Abstract
Of the neurogenic zones in the adult brain, adult hippocampal neurogenesis attracts the most attention, because it is involved in higher cognitive function, most notably memory processes, and certain affective behaviors. Adult hippocampal neurogenesis is also found in humans at a considerable level and appears to contribute significantly to hippocampal plasticity across the life span, because it is regulated by activity. Adult hippocampal neurogenesis generates new excitatory granule cells in the dentate gyrus, whose axons form the mossy fiber tract that links the dentate gyrus to CA3. It originates from a population of radial glia-like precursor cells (type 1 cells) that have astrocytic properties, express markers of neural stem cells and divide rarely. They give rise to intermediate progenitor cells with first glial (type 2a) and then neuronal (type 2b) phenotype. Through a migratory neuroblast-like stage (type 3), the newborn, lineage-committed cells exit the cell cycle and enter a maturation stage, during which they extend their dendrites into a the molecular layer and their axon to CA3. They go through a period of several weeks, during which they show increased synaptic plasticity, before finally becoming indistinguishable from the older granule cells.
Adult hippocampal neurogenesis plays a key role in certain cognitive functions (e.g., memory). It is a complex, multistep process that generates new excitatory granule cells in the dentate gyrus.
Because it has turned out that adult neurogenesis not only exists in the human hippocampus but even seems to be restricted to it (see Spalding et al. 2013; Bergmann et al. 2015), public and scientific attention to the phenomenon is soaring. In PubMed, search results for “adult neurogenesis” and “hippocampus” outnumber those for “adult neurogenesis” and “olfactory bulb” or “subventricular” by ∼3:1. This is no reason to neglect research on adult neurogenesis in the olfactory system, which is a necessary part of any holistic view on adult neurogenesis, but reason enough to ask for the motifs behind this interest. The answer, presumably, is “function.” Adult hippocampal neurogenesis adds particular functionality to the mammalian hippocampus and presumably is involved in cognitive functions that we consider to be essential for humans. There is a price to pay for this type of plasticity. Adult neurogenesis is a complex multistep process, not a simple event. This review deals with the description of this process and the restriction points at which regulation occurs.
Adult neurogenesis is brain development recapitulated in the adult and comprises a series of sequential developmental events that are all necessary for the generation of new neurons. In the original publications on adult neurogenesis, the precursor cell population, from which neurogenesis originates, could be identified only through the detection of their proliferative activity and the absence of morphological characteristics of mature neurons and later neuron-specific antigens, such as NeuN or calbindin (Altman and Das 1965; Kaplan and Hinds 1977; Cameron et al. 1993; Kuhn et al. 1996). The new neurons, in contrast, were identified by the presence of mature neuronal markers in cells that had been birthmarked with the thymidine or BrdU method (see Kuhn et al. 2015) a couple of weeks earlier. The expression of polysialilated neural-cell-adhesion molecule (PSA-NCAM) with neurogenesis has been noted early but could not be clearly linked to either proliferation or the mature stage (Seki and Arai 1993a,b). PSA-NCAM expression was the first indication of the developmental events that take place, filling the gaps between the start and endpoint of development. Today, we have quite detailed knowledge about the course of neuronal development in the adult hippocampus and, although many detailed questions are open, a clear overall picture has emerged (Kempermann et al. 2004; Abrous et al. 2005; Ming and Song 2005; Lledo et al. 2006). We often even use doublecortin (DCX), which shows a complete overlap in expression with PSA-NCAM in the hippocampus, as surrogate markers for adult neurogenesis. This is sometimes questionable because the process is not identical to the end result, the existence of mature new neurons, but it is also telling. A plasticity marker is widely considered as representative of the whole process and its result.
Although we often simply talk of neurogenesis in the hippocampus, precisely, neurogenesis occurs only in the dentate gyrus, not in other subregions; and, in an older nomenclature, the dentate gyrus is not even part of the hippocampus proper (but the “hippocampal formation”). Although there are justifications to exclude the dentate gyrus from the hippocampus, we believe that, from any functional perspective, this distinction is awkward. Arguably, the contribution of the dentate gyrus and the new neurons within it is critically important to overall hippocampal function. As experiments suggest, one can do quite well without adult neurogenesis, but certain advanced features, which might explain the “evolutionary success” of the mammalian dentate gyrus, depend on the new neurons (see Amrein 2015; Kempermann 2015). The vote has, anyway, long been made by the scientific audience. We talk about adult hippocampal neurogenesis, when we mean neurogenesis in the adult dentate gyrus.
Adult hippocampal neurogenesis generates only one type of neuron: granule cells in the dentate gyrus. To date, there is no conclusive evidence that other neuronal cell types could be generated under physiological conditions, although some as-yet unconfirmed claims have been made (Rietze et al. 2000; Liu et al. 2003). Granule cells are the excitatory principal neurons of the dentate gyrus. They receive input from the entorhinal cortex and send their axonal projection along the mossy fiber tract to area CA3, where they terminate in large synapse- and interneuron-rich structures, the so-called “boutons.” They provide excitatory input to the pyramidal cells of CA3. They fire very sparsely and their activity is modulated by a large number of interneurons in the dentate gyrus and hilus area. The precursor cells, from which adult neurogenesis originates, reside in a narrow band of tissue between the granule cell layer and the hilus, the so-called subgranular zone (SGZ). The term was coined by the discoverer of adult hippocampal neurogenesis, Joseph Altman in 1975. The original description of adult neurogenesis in the rodent brain was published in 1965 by Joseph Altman and his colleague Gopal Das (Altman and Das 1965).
The SGZ contains the microenvironment that is permissive for neuronal development to occur. Analogous to other stem cell systems in the body, this microenvironment is called the neurogenic “niche.” The niche comprises the precursor cells themselves, their immediate progeny and immature neurons, other glial cells and endothelia, very likely immune cells, microglia, and macrophages, and an extracellular matrix. According to one study, the niche is surrounded by a common basal membrane (Mercier et al. 2002). Because of the prominent role that the vasculature appears to play in this context, the neurogenic niche has also been called the “vascular niche” (Palmer et al. 2000).
