Abstract
Tumor-derived and bacterial phosphoantigens are recognized by unconventional lymphocytes that express a Vγ9Vδ2 T cell receptor (Vδ2 T cells) and mediate host protection against microbial infections and malignancies. Vδ2 T cells are absent in rodents but readily populate the human intestine, where their function is largely unknown. Here, we assessed Vδ2 T cell phenotype and function by flow cytometry in blood and intestinal tissue from Crohn’s disease patients (CD patients) and healthy controls. Blood from CD patients included an increased percentage of gut-tropic integrin β7–expressing Vδ2 T cells, while “Th1-committed” CD27-expressing Vδ2 T cells were selectively depleted. A corresponding population of CD27+ Vδ2 T cells was present in mucosal biopsies from CD patients and produced elevated levels of TNFα compared with controls. In colonic mucosa from CD patients, Vδ2 T cell production of TNFα was reduced by pharmacological blockade of retinoic acid receptor-α (RARα) signaling, indicating that dietary vitamin metabolites can influence Vδ2 T cell function in inflamed intestine. Vδ2 T cells were ablated in blood and tissue from CD patients receiving azathioprine (AZA) therapy, and posttreatment Vδ2 T cell recovery correlated with time since drug withdrawal and inversely correlated with patient age. These results indicate that human Vδ2 T cells exert proinflammatory effects in CD that are modified by dietary vitamin metabolites and ablated by AZA therapy, which may help resolve intestinal inflammation but could increase malignancy risk by impairing systemic tumor surveillance.
Introduction
Tumor cells and bacteria produce nonpeptide metabolites known as phosphoantigens (PAg), which are uniquely recognized by a population of unconventional lymphocytes that express a Vγ9Vδ2 T cell receptor (Vδ2 T cells). Unusually among lymphocytes, Vδ2 T cells are found only in humans and higher primates, where they mediate host protection against a wide range of microbial infections, lymphoproliferative disorders, and solid cancers (1, 2). Although numerous constituents of the gut microbiota are thought to be obligate producers of PAg (1), the absence of Vδ2 T cells in rodent models has so far prevented detailed investigation of their role in mucosal inflammation.
Nonpeptide products of the gut microbiota have been shown to influence the balance of pro- and antiinflammatory lymphocytes in the intestine (3), and studies in macaques have demonstrated that injection of nonpeptide PAg stimulates circulating Vδ2 T cells to proliferate and accumulate in mucosal tissues (4). PAg are produced by a wide range of bacteria that can colonize the gut (1) and can also accumulate in host cells due to dysregulation of the mevalonate kinase metabolic pathway during malignant transformation or microbial infection (5, 6). Intriguingly, human patients with mutations in the mevalonate kinase gene exhibit a severe neonatal colitis that can be successfully treated with bisphosphonate drugs, which modulate PAg synthesis and alter Vδ2 T cell function in vivo (7–10). We recently reported that PAg exposure stimulates human blood Vδ2 T cells to upregulate the gut-homing integrin α4β7, and we identified Vδ2 T cells in human colonic biopsies that produced proinflammatory cytokines and enhanced IFNγ synthesis by intestinal CD4+ T cells (11). These data indicate a potential role for Vδ2 T cells in the pathology of Crohn’s disease (CD), which is characterized by enhanced effector function of CD4+ T cells directed against components of the gut microbiota. In addition to our own detection of Vδ2 T cells in human colonic lamina propria in situ (11), these cells have also been observed in gastrointestinal lymphoid tissues (12) and were previously identified in the gut in a small number of CD patients (13, 14), but the role played by these cells in mucosal inflammation in CD is currently unknown.
The early pathogenesis of CD is thought to involve increased intestinal permeability and altered innate responses to bacterial products that cross the gut barrier, leading to the establishment of a disease-permissive environment in the intestine (15–17). In healthy humans, activation of intestinal Vδ2 T cells by bacterial PAg is likely to be restricted by the gut barrier, but increased intestinal permeability and/or dysbiosis of the gut microbiota in CD could permit increased activation of Vδ2 T cells that are capable of enhancing CD4+ T cell function in the gut (11, 18). We therefore investigated whether human Vδ2 T cells contribute to mucosal inflammation in CD by assessing Vδ2 T cell phenotype, frequency, gut-homing potential, and cytokine production in peripheral blood and colonic biopsy tissue from CD patients and healthy controls. We observed that Vδ2 T cells from CD patients exhibited increased expression of the gut-homing integrin β7 in blood together with a selective depletion of CD27+ “Th1-committed” cells from the circulation, while also displaying a corresponding population of CD27+ Vδ2 T cells in colonic biopsy tissue that produced elevated levels of TNFα relative to healthy controls. Furthermore, manipulation of Vδ2 T cell function by inhibition of retinoic acid receptor–α (RARα) signaling or exposure to the thiopurine drug azathioprine (AZA) exerted potent effects on Vδ2 T cell frequency and cytokine production both in vitro and in vivo. These data could have substantial implications for the management of malignancy risk in patients with CD and other chronic inflammatory disorders, and may lead to the development of therapies that target Vδ2 T cells to reduce inflammation in the human intestine.
Results
Enhanced gut-tropism and depletion of circulating CD27+ Vδ2 T cells in patients with CD.
