-33/ST2 pathway is non-redundant for initiating uncontrolled myelopoiesis in styx mutants. In addition, IL-33 contributes to the development of JAK2V617F-dependent MPNs in mice and promotes colony formation of human primary CD34+ MPN stem/progenitor cells. IL-33 is also increased in BM biopsies of MPN patients. Together, these results reveal a central functional involvement of the IL-33/ST2 pathway in MPN pathogenesis.
Results
Development of MPN-like disease is directed by MyD88 but independent of microbiota-derived signals.
As a model of MPN, we used a Inpp5d styx allele that was identified in an N-ethyl-N-nitrosourea (ENU) genetic screen. The styx point mutation results in a thymine-to-adenine transversion in the donor splice site of intron 5 of the Inpp5d gene, causing an aberrant transcript lacking exon 5, which in turn leads to loss of SHIP protein expression (Supplemental Figure 1, A–D; supplemental material available online with this article; doi:10.1172/JCI77347DS1). SHIP is a negative regulator of the PI3K pathway in hematopoietic cells, and its deficiency leads to increased numbers of granulocyte-macrophage progenitors (GMPs) in the BM and spleen (23). Homozygous styx mice entirely recapitulate the myeloproliferative-like phenotype of the described Inpp5d KO strains (23, 24), showing hyperproliferative BM, associated splenomegaly, and myeloid cell infiltration into several organs, including the spleen, the ileum, and particularly the lung (Supplemental Figure 1E). As a consequence, styx mutants display a chronic progressive and fatal wasting disease with kinetics similar to Inpp5d KO strains (Figure 1A; ref. 23).
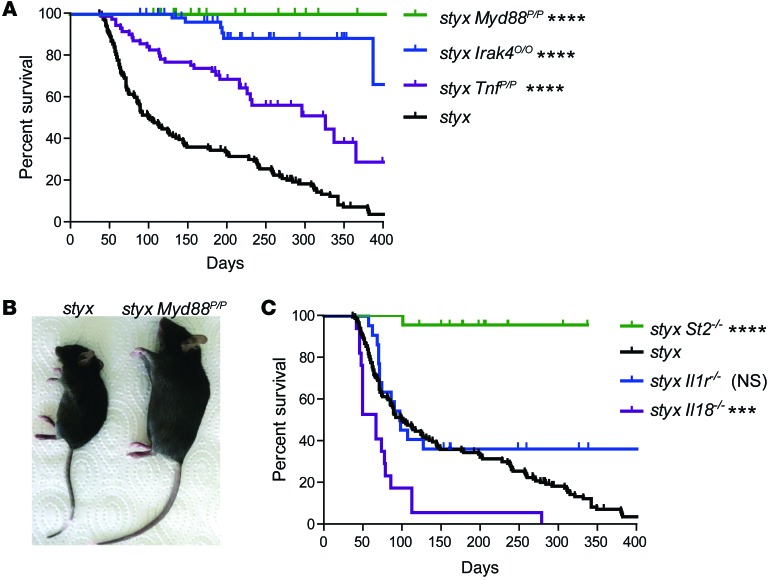
Figure 1. MyD88/IRAK4-dependent IL-33/ST2 signaling promotes MPN-like disease.
(A) Survival of single styx mutants (n = 142) and of styx mice backcrossed on a Myd88- (n = 32), Irak4- (n = 61), and Tnf- (n = 68) deficient background. (B) Macroscopic phenotype of 14-week-old styx and styx Myd88poc/poc male mice that are Myd88 deficient. (C) Survival of styx (n = 142), styx St2–/– (n = 22), styx Il18–/– (n = 8), and styx Il1r1–/– (n = 22) mice. Survival curves for styx mice in A and C represent the same group of mice. Log-rank (Mantel-Cox) test was used, using styx mice as a reference group. ***P < 0.001; ****P < 0.0001.
Given the possible association between certain infections and malignant transformation of the myeloid lineage (14), we investigated the putative role for environmental inflammatory cues in the development of dysregulated myeloproliferation in the styx model. We followed a genetic strategy to systematically disrupt signaling molecules relevant for microbe sensing, focusing on TLRs. Because of its pure C57BL/6J background, the styx strain appeared particularly suitable for such an approach, in contrast to the non–C57BL/6 Inpp5d KO strains generated so far (23–25).
We found that genetic disruption of Myd88, a central protein adapter that relays signals from most TLRs, abrogated fatal MPN-like disease in styx mutants (Figure 1, A and B). Accordingly, styx mice lacking IRAK4, a protein directly downstream of MyD88 in the signaling cascade, were also rescued from the MPN phenotype (Figure 1A). However, single deletion of Tlr2 or Tlr4, critical sensors of microbial products derived from commensal bacteria, failed to prevent disease progression on the styx background. styx Unc93b13d/3d double mutants with impaired endosomal TLR signaling died earlier compared with styx mice, likely from an increased susceptibility to opportunistic infections (Supplemental Figure 2A).
We further hypothesized that MPN development may be provoked by synergistic signaling via multiple TLRs converging toward MyD88 and IRAK4. However, styx mice rederived into a germ-free environment, and consequently exposed to a greatly reduced quantity of TLR ligands, developed MPN-like disease at the same rate and severity as mice kept under specific pathogen–free (SPF) conditions (Supplemental Figure 2).
TLR signaling triggers host cell responses with production of effector cytokines such as TNF. Introduction of a mutation disrupting TNF binding to its receptor significantly extended lifespan but failed to completely rescue styx mice. This reveals a partial contribution of TNF to disease development and associated immunopathology in our model (Figure 1A).
In brief, progression of MPN-like disease in styx mutants is controlled by signaling through MyD88 and IRAK4, but not TLRs. Moreover, the commensal microbiota is not required to induce the observed phenotype.
A non-redundant contribution of the IL-33/ST2 pathway to MPNs.
MyD88 and IRAK4 not only transduce signaling from TLRs, but they are also located downstream of the receptors for the cytokines IL-1, IL-18, and IL-33. Abrogation of signaling through the IL-1 or IL-18 pathways did not limit SHIP-controlled myeloproliferation (Figure 1C).
However, styx mice with bi-allelic deficiency in the MyD88-dependent IL-33 receptor ST2 were found to be protected from disease development (Figure 1C), demonstrating that signaling from ST2 was necessary to induce MPN-like disease in styx mutants.
Dysregulated BM hematopoiesis is a key feature of MPNs in humans and is also the primary etiology for the myeloproliferative disease accompanying SHIP deficiency (23). We thus assessed hematopoietic stem cell (HSC) and myeloid progenitor cell populations in homozygous styx, styx St2–/–, WT, and St2–/– mice by flow cytometry, according to Wilson et al. (Supplemental Figure 3, A and B; ref. 26). Percentages of HSCs (Figure 2, A and B) and myeloid progenitors (Figure 2, C and D) were significantly increased in the BM of styx mice, compared with WT mice. However, genetic blockade of IL-33/ST2 signaling prevented the aberrant hematopoiesis associated with SHIP deficiency, since percentages of BM HSC and myeloid progenitor cell populations were comparable in styx St2–/– mice and WT or St2–/– controls (Figure 2, A–D).
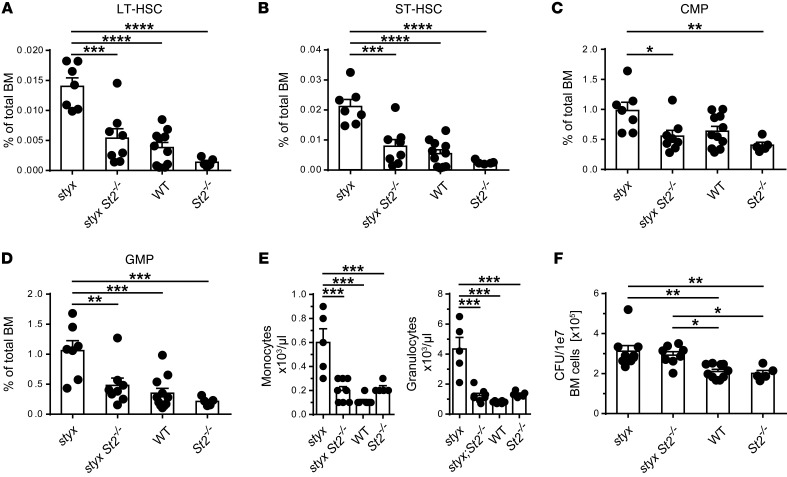
Figure 2. IL-33/ST2 signaling alters BM HSC and myeloid progenitor cell populations, promoting systemic myeloproliferation.