The type 1 precursor cells, from which adult neurogenesis originates, have endfeet on the vasculature in the SGZ (Filippov et al. 2003), vascular endothelial growth factor (VEGF) is a potent regulator of adult neurogenesis (Jin et al. 2002; Schänzer et al. 2004), and a complex relationship exists between endothelial cells and hippocampal precursor cells (Wurmser et al. 2004). The niche provides a unique milieu consisting of extracellular matrix, short- and long-range humoral factors, and cell-to-cell contacts, which allow neuronal development to occur in a controlled fashion (“neurogenic permissiveness”). Local astrocytes play a key role in promoting neurogenesis. In vivo, the developing cells show a close spatial relationship with astrocytes (Shapiro et al. 2005; Plümpe et al. 2006). Ex vivo, astrocytes and astrocyte-derived factors were potent inducers of neurogenesis from hippocampal precursor cells (Song et al. 2002; Barkho et al. 2006).
The SGZ is also special in that it receives synaptic input from various other brain regions: dopaminergic fibers from the ventral tegmental area, serotonergic projections from the raphe nuclei, acetylcholinergic input from the septum, and γ-aminobutyric acid (GABA)ergic connections from local interneurons. In addition, there are commissural fibers from the contralateral side. Manipulations of all the different neurotransmitter and input systems, for example, by lesioning studies to the input structures or pharmacological intervention, have revealed a regulatory effect on adult neurogenesis, although the level of resolution is still too low to identify the relative specific contributions of the individual systems to the control of adult neurogenesis (Bengzon et al. 1997; Cooper-Kuhn et al. 2004; Dominguez-Escriba et al. 2006) and to understand how the variety of stimuli is integrated. Nevertheless, the role of interneurons is critical in more than one regard. First, ambient and synaptic GABA drives neuronal development (Ge et al. 2007a), but, at a later stage, the balance between inhibition and excitation also determines that the new neurons preferentially respond to incoming stimuli, biasing activity toward the new neurons (Marin-Burgin et al. 2012).
DISTINCT STEPS OF NEURONAL DEVELOPMENT
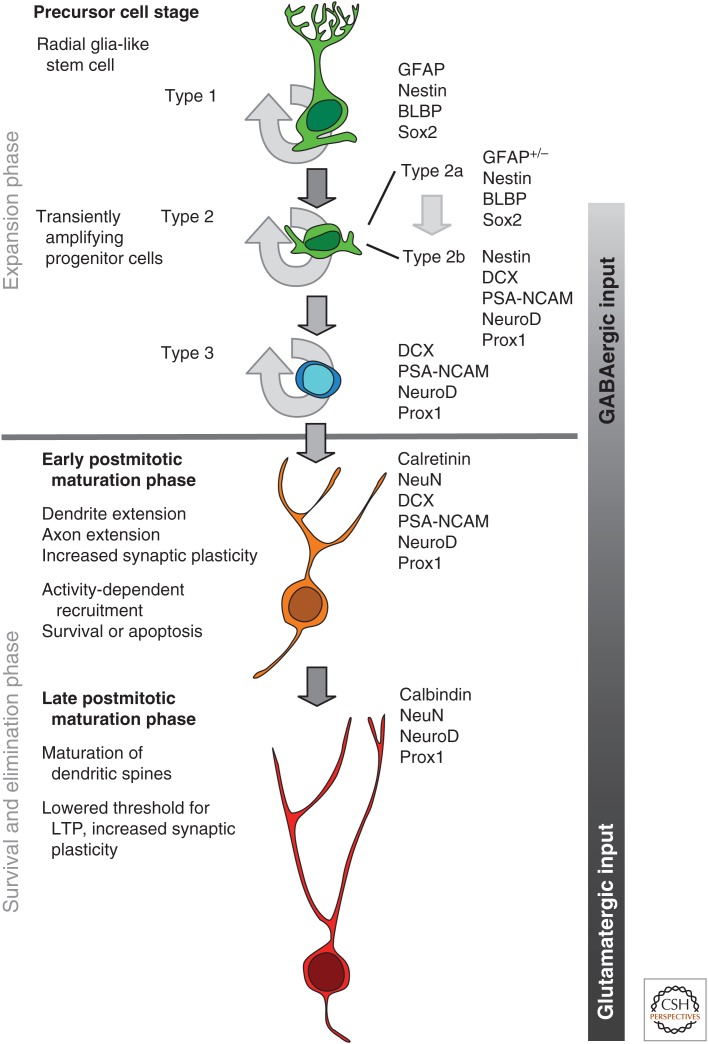
Adult neurogenesis can be divided into four phases: a precursor cell phase, an early survival phase, a postmitotic maturation phase, and a late survival phase. Based on cell morphology and a set of marker proteins, six distinct milestones can be identified, which to date still somewhat overemphasize the precursor cell stages of adult neurogenesis (Fig. 1) (Kempermann et al. 2004; Steiner et al. 2006). From a radial glia-like precursor cell, adult neurogenesis progresses over three identifiable progenitor stages associated with high proliferative activity to a postmitotic maturation phase and, finally, the existence of a new granule cell (Brandt et al. 2003; Filippov et al. 2003; Fukuda et al. 2003; Encinas et al. 2006; Steiner et al. 2006). Although on the precursor cell stage and early after cell-cycle exit, large changes in cell numbers occur and the effects of development become more qualitative at later times.
Figure 1.
Developmental stages in the course of adult hippocampal neurogenesis (see text for details). GFAP, Glial fibrillary acidic protein; BLBP, brain lipid-binding protein; DCX, doublecortin; PSA-NCAM, polysialilated neural-cell-adhesion molecule; LTP, long-term potentiation.
The precursor cell phase serves the expansion of the pool of cells that might differentiate into neurons. The early survival phase marks the exit from the cell cycle. Most newborn cells are eliminated within days after they are born. The postmitotic maturation phase is associated with the establishment of functional connections, the growth of axon and dendrites, and synaptogenesis. The late survival phase represents a period of fine-tuning. It has been estimated that the entire period of adult neurogenesis takes ∼ 7 wk. Characteristic electrophysiological patterns allow the assignment of functional states to the morphologically distinguishable steps of development.
