Abstract
The control of organism and organ size is a central question in biology. Despite the attention it has received, our understanding of how adult organ size is determined and maintained is still incomplete. Early work has shown that both autonomous and regulated mechanisms drive vertebrate organ growth, and both intrinsic and extrinsic cues contribute to organ size. The molecular nature of organ-size determinants has been the subject of intense study, and major pathways, which underlie cell interactions controlling cell compartment size, have been identified. In this work, we review these data as well as the future perspectives of research in this important area of study.
All mammals—from Etruscan shrews to blue whales—develop from eggs that are roughly the same size. The morphogenetic and homeostatic growth pathways that control the sizes of their organs are tightly regulated.
Adult body mass in mammalian species ranges from ∼1.5 grams in adult Etruscan shrews (Suncus etruscus) to ∼150,000,000 grams in blue whales (Balaenoptera musculus), yet they all develop from eggs of roughly the same size (Austin 1961). And, although all mammals conform to a similar body plan, the proportions of different organs and tissue compartments vary tremendously among species. These simple observations exemplify the enormous potential for growth of mammalian organisms, but also the fact that size is no accident because it is the result of a tightly controlled process. However, our understanding of this critical feature of animal biology remains incomplete.
Animal organ size must comply with a complex set of biological and ecological constraints to ensure survival. Organ morphogenesis and growth must be tightly coordinated to ensure that the body plan is correctly established, as a deformed animal would have its survival severely compromised. Yet, organ growth must also be flexible enough to adapt to environmental challenges, such as injury or changing food availability. There are also constraints imposed by the animal’s life cycle, including growth associated with sexual maturation and dimorphism. And, finally, each organ must meet needs specific to its function, which requires rapid cell turnover in some tissues, but virtually none in others. Not surprisingly, under these constraints, organ-size determination in mammals has evolved to be a complex process. In this article, we will review the progress made toward understanding how mammalian body and organ size is determined, first, by examining how early experiments have identified different modes of size determination in mammals, and then by reviewing the expanding body of work characterizing a network of molecular mechanisms underlying them.
CHARACTERISTICS OF MAMMALIAN GROWTH
Study of Animal Growth: Autonomous versus Regulated Growth
Examination of growth properties has historically involved two major approaches. The first aims to determine whether a tissue compartment is subject to a size “set point” by characterizing its response to cell number alteration. Compensation by changes in cell growth, proliferation, or survival is evidence of “regulated” growth: the compartment size is “sensed” and correspondingly adjusted. Lack of a set point indicates that growth is driven by an “autonomous” program, which is insensitive to compartment size. Even then, final size is not necessarily fixed, as the execution of the program might be modulated by intrinsic and external factors. This type of approach was initially limited to surgical ablation and engraftment, but the advent of genetic manipulation has considerably increased its scope and value by allowing precisely targeted cell number alterations in almost any tissue in a temporally controlled manner and with the added possibility of cell lineage tracking. The second approach aims to determine the nature of signals regulating growth by characterizing compartment response to a change in the external or intrinsic environment. Heterotypic and heterochronic graft, parabiosis, and nutrient restriction could all be included in this group, along with alteration of cell microenvironment via gene expression and protein function manipulation. Taken together, these approaches have succeeded in uncovering major principles of organ-size determinations, as we will discuss below.
Whole Body Size Control in Mammals
Growth Control during Early Embryonic Development
Because conformation of the animal body plan is a major constraint size for all organs, it is useful to review what is known about how body size is determined in mammals. Control of cell number in preimplantation embryos was among the first features of size determination to be studied. Blastomere ablation or embryo scission up to the third cleavage stage results in smaller blastocysts containing fewer cells. However, increased proliferation is observed starting shortly after implantation, at embryonic day 7.5 in mice, resulting in normalization of embryo size by midorganogenesis (E10.5) (Tarkowski and Wróblewska 1967; Rands 1986). Conversely, postimplantation mouse embryos, resulting from morula aggregation, grow more slowly and their size is normalized by E7.5 (Lewis and Rossant 1982). Hence, embryo size in mammals is governed by an autonomous growth program early in development, but then switches to a program whereby size is tightly regulated to a predetermined set point, at least until mid- or late organogenesis.
Control of Late Embryonic and Postnatal Growth by Regulative and Autonomous Mechanisms
The existence of “extrinsic” (regulative) mechanisms modulating late embryonic and postnatal growth was also established decades ago, based on the observation that a transient deficit of amino acids results in accelerated “catch-up” growth in mammals after normal nutrition is restored (Osborne and Mendel 1914; Tanner 1963). The observation of the effect of pituitary gland ablation on bodily growth in 1909 led to the discovery of growth hormone (GH) in the 1920s and its purification in the 1940s (Aschner 1909; Kopchick 2003). GH deficiency results in slowed postnatal growth and reduced body size, whereas transgenic animals overexpressing GH display increased growth and size (Palmiter et al. 1982; Hull and Harvey 1999). However, linear growth rates in GH-deficient animals still decline with age at a pace identical to that of age-matched controls (Lupu et al. 2001). Similarly, GH-treated animals display normal age-related growth deceleration if the ratio of GH to body mass is kept constant (Mathews et al. 1988). This indicates that age-related growth deceleration is not controlled by GH levels, whereas the phenomenon of catch-up growth further suggests that growth deceleration is driven by how much growth has already taken place, rather than by the chronological age of the animal.