Flow cytometric analysis of lin– BM cells show different frequencies of (A) long-term HSC (LT-HSC) and (B) short-term HSC (ST-HSC), (C) common myeloid progenitors (CMP) and (D) GMPs in the indicated mouse strains. (E) CBC analysis of myeloid cells. (F) CFU of the indicated lin– BM cells after 7 days of culture in the presence of IL-3, FLT3-ligand, and SCF. Data are mean ± SEM. Pooled data from 3 (A and D) or 2 (E and F) independent experiments are represented (n = 5–10 mice per group). (A–F) One-way ANOVA with Bonferroni post hoc test was used; only statistically significant data are presented. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
In the periphery, effective hematopoiesis with granulocytosis and monocytosis were observed in styx but not styx St2–/– mice, as measured by complete blood count (CBC) analysis (Figure 2E). We next functionally analyzed styx, styx St2–/–, WT, and St2–/– BM lineage–negative (lin–) cells for their capacity to form myeloid colonies. SHIP-deficient HSCs are known for their increased capacity to form myeloid colonies due to their enhanced sensitivity to cytokines (23, 24). Accordingly, styx lin– cells exhibited markedly increased colony formation compared with St2–/– and WT controls. However, colony numbers were similarly augmented for styx St2–/– cells (Figure 2F). This implies that although disruption of IL-33/ST2 signaling normalizes hematopoietic subsets in styx mice, styx St2–/– cells retain an intrinsic hyperproliferative ability.
Taken together, these results reveal that steady-state engagement of the IL-33/ST2 pathway acts as a non-redundant trigger for the accumulation of BM HSCs and myeloid progenitors, and for the concomitant MPN-like phenotype observed upon SHIP loss of function.
Stromal IL-33 engages ST2 signaling on both the radio-resistant and the radio-sensitive compartments to induce MPN-like disease.
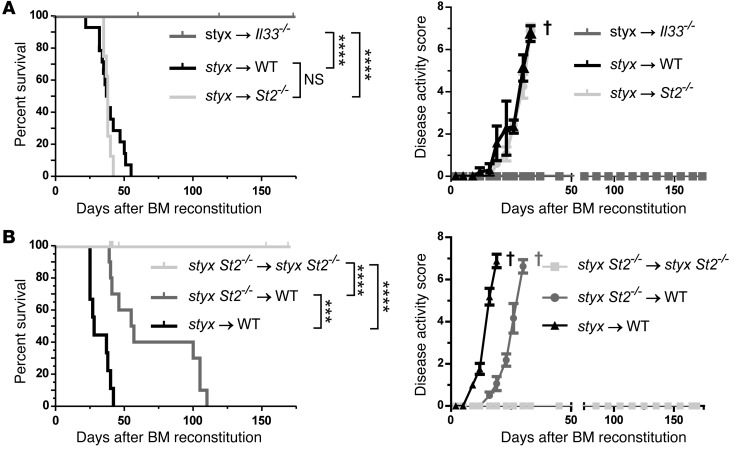
To determine the source of IL-33 and the compartment(s) expressing ST2 in our model system, we next generated various sets of BM chimeric mice. Lethally irradiated St2–/– or control WT hosts reconstituted with styx BM developed MPN-like disease with similar kinetics (Figure 3A). However, genetic abrogation of IL-33/ST2 signaling on a hematopoietic SHIP-deficient compartment significantly delayed, but did not prevent, disease development (Figure 3B and Supplemental Table 1). These findings demonstrate that ST2 can be engaged on both stromal and hematopoietic cells and that the 2 compartments can compensate for each other to induce MPN-like disease in our model.
Figure 3. Stromal cell–derived IL-33 engages ST2 signaling on both the stromal and the hematopoietic compartment to induce MPN-like disease.
(A) Contribution of IL-33 and IL-33/ST2 signaling in radio-resistant cells. (B) Contribution of IL-33/ST2 signaling in radio-sensitive cells. Survival curves and disease activity index are shown for the indicated groups of BM chimeric mice. Pooled data from 2 independent experiments are represented each for (A) styx→WT (n = 14), styx→St2–/– (n = 8), styx→Il33–/– (n = 9), and (B) styx→WT (n = 9), styx St2–/–→styx St2–/– (n = 10), and styx St2–/–→WT (n = 10). Log-rank (Mantel-Cox) test was used. † indicates euthanasia of animals. ***P < 0.001; ****P < 0.0001.
Importantly, lethally irradiated Il33–/– mice reconstituted with styx BM did not develop MPN-like disease (Figure 3A and Supplemental Table 1), thereby indicating a non-hematopoietic origin of IL-33 for uncontrolled myelopoiesis. Since the BM is the primary site of hematopoiesis in adult mice, we investigated whether BM stromal cells produce IL-33. IHC analysis showed that IL-33 protein in BM was expressed at similar levels in styx, styx St2–/–, WT, and St2–/– mice. Furthermore, IL-33 expression was restricted to few cells — most of which had characteristic histological features of endothelial cells (ECs) — lining the BM sinusoids (Supplemental Figure 3). Thus, although IL-33 is not constitutively expressed in ECs along the vascular tree (17), our data demonstrate that it is expressed in some BM sinusoidal ECs.
ST2 is expressed on BM stromal and hematopoietic cells, but not on myeloid progenitors.
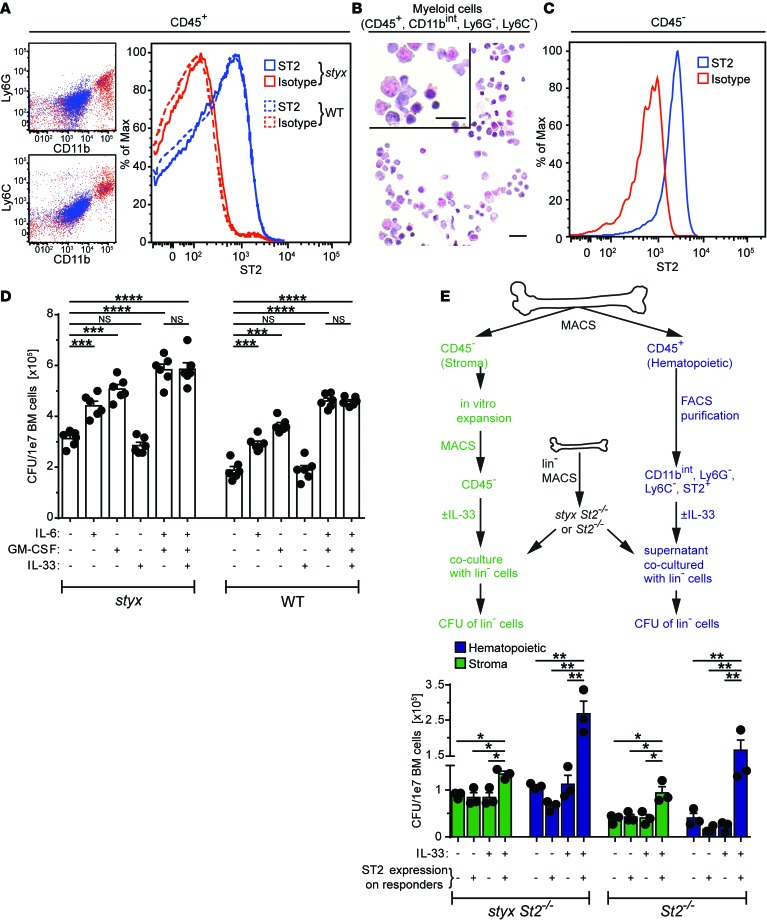
To assess how IL-33 produced by radio-resistant cells induces MPNs in styx mutants, we analyzed the expression pattern of the ST2 receptor on BM cells of styx, styx St2–/–, WT, and St2–/– mice. While surface ST2 was not detectable on BM HSCs and myeloid progenitors (Supplemental Figure 4), we identified a heterogeneous lin+ population of myeloid origin cells that expressed ST2 that was CD11bint, Ly6C–, and Ly6G– (Figure 4, A and B). Further phenotypic analysis revealed that the majority of these cells most likely represented early myeloid cells and were CD11bint, FcεRI–, and c-KIT–, while the minority were basophils, defined as CD11bint, FcεRI+, c-KIT–, and CD49b+ (Supplemental Figure 5A). These two ST2+ populations accounted for 5%–6% and 0.7% of total hematopoietic cells in the BM, respectively (data not shown). Importantly, ST2+ BM cells in styx versus WT mice did not differ in their relative distribution or their ST2 expression levels (data not shown).