One central question in research on adult hippocampal neurogenesis is how far it is similar to or distinct from embryonic and early postnatal neurogenesis in the dentate gyrus. The dentate gyrus develops in three distinctive waves of development, of which adult neurogenesis is the last (Altman and Bayer 1990a,b). The bulk of dentate gyrus neurons is produced at around P7. From a functional perspective, Laplagne et al. (2006) have argued that adult-generated neurons behave highly similar to those produced during the neonatal period, suggesting a homogenous population. On the other hand, quality and quantity of extrinsic stimuli and memory contents that pass the dentate gyrus will be dramatically different between postnatal and adult periods. Also, the speed of maturation might differ (Overstreet-Wadiche et al. 2006a), although with respect to the influence of extrinsic stimuli (here, seizures) on differentiation speed, the data are not consistent (Jakubs et al. 2006; Overstreet-Wadiche et al. 2006b; Plümpe et al. 2006).
THE PRECURSOR CELL PHASE
A number of morphologically identifiable “types” of precursor cells are involved in the course of adult hippocampal neurogenesis. Such cell types do not actually constitute distinct populations of cells but rather reflect milestones of a developmental process.
Adult hippocampal neurogenesis originates from a population of precursor cells with glial properties. A subset of these shows morphological and antigenic characteristics of radial glia. Their cell body is found in the SGZ and the process extends into the molecular layer. Not all radial elements show the same marker expression and some markers for radial glia during embryonic development are absent. The astrocytic nature of hippocampal precursor cells was first shown by Seri, Alvarez-Buylla, and colleagues (2001), when they suppressed cell division by application of a cytostatic drug and found that the first cells that reappeared were proliferative astrocyte-like cells with radial morphology. The second line of evidence came from experiments in which the receptor for an avian virus was expressed under the promoter of glial fibrillary acidic protein (GFAP) or nestin, so that astrocyte-like or nestin-expressing cells could specifically be infected by an otherwise inert virus. Transduced cells generated new neurons in vivo, demonstrating the developmental potential in vivo (Seri et al. 2001, 2004). The study related to similar experiments in the subventricular zone (SVZ)/olfactory bulb (OB) system (Doetsch et al. 1999a,b; Laywell et al. 2000).
Ex vivo, hippocampal precursor cells were first isolated by Ray et al. (1993) from the embryonic brain and by Palmer et al. (1995) from the adult rat brain. In culture, the precursor cells show signs of stemness (self-renewal and multipotency) (Palmer et al. 1997). To which degree these cells are true stem cells, in the sense that their capacity for self-renewal is “unlimited,” has been disputed by others (Seaberg and van der Kooy 2002; Bull and Bartlett 2005), but methodological and strain differences between the studies prevented closing the case. After careful microdissection of dentate gyrus tissue and by the use of an enrichment procedure, it was found that the murine dentate gyrus in fact contained “stem cells” in the stricter sense of the definition (Babu et al. 2007). A similar discussion arose in vivo, in which two studies asked whether the radial glia-like type 1 cells are capable of both asymmetric and symmetric divisions. Encinas and colleagues presented a model in which the potential of the precursor cells is fixed and the precursor cell population becomes exhausted with advancing age (Encinas et al. 2011). This finding was contrasted by a study by Bonaguidi et al. (2012), which discovered that the range of possible behaviors is actually much larger at the level of individual cells. Both studies might be correct, but show different aspects of the same issue. If adequately stimulated, the precursor cells might switch their program and allow the long-term maintenance of the precursor cell pool, which is lost in the case of inactivity. This idea is plausible in the context of other aspects of the activity-dependent regulation of adult neurogenesis but remains to be tested (Kempermann 2011a). Precursor cells in the adult hippocampus, however, are heterogeneous in their properties even at the apparent “stages” that can be more or less readily identified (Bonaguidi et al. 2012).
With that caveat in mind, the radial glia-like type 1 cells of the hippocampus give rise to intermediate progenitor cells, type 2 cells. These show a high proliferative activity. A subset of these cells still expresses glial markers but lack the characteristic morphology of radial cells (type 2a). On the level of type 2 cells that, together with type 1 cells, express intermediate filament nestin, first indications of neuronal lineage choice appear. These markers comprise, among others, transcription factors NeuroD1 and Prox1. This cellular phenotype has been called a type 2b cell (Steiner et al. 2006). Of these, Prox1 is specific to granule cell development. Manipulation of Prox1 abolishes adult neurogenesis at this stage (Karalay et al. 2011). Type 2 cells are also characterized by their expression of Eomes (Tbr2), a transcription factor that, during embryonic cortical development, identifies the basal progenitor cells, which maintain self-renewing properties and can differentiate into neurons (Hodge et al. 2008). Tbr2 appears to suppress Sox2 and is critical for the transition from stem cells to intermediate progenitor cells (Hodge et al. 2012b).
A point-by-point comparison between adult neurogenesis and fetal and early postnatal neurogenesis in the dentate gyrus is still lacking, but many transcription factors involved in embryonic cortical and hippocampal development are also involved in adult hippocampal neurogenesis (Li and Pleasure 2005; Hodge et al. 2012a). Insight into the transcriptional control of the initiation of neuronal differentiation is scarce. From the available data, however, it is obvious that if a fate choice decision is made at all, it must occur on the level of the type 2a cells. All later cells express NeuroD1 and Prox1 and there is no overlap between NeuroD1 and Prox1 and astrocytic markers at any time point. Tailless (Tlx) is a key candidate for a transcription factor involved in controlling the transition between glial and neuronal phenotypes of the precursor cells (Shi et al. 2004), and so are Prox1 and NeuroD1 themselves (Liu et al. 2000; Gao et al. 2009; Karalay et al. 2011). Beckervordersandforth et al. (2015) covers the transcriptional control of hippocampal neurogenesis in greater detail.
