Abstract
Inflammasome activation and caspase-1–dependent (CASP1-dependent) processing and secretion of IL-1β and IL-18 are critical events at the interface of the bacterial pathogen Helicobacter pylori with its host. Whereas IL-1β promotes Th1 and Th17 responses and gastric immunopathology, IL-18 is required for Treg differentiation, H. pylori persistence, and protection against allergic asthma, which is a hallmark of H. pylori–infected mice and humans. Here, we show that inflammasome activation in DCs requires the cytoplasmic sensor NLRP3 as well as induction of TLR2 signaling by H. pylori. Screening of an H. pylori transposon mutant library revealed that pro–IL-1β expression is induced by LPS from H. pylori, while the urease B subunit (UreB) is required for NLRP3 inflammasome licensing. UreB activates the TLR2-dependent expression of NLRP3, which represents a rate-limiting step in NLRP3 inflammasome assembly. ureB-deficient H. pylori mutants were defective for CASP1 activation in murine bone marrow–derived DCs, splenic DCs, and human blood-derived DCs. Despite colonizing the murine stomach, ureB mutants failed to induce IL-1β and IL-18 secretion and to promote Treg responses. Unlike WT H. pylori, ureB mutants were incapable of conferring protection against allergen-induced asthma in murine models. Together, these results indicate that the TLR2/NLRP3/CASP1/IL-18 axis is critical to H. pylori–specific immune regulation.
Introduction
Persistent infection of the gastric mucosa with H. pylori causes gastritis (1) and represents a major risk factor for the development of gastric cancer (2) but has also been inversely linked to the risk of allergic and chronic inflammatory diseases (3, 4). The outcome of the H. pylori/host interaction is determined both by host and bacterial genetic factors (5) as well as the infected individual’s predominant T cell response to H. pylori: whereas asymptomatic carriers generate H. pylori–specific Tregs, patients with peptic ulcer are characterized by Th1/Th2-biased, pathogenic T effector responses (6). Treg-predominant responses are particularly pronounced in children (7) and can be recapitulated in experimental models of neonatal H. pylori infection (8), in which they are required for protection against allergen-induced asthma (9). H. pylori activates caspase-1 (CASP1) in infected macrophages and DCs and induces the processing and secretion of the CASP1-dependent cytokines IL-1β and IL-18 by triggering the assembly and activation of an ASC- and NLRP3-containing inflammasome (10–12). CASP1 activation by H. pylori has both proinflammatory and antiinflammatory consequences that are differentially mediated by its cytokine substrates (10). IL-1β exerts proinflammatory effects that promote Th1- and Th17-driven H. pylori control and gastric immunopathology (10, 11); in contrast, IL-18 signaling restricts severe gastric immunopathology and contributes to asthma protection by promoting Treg differentiation (10, 13). Here, we show using in vitro and in vivo infection models that NLRP3 inflammasome activation by H. pylori requires licensing through TLR2. A saturating transposon (tn) library screen revealed a critical and previously unrecognized role for H. pylori’s urease enzyme in promoting the TLR2-dependent transcriptional activation of NLRP3 expression, CASP1 activation, and cytokine processing as well as Treg differentiation and asthma protection.