Figure 4. ST2 expression and function in the BM.
(A) ST2 expression on hematopoietic BM cells is restricted to committed myeloid cells (CD11bint, Ly6G–, and Ly6C–). Representative histogram of one styx and one WT mouse is shown. (B) Cytospin of FACS-purified BM CD11bint, Ly6G–, Ly6C–, ST2+ cells showing phenotypical heterogeneity (scale bars: 20 μm). (C) ST2 expression on BM endothelial cells (defined as CD45–, lin–, CD51–, and CD31+). (D) Lin– BM cells from styx and WT mice were cultured in methylcellulose with the indicated cytokines. Colony numbers were assessed after 7 days. (E) BM primary stromal cells were grown in vitro (46). MACS-purified CD45– cells from these cultures were stimulated with IL-33 for 24 hours, before addition of lin– cells for coculture. Alternatively, lin– cells were supplemented with conditioned medium from FACS-purified CD11bint, Ly6G–, and Ly6C– cells previously stimulated with IL-33 for 24 hours. Then, styx St2–/– or St2–/– lin– cells incubated for 48 hours under these respective conditions were cultured in methylcellulose for 7 days, and colony formation was assessed. Conditions for culture (± IL-33) and genotypes of stromal or hematopoietic stimulation responders (+: styx St2+/+; -: styx St2–/–) are indicated. Data are mean ± SEM and are representative of (A) n = 8 mice per group; (B) n = 2 mice per group; (C) pooled cells from 4 WT mice, repeated twice; (D) pooled data of 2 experiments, n = 6 mice per group; and (E) 1 experiment where each data point represents 2 pooled mice (n = 6 mice per group). Stromal and hematopoietic responders were pooled from 5 donors. (D and E) One-way ANOVA with Bonferroni post hoc test was used. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
Since our BM chimera experiments indicated that the radio-resistant compartment was also able to promote MPN-like disease in an ST2-dependent manner, we correspondingly assessed ST2 expression on BM stromal cells. We found that ECs (defined as CD45–, lin–, CD51–, and CD31+) and mesenchymal stromal cells (MSCs; specified as CD45–, lin–, CD31–, CD51+, and CD140a+; ref. 27) in the BM expressed ST2, although ST2 expression was marginal on MSCs (Figure 4C and Supplemental Figure 5B).
Together, our data suggest that the BM microenvironment contains the minimal requirements to promote myeloproliferative disease on a SHIP-deficient background via activation of IL-33/ST2 signaling. Furthermore, the expression pattern of ST2 indicates that IL-33/ST2 signaling likely has an indirect effect on styx HSCs and myeloid progenitors.
IL-33 acts indirectly on HSCs and myeloid progenitors.
In order to determine a potential indirect mode of action of IL-33/ST2 signaling in HSCs and myeloid progenitor cells, we used magnetic cell separation to purify lin– cells from styx or WT BM and assessed their capacity to form colonies in the presence or absence of IL-33 (Figure 4D). IL-33 alone did not affect colony formation of lin– BM cells. However, stimulation with IL-6 and/or GM-CSF, factors known to promote myeloproliferation and contribute to the dysregulated hematopoiesis characteristic of SHIP-deficient mice (23, 24, 28), increased the number of colonies from styx and WT lin– BM cells. Moreover, addition of IL-33 to IL-6 and GM-CSF did not further modulate the colony-forming ability of lin– cells (Figure 4D). These results show that IL-33 does not promote colony formation of mouse HSCs and myeloid progenitor cells directly, and it does not synergize with other known myeloproliferative factors. Also, and in agreement with our ST2 expression analysis, SHIP-deficiency is not associated with an increased sensitivity of HSCs and myeloid progenitors to IL-33, as observed for IL-6 and GM-CSF (Figure 4D).
We next performed a coculture assay to dissect how IL-33/ST2 signaling indirectly supports development of MPN-like disease in styx BM. We assessed the contribution of stromal cells, as well as the role of the hematopoietic cell population we previously identified as constitutively expressing ST2. To this end, we measured colony formation by ST2– lin– cells following coculture with IL-33–stimulated primary stromal cells (MACS-purified CD45– BM cells) or after supplementation with conditioned medium from IL-33–stimulated, ST2+ BM hematopoietic cells. Under these experimental settings, we observed an increase in colony formation of styx St2–/– or St2–/– lin– BM cells solely in the combined presence of exogenous IL-33 and ST2-expressing stromal or hematopoietic responders (Figure 4E).
Hence, we concluded that IL-33 can indirectly support development of MPN-like disease in styx mutants via the activation of the radio-resistant compartment, as also indicated by our BM chimera experiments (Figure 3). Our results also suggest that the ST2-expressing hematopoietic population in the BM is able to sustain myeloproliferation, likely via the secretion of soluble factors. In addition, the contribution of ST2+ hematopoietic cells to development of myeloproliferation in styx mice appears to be superior to the one provided by ST2+ stromal cells, in line with our findings from the BM chimeras.
IL-33 induces cytokine and growth-factor secretion by BM cells.
Increased levels of several growth and survival factors in the peripheral blood have been proposed to contribute to the dysregulated hematopoiesis observed in SHIP-deficient mice (29). Accordingly, analysis of the sera of 40-day-old styx mice that were healthy in appearance showed that the levels of cytokines critical in supporting myelopoiesis — including G-CSF, IL-6, and to a lesser extent GM-CSF, M-CSF, IL-3, and IL-5 — were already elevated in several mutants at this age (Supplemental Figure 6, A–F). Conversely, the same cytokines were present at low concentrations in age-matched styx St2–/–, WT, or St2–/– mice. Although we previously identified TNF as a partial mediator of MPN-like disease, it was not elevated in the serum of young styx mice (Figure 1A and Supplemental Figure 6G). However, serum IL-33 levels were significantly augmented in the mutants (Supplemental Figure 6H).
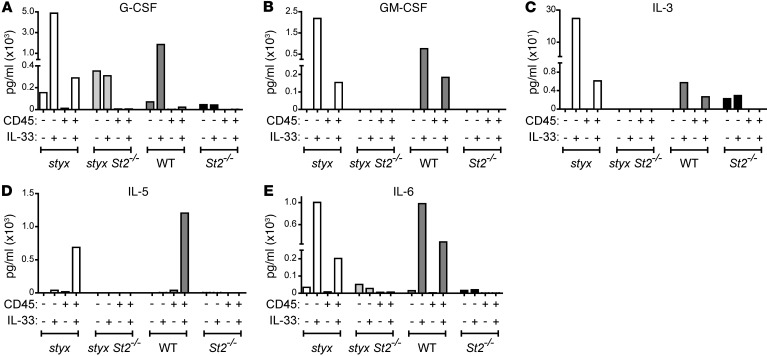
SHIP has been suggested to regulate the production of soluble factors produced by the BM niche (29). To assess the ability of the BM microenvironment to secrete cytokines and growth factors in response to IL-33 and thus promote myelopoiesis, we isolated BM cells from styx and WT mice, and we MACS-purified them into CD45– non-hematopoietic and CD45+ hematopoietic cell fractions. Ex vivo stimulation with IL-33 increased the levels of G-CSF, GM-CSF, IL-3, and IL-6 in the supernatants of both CD45– and CD45+ cell fractions (Figure 5). IL-5 was only secreted by CD45+ hematopoietic cells. Engagement of IL-33/ST2 signaling was required, as specific cytokine secretion was abrogated in the absence of ST2 (Figure 5). Therefore, we deduced that the BM microenvironment is able to produce growth factors in response to secreted IL-33 to provoke uncontrolled myeloproliferation in styx mice.