On the level of type 2 cells, the developing cells also receive first synaptic input, which is GABAergic (Tozuka et al. 2005; Wang et al. 2005). A more recent study, however, suggests that type 1 cells also express GABAA receptors throughout and AMPA receptors only in their processes with no ionotropic glutamate receptors (Renzel et al. 2013). Although type 1 cells can respond to extrinsic stimuli by increasing cell proliferation (Huttmann et al. 2003; Kunze et al. 2006; Weber et al. 2013), the burden of expansion lies on the type 2 cells. The radial cells represent the largely quiescent compartment, and the control of quiescence is also under the control of GABA that comes from the local parvalbumin-expressing interneurons (basket cells) (Song et al. 2012; Moss and Toni 2013). In any case, during their development, the new cells are first responsive to ambient GABA, and more respond to synaptic excitatory GABAergic input.
Type 2 cells respond to physiological stimuli, such as voluntary wheel running (Kronenberg et al. 2003), or pharmacological stimulation via serotonin-dependent mechanisms (Encinas et al. 2006). Again, it seems to be GABA that sets the pace for this regulation (Ge et al. 2007a).
Among the neuronal lineage markers first appearing at the type 2b stage is DCX. DCX is expressed at the proliferative stage, even after nestin has been down-regulated (type 3 cells). Normally, type 3 cells show only little proliferative activity. Under pathological conditions, however, such as experimental seizures, they can show a disproportional increase in cell division (Jessberger et al. 2005). DCX expression extends from a proliferation stage, through cell-cycle exit, to a period of postmitotic maturation that lasts ∼2 to 3 wk (Brandt et al. 2003; Rao and Shetty 2004; Couillard-Despres et al. 2005; Plümpe et al. 2006). DCX shows an almost complete overlap with PSA-NCAM and is a widely used surrogate marker for adult neurogenesis. Strikingly, despite its prominent expression, DCX does not seem to be required for normal neuronal development in the adult hippocampus (Merz and Lie 2013).
THE EARLY SURVIVAL PHASE
Very early after cell-cycle exit, the new neurons express postmitotic markers, such as NeuN (RbFox3), and the transient marker calretinin (Brandt et al. 2003). Because type 3 cells are still proliferative, NeuN can be found in some cells as early as 1 d after the injection of the proliferation marker. The protein is a splice factor of unknown specific function in this context. The number of NeuN-positive new neurons is highest at very early time points and decreases dramatically within a few days. This elimination process is apoptotic (Biebl et al. 2000; Kuhn et al. 2005). Thus, the majority of cells is eliminated well before they have made functional connections in the target area in CA3 or received correct dendritic input from the entorhinal cortex in the molecular layer of the dentate gyrus. Besides BDNF signaling as the main suspect and the contribution of several neurotransmitter systems (Tashiro et al. 2006), a number of other “survival factors” have been identified (e.g., among others, p63 [Cancino et al. 2013], Hspb8 [Ramírez-Rodríguez et al. 2013], and NF-κB [Imielski et al. 2012]), so that the overall mechanistic picture is far from clear.
The initiation of dendrite development after the time point of cell-cycle exit appears to be highly variable. The time course of dendritic development itself, in contrast, seems to follow a rather fixed temporal course. Within days after cell-cycle exit, the new cells send their axon to target area CA3, where they form appropriate synapses (Sun et al. 2013). Accordingly, this phase is associated with the expression of collapsing-response mediator protein (Crmp, also known as TOAD-64 or TUC-4), a molecule involved in axon path finding. The newborn neurons also express the embryonic tau isoform, which is otherwise not present in the adult brain (Bullmann et al. 2010). The axons of the new neurons are part of the mossy fiber tract, whose high level of plasticity has been noted early on but had not been brought into connection with adult neurogenesis. Today, we know that the axonal plasticity that characterizes the mossy fiber tract to a large degree depends on the new neurons (Römer et al. 2011).
The main synaptic input to the new cells is still GABAergic at this stage and GABA remains excitatory. GABA switches to its inhibitory function only, when sufficient glutamatergic contact has been made and, presumably, when the cells have begun to develop their own glutamatergic neurotransmitter phenotype (Tozuka et al. 2005). GABA action itself drives neuronal maturation in these cells and steers the synaptic integration (Ge et al. 2006).
Quantitatively, most of the regulation occurs at this stage of neuronal development, not in the expansion phase as it is often assumed (Kempermann et al. 2006). The reason is that precursor cell proliferation generates a vast surplus of new neurons, and that only a very small proportion survives for long periods of time (Kempermann et al. 2003). It seems that cells that have survived the first 2 wk will be stably and persistently integrated into the network of the dentate gyrus for a very long time. After this time point, only very small changes in cell number occur. One consequence of this observation is that adult neurogenesis, lifelong, contributes to growth of the dentate gyrus and does not replace older cells (Crespo et al. 1986), although this growth has not been proven at later life stages, when the levels of adult neurogenesis are very low. For the first year in the life of a rodent, growth has been shown in several studies (Altman and Das 1965; Bayer et al. 1982; Boss et al. 1985), but a modern stereological account is still lacking. A study based on genetic lineage tracing suggested that, in mice, ∼30% of the granule cells are generated after birth and during adulthood (Ninkovic et al. 2007). This number is close to the estimated turnover fraction in the human hippocampus (Spalding et al. 2013).
Along similar lines of reasoning, it seems that stimuli that control the expansion phase tend to be rather nonspecific (e.g., the pansynaptic activation in seizures, physical activity), whereas stimuli that are more specific to the hippocampus in that they reflect hippocampus-dependent function affect the survival phases. On a quantitative level, this has been shown only for the early postmitotic period. Exposure to the complexity of an enriched environment or, at least in some studies, to learning stimuli of hippocampus-dependent learning tasks increase survival at this stage (Gould et al. 1999; Dobrossy et al. 2003). Presumably, similar effects are found at later stages as well but so far have not been measurable with the available methods. (For more details on the mechanisms underlying cell-cycle exit, migration, and early maturation, please refer to Toni and Schinder 2015.)
POSTMITOTIC MATURATION PHASE
Serendipitously, it was found that the maturing cells up-regulate promoter activity of pro-opiomelanocortin (POMC), although the protein is not detectable in these cells. A transgenic mouse line expressing green fluorescent protein (GFP) under the POMC promoter has become a useful tool to study the electrophysiology of the immature neurons (Overstreet et al. 2004). At this stage, depolarizing GABA is required to allow the formation of glutamatergic synapses (Chancey et al. 2013), comparable to the situation in the developing cortex (Wang and Kriegstein 2008). The exact timing of the maturation is dependent on the activity in local circuits, further supporting the idea that adult hippocampal neurogenesis is controlled by activity at numerous stages of neuronal development (Piatti et al. 2011).