Results and Discussion
H. pylori activates CASP1 and induces IL-1β secretion by DCs in an ASC-, NLRP3-, and TLR2-dependent manner.
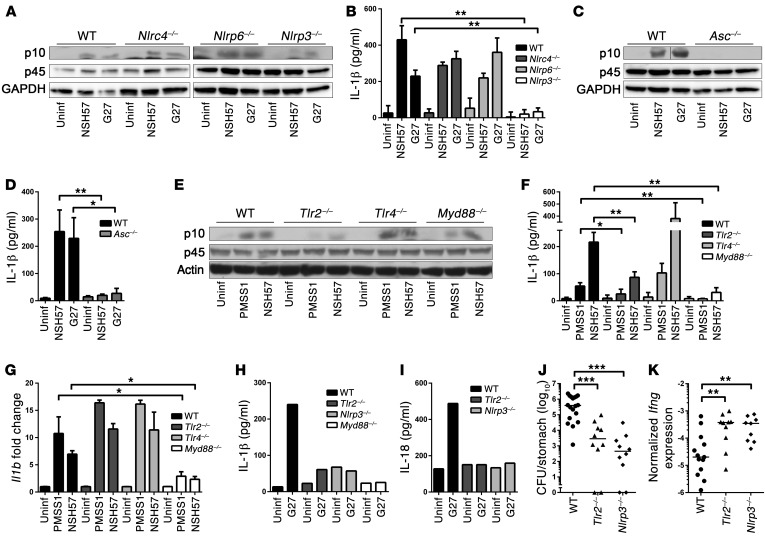
Having shown previously that H. pylori exposure activates CASP1 and induces the secretion of mature IL-1β and IL-18 in bone marrow–derived DCs (BMDCs) (10), we sought to identify host determinants of inflammasome activation by H. pylori. BMDCs from WT, Nlrc4–/–, Nlrp6–/–, Nlrp3–/–, Aim2–/–, and Asc–/– mice, and from mice lacking various TLRs and adaptor molecules, were cocultured with two different strains of H. pylori and examined with respect to CASP1 activation and IL-1β secretion. Whereas the inflammasome sensors NLRC4, NLRP6, and AIM2 were dispensable for CASP1 activation and IL-1β secretion, both processes were found to be dependent on the bipartite adaptor protein ASC as well as the cytoplasmic inflammasome sensor NLRP3 (Figure 1, A–D, and Supplemental Figure 1, A–E; supplemental material available online with this article; doi:10.1172/JCI79337DS1). Surprisingly, we found that CASP1 activation and IL-1β secretion, but not the transcriptional activation of IL-1β, required surface-exposed TLR2 (Figure 1, E–G). Other surface and endosomal TLRs known to contribute to innate immune recognition of Gram-negative pathogens, i.e., TLR4, TLR5, and TLR9 or the IL-1 receptor, were not required for IL-1β secretion (Figure 1, E–G, and Supplemental Figure 2, A–I). Similarly, we could not detect a contribution of the Nod-like receptor NOD2 or of the ATP sensor P2X7R to these processes (Supplemental Figure 2, J–L). Of two known adaptor molecules relaying signals downstream of the TLRs, only MyD88, but not TRIF, was involved in IL-1β expression and secretion (Figure 1, E–G, and Supplemental Figure 2, M and N). A recently identified noncanonical inflammasome activation pathway involving TRIF- and IRF3/7-mediated type I IFN production and signaling (14) was dispensable for H. pylori–induced inflammasome activation (Supplemental Figure 2, N–R). The critical role of TLR2, MyD88, and NLRP3 in H. pylori–induced IL-1β secretion was confirmed with immunomagnetically isolated CD11c+ splenic DCs (Figure 1H and Supplemental Figure 2S). IL-18 secretion by splenic DCs also required TLR2 and NLRP3 (Figure 1I).
Figure 1. CASP1 activation by H. pylori depends on NLRP3, ASC, and TLR2.
(A–G) BMDCs from mice of the indicated genotypes were infected overnight with H. pylori NSH57, G27, and/or PMSS1. (A, C, and E) Western blot analysis of CASP1 activation (p10) in the cell supernatant compared to full-length CASP1 p45 and GAPDH in the extract. Representative results of 3 independent experiments are shown (n = 3). (B, D, and F) IL-1β ELISA of culture supernatants; cells were prestimulated with E. coli LPS prior to infection. Uninf, uninfected. (G) Il1b transcription, as measured by qRT-PCR (normalized to Gapdh and to uninfected controls). Mean + SD of 3 independent experiments is shown (n = 3). (H and I) CD11c+ splenic DCs were infected overnight with G27. (H) IL-1β and (I) IL-18 secretion was measured by ELISA. Representative data of 3 independent experiments are shown (n = 3). (J and K) Mice were infected for 1 month with PMSS1 prior to the quantification of (J) gastric colonization and (K) Ifng expression. Pooled data from 2 studies are shown (n = 2). Horizontal lines indicate medians. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, Mann-Whitney U test. Error bars represent mean + SD.
CASP1–/– mice, and mice lacking either IL-18 or its receptor, control Helicobacter infections more effectively than WT animals, because they fail to peripherally induce Tregs (10, 13). Experimental infection of Nlrp3–/– and Tlr2–/– mice with the H. pylori isolate PMSS1 recapitulates this phenotype: both strains were colonized at lower levels and exhibited higher gastric mucosal IFN-γ expression (Figure 1, J and K), which correlates well with higher frequencies of IFN-γ–expressing CD4+ T cells in the mesenteric lymph nodes (MLNs) of Tlr2–/– mice relative to those in WT mice (Supplemental Figure 2T). The combined results suggest that H. pylori activates the inflammasome in a TLR2- and NLRP3-dependent manner and benefits from this process because it promotes H. pylori persistence.