Figure 5. IL-33 is a potent inducer of cytokines and growth factors promoting myelopoiesis.
BM cells from the indicated strains were MACS-purified into CD45+ (enriched for hematopoietic) and CD45– (enriched for stromal) cells and cultured ± IL-33. After 24 hours, supernatant was collected, and concentrations of (A) G-CSF, (B) GM-CSF, (C) IL-3, (D) IL-5, and (E) IL-6 were measured by LASER Bead Technology. Each condition represents the value measured from pooled cells from 7 (styx) or 10 (styx St2–/–, WT, and St2–/–) mice. A comparable trend was found after 12 hours of culture ± IL-33 (not depicted).
SHIP-deficient myeloid cells display a higher responsiveness to cytokines, such as GM-CSF and IL-3, that leads to a reduced susceptibility to apoptotic stimuli (23, 24). Consequently, we next evaluated whether IL-33/ST2 signaling may affect the viability of styx BM cells. We found that ex vivo CD11b+ BM styx St2–/– cells cultured for short term (6 hours) without cytokine supplementation showed survival rates similar to control cells but reduced compared with ST2-proficient styx cells (Supplemental Figure 7A). Correspondingly, in vitro stimulation with IL-33 promoted the survival of styx but not styx St2–/– CD11b+ BM cells (Supplemental Figure 7B).
In summary, these data support the notion that IL-33 is an important trigger for the production of effector cytokines by the stromal and hematopoietic compartments. These cytokines, in turn, promote enhanced survival and proliferation of myeloid cells, eventually resulting in MPN-like disease in styx mutants.
Stromal IL-33 supports the development of JAK2V617F-dependent MPNs in vivo.
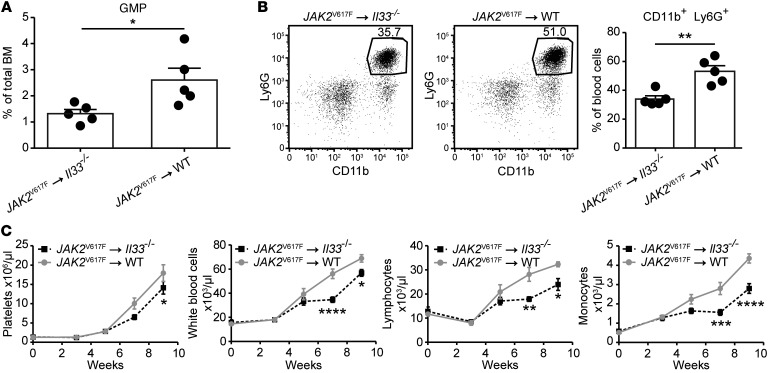
To assess the contribution of IL-33/ST2 signaling to dysregulated myelopoiesis in a different model of MPN, we adoptively transferred BM cells transgenic for the JAK2V617F mutation (30) into Il33–/– recipient mice. In these chimeras, we found a lower percentage of JAK2V617F+ GMPs in the BM and a reduced frequency of blood neutrophils (defined as CD11b+ and Ly6G+) compared with control mice expressing IL-33 in the stroma (Figure 6, A and B). IL-33–deficiency in the radio-resistant compartment also correlated with delayed expansion of several hematopoietic lineages from donor JAK2V617F+ BM cells (Figure 6C). Taken together, these data suggest that stromal cell–derived IL-33 supports the development of JAK2V617F-dependent MPNs in vivo.
Figure 6. Stromal cell–derived IL-33 supports the development of JAK2V617F-dependent MPNs in vivo.
BM cells from Scl-Cre JAK2V617F transgenic donor mice were injected into lethally irradiated Il33–/– or WT recipient mice. After 7 weeks, (A) percentage of GMPs in the BM and (B) frequency of neutrophils in the blood were assessed. (C) Alternatively, indicated blood parameters were evaluated every other week by CBC, starting 3 weeks after reconstitution. n = 5 mice per group. For C, 1 representative of 2 independent experiments is shown. (A and B) Standard Student’s t test, and (C) 2-way ANOVA with Bonferroni post hoc test were used. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
Increased levels of IL-33 protein in the BM of MPN patients.
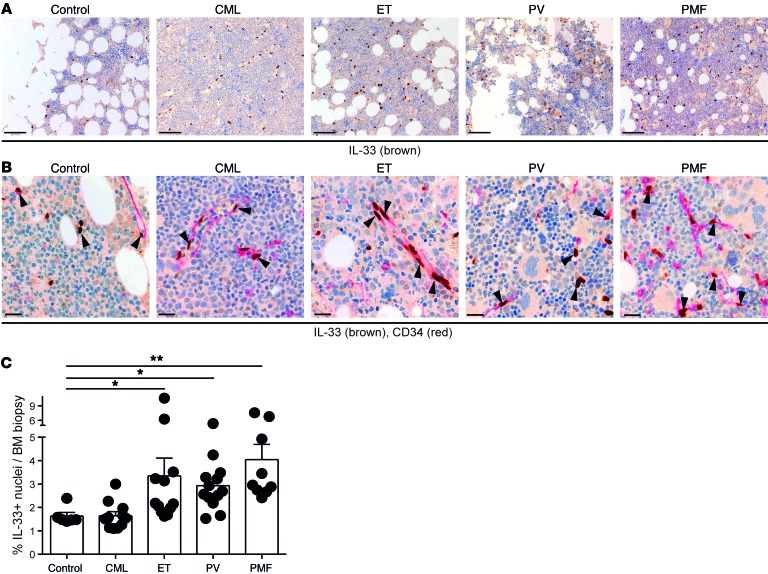
To address the general relevance of the findings from our mouse models of MPN, we assessed the presence of IL-33 in the BM of MPN patients. IL-33 was also expressed in human BM biopsies and was localized in the nucleus of cells that were mainly but not solely endothelial, based on their histomorphological presentation and expression of CD34 (Figure 7, A and B). Automated quantification of cells displaying nuclear IL-33 expression indicated that MPN patients newly diagnosed with ET, PMF, and PV, but not CML, display a higher percentage of IL-33+ BM cells compared with controls (Figure 7C). This increase seemed specific to BCR-ABL1–negative MPN patients, as it was not detected in other types of chronic or acute leukemia affecting the myeloid or lymphoid lineages (Supplemental Figure 8A). Hence, these data suggest that the IL-33/ST2 pathway may also play a role in the pathogenesis of human MPNs.
Figure 7. Increased levels of IL-33 protein in trephine biopsies of MPN patients.
IHC for (A) IL-33 (brown) (scale bar: 100 μm) and for (B) IL-33 (brown) and CD34 (red) in normal and MPN BM (scale bar: 20 μm). (C) Percentage of IL-33–positive nuclei in BM of controls (n = 6) and of patients newly diagnosed with the indicated hematopoietic malignancies. CML (n = 11), ET (n = 12), PV (n = 13), and PMF (n = 9). Data are mean ± SEM. Kruskal-Wallis with Dunn’s post hoc test was used; control biopsies were compared with all other groups. *P < 0.05; **P < 0.01.
MPN cell lines and CD34+ stem/progenitor cells from MPN patients directly respond to IL-33.