The question of whether late embryonic size may also be subject to “set point control” requires similar methods of unbiased, body-wide cell ablation and assessing for catch-up growth. Although such an approach has not been taken experimentally, a similar situation has been found to result naturally from mutation of the pericentrin gene in humans. The pericentrin protein is a component of centrosomes and loss of its function results in an increased frequency of abnormal mitoses, leading to cell arrest or death, thus reducing growth efficiency. Pericentrin mutations cause primordial dwarfism, a rare condition characterized by slowed growth, starting during gestation. Organ and body size are reduced, but proportions are preserved except for some craniofacial bones (Delaval and Doxsey 2010). Continually impaired cell proliferation and loss are, therefore, not compensated for during late embryonic and postnatal growth in humans, arguing against the existence of a mammalian set point for body size.
Vertebrate Organ-Growth Properties
Organ Size Is Determined by Extrinsic and Intrinsic Cues
The existence of overlapping mechanisms of size regulation in vertebrate organs was also first revealed by early work, most notably the studies of amphibian limb growth performed by Harrison in the 1920s (Harrison 1924). Harrison took advantage of the differences in growth in two closely related species of salamander, Ambystoma punctatum (now maculatum) and Ambystoma tigrinum. These species are similar in size at the time of metamorphosis, but punctatum larvae develop their limbs earlier and reach smaller adult sizes than tigrinum. Harrison cross-transplanted limb buds between the species to surprising results: punctatum limbs grafted on tigrinum larvae grew to be smaller than corresponding donor limbs, whereas tigrinum buds grafted in punctatum larvae reached gigantic proportions. Harrison concluded that size was determined by integration of a limb-intrinsic “potential,” which was greater in tigrinum, and a systemic “regulator” more active in punctatum (Harrison 1924).
Size-Control Mechanisms Differ across Mammalian Organs
Adult organs in mammals display dramatically varying degrees of plasticity. Liver mass, for example, is tightly regulated to a set point. Surgical ablation of liver lobes results in rapid growth of the remaining lobes, bringing back the organ within 5% of its original mass in a few days. Liver transplantation to a recipient larger than the donor also induces growth, resulting in rapid normalization of liver–body ratio. Conversely, large-for-host transplanted liver mass decreases as a result of increased apoptosis (Fausto et al. 2006). In animals with linked blood circulation (parabiosis), partial hepatectomy induces compensatory growth of both livers, showing that liver size is influenced by systemic factors still unidentified (Bucher et al. 1951; Moolten and Bucher 1967). In contrast, the thymus, kidney, gut, and cartilage growth plates do not alter their growth on transplantation (Lui and Baron 2011). Regenerative capacity is very limited in most mammalian organs; however, responses depend on the nature of the injury. For example, obstruction of the main pancreatic duct results in acute inflammation and pancreatic tissue destruction, but full regeneration occurs once the obstruction is resolved. Partial resection of the pancreas, in contrast, elicits no response. Resected small intestine does not increase in length even in juvenile individuals; instead, enteric villi increase their length to compensate for lost absorption surface (Stanger 2008).
Developing and Mature Organ Size Is Controlled by Different Processes
Differences in organ plasticity arise early during organ development. Cell ablation via targeted expression of the diphtheria toxin in liver progenitor cells of transgenic mice results in compensatory proliferation and restoration of normal liver by the time of birth. In contrast, ablation of pancreatic progenitors using the same approach results in a proportional reduction of pancreas size. The two organ primordia, therefore, show different size regulation despite the fact that both arise from a shared pool of progenitors in the early foregut (Stanger et al. 2007). Further complicating this picture, some organs display different plasticity during embryonic and postnatal growth. The early limb bud, for instance, is capable of regeneration on partial ablation. However, it rapidly loses as development proceeds, indicating a switch from regulated to autonomous growth (Müller et al. 1999). In others, both types of regulation seem to co-exist: while the hematopoietic bone marrow exhibits a high degree of plasticity, the number of hematopoietic stem cells is determined by an autonomous mechanism (Müller-Sieburg et al. 2000).
Characterizing Mammalian Organ Growth at the Cell Level: Morphogenetic versus Homeostatic Growth?