Genome-wide screening for factors involved in IL-1β secretion reveals a role for H. pylori LPS and urease.
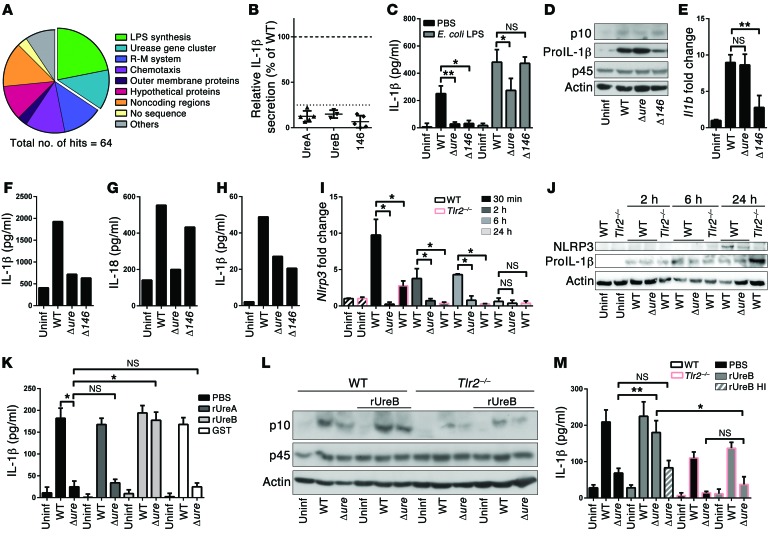
Known activators of the NLRP3 inflammasome include both foreign and endogenous compounds, with the best-understood being urate crystals, asbestos, ATP, and bacterial pore-forming toxins (15). We were able to exclude a role for the H. pylori immunomodulator γ-glutamyl-transpeptidase GGT and the Cag pathogenicity island in CASP1 activation and IL-1β secretion (Supplemental Figure 3, A and B). To search for H. pylori factors involved in inflammasome activation in a genome-wide manner, we took advantage of a previously described tn mutant library (16). As IL-1β secretion by H. pylori–exposed DCs is dependent on CASP1 (10), we opted for IL-1β ELISA as a screening readout (Supplemental Figure 3C). The insertion sites of 64 mutants with defects in inducing IL-1β secretion (<25% of the corresponding WT infection) were sequenced and mapped to 32 different loci (Supplemental Table 1). Loci belonging to two mutant categories were identified repeatedly; these harbored tn insertions in genes involved in LPS biosynthesis and in the urease gene cluster (Figure 2, A and B). The LPS synthesis gene hit most often was LPS-1,2-glycosyltransferase (HPG27_146). LPS from H. pylori deficient for this gene lack the O-side chain and therefore Lewis antigens (Supplemental Figure 4A). A gene-specific deletion mutant (Δ146) in strain G27 recapitulated the tn mutant phenotype, as it failed to induce IL-1β secretion in BMDCs (Figure 2C). The phenotype of this mutant was attributable to its failure to induce pro–IL-1β expression at the transcriptional level, rather than to a defect in CASP1 activation, and could be rescued by E. coli or H. pylori LPS (Figure 2, C–E, and Supplemental Figure 4, B and C). IL-1β expression upon stimulation with both types of LPS was MyD88- and TLR4-dependent (Supplemental Figure 4D).
Figure 2. IL-1β secretion upon H. pylori infection requires LPS-induced transcriptional activation of pro–IL-1β and urease- and TLR2-dependent expression of NLRP3.
(A) Genome-wide screening for H. pylori tn mutants incapable of IL-1β secretion identifies the indicated mutant categories. R-M, restriction modification. (B) Relative IL-1β secretion of individual tn clones with insertions in ureA, ureB, and HPG27_146. (C–E and I–M) Murine BMDCs, (F and G) splenic CD11c+ DCs, and (H) human blood-derived DCs were infected overnight or for the indicated time points with G27 WT, Δure, or Δ146 strains of H. pylori and analyzed by (C, F, H, K, and M) IL-1β ELISA; (G) IL-18 ELISA; (D, J, and L) CASP1 p10 and p45, pro–IL-1β, and NLRP3 Western blotting; and (E and I) qRT-PCR (normalized to Gapdh and to uninfected controls). BMDCs were prestimulated with E. coli LPS where indicated, and 1 μg/ml recombinant GST-tagged UreA, UreB (native or heat-inactivated [HI] for 10 minutes at 70°C), or GST was added to cocultures as noted. Pooled data from 3 to 4 independent experiments are show in C, E, I, K, and M; a representative of 2 experiments is shown in F–H. *P ≤ 0.05, **P ≤ 0.01, Mann-Whitney U test. Error bars represent mean + SD.