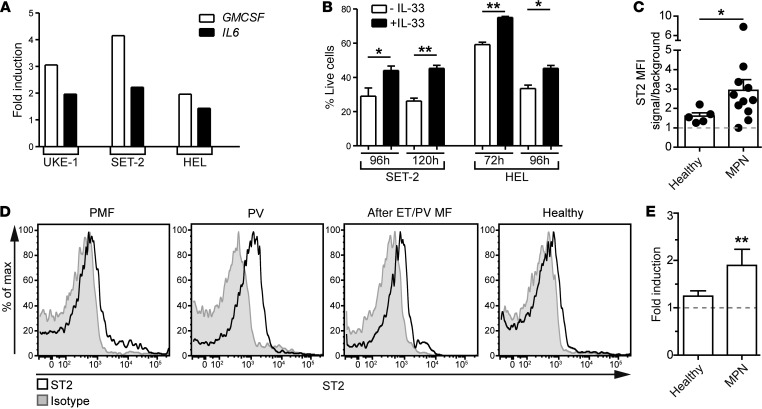
The IHC findings from patient BM biopsies raised the question as to whether IL-33 also exerts a proproliferative effect on human MPN cells. In particular, the increase in IL-33 protein expression in BCR-ABL1–negative MPNs prompted us to examine the effect of IL-33 on human JAK2V617F+ cells. In vitro experiments showed that IL-33 promoted the upregulation of CSF2 (GMCSF) and IL6 transcripts in several JAK2V617F+ cell lines (Figure 8A). These cell lines constitutively expressed ST2 on the surface (Supplemental Figure 8B). IL-33 did not stimulate the growth of MPN cell lines; however, it improved their survival (Figure 8B). Human BCR-ABL1+ CML cell lines expressed ST2 at various intensities, and they also upregulated the transcription of growth factors when stimulated with IL-33 (Supplemental Figure 8, C and D). However, IL-33 did not influence the proliferation or the survival of CML cell lines (Supplemental Figure 8E). These results suggest that JAK2V617F+ cells are more sensitive to the effects of IL-33/ST2 signaling compared with BCR-ABL1+ CML cells.
Figure 8. IL-33/ST2 signaling promotes MPN development.
(A) Indicated JAK2V617F+ cell lines were incubated for 24 hours ± IL-33, and expression levels were measured for the transcripts of the indicated genes. Mean expression levels of technical duplicates are represented as fold induction and have been normalized to conditions without IL-33. (B) The MPN cell lines SET-2 and HEL were cultured ± IL-33, and percentage of live cells (annexin V– DAPI–) was assessed at the indicated time points. Results show means ± SEM. (C) Relative median fluorescence intensity (MFI) of ST2 expression on blood CD34+ stem/progenitor cells from MPNs (PMF [n = 3], PV [n = 5], and after ET/PV myelofibrosis [n = 3]) and control (n = 5) donors. (D) Representative histograms showing ST2 expression on blood CD34+ stem/progenitor cells from the indicated donors. (E) Colony formation assay (CFA) from MACS-purified blood CD34+ stem/progenitor cells from controls and MPN patients cultured in standard CFC medium ± IL-33 for 2 weeks. Data are mean ± SEM and show fold changes in colony numbers after normalization to conditions without IL-33. Healthy (n = 5) and 10 MPN donors were used. (A) One representative of 2 independent experiments, and (B) pooled values from 3 individual experiments are shown. (C and D) Pooled data from 2 independent experiments are shown. (B) Standard Student’s t test, (C) Standard Student’s t test with Welch’s correction, and (D) Wilcoxon signed-rank test were used. *P < 0.05; **P < 0.01.
Primary human CD34+ hematopoietic stem/progenitor cells (HSPCs) express ST2 receptor and secrete cytokines and chemokines when stimulated with IL-33 (31). We detected increased ST2 expression on CD34+ stem/progenitor cells from BCR-ABL1–negative MPN patients (Figure 8, C and D). In agreement with our data from cell lines, GMCSF expression was significantly upregulated in circulating CD34+ stem/progenitor cells from CML patients and CD34+ HSPCs from controls following IL-33–mediated stimulation (Supplemental Figure 8F). Furthermore, IL-33 promoted colony formation of CD34+ MPN stem/progenitor cells but not of CD34+ control HSPCs (Figure 8E).
Collectively, these observations extend our in vivo data in SHIP-deficient mice and substantiate their relevance to human MPNs. Our findings also point toward differences as to how the IL-33/ST2 pathway may exert an effect on MPN progenitors — either via the intermediate of differentiated cells that are ST2+ in the mouse model or directly on ST2-expressing CD34+ stem/progenitor cells in MPN patients (Figure 9).
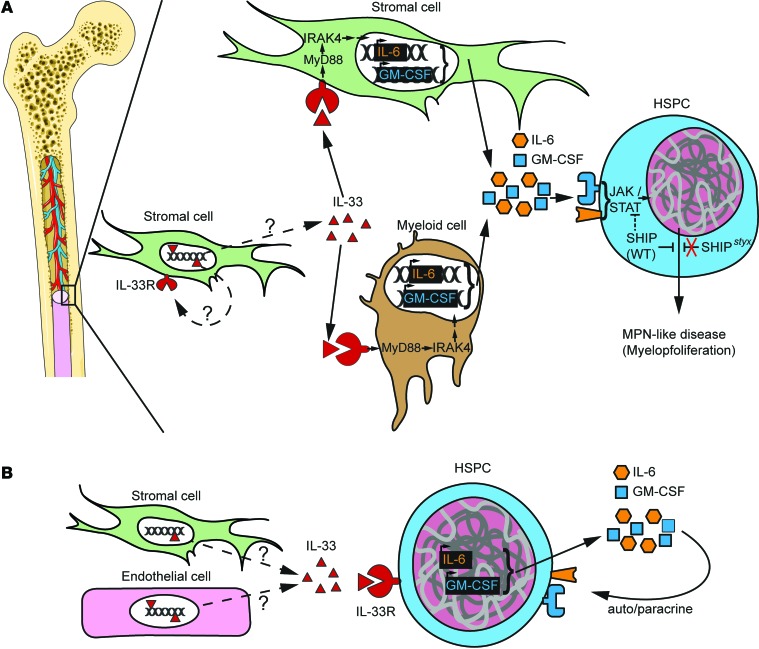
Figure 9. Models for the development of MPN in the BM microenvironment.
(A) Model for SHIP-deficient mice. Stromal cell–derived IL-33 is released under stress or steady-state conditions through a pathway that is yet to be determined. IL-33 engages its receptor ST2 on stromal cells or, preferentially, on a committed population of myeloid cells. Signal transduction via the ST2→MyD88→IRAK4 axis promotes the secretion of several cytokines and growth factors promoting myoproliferation — among others GM-CSF and IL-6 — that bind to their respective receptors and activate the JAK2/STAT5 pathway (49). SHIP is a negative regulator in this process, and impaired SHIP-mediated inhibition results in MPN-like disease. (B) IL-33 released by stromal cells in the human BM engages ST2 receptor on CD34+ HSPCs. Activation of cell signaling downstream of ST2 leads to the production of autocrine cytokines and growth factors that support proliferation.
Discussion
IL-33 has hitherto essentially been described as a nuclear alarmin that is released following necrotic cell death or under conditions of cellular stress. On the other hand, IL-33 undergoes caspase-mediated inactivation during apoptosis (reviewed in ref. 16). Inflammatory cues such as IFNγ and IL-4, as well as microbial products such as LPS, strongly induce expression of endogenous IL-33 (17, 32). Here we present evidence that IL-33 signaling can also be engaged in vivo to drive tumorigenesis and immunopathology under sterile, steady-state conditions in mice.
Although we can merely speculate about the initial source of IL-33 to induce MPN-like disease in styx mice, the BM stroma emerges as a valid local provider of this cytokine, since IL-33 is expressed in the niche. Exposure to fungus allergens leads to the ATP-dependent release of IL-33 in the airways of challenged mice (33). However, no mechanism has been proposed so far as to whether and how active IL-33 may be released or secreted from healthy, non-stressed cells in vivo. Nevertheless, low amounts of the active form of IL-33 can be detected in the serum of healthy human donors (34) and in 2 of the 8 naive WT mice we tested. Serum IL-33 can likely transit through the BM. Notably, St2–/– mice displayed higher levels of serum IL-33 than WT controls (122 ± 42 versus 3 ± 1 pg/ml, P < 0.01). This suggests that IL-33 is released and circulates even under steady-state conditions and that ST2 regulates its availability. Thus, it is conceivable that steady-state levels of IL-33 may trigger a self-amplifying inflammatory milieu favorable to tumor development in genetically susceptible hosts.