Organ Growth Is Driven by Two Distinct Cellular Processes
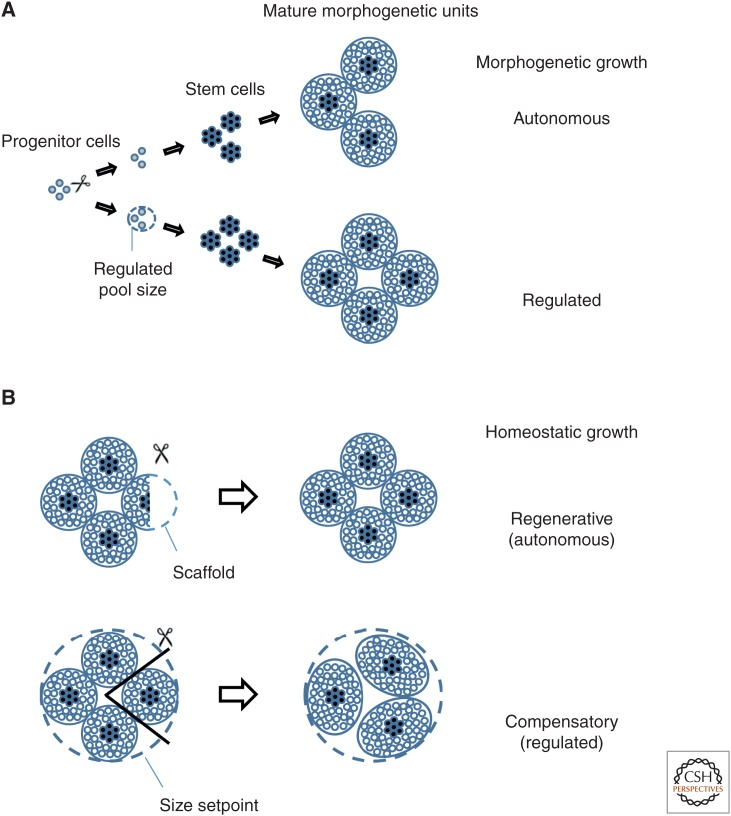
Although the results discussed above paint a complex picture, a look at the cellular mechanisms driving organ growth might provide some insight into the process of organ-size regulation. Most tissue compartments in vertebrates are constituted around a self-renewing stem-cell population, giving rise to one or more differentiated cell types. Stem cells reside in a variable number of anatomically discrete niches acting as independent morphogenetic units. Enteric crypts, pancreatic acini, skeletal growth plates, and the entire hematopoietic cell compartment could be considered each a morphogenetic unit. In some cases, such as the liver, differentiated cells are capable of self-renewal without the need for a stem cell (Yanger et al. 2013). However, liver lobes behave as a discrete morphogenetic unit as they are anatomically isolated from each other. It is, therefore, reasonable to consider organ size to be the coordinated outcome of two modes of growth control: “morphogenetic growth,” involving creation of morphogenetic units, and “homeostatic growth,” resulting from increase in morphogenetic unit size (Fig. 1).
Figure 1.
Response of mammalian organs to cell ablation during morphogenesis and homeostasis. Organs are represented as clusters of independent morphogenetic (see text for details) units each containing stem (black dots) and differentiated cells (white dots). (A) Morphogenetic unit number is determined during embryonic development. Progenitor pool size can be determined by an autonomous program (upper path) that cannot compensate for progenitor-cell loss, or by ongoing intrinsic and/or extrinsic cues (dotted circle) allowing for compensatory proliferation (lower path) in response to cell ablation. (B) Mature morphogenetic unit size is, in most cases, limited by extrinsic and intrinsic cues and might regenerate as long as stem cells are spared (top). Lost morphogenetic units cannot, in most cases, be recreated. Instead, the remaining units may undergo compensatory growth. Total organ size is then limited by external and/or internal cues determining a “set point” (bottom).
Morphogenetic growth is largely an autonomous process. Morphogenetic unit formation in mammals occurs mostly during embryonic development. The number of units produced in organ primordia is dependent on progenitor pool size, which is regulated in the early embryo, but becomes autonomously determined in specific organ lineages. The reason for this is not fully understood, but growth and differentiation themselves may act as factors restricting progenitor pool plasticity. This is illustrated in the limb bud in which growth is driven by signals from the apical ectodermal ridge that are, in turn, disrupted by growth (as further discussed below) (Cohn et al. 1995; Tanaka et al. 1997). In some cases, however, new morphogenetic unit formation might extend beyond embryonic development. This is the case in the intestine in which new crypts are produced by fission of existing ones. This phenomenon likely plays a role in the elongation of the bowel during postnatal growth, but it is not observed in response to injury, suggesting that it might be under control of GH levels, an autonomous program, or both (Cummins et al. 2006).
Homeostatic growth can be autonomous or regulated. Morphogenetic unit size, on the other hand, is clearly regulated in most mature organs, but the mode of size determination varies, arguably as result of evolutionary adaptation to the function of each organ. Organs with functions requiring rapid adaptation and variable functional output, such as the liver, muscles, or the hematopoietic stem-cell compartment, are tightly regulated to a set point, which supposes the existence of “thermostats” by which the organ can sense physiological stress. The size of organs that do not need to meet changing demands is determined either autonomously or by interaction with a “scaffold” compartment the size of which is, in turn, autonomously determined. This paradigm is illustrated by the acinar pancreas, which regenerates as long as the subjacent ductal compartment is maintained (Stanger 2008). Size of the ductal compartment, in turn, is set early during development and its subsequent growth is not regulated. Consistent with this idea, the mass of the endocrine β-cell compartment, which plays a critical role in metabolic regulation, is subject to regulation and compensatory growth, despite sharing the same progenitor pool as the acinar and duct compartments (Bouwens and Rooman 2005).
MOLECULAR MECHANISMS OF GROWTH AND SIZE CONTROL
The work performed in the premolecular age of biology established the complex character of size regulation in mammals, and led to the proposal of a number of potential mechanisms driving this process. The molecular basis of size regulation is currently an active and expanding field of research. Although a complete picture has yet to emerge, critical findings have not only provided a molecular basis for some of the previous results, but have, in addition, identified new modes of size regulation.