Remarkably, of the 8 tn insertions mapping to the urease gene cluster, all affected 2 genes encoding the structural urease subunits, ureA or ureB (Supplemental Table 1). A gene-specific deletion mutant lacking both UreA and UreB proteins (G27Δure, Supplemental Figure 5A) phenocopied the effect of the tn insertion mutants, which could be attributed to its failure to activate CASP1 (Figure 2, C and D). In contrast, Il1b transcription was normal (Figure 2E). Coculturing of murine splenic CD11c+ DCs and human blood-derived DCs confirmed the defect of the H. pylori Δure and Δ146 mutants with respect to IL-1β secretion; in contrast, the secretion of IL-18, which does not require transcriptional activation, was almost at WT levels in the case of the Δ146 mutant (Figure 2, F–H). In summary, our screen identified H. pylori factors regulating CASP1-dependent cytokine secretion at two distinct levels, one transcriptional and one posttranslational.
Given the similarities in outcome of TLR2 deficiency of the host on the one hand and urease deficiency of the bacteria on the other (i.e., lack of CASP1 activation), we hypothesized that H. pylori urease might provide a TLR2-mediated signal to promote inflammasome/CASP1 activation. As NLRP3 expression can be primed by TLR signaling, we asked whether NLRP3 transcript and protein levels in BMDCs are affected by H. pylori exposure. Indeed, WT H. pylori infection efficiently induced NLRP3, but not AIM2 or NLRC4, expression at the transcript and protein levels in a TLR2-, MyD88-, and NF-κB–dependent manner; this was not observed in BMDCs infected with Δure H. pylori (Figure 2, I and J; Supplemental Figure 5, B–H; and data not shown). Interestingly, the defect of the Δure mutant with respect to CASP1 activation and IL-1β secretion could be rescued by recombinant UreB, but not UreA or the GST tag control; this effect was only seen in WT, but not in Tlr2–/–, BMDCs (Figure 2, K–M). Heat-inactivated UreB had no effect on the two processes (Figure 2M and data not shown). Taken together, the results suggest that UreB signals via TLR2 to prime NLRP3 expression, which appears to be a rate-limiting step in H. pylori–induced inflammasome activation and IL-1β processing. This is particularly interesting because TLR2 has not yet been described to be activated by bacterial (non-lipo-) proteins.
H. pylori urease is required for CASP1 activation, Treg responses, and asthma protection in vivo.
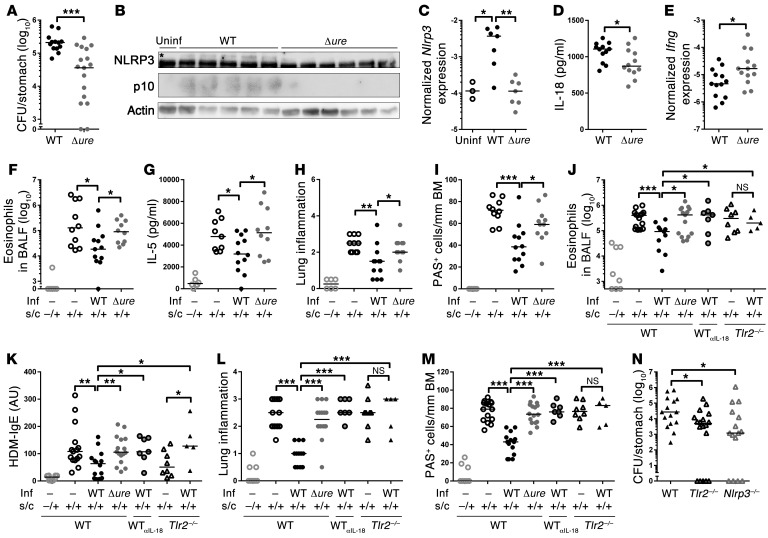
To investigate the consequences of LPS and urease deficiency in vivo, Δure and Δ146 mutants were generated in the mouse-colonizing strains PMSS1 and/or SS1 and used for experimental infections of adult or neonatal C57BL/6 mice. Whereas Δ146 failed to colonize under all circumstances (data not shown), the PMSS1Δure mutant colonized adult infected mice at WT levels for at least 3 months, without evidence of having regained urease expression (Supplemental Figure 6, A and B). In contrast, SS1Δure consistently failed to colonize (data not shown), confirming that urease proficiency is required for mouse colonization in certain strain backgrounds (17). Interestingly, PMSS1Δure induced significantly less gastric production of IL-1β and IL-18 and less active CASP1 than the parental WT strain (Supplemental Figure 6, C–E). In line with the increased gastric IFN-γ expression of Tlr2–/– and Nlrp3–/– animals (Figure 1K), Δure-infected mice exhibited higher gastric mucosal IFN-γ expression and more IFN-γ+CD4+ cells in the MLNs than infected WT animals (Supplemental Figure 6, F and G). A similar pattern was observed in neonatally infected animals, in which CASP1 activation, NLRP3 expression, and IL-18 secretion were also found to depend on urease proficiency of