Upon disease progression in SHIP-deficient animals, infiltrating immune cells progressively cause tissue damage to the lung and intestine — organs that contain relatively high numbers of IL-33–expressing cells (Supplemental Figure 9; ref. 17). This immunopathology likely leads to rising concentrations of IL-33 in the serum, as detected in styx mutants. The evidence that altered hematopoiesis develops spontaneously in our model is still reconcilable with our initial hypothesis proposing that external stimuli may promote MPN development. IL-33 released during infection with pathogens (19, 20) may indeed further precipitate uncontrolled myeloproliferation. Also, opportunistic infections may be the cause of the accelerated disease observed in styx mice deficient in Tlr4, Unc93b1, or Il18 (Figure 1, A and B, Supplemental Figure 2A, and Figure 1C). Accordingly, the irradiation-induced increase in proinflammatory cytokines such as IL-6 and G-CSF, but also IL-33 (35, 36), likely accounts for the accelerated disease kinetics observed in chimeric mice reconstituted with styx BM compared with nonmanipulated styx mutants (Figures 1 and 3). Irradiation-induced systemic inflammation may also mask the relative contribution of radio-resistant cells to MPN-like disease in our model. This may explain why the styx→WT and styx→St2–/– sets of chimeras develop MPN-like disease with similar kinetics (Figure 3); however, our data show that BM stromal cells can engage ST2, for example to promote colony formation in vitro (Figure 4E). Increased availability of IL-33 in the BM may similarly support disease pathogenesis in BCR-ABL1–negative MPN patients.
Critically, we observed a different ST2 expression pattern in mice compared with humans. In the BM of styx and WT mice, ST2 expression was restricted to non-hematopoietic stromal and committed hematopoietic cells. ST2 protein was undetectable on murine HSCs and myeloid progenitors, and addition of IL-33 to these cells did not modulate their capacity to form colonies. However, human CD34+ stem/progenitor cells from controls and MPN patients were able to engage IL-33/ST2 signaling to produce growth factors and proinflammatory cytokines, an observation previously made by others (31). Also, a recent study reported that ST2 is upregulated on CD34+ stem/progenitor cells from CML patients (37). Therefore, it remains to be addressed whether these differences in ST2 expression patterns rely on species-dependent divergences and whether ST2 can be also engaged on human BM stromal cells. This is relevant because humoral factors secreted by BM stromal cells may also support MPN pathogenesis by impairing compound-mediated JAK2 inhibition (11).
Our data indicate an indirect effect of the IL-33/ST2 pathway for the development of MPN-like disease in the styx model, via the production of soluble factors. Several of the cytokines released by ST2+ BM cells have previously been shown to support dysregulated myeloproliferation in Inpp5d KO mice (23) and also in other murine models of MPN (38). These IL-33–induced cytokines likely also promoted the aberrant expansion of JAK2V617F+ hematopoietic cells in BM recipient mice. However, IL-33–induced cytokines appear to be redundant for MPN development. For instance, blockade of IL-6 signaling only partially corrects the aberrant hematopoiesis in SHIP-deficient (28) or transgenic BCR-ABL1 mice (4).
IL-33 has a dual function, both as an inhibitor of transcription when residing in the nucleus (39) or as a proinflammatory cytokine upon release (16). Our results suggest that in its extracellular function and through ST2 binding, IL-33 promotes dysregulated hematopoiesis in the styx and transgenic JAK2V617F models, as well as cytokine production in stimulated MPN cells. Interestingly, plasma levels of IL-33 are not altered in CML patients (37) and are even reduced in patients suffering from PV or ET (34). Although availability of secreted IL-33 in the niche has yet to be demonstrated, we clearly show that nuclear IL-33 is expressed to a greater extent in the BM of newly diagnosed BCR-ABL1–negative MPN patients compared with controls. Since MPN stem/progenitor cells display hypersensitivity to cytokines and growth factors, similarly to SHIP-deficient HSCs and myeloid progenitor cells (2, 23), it is conceivable that even low amounts of secreted IL-33 within the niche may suffice to initiate ST2-dependent inflammation that supports MPNs.
While the soluble form of ST2 appears to be a predictive marker for clinical stage and outcome in CML (37), no such data exist on the role for IL-33/ST2 signaling in the progression of BCR-ABL1–negative MPNs. In particular, its potential contribution to the progression to end-stage myelofibrosis is unclear. IL-33 has a known direct proangiogenic effect on ECs (40), and increased microvessel density has been associated with fibrosis in JAK2V617F mutation-bearing MPN patients (41). Furthermore, abnormal expression and activity of a number of proinflammatory cytokines are linked to myelofibrosis (10), and our results indicate that IL-33 promotes the secretion of various cytokines in the BM microenvironment. Therefore, it may be relevant in the future to address whether therapeutic blockade of the IL-33/ST2 axis may prevent myelofibrosis or halt its progression.
In summary, our data provide evidence for a role of IL-33/ST2 signaling for dysregulated hematopoiesis in mouse models of MPN. They also indicate that IL-33 expression is specifically increased in the BM of BCR-ABL1–negative MPN patients. Our results warrant further investigation on the contribution of the IL-33/ST2 axis to disease pathogenesis in MPN patients.
Methods
Patients.
PBMCs from CML or JAK2V617F-positive patients at diagnosis — or from donors who underwent peripheral blood stem and progenitor cell mobilization and collection for reasons other than hematological neoplasms — were obtained from the Department of Hematology of the University Hospitals of Bern and Zurich, Switzerland, and were enriched for CD34+ stem/progenitor cells by MACS. Samples from BM biopsies were retrieved from the archives of the Institute of Pathology, University of Bern, Switzerland; control samples were obtained from non-leukemic individuals showing minimal reactive changes. All CML patients were positive for the BCR-ABL1 transcript.
Animals.
All strains used were on a C57BL/6J background. C57BL/6J mice bred at The Scripps Research Institute (TSRI) were injected with ENU, as described (42). The Inpp5dstyx/styx (styx mice), Myd88poc, Irak4otiose, Tlr2languid, Tlr4lps3, TnfPanR1, and Unc93b13d strains are described at the Southwestern Medical Center Mutagenetix database (http://mutagenetix.utsouthwestern.edu). B6.129S7-Il1r1tm1Imx/J, B6.129P2-Il18tm1Aki/J, and B6.SJL-Ptprca Pep3b/BoyJ mice were obtained from The Jackson Laboratory. Il33–/– mice (43) were obtained through the RIKEN Center for Developmental Biology (Acc. No. CDB0631K; http://www.cdb.riken.jp/arg/mutant%20mice%20list.html). Il1rl1–/– (St2–/–) mice have been described (44). The styx mice were rederived to germ-free status by 2-cell embryo transfer and were maintained in flexible film isolators in the Clean Mouse Facility, University of Bern, Switzerland. BM cells from transgenic JAK2V617F (Scl-Cre FF1; ref. 45) mice were provided by Radek C. Skoda (University Hospital Basel, Basel, Switzerland). For survival analyses, data were collected during the entire course of the study and pooled for graphic representation. Unless indicated in the corresponding figure, animals used in this study were 6–12 weeks old and age/sex-matched.
Flow cytometry.
BM cells were resuspended and filtered through 70 μm cell strainers (Falcon Technologies BD) to obtain single-cell suspensions. Following rbc lysis, FcR blockade, and lineage depletion, HSCs and myeloid progenitors were stained as described previously (26), using the antibodies indicated in Supplemental Table 2. Live/dead cell discrimination was performed using DAPI (BioLegend) and annexin V (BioLegend). The gating strategy used to discriminate HSC and myeloid progenitor cell populations is shown in Supplemental Figure 3, A and B.
Blood-derived, MACS-purified CD34+ human stem/progenitor cells were carefully thawed in HBSS containing 10% human serum. After centrifugation, cells were resuspended in IMDM and analyzed for colony formation or ST2 surface expression. The gating strategy used to assess ST2 expression on primary human CD34+ stem/progenitor cells is shown in Supplemental Figure 10.
Colony formation assay.