Regulation by Soluble Growth Factors
The GH/insulin growth factor (IGF) axis is the main modulator of body size in mammals. Early in vitro studies into the function of the mammalian GH showed that, although GH treatment induces cell proliferation in cartilage in vivo, it had no effect on cultured cells. However, cultured cartilage does respond to normal serum, indicating that GH effects were mediated by at least a second blood-borne factor (Salmon and Daughaday 1957). This factor was later identified as IGF-1. IGF-1 is a potent mitogen in most cell types and its deficiency results in dwarfism that does not respond to GH treatment. Allelic IGF-1 polymorphisms, resulting in varying serum protein levels, have been shown to account for the body size diversity in domestic canine breeds, further demonstrating its key role in adult body size determination. In contrast to GH, reduced IGF activity affects prenatal growth, indicating that additional mechanisms maintain IGF levels during development. IGF-1 is produced in many cell types in response to GH, but the main source of circulating IGF protein is the liver (∼75%), suggesting that the mechanisms regulating liver primordium size may also play an important part in body size determination during late development (D’Ercole 1993; Puche and Castilla-Cortázar 2012).
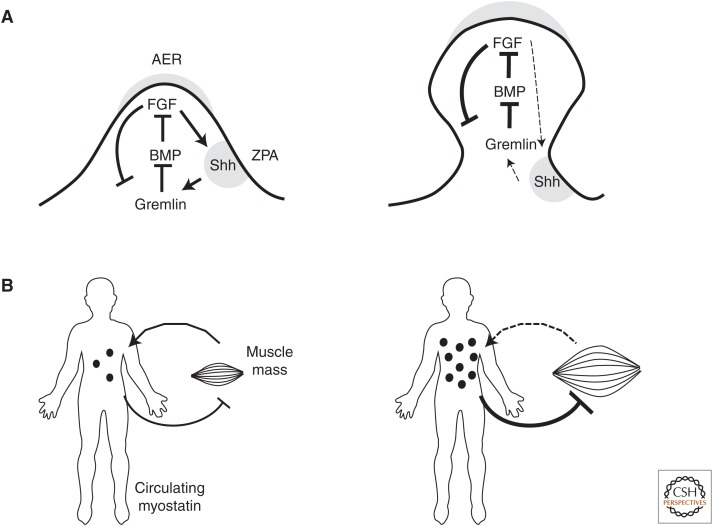
Soluble factors regulate both morphogenetic and homeostatic organ growth. Progenitor pool size in many embryonic organ primordia is affected by growth factors produced by surrounding cells, but also by the progenitor pool itself. For example, early limb bud growth is driven by members of the fibroblast growth factor (FGF) family, secreted by the apical limb bud cells (Cohn et al. 1995). FGF expression is maintained through a feedback loop, involving bone morphogenetic proteins (BMPs), produced by the flank mesenchyme, which antagonize FGF (Fig. 2). BMPs are inhibited by the Gremlin protein, produced in response to low FGF levels. High FGF levels, however, repress Gremlin expression. As the limb grows, FGF levels increase and disrupt the loop, terminating growth (Verheyden and Sun 2008). Dissecting the role of growth factors in embryo size control, however, is difficult as cell fate decision and growth decision are often linked, as shown by the fact that FGF reduction in the limb bud leads to truncated limbs rather than smaller, but well-formed limbs (Sun et al. 2002).
Figure 2.
Intrinsic regulation of organ size by soluble factors. (A) Regulation of limb bud growth by the fibroblast growth factor (FGF)-Sonic hedgehog (Shh)-Gremlin loop. FGF factors secreted by the apical ectodermal ridge (AER) and underlying apical mesemchyme drive limb bud growth. They also maintain expression of Shh in the zone of polarizing activity (ZPA). Shh drives expression of the bone morphogenetic protein (BMP) inhibitor Gremlin. BMPs are secreted by the proximal mesenchyme and inhibit FGF expression. Gremlin expression is also negatively regulated by FGFs. As the limb bud grows, ZPA cells become exposed to decreasing FGF levels, resulting in disruption of the signaling loop and growth arrest. (B) Muscle mass regulation by myostatin, a TGF-β superfamily member secreted by skeletal muscles. Increased muscle mass leads to higher circulating myostatin levels, which inhibit both myogenesis and muscle fiber growth. Myostatin thus functions as “chalone,” an organ-specific growth inhibitor that acts as a “thermostat” of organ-to-body mass ratio.