H. pylori; moreover, colonization levels of the Δure mutant were strongly reduced relative to the WT strain in neonatally infected mice (Figure 3, A–D). As in adult infected mice, the Δure mutant elicited higher Ifng expression (Figure 3E). Interestingly, H. pylori urease was further required for the efficient protection against allergen-induced asthma that is a hallmark of neonatally infected mice. All examined parameters of ovalbumin-induced allergic asthma, i.e., bronchoalveolar eosinophilia, lung inflammation, and goblet cell metaplasia as well as pulmonary Th2 cytokine production, were clearly reduced in infected WT animals but not in Δure-infected animals (Figure 3, F–I, and Supplemental Figure 7A). Similar results were obtained in the house dust mite model of allergic asthma (Figure 3, J–M). Moreover, protection was abrogated by a blocking antibody targeting IL-18 and in Tlr2–/– mice (Figure 3, J–M), which, similar to Nlrp3–/– mice, had a lower bacterial burden than WT mice (Figure 3N). Protection against ovalbumin-induced asthma could be adoptively transferred via immunomagnetically purified CD25+ Tregs from H. pylori infected WT donors but not Δure-infected donors; Tregs from H. pylori infected WT Tlr2–/– and Nlrp3–/– animals also failed to confer protection (Supplemental Figure 7, B and C). The quantification of CD25+FoxP3+ Tregs in MLNs further revealed lower Treg frequencies in Δure-infected mice relative to infected WT mice and Tlr2–/– mice relative to WT mice (Supplemental Figure 7, D and E). In summary, the findings described here document a previously unrecognized role of H. pylori urease in innate immune recognition and H. pylori persistence that presumably is unrelated to its function in acid resistance. Here, we show that UreB promotes the TLR2-dependent expression of NLRP3, a critical component of the inflammasome that is required for CASP1 activation and IL-1β/IL-18 processing (see model in Supplemental Figure 8). Infection with urease gene deletion mutants phenocopies the effects of TLR2 and NLRP3 deficiency. The combined results confirm a critical contribution of the TLR2/NLRP3/CASP1/IL-18 axis to microbially induced immune regulation and introduce the H. pylori urease as a novel immunomodulator of this important human pathobiont.
Figure 3. H. pylori urease is required for CASP1 activation, persistence, and asthma protection in neonatally infected mice.
(A–E) Neonatal C57BL/6 mice were infected for 1 month with WT or Δure H. pylori PMSS1 and assessed with respect to (A) gastric colonization, (B) gastric mucosal NLRP3 and CASP1 (asterisk indicates the NLRP3-specific band), (C) Nlrp3 expression, (D) IL-18, and (E) Ifng expression, as analyzed by (B) Western blotting, (D) ELISA, and/or (C and E) qRT-PCR. (F–M) Neonatally infected mice were additionally sensitized and challenged (s.c.) with (F–I) ovalbumin or (J–M) house dust mite (HDM) allergen starting at 4 weeks after infection to induce allergic asthma; αIL-18 mAb was administered weekly starting at the time of infection. Inf, infection. (F and J) Eosinophils in 1 ml of bronchoalveolar lavage fluid (BALF). (G) IL-5 ELISA of ovalbumin-restimulated lung single cell preparations. (H and L) Lung inflammation, as assessed on H&E-stained sections. (I and M) Goblet cell metaplasia, as quantified on PAS-stained sections. BM, basement membrane. (K) House dust mite–specific serum IgE, as determined by ELISA. (N) H. pylori colonization of WT, Tlr2–/–, and Nlrp3–/– mice 1 month after infection. Symbols represent individual animals, and horizontal lines indicate the medians. Pooled data from 2 (A–E and N) and 3 (F–M) independent experiments are shown. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, Mann-Whitney U test.