Vδ2 T cells are unconventional blood lymphocytes that are uniquely able to recognize PAg produced by tumor cells and microbes (1, 5, 6). We recently demonstrated that PAg exposure stimulates human blood Vδ2 T cells to upregulate the gut-homing integrin α4β7 and increase binding to the intestinal addressin MAdCAM-1 (11). To test the hypothesis that human Vδ2 T cells contribute to mucosal inflammation in CD, we first used flow-cytometry to assess the gut-homing potential of blood Vδ2 T cells in a cohort of CD patients who had moderately active disease but who were not receiving immunosuppressive therapy. Since blood Vδ2 T cells uniformly lack integrin αE/CD103 (11, 12, 19), expression of β7 integrin by these cells corresponds to the gut-homing heterodimer α4β7 and accurately reflects potential to bind MAdCAM-1 (11, 20). Blood Vδ2 T cells exhibited a wide range of frequencies in the demographically diverse cohorts of CD patients and controls used for this initial analysis (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI80840DS1), but there was a consistent increase in the proportion of gut-tropic β7+ Vδ2 T cells detected in CD (n = 12, median 80.8% β7+) when compared with healthy volunteers (n = 26, 64.9% β7+; P = 0.010), suggesting increased activation and enhanced gut-homing potential of blood Vδ2 T cells in active disease (refs. 11, 12, and Figure 1A). While it is difficult to quantify the physiological impact of this increased β7 expression among blood Vδ2 T cells in CD, we and others have observed that the high proportions of β7+ Vδ2 T cells present in healthy human blood (~65%–75%) can be further upregulated by exposure to PAg in vitro (~80%–90%) (11, 12), suggesting that these cells may be similarly activated in CD patients in vivo (80.8% in the current report), perhaps due to PAg translocation from the gut (17, 21).
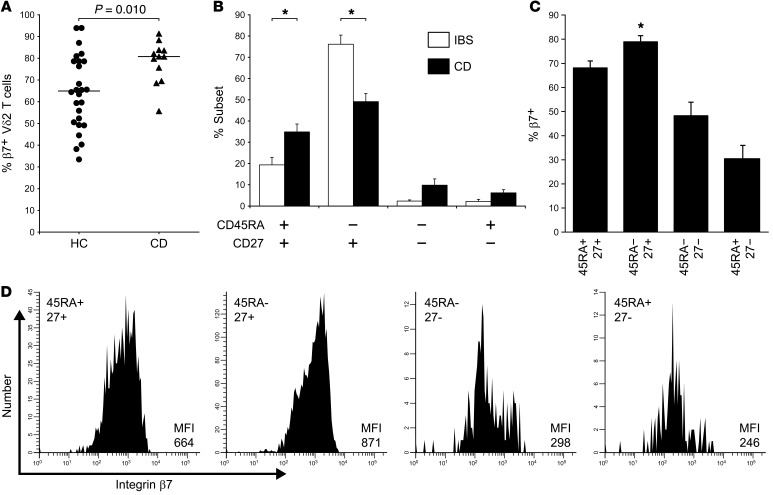
Figure 1. Enhanced gut-homing potential and depletion of circulating CD27+ Vδ2 T cells in CD.
(A) In patients with moderately active CD about to receive de novo AZA therapy (n = 12), flow-cytometric analysis of blood lymphocytes revealed a significant increase in the proportion of β7+ gut-homing cells within the CD3+ Vδ2 T cell population when compared with healthy controls (n = 26). (B) In a separate analysis of pediatric CD patients (mean age 13 years), we observed that the overall proportion of β7+ Vδ2 T cells in blood was comparable with adult CD patients (NS, not shown), but the CD45RA–CD27+ subset was selectively depleted compared with sex/age/ethnicity-matched IBS controls (*P < 0.001; n = 8 per group), while the subset distribution of conventional αβ T cells as defined by these markers was unaltered in CD (data not shown). (C) The CD27+ population of circulating Vδ2 T cells expressed significantly higher levels of β7 integrin than did any other Vδ2 T cell subset (*P < 0.05; n = 8), consistent with increased trafficking of these cells to the gut and a corresponding depletion from the blood in CD. (D) Example histograms showing integrin β7 expression levels in the different Vδ2 T cell subsets detected in blood from an IBS control (representative of n = 8). Significant differences between groups were determined by Mann-Whitney rank-sum test (A), or repeated-measures ANOVA (B and C).
We next sought to determine whether the enhanced gut-tropic phenotype of Vδ2 T cells in CD patients could be attributed to a specific subpopulation of these cells. Human Vδ2 T cells are commonly divided into subsets of putative naive (CD45RA+CD27+), central-memory (CD45RA–CD27+), effector-memory (CD45RA–CD27–), and terminally differentiated effector-memory cells or TEMRA (CD45RA+CD27–) that exhibit differential sensitivity to PAg, variable cytokine production, and distinct proliferative potential (22, 23). Since the population size and subset balance of blood Vδ2 T cells is shaped by age, sex, ethnicity, historical pathogen exposure, and concomitant therapies (24, 25), we sought to minimize these potential influences on our analyses by recruiting a cohort of pediatric CD patients with untreated, moderately active disease (predominantly new diagnoses) and compared these with a closely matched control population of patients with irritable bowel syndrome (IBS). Using this approach, we observed that blood Vδ2 T cell numbers were significantly reduced in pediatric CD patients compared with the matched IBS controls (CD median 23/μl, IBS 74/μl; P = 0.04, data not shown), and that CD45RA–CD27+ cells were selectively depleted in CD (P < 0.001; Figure 1B). These data suggested that circulating CD45RA–CD27+ Vδ2 T cells, which have previously been identified as Th1-committed cells with enhanced survival characteristics (26, 27), are preferentially recruited to the intestine in patients with CD. Consistent with this concept, we also observed that CD27+ Vδ2 T cells expressed higher levels of β7 integrin than were detected in any other subset of Vδ2 T cells in the circulation (P < 0.05; Figure 1, C and D).