Purified, lin– murine BM cells were cultured at a starting density of 2 × 103 to 5 × 103 for 7 days in MethoCult-based medium (StemCell Technologies Inc.) supplemented with 15% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 100 μM 2-mercaptoethanol, and 20% BIT (50 mg/ml BSA in IMDM, 1.44 U/ml rh-insulin, 250 ng/ml human holo transferrin). The following cytokines were always included: 50 ng/ml rm SCF, 10 ng/ml, 50 ng/ml rm Flt3-ligand, and 10 ng/ml rm IL-3. The following cytokines were added when specified: 10 ng/ml rh IL-6, 10 ng/ml, rm GM-CSF, and 100 ng/ml rm IL-33 (all from ProSpec). Colonies were counted using a DMIL inverted microscope (Leica Microsystems) mounted with an Intensilight C-HGFI unit (Nikon). Alternatively, 1 × 103 primary blood CD34+ stem/progenitor cells from MPN patients or healthy donors were plated in MethoCult H4435 Enriched medium (StemCell Technologies Inc.) with or without 100 ng/ml rh IL-33 (ProSpec). Colonies were counted after 14 days.
Coculture experiment.
Regarding preparation of BM stromal responders, BM stromal cell (BMSC) cultures were set up from epiphyses from 5 mice per group, as described previously (46). CD45– BMSCs were MACS-purified after 3 weeks of expansion, and ST2 expression was confirmed by flow cytometry (data not shown). Then, 5 × 104 purified BMSC were stimulated with 100 ng/ml rm IL-33 for 24 hours. In preparation of supernatant from BM hematopoietic responders, 5 × 104 FACS-purified CD11bint Ly6G– Ly6C– hematopoietic BM cells pooled from 5 mice per group were stimulated with our without 100 ng/ml rm IL-33. Supernatant was collected after 24 hours and stored frozen. Next, 5 × 105 lin– MACS-purified BM cells pooled from 6 mice per group were added to IL-33–stimulated BMSCs for coculture or supplemented with supernatant from IL-33–stimulated hematopoietic cells. After 48 hours of culture, 5 × 103 lin– cells were seeded for colony formation assay as described above.
CBC.
Blood was obtained through retro orbital bleeding of anesthetized mice and collected in Microvette (Sarstedt) tubes. CBC was assessed on a Vet abcTM Animal Blood Counter (Scil Animal Care Company GmbH).
Chimeric experiments.
Mice were irradiated twice with an individual dose of 650 cGy with 4 hours recovery time between. Mice were then adoptively transferred i.v. with 1 × 107 cells from whole BM of donor mice.
Scoring of Disease Activity Index (DAI).
DAI was determined twice every week using a score index approved by the local authorities and based on previous reports (47, 48). Score was determined according to specific criteria. Behavior scores were as follows: apathy (2.8); hiding, not active, aggressive upon touch (1.8); hiding, calm, active upon touch (0.5); and active, attentive (0). Breathing scores were as follows: impaired, heavy breathing (2.8); and normal breathing (0). Body conditioning scores were as follows: emaciated (1.8); under-conditioned (0.5); and well-conditioned (0). Exercise and posture scores were as follows: paralysis (2.8); bent posture, strong bristling of hairs, motoric disorder (1.8); uncoordinated upon touch, bent posture, mild bristling of hairs (0.5); and normal (0).
Histology and IHC.
Mouse and human tissues were fixed in 4% formaldehyde for 24 hours prior to dehydration and paraffin embedding. Sections were deparaffinized and stained with hematoxylin and eosin (H&E) or PAP under standard conditions. Antigen retrieval for IHC was performed with treatment in a 1 mM Tris/1 mM EDTA pH 9.0 solution for 18 minutes or in Bond Epitope Retrieval Solution 2 (Leica Biosystems) for 30 min at 95°C, for murine (IL-33) or human tissue (IL-33 and CD34), respectively. After an endogenous peroxidase block, primary goat anti-mouse IL-33, goat anti-human IL-33 (both from R&D Systems), and mouse anti-human CD34 (Dako) antibodies were used at a dilution of 1:100, 1:400, and 1:200, respectively. A secondary rabbit anti-goat antibody (Dako) or rabbit anti-rat antibody (Leica Biosystems) (1:100), followed by a tertiary horseradish peroxidase–linked (HRP-linked) anti-rabbit antibody (Dako or Leica Biosystems), was used undiluted to detect positive cells.
IHC of tissues from Il33–/– mice was performed to exclude unspecific staining of the murine IL-33 antibody used in this study. As another negative control for antibody specificity, we incubated the goat anti-mouse IL-33 antibody with rm IL-33 prior to the staining of tissue from Il33+/+ mice. We also stained tissue from Il33+/+ mice with a goat anti-rabbit isotype control antibody (Dako). Slides were quantified with Aperio Group LLC. Settings used for analysis were adjusted and controlled by a board certified pathologist in a blinded fashion.
Quantitative PCR assay.
Mouse primary BM cells were MACS-purified with a murine CD45 micro-bead assay (Miltenyi Biotec) according to the manufacturer’s protocol. RNA from human cell lines or primary MACS-purified CD34+ stem/progenitor cells from donors was purified using TRI-reagent (Sigma-Aldrich). RNA was transcribed into cDNA using M-MLV reverse transcriptase (Promega). FastStart SYBR Green Master (Roche Diagnostics) was used to detect the target genes GCSF, GMCSF, IL6, and GAPDH (QIAGEN). All reactions were run in a StepOnePlus Real-Time PCR System (Invitrogen). Expression levels of genes were normalized to GAPDH mRNA, and IL-33–stimulated versus unstimulated groups were compared applying the 2–ΔΔCT method.
Human MPN cell lines.
The human CML cell lines JURL-MK1, KU812, LAMA-84, and NALM-1 were provided by Patrick Ziegler (University Hospital Aachen, Aachen, Germany). The human JAK2V617F cells lines HEL 92.1.7 (HEL), SET-2, and UKE-1 were provided by Radek C. Skoda (University Hospital Basel, Basel, Switzerland).
Cytokine measurement.
MACS-purified primary cells were stimulated with 100 ng/ml rm IL-33 for 24 hours ex vivo. Cells were centrifuged for 5 minutes at 500g, and supernatant was removed. Cell culture supernatant and serum cytokines were analyzed by Multiplexing LASER Bead Technology (Eve Technologies).
Statistics.
Two-tailed standard Student’s t test with or without Welch’s correction and Wilcoxon signed-rank tests were used as indicated for single comparisons. For datasets where multiple comparisons were performed, one-way ANOVA was performed with Bonferroni post hoc test. For survival curves, log-rank (Mantel-Cox) test was used. For all statistical analyses, P values of less than 0.05 were considered significant.
Study approval.
Patients and donors gave written informed consent, and the analyses of samples were approved by the cantonal Ethics Committee from Bern (Ethics Committee Bern, Bern, Switzerland) and Zurich (Ethics Committee Zurich, Zurich, Switzerland).
All animal experiments were performed in accordance with institutional and federal regulations governing animal care and use ,and were approved by TSRI Institutional Animal Care and Use Committee (La Jolla, California, USA) and the Cantonal Veterinary Office of Bern (Bern, Switzerland).
Supplementary Material
Acknowledgments
We thank Andrew McKenzie, Padraic Fallon, Daniel Pinschewer, and Johann Wojta for providing us with the St2–/– and Il33–/– mice. Anne-Laure Huguenin, Daniela Korner, Olivia Jana Steinsiepe, Inti Zlobec, and the team of the Translational Research Unit gave excellent technical support. We thank Christoph Müller and Mario Noti for critical comments. This work was supported by grants from the Swiss National Science Foundation (310030_138188), the Bern University Research Foundation (to P. Krebs), the “Stiftung für klinisch-experimentelle Tumorforschung” (to P. Krebs and Y. Banz) by NIH grant 5P01AI070167 and Contract 5U19A100627 (to B. Beutler), a Boehringer-Ingelheim Fonds fellowship (to L.F. Mager), and the University of Zurich Clinical Research Priority Program (to A.P. Theocharides and M.G. Manz).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(7):2579–2591. doi:10.1172/JCI77347.