Methods
Animal experimentation.
C57BL/6 WT, Casp1–/–, Tlr2–/–, Tlr4–/–, Tlr5–/–, Tlr9–/–, Myd88–/–, Trif–/–, Nlrp3–/–, Ifnar–/–, Irf7–/–, Nod2–/–, P2rx7–/–, and Aim2–/– mice were originally obtained from Charles River Laboratories. Nlrc4–/– and Asc–/– mice were provided by Genentech. Nlrp6–/– mice were provided by Millenium Pharmaceuticals. Il1r–/– mice were provided by Manfred Kopf. Mice were infected orally with 108 CFU H. pylori PMSS1 at 6 weeks or 7 days of age. Bacterial colonization was assessed by colony counting. The procedures used for asthma induction and cytokine quantification by qPCR, ELISA, and FACS are described in the Supplemental Methods.
H. pylori strains, infection of DCs, and tn library screening.
H. pylori strains and culture conditions as well as the procedures used for the differentiation and immunomagnetic isolation of DCs and for tn library screening are described in the Supplemental Methods, along with protocols for Western blotting and purification of recombinant proteins.
Statistics.
GraphPad Prism (GraphPad Software) was used for statistical analyses. All P values were calculated by Mann-Whitney U test.
Study approval.
All animal experimentation was reviewed and approved by the Veterinary Office of the canton of Zurich (Zurich, Switzerland) (licenses 24/2013 and 170/2014 to A. Müller).
Supplementary Material
Acknowledgments
This study was funded by Swiss National Science Foundation grants 310030-143609 and BSCGIO_157841/1 to A. Müller. Additional funding was obtained from German Research Foundation grants CRC-796 (project B10) and CRC-1181 (project A04) to S. Backert. We thank Nina Salama for the tn library, Raffaela Semper for help with screening, and Ben Appelmelk for valuable advice on H. pylori LPS.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(8):3297–3302. doi:10.1172/JCI79337.
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Parsonnet J, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198(4):553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010;16(6):1077–1084. doi: 10.1002/ibd.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pritchard DM, Crabtree JE. Helicobacter pylori and gastric cancer. Curr Opin Gastroenterol. 2006;22(6):620–625. doi: 10.1097/01.mog.0000245539.50765.f6. [DOI] [PubMed] [Google Scholar]
- 6.Robinson K, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57(10):1375–1385. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 7.Harris PR, et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134(2):491–499. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Arnold IC, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140(1):199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold IC, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121(8):3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hitzler I, et al. Caspase-1 has both proinflammatory and regulatory properties in helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. J Immunol. 2012;188(8):3594–3602. doi: 10.4049/jimmunol.1103212. [DOI] [PubMed] [Google Scholar]
- 11.Kim DJ, Park JH, Franchi L, Backert S, Nunez G. The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1beta production in Helicobacter pylori-infected dendritic cells. Eur J Immunol. 2013;43(10):2650–2658. doi: 10.1002/eji.201243281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semper RP, et al. Helicobacter pylori-induced IL-1β secretion in innate immune cells is regulated by the NLRP3 inflammasome and requires the cag pathogenicity island. J Immunol. 2014;193(7):3566–3576. doi: 10.4049/jimmunol.1400362. [DOI] [PubMed] [Google Scholar]
- 13.Oertli M, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122(3):1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathinam VA, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150(3):606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev. 2011;243(1):174–190. doi: 10.1111/j.1600-065X.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salama NR, Shepherd B, Falkow S. Global transposon mutagenesis and essential gene analysis of Helicobacter pylori. J Bacteriol. 2004;186(23):7926–7935. doi: 10.1128/JB.186.23.7926-7935.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eaton KA, et al. In vivo complementation of ureB restores the ability of Helicobacter pylori to colonize. Infect Immun. 2002;70(2):771–778. doi: 10.1128/IAI.70.2.771-778.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.