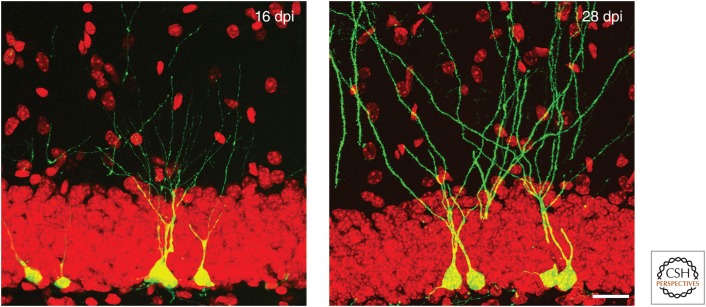
Details of spine development have largely been investigated by transducing a proliferating cell with a GFP-expressing retrovirus (Fig. 2) (Zhao et al. 2006). From these experiments, we know that axon elongation precedes spine formation on the dendrites and both are orchestrated in a precise and complex way (Sun et al. 2013). Although axonal contact to CA3 is made ∼10 d after labeling the proliferative cells, the first spines appear almost a week later. To connect to the target cells in CA3, the axons of the new neurons enter a competition with existing synapses in the mossy fiber boutons (Toni et al. 2008).
Figure 2.
Dendrite development of newborn neurons in the dentate gyrus. Proliferating precursor cells in the subgranular zone (SGZ) were labeled with a green fluorescent protein (GFP)-expressing retrovirus and analyzed at later time points. Here, GFP-positive new granule cells can be seen at ∼2 wk (16 d after injection, left) and 4 wk (28 d after injection, right). During the early postmitotic maturation phase, the cells develop the full morphology of hippocampal granule cells. It is noteworthy that the cells might show a slightly different pace of maturation. After 4 wk, many cells have extended its dendritic tree far into the molecular layer. First dendritic spines can be seen on the dendrites (see Zhao et al. 2006, for details of dendritic development in adult hippocampal neurogenesis). Scale bar, 15 µm. (The figure is contributed by Chunmei Zhao, Salk Institute.)
Functional maturation of the new neurons has now been characterized to a considerable degree (van Praag et al. 2002; Ambrogini et al. 2004; Schmidt-Hieber et al. 2004; Esposito et al. 2005; Couillard-Despres et al. 2006; Marin-Burgin et al. 2012). The cells progress from a state with high input resistance to the normal membrane properties of mature granule cells. (For more details on the functional maturation of the new neurons, please refer to Toni and Schinder 2015 and Song et al. 2015.)
LATE MATURATION PHASE
We presently know the least about the adaptive changes that occur late in neuronal development of adult neurogenesis. The period of calretinin expression lasts only ∼3 to 4 wk, roughly consistent with the temporal pattern of dendritic maturation. Presumably, after full structural integration into the existing network, the new cells switch their calcium-binding protein from calretinin to calbindin (Brandt et al. 2003). Still, it takes several more weeks until the new cells have become electrophysiologically indistinguishable from their older neighbors (van Praag et al. 2002; Ambrogini et al. 2004). Once glutamatergic synaptic connections have been made, the new neurons go through a phase of increased synaptic plasticity. The threshold to induce long-term potentiation (LTP) in the immature neurons is lower than in mature granule cells (Wang et al. 2000; Schmidt-Hieber et al. 2004). In fact, the only LTP measurable from the dentate gyrus under normal (i.e., inhibited) conditions originates from the newborn neurons, which are not yet inhibited by the local interneurons (Saxe et al. 2006; Garthe et al. 2009). These particular properties bias the input toward the new neurons (Marin-Burgin et al. 2012).
This critical period lasts from ∼1 to 1.5 mo after the cells were generated (Ge et al. 2007b). Some theories about the potential function of the new granule cells build on this fact by arguing that the altered plastic properties help the dentate gyrus to encode temporal information into memories to be stored (Aimone et al. 2006). Alternatively, the increased plasticity might serve the purpose of facilitating preferential integration of the new cells to achieve long-term changes in the network (Wiskott et al. 2006). Possibly, both ideas are correct, and a specific transient function prepares the ground for an equally specific long-term function. Presumably, important regulatory events take place at this stage, but they will be effective more on a qualitative level than on a quantitative one.
CONTROL OF NEURONAL DEVELOPMENT
The inherent mechanisms that constitute the process of neuronal development have to be distinguished from regulatory events that act on these mechanisms. Transcriptional control of adult neurogenesis represents the backbone of neuronal development. Regulatory events do not change this backbone but modulate it and rely on its maintained integrity. Transcriptional control thereby represents the shared target of regulation. These mechanisms are described and discussed in Beckervordersandforth et al. (2015). In the following paragraph, the attempt is made to tie these distinct molecular mechanisms to the identifiable stages of development.
On the level of the precursor cells, basic helix–loop–helix factor Sox2 characterizes the stem-like cells with glial properties (D’Amour and Gage 2003; Steiner et al. 2006). Overlap between Sox2 and early neuronal markers is minimal. However, Sox2 is also found in S100β-positive astrocytes without precursor cell function. Sox2 expression is tightly regulated and critical for the balance between proliferation and differentiation (Julian et al. 2013).
The transition between glial and neuronal phenotypes might be controlled by Tlx (Shi et al. 2004) and Ascl1. The earliest known neuronal factor is NeuroD1, which is recognized by a binding motif in the promoter region of the Dcx gene (Steiner et al. 2006). Parallel to NeuroD1, Prox1 is found. Prox1 is highly specific to granule cells (Pleasure et al. 2000).
This set of transcription factors is different from the subventricular system, in which Pax6, Dlx, and Olig2 play prominent roles. Expression of Pax6 has been noted in the dentate gyrus as well (Nacher et al. 2005), but its function is not clear yet. Olig2 is expressed in the dentate gyrus but in cells outside the lineage, which leads to granule cell development. It is, thus, assumed that new oligodendrocytes in the adult dentate gyrus, which are very rare anyway (Kempermann et al. 2003; Steiner et al. 2004), originate from a distinct pool of precursor cells that are characterized by their expression of proteoglycan NG2.