In adult organs, growth factors act as “thermostats,” regulating organ size in response to physiological stress. One of the best-characterized examples is that of erythropoietin, which drives hematopoietic cell proliferation and differentiation, and is produced by liver and kidney cells in response to hypoxia (Jelkmann 2011). Alternatively, organs may regulate their own size by producing organ-specific growth inhibitors. The existence of these factors or “chalones” was proposed by Bulloughs when he observed that wounding the skin on one side of rat ears induced proliferation on the uninjured side (Bullough 1965; Gamer et al. 2003). Chalones remained hypothetical until identification of myostatin, a protein of the TGF-β family produced by myoblasts. Animals and humans lacking myostatin display increased muscle mass, showing that it acts as muscle-specific growth inhibitor (Schiaffino et al. 2013). Another TGF-β family member, GDF11, is produced by embryonic olfactory epithelium cells and determines the olfactory epithelium size by inhibiting progenitor-cell proliferation (Wu et al. 2003). Surprisingly, no other chalones have been identified, even in organs with a size set point, such as in the liver. One possible reason is that the nature of chalones may not be limited to peptide growth factors. There is evidence suggesting that liver size might be regulated by biliary salts, small lipid molecules produced by the liver and recaptured by the portal circulation. They regulate transcription by binding to the nuclear farnesoid X receptor (FXR). FXR activation is required for liver regeneration after partial hepatectomy (Huang et al. 2006). Another possibility is that adult organ sizes might be regulated through cascades and signaling loops, rather than single factors, in a manner similar to that described in the embryonic limb bud (Fig. 2).
The Akt/mammalian target of rapamycin (mTOR) pathway integrates extracellular signals regulating growth. Growth factors act by binding to specific receptors that trigger intracellular signaling cascades modulating the processes of cell proliferation, growth, and survival. These cascades are often interconnected or overlapping, allowing for integration of multiple environmental inputs into a coherent cell response. In vertebrates, a considerable amount of work has shown the central role of the Akt/mTOR signaling pathway in the transduction and integration of a wide range of growth stimuli.
The central element of the pathway is the Akt serine/threonine kinase. Activation of Akt is initiated by translocation of the protein to the plasma membrane, mediated by binding of its pleckstrin homology domain to membrane phosphatidylinositol (3,4,5)-triphosphate (PIP3). Membrane-bound Akt is then phosphorylated and activated by the PI-dependent kinase (PDPK1) and the mammalian target of rapamycin complex (mTORC) 2. PIP3 is synthesized by the PI3 kinase (PI3K) and degraded by the PTEN phosphatase. PI3K is, in turn, activated by several major classes of growth factor receptors, including tyrosine kinase, cytokine, TGF-β, and G protein coupled receptors. The most potent activator of Akt in mammalian cells is the IGF receptor. Akt activity is also regulated by the cell energy metabolism via mTORC2, which is activated by the AMP-dependent kinase (AMPK) (Altomare and Testa 2005; Memmott and Dennis 2009; Tumaneng et al. 2012).
Akt activation promotes growth, proliferation, and survival in most vertebrate cell types. Its effects are mediated in several ways. mTORC1 mediates most of the effects on cell growth by promoting ribosomal output via the S6 ribosomal kinase. mTORC1 is also regulated by amino acid availability and hypoxia. Akt can suppress apoptosis directly by phosphorylating and inactivating the proapoptotic protein Bad, and also indirectly by down-regulating FasL expression and activating nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signaling. Finally, Akt induces cell proliferation by inhibiting the activity of the glycogen synthase kinase-3 (GSK3), resulting in stabilization of the myc and cyclin D1 proteins, which promote cell cycle progression from the G1 to the S phase. In addition, Akt also phosphorylates cyclin inhibitors p27Kip and p21WAF, resulting in their exclusion from the nucleus and release of cyclin inhibition. Activation of PI3K can also promote progression through the G2/M transition in vertebrate oocytes, but the mechanism behind this effect is not well understood, and it is unclear whether the Akt pathway plays a role in G2/M progression in somatic cells (Altomare and Testa 2005; Memmott and Dennis 2009; Tumaneng et al. 2012).
Several lines of evidence indicate that the Akt pathway plays a central role in mammalian growth regulation in vivo. Akt1 deletion in mice results in dwarfism (Chen et al. 2001; Peng et al. 2003). In humans, mutations resulting in Akt activation cause Proteus syndrome, a condition characterized by localized excessive growth of bone, muscle, skin, and connective tissue. The characteristically variable distribution of abnormal growth results from a mosaic distribution of the mutations, which are acquired spontaneously during early development. Similarly, PTEN mutations have been implicated in Bannayan–Riley–Ruvalcaba syndrome, characterized by emergence of benign neoplasms (hamartomas) in multiple tissues. Perhaps the most compelling evidence of the central role of the Akt pathway is, however, the fact that many Akt pathway mutations display oncogenic or tumor suppressor activity and that they are found in most malignant neoplasms (Cheung and Testa 2013).
Regulation by Localized Cell Interactions
Cell competition is a potential size regulation mechanism. Because early embryos develop in isolation and are composed of undifferentiated cells, their size must be regulated by horizontal cell interactions. A growing body of work suggests that the phenomenon of cell competition could be one such mechanism. Cell competition is triggered by differences in growth rates across cells. It results in apoptosis of the slower growing cells and compensatory growth of the advantaged cells, thus, preserving total population size. Cell competition was initially identified in mosaic Drosophila imaginal discs carrying “minute” mutations, which impair ribosomal synthesis and cell growth (Morata and Ripoll 1975). The molecular mechanism involved is not fully understood. In addition to “minutes,” differences in Myc expression levels have been shown to result in cell competition, and Myc overexpression can turn imaginal disc cells into “supercompetitors,” which drive wild-type cell elimination from the disc (de la Cova et al. 2004; Moreno and Basler 2004; Johnston 2009). Cell competition in mammals was initially suggested by the observation that embryonic rat liver progenitors grafted into adult livers increase their number over time, whereas total liver mass is preserved. This is accompanied by increased apoptosis of recipient cells in the vicinity of the grafts (Dabeva et al. 2000). Two recent studies elegantly showed that induced and endogenous differences in Myc expression levels result in cell competition in mouse embryonic stem-cell cultures, as well as in the embryonic epiblast in vivo, raising the possibility that early mammal embryo size might be regulated, at least in part, through cell competition (Clavería et al. 2013; Sancho et al. 2013).