Taken together with our recent report that PAg exposure stimulates Vδ2 T cells to upregulate the gut-homing integrin α4β7 and confers increased binding to MAdCAM-1 in vitro (11), as well as with previous observations that MAdCAM-1 expression is upregulated on the vascular endothelium in CD patients in vivo (28), these data suggest that CD is associated with Vδ2 T cell activation, increased gut-tropism, and preferential recruitment of β7hiCD27+ cells to the intestinal mucosa.
TNFα production by intestinal Vδ2 T cells is increased in CD and impaired by disruption of RARα signaling.
We next assessed whether increased β7 expression and depletion of CD27+ Vδ2 T cells from the circulation in active CD was accompanied by the presence of a corresponding Vδ2 T cell population in the intestinal mucosa. Analysis of lamina propria mononuclear cells by flow-cytometry confirmed that mucosal tissue from CD patients contained a distinct population of CD27+ Vδ2 T cells that closely resembled those cells depleted from the blood (Figure 2, A and B). We have previously reported that PAg addition to healthy gut biopsies stimulates CD103– Vδ2 T cells to produce large quantities of IFNγ, leading to increased Th1 commitment of colonic αβ T cells, whereas CD103+ Vδ2 T cells in this tissue were relatively poor cytokine producers (11). Given the limited amount of intestinal tissue available at endoscopy, we instead used unstimulated biopsy cultures in the current report, enabling us to compare Vδ2 T cell cytokine responses to endogenous factors between CD patients and controls rather than risk abolishing differences between groups by adding a potent PAg stimulus throughout. First, we assessed whether CD103– Vδ2 T cells were enriched in the colonic mucosa in CD, but instead we observed no significant difference in subset balance when compared with healthy controls (Figure 2C). However, when we assessed cytokine production profile, mucosal Vδ2 T cells from CD patients produced significantly higher levels of TNFα and IL-17A than those from healthy volunteers (Figure 3A). There was a similar trend for IFNγ production, but this did not reach statistical significance. We further observed that mucosal Vδ2 T cells from CD patients displayed high levels of cytokine synthesis irrespective of CD103 expression (Figure 3B), suggesting that both major Vδ2 T cell subsets in the CD mucosa are strongly activated by endogenous factors. Thus, unlike healthy biopsy tissue (11), the relative frequencies of CD103– and CD103+ cells in CD colon did not influence cytokine production by conventional colonic T cells (data not shown).
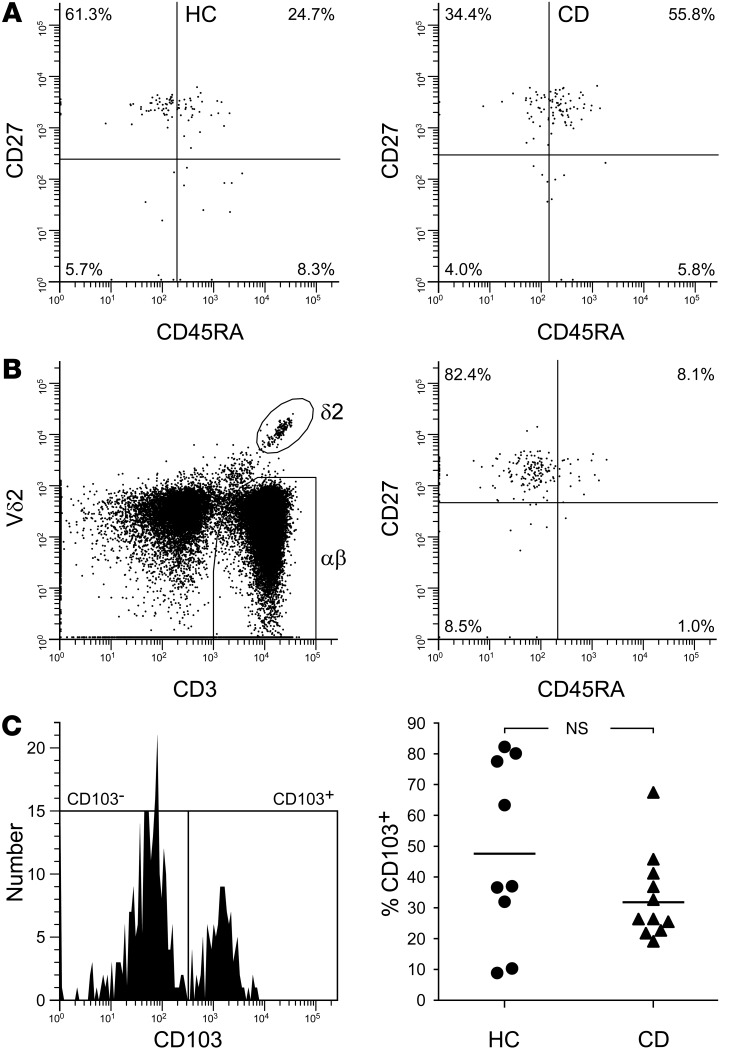
Figure 2. Intestinal Vδ2 T cells in CD patients express CD27 and incorporate both CD103– and CD103+ subsets.