REGULATION AND FUNCTION OF ADULT HIPPOCAMPAL NEUROGENESIS
Although there is no consistent use of the terminology, “control” and “regulation” of a biological process are not identical (Kempermann 2011b). Regulation means those processes that act on the basic mechanisms that control neurogenesis. Regulation thus encompasses processes on many conceptual levels, from behavioral down to molecular. Quantitatively, regulation of adult hippocampal neurogenesis mostly occurs on the level of survival of the newborn cells. Between 30 inbred strains of mice, the genetically determined level of survival explained 85% of the variance found in net neurogenesis, whereas cell proliferation explained only 19% (Kempermann et al. 2006). On the other hand, numerous studies reported examples of factors that influence cell proliferation. The current hypothesis is that this broad sensitivity of precursor cell proliferation is nonspecific, whereas survival-promoting effects depend on specifically hippocampal functional stimuli. Lucassen et al. (2015), Kuhn (2015), and Song et al. (2015) expand on this idea.
The key point is, as already alluded to at various points during this review, that adult hippocampal neurogenesis is an activity-dependent process, during which macroscopic behavior of the individual is translated into changes at the systems and network level, which in turn affects local circuitry and humoral and other signaling systems that affect the control of adult neurogenesis. The complexity of this cellular plasticity is amazing and hardly understood to date. The consequential insight, however, is inevitable. Functions of the new neurons, as far as they result in any meaningful changes in behavior or “activity,” cannot be separated from regulation and vice versa. The link between the two lies at the heart of adult neurogenesis and is the very essence of “plasticity.”
SUMMARY
Adult hippocampal neurogenesis is a multistep process that originates from a sequence of proliferative precursor cells and leads to the existence of a new granule cell in the dentate gyrus. An expansion phase on the level of the precursor cells, during which proliferation is regulated by many nonspecific stimuli, gives way to a postmitotic maturation phase, during which only a subset of the newly generated cells survive. On the precursor cell level, the cascade originates in a radial glia-like type 1 cell, presumably the highest ranking stem cell in this system. It gives rise to highly proliferative type 2 cells, which can be divided into a more glial-like (type 2a) and a neuronally determined phase (type 2b). Finally, a proliferative late precursor cell, type 3, exists that marks the exit from the cell cycle. The selective postmitotic survival is dependent on specific, hippocampus-dependent stimuli and accounts for the greatest part of the neurogenic regulation. Morphological maturation finds its most visible expression in the extension of the dendrites and the emergence of dendritic spines. GABAergic input, first ambient, later synaptic, promotes neuronal maturation until regular glutamatergic input from the entorhinal cortex sets in. In a brief postmitotic interval, during which the new cells express calcium buffering protein calretinin, the new neurons also extend their axon to area CA3. This phase of early synaptic integration is also characterized by increased synaptic plasticity, presumably facilitating the survival-promoting effects of functional integration. At present, little is known about the details of neuronal maturation, but it seems that after a period of ∼7 wk, the new neurons become indistinguishable from their older neighbors. A number of transcription factors have been identified that can be linked to particular stages of neuronal development in the adult hippocampus, for example, granule-cell-specific factor Prox1 that is expressed very early on the level of type 2 progenitor cells and remains expressed in mature granule cells.
Footnotes
Editors: Fred H. Gage, Gerd Kempermann, and Hongjun Song
Additional Perspectives on Neurogenesis available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Abrous DN, Koehl M, Le Moal M. 2005. Adult neurogenesis: From precursors to network and physiology. Physiol Rev 85: 523–569. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. 2006. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci 9: 723–727. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. 1990a. Migration and distribution of two populations of hippocampal progenitors during the perinatal and postnatal periods. J Comp Neurol 301: 365–381. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. 1990b. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol 301: 325–342. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. 2004. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res 1017: 21–31. [DOI] [PubMed] [Google Scholar]
- *.Amrein I. 2015. Adult hippocampal neurogenesis in natural populations of mammals. Cold Spring Harb Perspect Biol 7: a021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu H, Cheung G, Kettenmann H, Palmer TD, Kempermann G. 2007. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS ONE 2: e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X. 2006. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev 15: 407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Yackel JW, Puri PS. 1982. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science 216: 890–892. [DOI] [PubMed] [Google Scholar]
- *.Beckervordersandforth R, Zhang C-L, Lie DC. 2015. Transcription-factor-dependent control of adult hippocampal neurogenesis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmér E, Nanobashvili A, Kokaia M, Lindvall O. 1997. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci 94: 10432–10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bergmann O, Spalding KL, Frisén J. 2015. Adult neurogenesis in humans. Cold Spring Harb Perspect Biol 7: a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebl M, Cooper CM, Winkler J, Kuhn HG. 2000. Analysis of neurogenesis and programmed cell death reveals a self- renewing capacity in the adult rat brain. Neurosci Lett 291: 17–20. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Song J, Ming GL, Song H. 2012. A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr Opin Neurobiol 22: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss BD, Peterson GM, Cowan WM. 1985. On the number of neurons in the dentate gyrus of the rat. Brain Res 338: 144–150. [DOI] [PubMed] [Google Scholar]
- Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, von der Behrens W, Kempermann G. 2003. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci 24: 603–613. [DOI] [PubMed] [Google Scholar]
- Bull ND, Bartlett PF. 2005. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci 25: 10815–10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmann T, Hartig W, Holzer M, Arendt T. 2010. Expression of the embryonal isoform (0N/3R) of the microtubule-associated protein tau in the adult rat central nervous system. J Comp Neurol 518: 2538–2553. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. 1993. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56: 337–344. [DOI] [PubMed] [Google Scholar]
- Cancino GI, Yiu AP, Fatt MP, Dugani CB, Flores ER, Frankland PW, Josselyn SA, Miller FD, Kaplan DR. 2013. p63 regulates adult neural precursor and newly born neuron survival to control hippocampal-dependent behavior. J Neurosci 33: 12569–12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, Overstreet-Wadiche LS. 2013. GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci 33: 6614–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Winkler J, Kuhn HG. 2004. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res 77: 155–165. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. 2005. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21: 1–14. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Karl C, Lindemann G, Schmid P, Aigner R, Laemke J, Bogdahn U, Winkler J, Bischofberger J, et al. 2006. Targeted transgene expression in neuronal precursors: Watching young neurons in the old brain. Eur J Neurosci 24: 1535–1545. [DOI] [PubMed] [Google Scholar]