The Hippo pathway regulates the size of some mammalian organs. In flies, the Hippo pathway has been implicated as a driver of cell competition upstream of myc (Neto-Silva et al. 2010; de Beco et al. 2012). The pathway consists of a kinase cascade (consisting of the Hippo and Warts kinases), which negatively regulates a transcriptional coactivator (Yorkie). The pathway is highly conserved throughout evolution, with mammalian orthologs, including the Mst1 and Mst2 kinases and the down transcriptional coactivator, Yes-associated protein (YAP). Phosphorylated YAP protein is retained in the cytoplasm and degraded. Pathway activation involves repression of Mst1/2, resulting in YAP translocation to the nucleus where it regulates target gene transcription. The Hippo pathway has been shown to be a key regulator of organ size in mammals. Activation of YAP results in increased cell proliferation and decreased apoptosis and many mammalian cell types. In vivo inactivation of YAP results in reduced liver size, whereas activation results in liver hypertrophy and tumor formation (Zeng and Hong 2008). In the gut, YAP activation leads to overproliferation of enteric crypt cells, whereas, in the pancreas, it leads to dedifferentiation of acinar cells, suggesting that the Hippo pathway may influence organ size by regulating stem-cell niche size (Barry et al. 2013; Gao et al. 2013).
Cell polarity and cell–cell contact regulate Hippo signaling and cell competition. Evidence that identifies cell contact and polarity as the main drivers of Hippo pathway activation is rapidly accumulating. YAP activity in mammalian cells inversely correlates with cell density, whereas YAP activation increases stationary density in culture, implicating the pathway in the phenomenon of contact inhibition (Zeng and Hong 2008). In epithelial cells, acquisition of cell polarity is driven by the assembly of several membrane proteins complexes: Par3/Par6/aPKC in the apical membrane, Crb/PatJ/Pals1 in the zona adherens, and Dlg/Lgl/Scribble in the basal membrane (Assémat et al. 2008). The Par3 and Crb complexes have been shown to bind to members of the Hippo cascade, resulting in Yap activation, whereas assembly of the Dlg complex suppresses YAP activation (Varelas et al. 2010; Gumbiner and Kim 2014; Mohseni et al. 2014). Furthermore, Scribble knock-down results in cell competition in mammalian cells in vitro, raising the interesting possibility that cell adhesion, cell geometry, and/or mechanical constraints might be implicated in cell competition and organ-size regulation (Norman et al. 2012).
Cell-Autonomous Growth Control
Cell proliferation may control autonomous growth via epigenetic genome regulation. The size of organs that display unregulated growth is largely dependent on progenitor pool size; however, the mechanism driving growth arrest in these tissues remains poorly understood. Catch-up growth has been observed at the whole-body level, but also in some organs, such as the skeletal growth plates, suggesting that the number of cell divisions undergone is a determinant of autonomous growth cessation. This, in turn, suggests that autonomous growth cessation could be driven by cumulative changes to the genome resulting from successive replication, such as telomere shortening. However, there is little evidence to suggest telomere shortening controls size. Telomere shortening results in cell arrest after a critical amount of DNA damage accumulates (Davis et al. 2003). In contrast, early postnatal growth deceleration in mammals is characterized by a decrease in proliferation rates rather than by the accumulation of arrested cells. Consequently, telomerase deficiency results in accelerated aging in humans and mice, but normal early growth (Kipling 2001).
More recently, high-throughput transcriptional analysis has revealed that growth deceleration in juvenile mice is associated with genome-wide down-regulation of cell proliferation and growth gene expression. This suggests that growth deceleration may involve epigenetic regulation by DNA and histone modification (Lui and Baron 2011). Histone methylation and acetylation patterns, in particular, have been shown to vary with age. Mutations that affect histone modification affect aging and senesce in a manner similar to changes in telomere length (Berdasco and Esteller 2012; Huidobro et al. 2013). However, trimethylation of histone 3 K4 (H3K4), which is associated with active gene expression, decreases with age in the loci associated with the juvenile growth deceleration “program.” Furthermore, H3K4 methylation accumulation is delayed along with growth in response to nutrient deprivation, and it displays a catch-up increase on nutrient restitution (Lui et al. 2010). It is still, however, unclear whether H3K4 methylation is directly driven by cell proliferation and could, thus, be part of a cell-proliferation “counting” mechanism.
CONCLUSIONS AND PERSPECTIVES
The complexity of size regulation reflects the anatomical and physiological complexity of the mammalian organism. In this review, we have highlighted the different roles played by morphogenetic growth, which determines the size of progenitor and stem-cell pools, and homeostatic growth, which modulates their activity. The first provides a predetermined “template,” limiting organ-growth potential and ensuring conformation of a reproducible body plan, whereas the second “tunes” organ growth in response to intrinsic and extrinsic cues, reflecting the multiple physiological constraints acting on organ size.