References
- 1.Wadleigh M, Tefferi A. Classification and diagnosis of myeloproliferative neoplasms according to the 2008 World Health Organization criteria. Int J Hematol. 2010;91(2):174–179. doi: 10.1007/s12185-010-0529-5. [DOI] [PubMed] [Google Scholar]
- 2.Jedidi A, et al. Selective reduction of JAK2V617F-dependent cell growth by siRNA/shRNA and its reversal by cytokines. Blood. 2009;114(9):1842–1851. doi: 10.1182/blood-2008-09-176875. [DOI] [PubMed] [Google Scholar]
- 3.Jiang X, Lopez A, Holyoake T, Eaves A, Eaves C. Autocrine production and action of IL-3 and granulocyte colony-stimulating factor in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 1999;96(22):12804–12809. doi: 10.1073/pnas.96.22.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynaud D, et al. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20(5):661–673. doi: 10.1016/j.ccr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallipoli P, et al. Autocrine TNF-alpha production supports CML stem and progenitor cell survival and enhances their proliferation. Blood. 2013;122(19):3335–3339. doi: 10.1182/blood-2013-02-485607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121(1):396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhagwat N, Levine RL, Koppikar P. Sensitivity and resistance of JAK2 inhibitors to myeloproliferative neoplasms. Int J Hematol. 2013;97(6):695–702. doi: 10.1007/s12185-013-1353-5. [DOI] [PubMed] [Google Scholar]
- 8.Skov V, et al. Molecular profiling of peripheral blood cells from patients with polycythemia vera and related neoplasms: identification of deregulated genes of significance for inflammation and immune surveillance. Leuk Res. 2012;36(11):1387–1392. doi: 10.1016/j.leukres.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Fleischman AG, et al. TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood. 2011;118(24):6392–6398. doi: 10.1182/blood-2011-04-348144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasselbalch HC. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev. 2013;24(2):133–145. doi: 10.1016/j.cytogfr.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Manshouri T, et al. Bone marrow stroma-secreted cytokines protect JAK2(V617F)-mutated cells from the effects of a JAK2 inhibitor. Cancer Res. 2011;71(11):3831–3840. doi: 10.1158/0008-5472.CAN-10-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristinsson SY, Bjorkholm M, Hultcrantz M, Derolf AR, Landgren O, Goldin LR. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. 2011;29(21):2897–2903. doi: 10.1200/JCO.2011.34.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer. 2009;100(5):822–828. doi: 10.1038/sj.bjc.6604935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Titmarsh GJ, McMullin MF, McShane CM, Clarke M, Engels EA, Anderson LA. Community-acquired infections and their association with myeloid malignancies. Cancer Epidemiol. 2014;38(1):56–61. doi: 10.1016/j.canep.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischman AG, et al. Exaggerated response to toll-like receptor agonist contributes to excessive TNF production in myeloproliferative neoplasm. Blood. 2013;122(21):4097. [Google Scholar]
- 16.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10(2):103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 17.Pichery M, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188(7):3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 18.Pastorelli L, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A. 2010;107(17):8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonilla WV, et al. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335(6071):984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 20.Byers DE, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013;123(9):3967–3982. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croker BA, et al. Inflammation and autoimmunity caused by a SHP1 mutation depend on IL-1, MyD88, and a microbial trigger. Proc Natl Acad Sci U S A. 2008;105(39):15028–15033. doi: 10.1073/pnas.0806619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivas MN, et al. MyD88 is critically involved in immune tolerance breakdown at environmental interfaces of Foxp3-deficient mice. J Clin Invest. 2012;122(5):1933–1947. doi: 10.1172/JCI40591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helgason CD, et al. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12(11):1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, et al. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13(7):786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JW, et al. Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science. 2002;295(5562):2094–2097. doi: 10.1126/science.1068438. [DOI] [PubMed] [Google Scholar]
- 26.Wilson A, et al. Dormant and self-renewing hematopoietic stem cells and their niches. Ann N Y Acad Sci. 2007;1106:64–75. doi: 10.1196/annals.1392.021. [DOI] [PubMed] [Google Scholar]
- 27.Pinho S, et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7):1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda K, et al. IL-6 blocks a discrete early step in lymphopoiesis. Blood. 2005;106(3):879–885. doi: 10.1182/blood-2005-02-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazen AL, Smith MJ, Desponts C, Winter O, Moser K, Kerr WG. SHIP is required for a functional hematopoietic stem cell niche. Blood. 2009;113(13):2924–2933. doi: 10.1182/blood-2008-02-138008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubovcakova L, et al. Differential effects of hydroxyurea and INC424 on mutant allele burden and myeloproliferative phenotype in a JAK2-V617F polycythemia vera mouse model. Blood. 2013;121(7):1188–1199. doi: 10.1182/blood-2012-03-415646. [DOI] [PubMed] [Google Scholar]
- 31.Allakhverdi Z, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123(2):472–478. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Meephansan J, Tsuda H, Komine M, Tominaga S, Ohtsuki M. Regulation of IL-33 expression by IFN-γ and tumor necrosis factor-α in normal human epidermal keratinocytes. J Invest Dermatol. 2012;132(11):2593–2600. doi: 10.1038/jid.2012.185. [DOI] [PubMed] [Google Scholar]
- 33.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186(7):4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangemi S, et al. Decreased plasma levels of IL-33 could contribute to the altered function of Th2 lymphocytes in patients with polycythemia vera and essential thrombocythemia. Cancer Invest. 2013;31(3):212–213. doi: 10.3109/07357907.2013.764566. [DOI] [PubMed] [Google Scholar]
- 35.Singh VK, Fatanmi OO, Singh PK, Whitnall MH. Role of radiation-induced granulocyte colony-stimulating factor in recovery from whole body gamma-irradiation. Cytokine. 2012;58(3):406–414. doi: 10.1016/j.cyto.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Ha CT, et al. Circulating interleukin-18 as a biomarker of total-body radiation exposure in mice, minipigs, and nonhuman primates (NHP). PLoS One. 2014;9(10):e109249. doi: 10.1371/journal.pone.0109249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levescot A, et al. BCR-ABL-induced deregulation of the IL-33/ST2 pathway in CD34(+) progenitors from chronic myeloid leukemia patients. Cancer Res. 2014;74(10):2669–2676. doi: 10.1158/0008-5472.CAN-13-2797. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, et al. Primitive interleukin 3 null hematopoietic cells transduced with BCR-ABL show accelerated loss after culture of factor-independence in vitro and leukemogenic activity in vivo. Blood. 2002;100(10):3731–3740. doi: 10.1182/blood-2002-05-1324. [DOI] [PubMed] [Google Scholar]
- 39.Ali S, et al. The dual function cytokine IL-33 interacts with the transcription factor NF-κB to dampen NF-κB-stimulated gene transcription. J Immunol. 2011;187(4):1609–1616. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]
- 40.Choi YS, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114(14):3117–3126. doi: 10.1182/blood-2009-02-203372. [DOI] [PubMed] [Google Scholar]
- 41.Medinger M, et al. Angiogenesis and vascular endothelial growth factor-/receptor expression in myeloproliferative neoplasms: correlation with clinical parameters and JAK2-V617F mutational status. Br J Haematol. 2009;146(2):150–157. doi: 10.1111/j.1365-2141.2009.07726.x. [DOI] [PubMed] [Google Scholar]
- 42.Hoebe K, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424(6950):743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 43.Oboki K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107(43):18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191(6):1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiedt R, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111(8):3931–3940. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 46.Cheng CC, et al. Isolation and characterization of novel murine epiphysis derived mesenchymal stem cells. PLoS One. 2012;7(4):e36085. doi: 10.1371/journal.pone.0036085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ullman-Cullere MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci. 1999;49(3):319–323. [PubMed] [Google Scholar]
- 48.Paster EV, Villines KA, Hickman DL. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med. 2009;59(3):234–241. [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao W, Hong H, Kawakami Y, Lowell CA, Kawakami T. Regulation of myeloproliferation and M2 macrophage programming in mice by Lyn/Hck, SHIP, and Stat5. J Clin Invest. 2008;118(3):924–934. doi: 10.1172/JCI34013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.