- Crespo D, Stanfield BB, Cowan WM. 1986. Evidence that late-generated granule cells do not simply replace earlier formed neurons in the rat dentate gyrus. Exp Brain Res 62: 541–548. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Gage FH. 2003. Genetic and functional differences between multipotent neural and pluripotent embryonic stem cells. Proc Natl Acad Sci 100: 11866–11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrossy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. 2003. Differential effects of learning on neurogenesis: Learning increases or decreases the number of newly born cells depending on their birth date. Mol Psychiatry 8: 974–982. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. 1999a. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703–716. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. 1999b. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci 96: 11619–11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, Garcia-Verdugo JM, Canales JJ. 2006. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci 24: 586–594. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. 2006. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci 103: 8233–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. 2011. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8: 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. 2005. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 25: 10074–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. 2003. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci 23: 373–382. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. 2003. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci 23: 9357–9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. 2009. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci 12: 1090–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. 2009. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE 4: e5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. 2006. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. 2007a. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci 30: 1–8. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. 2007b. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. 1999. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2: 260–265. [DOI] [PubMed] [Google Scholar]
- Hodge RD, Kowalczyk TD, Wolf SA, Encinas JM, Rippey C, Enikolopov G, Kempermann G, Hevner RF. 2008. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J Neurosci 28: 3707–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, Kahoud RJ, Hevner RF. 2012a. Transcriptional control of glutamatergic differentiation during adult neurogenesis. Cell Mol Life Sci 69: 2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, Nelson BR, Kahoud RJ, Yang R, Mussar KE, Reiner SL, Hevner RF. 2012b. Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J Neurosci 32: 6275–6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhäuser C, Gray WP. 2003. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: Functional and immunocytochemical analysis. Eur J Neurosci 18: 2769–2778. [DOI] [PubMed] [Google Scholar]
- Imielski Y, Schwamborn JC, Lüningschrör P, Heimann P, Holzberg M, Werner H, Leske O, Püschel AW, Memet S, Heumann R, et al. 2012. Regrowing the adult brain: NF-κB controls functional circuit formation and tissue homeostasis in the dentate gyrus. PLoS ONE 7: e30838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. 2006. Environment matters: Synaptic properties of neurons born in epileptic brain develop to reduce excitability. Neuron 52: 1047–1059. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Romer B, Babu H, Kempermann G. 2005. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol 196: 342–351. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. 2002. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci 99: 11946–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian LM, Vandenbosch R, Pakenham CA, Andrusiak MG, Nguyen AP, McClellan KA, Svoboda DS, Lagace DC, Park DS, Leone G, et al. 2013. Opposing regulation of Sox2 by cell-cycle effectors E2f3a and E2f3b in neural stem cells. Cell Stem Cell 12: 440–452. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. 1977. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science 197: 1092–1094. [DOI] [PubMed] [Google Scholar]
- Karalay O, Doberauer K, Vadodaria KC, Knobloch M, Berti L, Miquelajauregui A, Schwark M, Jagasia R, Taketo MM, et al. 2011. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc Natl Acad Sci 108: 5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. 2011a. The pessimist’s and optimist’s views of adult neurogenesis. Cell 145: 1009–1011. [DOI] [PubMed] [Google Scholar]
- Kempermann G. 2011b. Seven principles in the regulation of adult neurogenesis. Eur J Neurosci 33: 1018–1024. [DOI] [PubMed] [Google Scholar]
- *.Kempermann G. 2015. Adult neurogenesis: An evolutionary perspective. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. 2003. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 130: 391–399. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. 2004. Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27: 447–452. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Chesler EJ, Lu L, Williams RW, Gage FH. 2006. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc Natl Acad Sci 103: 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. 2003. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467: 455–463. [DOI] [PubMed] [Google Scholar]
- *.Kuhn HG. 2015. Control of cell survival in adult mammalian neurogenesis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. 1996. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci 16: 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J. 2005. Increased generation of granule cells in adult Bcl-2-overexpressing mice: A role for cell death during continued hippocampal neurogenesis. Eur J Neurosci 22: 1907–1915. [DOI] [PubMed] [Google Scholar]
- *.Kuhn HG, Eisch AJ, Spalding K, Peterson DA. 2015. Detection and phenotypic characterization of adult neurogenesis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze A, Grass S, Witte OW, Yamaguchi M, Kempermann G, Redecker C. 2006. Proliferative response of distinct hippocampal progenitor cell populations after cortical infarcts in the adult brain. Neurobiol Dis 21: 324–332. [DOI] [PubMed] [Google Scholar]
- Laplagne DA, Espósito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. 2006. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol 4: e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. 2000. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci 97: 13883–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Pleasure SJ. 2005. Morphogenesis of the dentate gyrus: What we are learning from mouse mutants. Dev Neurosci 27: 93–99. [DOI] [PubMed] [Google Scholar]
- Liu M, Pleasure SJ, Collins AE, Noebels JL, Naya FJ, Tsai MJ, Lowenstewin DH. 2000. Loss of BETA2/NeuroD leads to malformation of the dentate gyrus and epilepsy. Proc Natl Acad Sci 97: 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wang J, Zhu D, Fu Y, Lukowiak K, Lu Y. 2003. Generation of functional inhibitory neurons in the adult rat hippocampus. J Neurosci 23: 732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. 2006. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7: 179–193. [DOI] [PubMed] [Google Scholar]
- *.Lucassen PJ, Oomen CA, Naninck EFG, Fitzsimons CP, van Dam AM, Czeh B, Korosi A. 2015. Regulation of adult neurogenesis and plasticity by (early) stress, glucocorticoids, and inflammation. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Burgin A, Mongiat LA, Pardi MB, Schinder AF. 2012. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science 335: 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. 2002. Anatomy of the brain neurogenic zones revisited: Fractones and the fibroblast/macrophage network. J Comp Neurol 451: 170–188. [DOI] [PubMed] [Google Scholar]