(A) Representative examples of Vδ2 T cell subset distribution in blood from an IBS control (HC) and a patient with CD showing selective depletion of the CD45RA–CD27+ subset in CD (each plot is representative of n = 8 independent experiments, and both display an equal number of cells). (B) Intestinal biopsy tissue from patients with new diagnoses of CD contained a distinct population of Vδ2 T cells that displayed a CD27+ phenotype analogous to that of the cells depleted from peripheral blood. (C) Mucosal Vδ2 T cells in CD patients included both CD103– and CD103+ subsets in proportions comparable to those present in the healthy intestine. Shown is an example flow-cytometry analysis of colonic biopsy tissue after extensive removal of the epithelium and showing positive identification of Vδ2 T cells (thus excluding Vδ1 T cells) among the egressed leukocytes in an 18-year-old patient with active CD (B; representative of n = 11 CD patients), as well as grouped data showing Vδ2 T cell subset balance in multiple individuals (C; n = 11 CD patients, n = 9 healthy controls; comparison by t test).
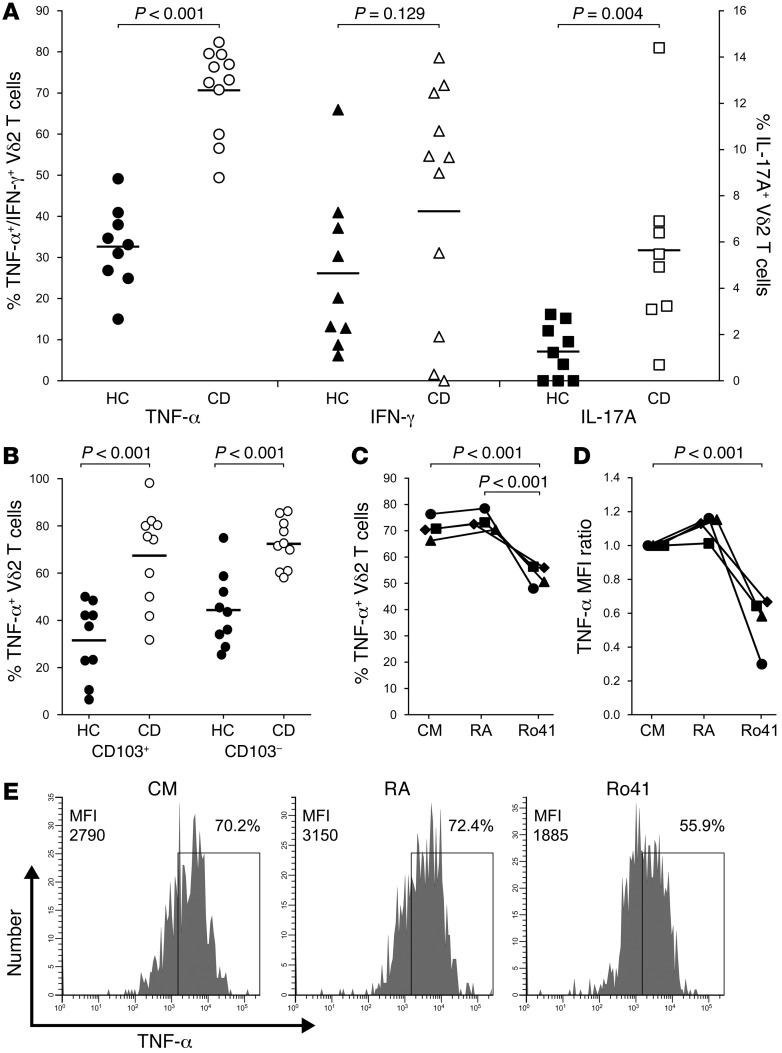
Figure 3. TNFα production by mucosal Vδ2 T cells is increased in CD and impaired by blockade of RARα signaling.
(A) Vδ2 T cells in biopsy tissue from CD patients (n = 11) displayed elevated production of TNFα and IL-17A compared with mucosal Vδ2 T cells from healthy controls (n = 9). Vδ2 T cells were identified as CD3+Vδ2+ gut lymphocytes, and in some assays, fixable live/dead dye was used to confirm the viability of cells in this gate. In some experiments, IL-17A staining was omitted due to the limited number of biopsies available. (B) Enhanced TNF synthesis by Vδ2 T cells in CD intestine was not restricted to a specific cell subset, since both CD103– and CD103+ populations produced comparable levels of this cytokine in the unstimulated biopsy cultures (n = 10 CD patients, n = 9 healthy controls). (C–E) Biopsy supplementation with 2nM all-trans RA over the 3d culture period exerted little effect on cytokine production by mucosal Vδ2 T cells from CD patients (n = 4), whereas inhibition of RARα signaling with the specific antagonist Ro41-5253 led to a substantial reduction in TNF synthesis. Significant differences between groups were determined by t test (A and B) or repeated-measures ANOVA (C and D). Example histograms in E are representative of n = 4 independent experiments.