However, critical aspects of the process of size regulation remain unclear. For instance, why is stem- and progenitor-cell plasticity “switched off” in most mature tissues? The greater regenerative capacity displayed by lower vertebrates suggests that this plasticity is lost as response to evolutionary constraints. What is the nature of those constraints? What is the molecular basis of plasticity changes? The involvement of cell competition in embryo size control represents an exciting recent development, but the process remains poorly understood. What are the determinants of cell “fitness” and how are they compared across cells? Finally, perhaps the least understood mechanisms are those driving programmed growth. How do cells keep track of growth? With continued attention to the problem, the advent of technologies that allow rapid characterization of genome-wide epigenetic, transcriptional, and posttranslational changes may provide answers to what is one of the oldest questions in animal biology.
Footnotes
Editors: Rebecca Heald, Iswar K. Hariharan, and David B. Wake
Additional Perspectives on Size Control in Biology: From Organelles to Organisms available at www.cshperspectives.org
REFERENCES
- Altomare DA, Testa JR. 2005. Perturbations of the AKT signaling pathway in human cancer. Oncogene 24: 7455–7464. [DOI] [PubMed] [Google Scholar]
- Aschner B. 1909. Presentation of dogs after extirpation of the pituitary. Wien Klin Wochenschr 22: 1730. [Google Scholar]
- Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. 2008. Polarity complex proteins. Biochim Biophys Acta 1778: 614–630. [DOI] [PubMed] [Google Scholar]
- Austin CR. 1961. General egg biology. In The mammalian egg, pp. 14–16. Blackwell, Oxford. [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et al. 2013. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493: 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdasco M, Esteller M. 2012. Hot topics in epigenetic mechanisms of aging: 2011. Aging Cell 11: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwens L, Rooman I. 2005. Regulation of pancreatic β-cell mass. Physiol Rev 85: 1255–1270. [DOI] [PubMed] [Google Scholar]
- Bucher NL, Scott JF, Aub JC. 1951. Regeneration of the liver in parabiotic rats. Cancer Res 11: 457–465. [PubMed] [Google Scholar]
- Bullough WS. 1965. Mitotic and functional homeostasis: A speculative review. Cancer Res 25: 1683–1727. [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, et al. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev 15: 2203–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Testa JR. 2013. Diverse mechanisms of AKT pathway activation in human malignancy. Curr Cancer Drug Targets 13: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavería C, Giovinazzo G, Sierra R, Torres M. 2013. Myc-driven endogenous cell competition in the early mammalian embryo. Nature 500: 39–44. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Izpisúa-Belmonte JC, Abud H, Heath JK, Tickle C. 1995. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80: 739–746. [DOI] [PubMed] [Google Scholar]
- Cummins AG, Jones BJ, Thompson FM. 2006. Postnatal epithelial growth of the small intestine in the rat occurs by both crypt fission and crypt hyperplasia. Dig Dis Sci 51: 718–723. [DOI] [PubMed] [Google Scholar]
- Dabeva MD, Petkov PM, Sandhu J, Oren R, Laconi E, Hurston E, Shafritz DA. 2000. Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am J Pathol 156: 2017–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T, Singhrao SK, Wyllie FS, Haughton MF, Smith PJ, Wiltshire M, Wynford-Thomas D, Jones CJ, Faragher RG, Kipling D. 2003. Telomere-based proliferative lifespan barriers in Werner-syndrome fibroblasts involve both p53-dependent and p53-independent mechanisms. J Cell Sci 116: 1349–1357. [DOI] [PubMed] [Google Scholar]
- de Beco S, Ziosi M, Johnston LA. 2012. New frontiers in cell competition. Dev Dyn 241: 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. 2004. Drosophila myc regulates organ size by inducing cell competition. Cell 117: 107–116. [DOI] [PubMed] [Google Scholar]
- Delaval B, Doxsey SJ. 2010. Pericentrin in cellular function and disease. J Cell Biol 188: 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ercole AJ. 1993. Expression of insulin-like growth factor-I in transgenic mice. Ann NY Acad Sci 692: 149–160. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS, Riehle KJ. 2006. Liver regeneration. Hepatology 43: S45–S53. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Nove J, Rosen V. 2003. Return of the chalones. Dev Cell 4: 143–144. [DOI] [PubMed] [Google Scholar]
- Gao T, Zhou D, Yang C, Singh T, Penzo-Méndez A, Maddipati R, Tzatsos A, Bardeesy N, Avruch J, Stanger BZ. 2013. Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology 144: 1543–1553, 1553.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM, Kim NG. 2014. The Hippo-YAP signaling pathway and contact inhibition of growth. J Cell Sci 127: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RG. 1924. Some unexpected results of the heteroplastic transplantation of limbs. Proc Natl Acad Sci 10: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. 2006. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312: 233–236. [DOI] [PubMed] [Google Scholar]
- Huidobro C, Fernandez AF, Fraga MF. 2013. Aging epigenetics: Causes and consequences. Mol Aspects Med 34: 765–781. [DOI] [PubMed] [Google Scholar]