- Merz K, Lie DC. 2013. Evidence that doublecortin is dispensable for the development of adult born neurons in mice. PLoS ONE 8: e62693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. 2005. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28: 223–250. [DOI] [PubMed] [Google Scholar]
- Moss J, Toni N. 2013. A circuit-based gatekeeper for adult neural stem cell proliferation: Parvalbumin-expressing interneurons of the dentate gyrus control the activation and proliferation of quiescent adult neural stem cells. Bioessays 35: 28–33. [DOI] [PubMed] [Google Scholar]
- Nacher J, Varea E, Blasco-Ibanez JM, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. 2005. Expression of the transcription factor Pax 6 in the adult rat dentate gyrus. J Neurosci Res 81: 753–761. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Mori T, Gotz M. 2007. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci 27: 10906–10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, Santangelo AM, Low MJ, Westbrook GL, Rubinstein M. 2004. A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci 24: 3251–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. 2006a. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci 26: 2326–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. 2006b. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci 26: 4095–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Ray J, Gage FH. 1995. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci 6: 474–486. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. 1997. The adult rat hippocampus contains premordial neural stem cells. Mol. Cell Neurosci 8: 389–404. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. 2000. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425: 479–494. [DOI] [PubMed] [Google Scholar]
- Piatti VC, Davies-Sala MG, Esposito MS, Mongiat LA, Trinchero MF, Schinder AF. 2011. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci 31: 7715–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure SJ, Collins AE, Lowenstein DH. 2000. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J Neurosci 20: 6095–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plümpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, Römer B, Rodriguez GR, Kronenberg G, Kempermann G. 2006. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci 7: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Rodríguez G, Babu H, Klempin F, Krylyshkina O, Baekelandt V, Gijsbers R, Debyser Z, Overall RW, Nicola Z, Fabel K, et al. 2013. The α crystallin domain of small heat shock protein b8 (Hspb8) acts as survival and differentiation factor in adult hippocampal neurogenesis. J Neurosci 33: 5785–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. 2004. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 19: 234–246. [DOI] [PubMed] [Google Scholar]
- Ray J, Peterson DA, Schinstine M, Gage FH. 1993. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci 90: 3602–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzel R, Sadek AR, Chang CH, Gray WP, Seifert G, Steinhauser C. 2013. Polarized distribution of AMPA, but not GABAA, receptors in radial glia-like cells of the adult dentate gyrus. Glia 61: 1146–1154. [DOI] [PubMed] [Google Scholar]
- Rietze R, Poulin P, Weiss S. 2000. Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol 424: 397–408. [PubMed] [Google Scholar]
- Römer B, Krebs J, Overall R, Fabel K, Babu H, Overstreet-Wadiche L, Brandt MD, WIlliams RW, Jessberger S, Kempermann G. 2011. Adult hippocampal neurogenesis and plasticity in the infrapyramidal bundle of the mossy fiber projection. I: Co-regulation by activity. Front Neurosci 5: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. 2006. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci 103: 17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schänzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. 2004. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol 14: 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. 2004. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429: 184–187. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. 2002. Adult rodent neurogenic regions: The ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci 22: 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y. 1993a. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci Res 17: 265–290. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. 1993b. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci 13: 2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. 2001. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21: 7153–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. 2004. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol 478: 359. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Korn MJ, Shan Z, Ribak CE. 2005. GFAP-expressing radial glia-like cell bodies are involved in a one-to-one relationship with doublecortin-immunolabeled newborn neurons in the adult dentate gyrus. Brain Res 1040: 81–91. [DOI] [PubMed] [Google Scholar]
- Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. 2004. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature 427: 78–83. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. 2002. Astroglia induce neurogenesis from adult neural stem cells. Nature 417: 39–44. [DOI] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, et al. 2012. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489: 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Song J, Olsen RHJ, Sun J, Ming Gl, Song H. 2015. Neuronal circuitry mechanisms regulating adult mammalian neurogenesis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, et al. 2013. Dynamics of hippocampal neurogenesis in adult humans. Cell 153: 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. 2004. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia 46: 41–52. [DOI] [PubMed] [Google Scholar]
- Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G. 2006. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia 54: 805–814. [DOI] [PubMed] [Google Scholar]
- Sun GJ, Sailor KA, Mahmood QA, Chavali N, Christian KM, Song H, Ming GL. 2013. Seamless reconstruction of intact adult-born neurons by serial end-block imaging reveals complex axonal guidance and development in the adult hippocampus. J Neurosci 33: 11400–14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. 2006. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442: 929–933. [DOI] [PubMed] [Google Scholar]
- *.Toni N, Schinder AF. 2015. Maturation and functional integration of new granule cells into the adult hippocampus. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. 2008. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 11: 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. 2005. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47: 803–815. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. 2002. Functional neurogenesis in the adult hippocampus. Nature 415: 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. 2008. GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci 28: 5547–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. 2000. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol 42: 248–257. [PubMed] [Google Scholar]
- Wang LP, Kempermann G, Kettenmann H. 2005. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci 29: 181–189. [DOI] [PubMed] [Google Scholar]
- Weber T, Baier V, Lentz K, Herrmann E, Krumm B, Sartorius A, Kronenberg G, Bartsch D. 2013. Genetic fate mapping of type-1 stem cell-dependent increase in newborn hippocampal neurons after electroconvulsive seizures. Hippocampus 23: 1321–1330. [DOI] [PubMed] [Google Scholar]
- Wiskott L, Rasch MJ, Kempermann G. 2006. A functional hypothesis for adult hippocampal neurogenesis: Avoidance of catastrophic interference in the dentate gyrus. Hippocampus 16: 329–343. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Nakashima K, Summers RG, Toni N, D’Amour KA, Kie DC, Gage FH. 2004. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature 430: 350–356. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG Jr, Ming GL, Gage FH. 2006. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]