We next sought to identify factors in the human intestinal environment that might increase proinflammatory cytokine production by Vδ2 T cells in CD patients. Intestinal antigen-presenting cells (APCs) metabolize dietary vitamin A into retinoic acid (RA), which exerts potent effects on T cell differentiation, effector function, gut tropism, and migration to inflamed tissues (29–31). Having recently reported that human intestinal APC display increased capacity for RA synthesis in inflammatory bowel disease (IBD) (32), and that human Vδ2 T cell function can be modified by exposure to RA in vitro (11), we hypothesized that RA signaling might influence Vδ2 T cell production of proinflammatory mediators in the CD intestine in vivo. To test this hypothesis, we collected biopsies of inflamed colonic mucosa from patients with new diagnoses of CD and assessed cytokine production by intestinal Vδ2 T cells after 3d culture with either exogenous RA or with the specific RARα antagonist Ro41-5253. Supplementation with 2nM all-trans RA appeared to induce a marginal increase in TNF production by Vδ2 T cells in CD biopsy tissue (the small effect likely being due to endogenous RA in the biopsy cultures; compare CM with RA in Figure 3, C and D; P = 0.357 and P = 0.209, respectively), but inhibition of RARα signaling with Ro41-5253 led to a substantial reduction in TNF synthesis by these cells (P < 0.001; Figure 3, C–E). Taken together with our previous finding that RA can increase the gut-homing potential of Vδ2 T cells in human peripheral blood (11), these data indicate that RA signaling in the local microenvironment influences Vδ2 T cell function and may alter cytokine production by these cells in the inflamed human intestine.
AZA selectively and reversibly ablates Vδ2 T cells in patients with CD.
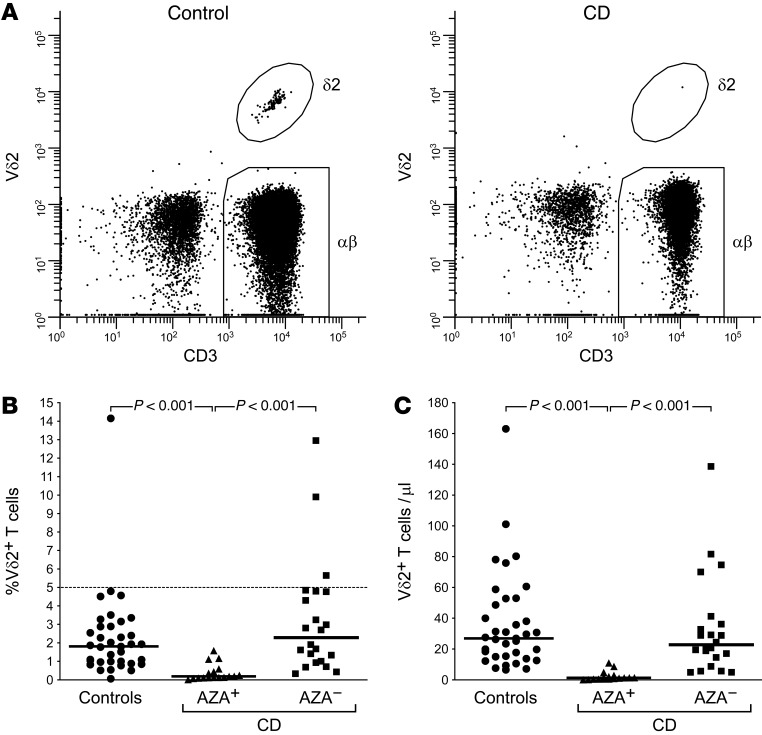
Having observed that exposure to pharmacological agents can substantially reduce TNF synthesis by intestinal Vδ2 T cells in CD patients, we next sought to determine whether the therapies routinely used to treat CD could similarly impair Vδ2 T cell function. Indeed, stimulation with microbial PAg induced rapid expansion of blood Vδ2 T cells over 5d culture in vitro, but even subtherapeutic concentrations of the thiopurine drug AZA (33) were sufficient to disrupt this response, while comparable effects on autologous conventional αβ T cells were only achieved at 100-fold higher doses (Figure 4). Staining with the viability dyes annexin V and 7-AAD confirmed that Vδ2 T cells and αβ T cells displayed comparable rates of drug-induced cell death in these assays, suggesting that AZA can selectively impair Vδ2 T cell proliferation (not shown). Accordingly, while Vδ2 T cells made up approximately 2% of total circulating T cells in both healthy volunteers and AZA-naive CD patients, these cells were essentially absent from the blood of CD patients established on AZA therapy (Figure 5A). There was a selective loss of circulating Vδ2 T cells in AZA-treated CD patients, whether numbers were assessed as a proportion of the total T cell pool (Figure 5B) or by calculating the absolute number of these cells per unit volume of blood (Figure 5C). In order to confirm the selective effects of AZA therapy on Vδ2 T cells, we next generated new data using a previously published cohort (16) of AZA-treated CD patients (n = 5) and healthy controls (n = 8), and determined the extent to which AZA depletes different populations of conventional blood T cells (CD4+ naive, CD4+ memory, CD8+ naive, CD8+ memory). This analysis confirmed that no major subset of αβ T cells in blood was depleted by more than 25% in AZA-treated CD, whereas circulating Vδ2 T cell numbers in the same individuals were reduced by >95% (Supplemental Figure 2). Indeed, an independent analysis of the blood γδ T cell compartment in newly recruited cohorts of AZA-treated CD patients and healthy controls (both n = 3), confirmed a selective loss of Vδ2+ cells only (<0.1% of total T cells in AZA+ CD, >1% in healthy controls) whereas Vδ1+ cells were preserved (~1% in AZA+ CD, ~1% in controls; data not shown), thus indicating that the drug ablates Vδ2 T cells in CD while sparing other major subsets of circulating γδ T cells. Consistent with these data, intestinal Vδ2 T cells were relatively frequent in biopsy tissue obtained from healthy controls (n = 4, mean 0.82% of total lamina propria T cells) or AZA-naive CD patients (n = 6, 0.78%), but numbers were significantly suppressed in CD patients receiving AZA (n = 5, <0.2%; P < 0.05).