- Hull KL, Harvey S. 1999. Growth hormone resistance: Clinical states and animal models. J Endocrinol 163: 165–172. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. 2011. Regulation of erythropoietin production. J Physiol 589: 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA. 2009. Competitive interactions between cells: Death, growth, and geography. Science 324: 1679–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipling D. 2001. Telomeres, replicative senescence and human ageing. Maturitas 38: 25–37; discussion 37–38. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ. 2003. History and future of growth hormone research. Horm Res 60: 103–112. [DOI] [PubMed] [Google Scholar]
- Lewis NE, Rossant J. 1982. Mechanism of size regulation in mouse embryo aggregates. J Embryol Exp Morphol 72: 169–181. [PubMed] [Google Scholar]
- Lui JC, Baron J. 2011. Mechanisms limiting body growth in mammals. Endocr Rev 32: 422–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JC, Forcinito P, Chang M, Chen W, Barnes KM, Baron J. 2010. Coordinated postnatal down-regulation of multiple growth-promoting genes: Evidence for a genetic program limiting organ growth. FASEB J 24: 3083–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. 2001. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 229: 141–162. [DOI] [PubMed] [Google Scholar]
- Mathews LS, Hammer RE, Behringer RR, D’Ercole AJ, Bell GI, Brinster RL, Palmiter RD. 1988. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology 123: 2827–2833. [DOI] [PubMed] [Google Scholar]
- Memmott RM, Dennis PA. 2009. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal 21: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK, et al. 2014. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat Cell Biol 16: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolten FL, Bucher NL. 1967. Regeneration of rat liver: Transfer of humoral agent by cross circulation. Science 158: 272–274. [DOI] [PubMed] [Google Scholar]
- Morata G, Ripoll P. 1975. Minutes: Mutants of Drosophila autonomously affecting cell division rate. Dev Biol 42: 211–221. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K. 2004. dMyc transforms cells into super-competitors. Cell 117: 117–129. [DOI] [PubMed] [Google Scholar]
- Muller TL, Ngo-Muller V, Reginelli A, Taylor G, Anderson R, Muneoka K. 1999. Regeneration in higher vertebrates: Limb buds and digit tips. Semin Cell Dev Biol 10: 405–413. [DOI] [PubMed] [Google Scholar]
- Müller-Sieburg CE, Cho RH, Sieburg HB, Kupriyanov S, Riblet R. 2000. Genetic control of hematopoietic stem cell frequency in mice is mostly cell autonomous. Blood 95: 2446–2448. [PubMed] [Google Scholar]
- Neto-Silva RM, de Beco S, Johnston LA. 2010. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev Cell 19: 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman M, Wisniewska KA, Lawrenson K, Garcia-Miranda P, Tada M, Kajita M, Mano H, Ishikawa S, Ikegawa M, Shimada T, et al. 2012. Loss of Scribble causes cell competition in mammalian cells. J Cell Sci 125: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne TB, Mendel LB. 1914. Amino acids in nutrition and growth. J Biol Chem 17: 329–349. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Brinster RL, Hammer RE, Trumbauer ME, Rosenfeld MG, Birnberg NC, Evans RM. 1982. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature 300: 611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, et al. 2003. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev 17: 1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puche JE, Castilla-Cortázar I. 2012. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J Transl Med 10: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands GF. 1986. Size regulation in the mouse embryo. II: The development of half embryos. J Embryol Exp Morphol 98: 209–217. [PubMed] [Google Scholar]
- Salmon WD, Daughaday WH. 1957. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med 49: 825–836. [PubMed] [Google Scholar]
- Sancho M, Di-Gregorio A, George N, Pozzi S, Sánchez JM, Pernaute B, Rodríguez TA. 2013. Competitive interactions eliminate unfit embryonic stem cells at the onset of differentiation. Dev Cell 26: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. 2013. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280: 4294–4314. [DOI] [PubMed] [Google Scholar]
- Stanger BZ. 2008. The biology of organ size determination. Diabetes Obes Metab 10: 16–22. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Tanaka AJ, Melton DA. 2007. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 445: 886–891. [DOI] [PubMed] [Google Scholar]
- Sun X, Mariani FV, Martin GR. 2002. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418: 501–508. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tamura K, Noji S, Nohno T, Ide H. 1997. Induction of additional limb at the dorsal-ventral boundary of a chick embryo. Dev Biol 182: 191–203. [DOI] [PubMed] [Google Scholar]
- Tanner JM. 1963. Regulation of growth in size in mammals. Nature 199: 845–850. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK, Wróblewska J. 1967. Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. J Embryol Exp Morphol 18: 155–180. [PubMed] [Google Scholar]
- Tumaneng K, Russell RC, Guan KL. 2012. Organ size control by Hippo and TOR pathways. Curr Biol 22: R368–R379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. 2010. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell 19: 831–844. [DOI] [PubMed] [Google Scholar]
- Verheyden JM, Sun X. 2008. An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature 454: 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. 2003. Autoregulation of neurogenesis by GDF11. Neuron 37: 197–207. [DOI] [PubMed] [Google Scholar]
- Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE, Stanger BZ. 2013. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev 27: 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Hong W. 2008. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13: 188–192. [DOI] [PubMed] [Google Scholar]