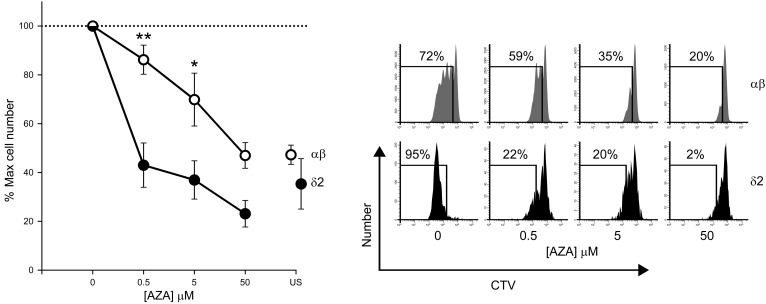
Figure 4. Activated human Vδ2 T cells are highly sensitive to AZA exposure.
Peripheral blood mononuclear cells were labeled with CellTrace Violet (CTV) dye, seeded into 96-well round-bottom plates in complete medium (4 × 105 cells/well) and stimulated with 1 nM HDMAPP phosphoantigen (1-hydroxy-2-methyl-2-buten-4-yl 4-diphosphate, tebu-bio, Peterborough, UK) together with anti-CD2/3/28 beads to activate conventional T cells (Miltenyi Biotec) in the presence of 0-50 μM AZA (Sigma-Aldrich) for 5 days at 37°C, 5% CO2. Both Vδ2 T cells and conventional αβ T cells expanded markedly over the 5-day culture, as assessed by flow-cytometry. Addition of low-dose AZA to these cultures significantly impaired the proliferation of Vδ2 T cells while exerting little effect on αβ T cell expansion. This difference in population growth was evident even at clinically relevant concentrations of AZA (5 μM) (33). Impairment of αβ T cell proliferation comparable to that observed for Vδ2 T cells was achieved only at high doses of AZA. Two-way repeated-measures ANOVA was used to test the influence of cell type and drug dose on proliferated cell number (*P = 0.022, **P = 0.005; compared with Vδ2 T cells subjected to the same concentration of AZA. There was a statistically significant interaction between cell type and drug dose; P = 0.018). Shown are grouped data from n = 6 independent experiments and a representative example of cell proliferation analysis by flow-cytometry. Cell frequencies were normalized to maximum proliferated cell number to control for intra/interindividual variability in expansion of the different cell types. US, Unstimulated cells in the absence of AZA.
Figure 5. Selective loss of circulating Vδ2 T cells in AZA-treated CD.
(A) Flow-cytometry enabled the identification of Vδ2 T cells in addition to conventional αβ T cells in direct ex vivo analyses of peripheral blood from healthy volunteers. In contrast, blood samples from CD patients receiving AZA therapy contained only trace numbers of Vδ2 T cells, whereas αβ T cell numbers were consistent with expected frequencies in AZA-treated patients. (B and C) Loss of Vδ2 T cells in AZA-treated CD (AZA+; n = 17) was extensive and selective, whether assessed as a proportion of the total T cell pool (B) or by calculating the absolute number of these cells per unit volume of blood (C). CD patients not receiving AZA therapy (AZA–; n = 20) exhibited Vδ2 T cell frequencies comparable with healthy controls (n = 36). Example dot plots in A are representative of n = 36 healthy volunteers and n = 17 AZA-treated CD patients. Bars in B and C indicate group median values. Significant differences between groups were determined using Kruskal-Wallis 1-way ANOVA on ranks.
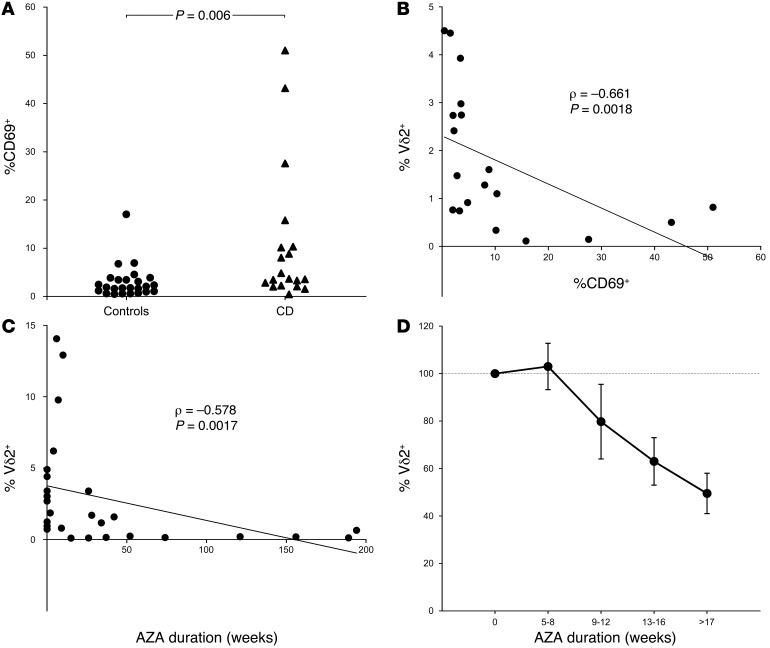
Previous data from a murine model have indicated that selective depletion of conventional memory T cells can be driven by repeated stimulation in the presence of AZA (33), which is known to disrupt purine biosynthesis, inhibit DNA replication, and induce apoptosis of highly activated T cell clones (34). Since human Vδ2 T cells are almost exclusively comprised of memory cells, we next sought evidence of ongoing Vδ2 T cell activation in CD that might drive the depletion of these cells in the presence of AZA. Indeed, the proportion of circulating Vδ2 T cells that displayed an activated CD69+ phenotype was significantly higher in CD patients than in healthy volunteers (Figure 6A). We therefore assessed whether the ablation of Vδ2 T cells was an AZA-specific phenomenon by analyzing the blood frequency of Vδ2 T cells in CD patients treated with the alternative immunosuppressant drug methotrexate (MTX), which exhibits a distinct mechanism of action from AZA but induces a similar inhibition of purine biosynthesis to restrict the proliferation of highly activated T cells (35). Using this approach, we observed that Vδ2 T cells comprised mean 2.13% of total blood T cells in MTX-treated CD patients (n = 3), which was comparable with healthy controls (2.25%; n = 36, NS), and significantly higher than that detected in CD patients receiving AZA therapy (0.39%; n = 17, P = 0.026). These data suggested that selective ablation of Vδ2 T cells in AZA-treated CD cannot be attributed solely to drug disruption of the purine biosynthetic pathways that sustain highly proliferative T cell clones. It remained unclear, therefore, whether Vδ2 T cells might be subject to increased activation in the blood of CD patients in vivo. In preclinical models, in vivo activation of circulating Vδ2 T cells stimulates rapid population expansion (4), so we next investigated whether CD69 expression correlated with Vδ2 T cell frequency in human CD. Instead, CD69 expression was associated with reduced Vδ2 T cell frequency (Figure 6B), perhaps indicating homeostatic proliferation in response to low cell numbers (36).
Figure 6. Activation and depletion of Vδ2 T cells in AZA-treated CD.
(A–B) An analysis of CD patients with inactive disease (CDAI < 150; n = 19) indicated that the proportion of CD69+-activated Vδ2 T cells in the circulation was significantly enhanced compared with healthy volunteers (n = 25) (A) and also correlated with reduced cell frequency in blood (B). These data suggested that Vδ2 T cell activation and recruitment to the gut might contribute to loss of these cells from the circulation in CD. (C and D) In a cross-sectional study of AZA-treated CD patients (n = 27), ablation of circulating Vδ2 T cells was observed after >1 year continuous therapy (C) and longitudinal followup analyses confirmed a progressive loss of Vδ2 T cells with rapid onset after commencing AZA treatment (D; mean ± SEM of n = 3–5 CD patients per time point). CD patients and control groups were compared using unpaired t tests. Correlations were assessed using Spearman rank tests.
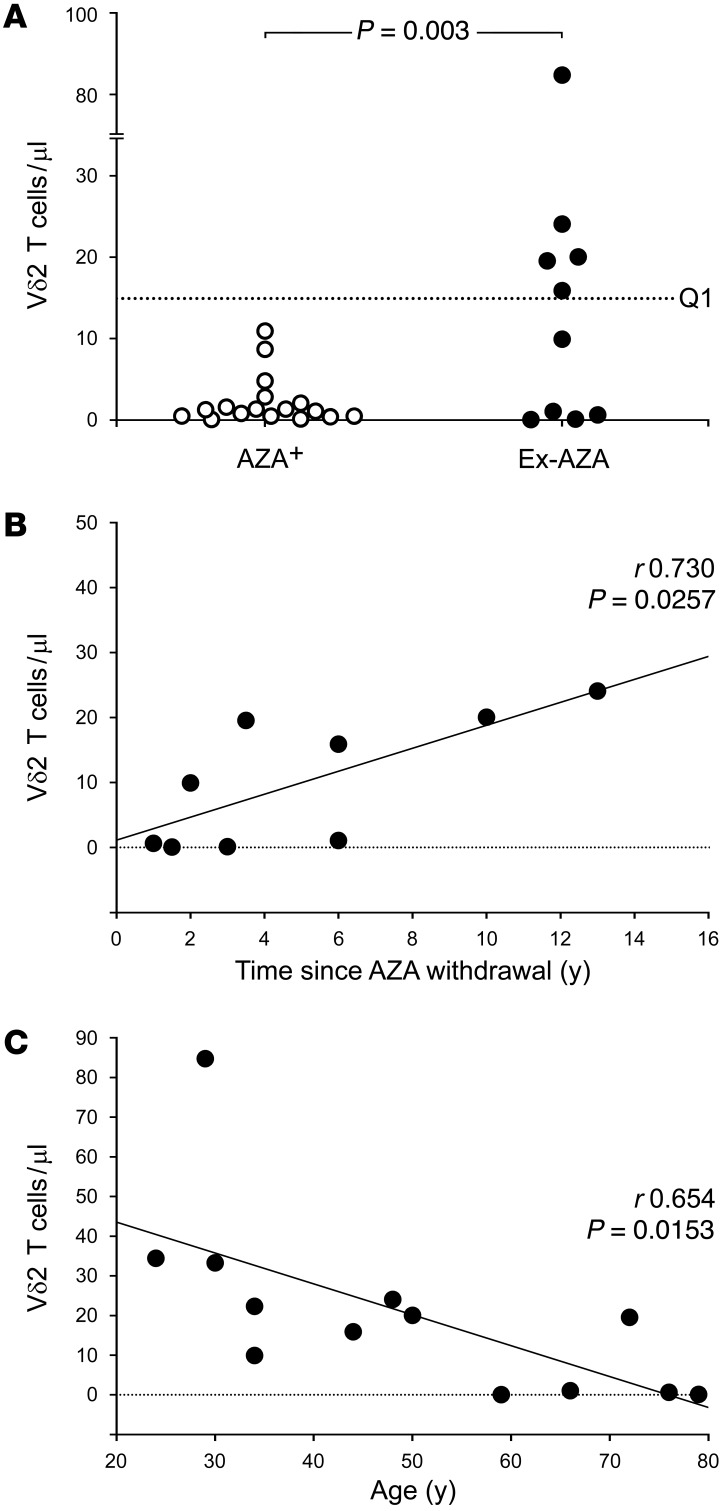
The clinical benefits of AZA are typically not seen until several months after initiation of therapy (37), and our cross-sectional analysis of AZA-treated CD patients revealed that blood Vδ2 T cell frequency was inversely correlated with treatment duration (Figure 6C). Accordingly, a longitudinal followup analysis of CD patients commencing de novo AZA therapy confirmed a rapid decline in Vδ2 T cell frequency after initiating treatment (Figure 6D). Since the long-term absence of Vδ2 T cells is likely to have detrimental effects on tumor surveillance (38–40), we next assessed whether Vδ2 T cells could be recovered after AZA withdrawal. To do this, we conducted a cross-sectional study of CD patients who had previously been treated with AZA for >1 year but were not receiving any thiopurine therapy at the time of sampling (ex-AZA). We observed evidence of blood Vδ2 T cell recovery in 5 of 10 ex-AZA CD patients — where the recovery threshold was defined as the 25th percentile of Vδ2 T cell frequency in AZA-naive CD patients — but not in any of the 17 CD patients on concurrent AZA therapy (Figure 7A; P = 0.003). Blood Vδ2 T cell frequency in the ex-AZA CD group was positively correlated with time elapsed after drug withdrawal (Figure 7B; r0.730, P = 0.0257), and was inversely correlated with patient age (Figure 7C; r0.654, P = 0.0153), suggesting that age-related decline in Vδ2 T cell numbers and function may impede recovery of their tumor-surveillance role after AZA cessation (24).
Figure 7. Post-AZA recovery of blood Vδ2 T cells is influenced by patient age and time elapsed since drug withdrawal.
(A) Cross-sectional study of CD patients previously treated with AZA for >1 year but not receiving thiopurine therapy at the time of sampling (ex-AZA; n = 10). Evidence of blood Vδ2 T cell recovery was observed in 5 of 10 ex-AZA CD patients (recovery threshold Q1 defined as the 25th percentile of Vδ2 T cell frequency in AZA-naive CD patients), but not in any of the CD patients on concurrent AZA therapy (AZA+; n = 17). Fisher exact test was used to assess Vδ2 T cell recovery after AZA withdrawal. (B and C) Blood Vδ2 T cell frequency in the ex-AZA CD patients was positively correlated with time elapsed since drug withdrawal (B; n = 9), and was inversely correlated with patient age (C; n = 13). Correlations were assessed using Pearson product-moment.
Discussion
This study provides evidence of enhanced gut-tropism and selective depletion of circulating CD27+Vδ2 T cells in patients with active CD, consistent with increased recruitment of these cells to the inflamed intestine. Accordingly, we detected a corresponding population of CD27+Vδ2 T cells in the lamina propria of CD patients that displayed elevated TNF production, which could be disrupted by pharmacological blockade of signaling through RARα. Furthermore, we observed that Vδ2 T cells in CD patients are highly sensitive to AZA exposure, leading to the selective ablation of these cells over about a year of continuous therapy. While impaired Vδ2 T cell function may contribute to the therapeutic effects of AZA in active CD, complete loss of this tumor surveillance population may also confer increased risk of malignancy, consistent with the increased rates of lymphoma observed in AZA-treated CD patients (41–43).
Due to ethical constraints, it is difficult to demonstrate cell recruitment to peripheral tissues in human patients in vivo, but our observation that CD27+Vδ2 T cells express high levels of β7 integrin in blood, are selectively depleted from the circulation in CD, and predominate in the inflamed colonic mucosa strongly suggest that these cells are activated and recruited to the intestine in patients with CD. Further investigation will now be required to identify the microbial/host-derived signals that may drive this process, although these are likely to include increased intestinal permeability (17), changes in the composition of the microbiota (1, 44), and systemic priming of innate leukocytes by bacterial products translocated from the gut (21). Indeed, it has already been reported that environmental exposures influence γδ T cell frequency in human peripheral blood (25) and that stable expansions of Vδ2 T cells can occur in the circulation of patients with IBD (45, 46). Consistent with these data, Vδ2 T cells have also been identified in the gut in a small number of CD patients (13, 14), although the role played by these cells in mucosal inflammation in CD has, until now, remained obscure.
In the current report, we observed a selective depletion of the CD27+ subset of blood Vδ2 T cells that reportedly displays high sensitivity to PAg, exhibits substantial proliferative potential, and can generate large numbers of IFNγ+ and TNFα+ cells upon sustained PAg exposure (22, 23). Accordingly, mucosal biopsies from CD patients contained a corresponding population of CD27+ Vδ2 T cells that produced elevated levels of TNFα and IL-17A, and incorporated both CD103– and CD103+ subsets, which displayed comparable levels of cytokine production. Indeed, we also observed high levels of TNF synthesis by intestinal Vδ2 T cells in a pilot analysis of patients with ulcerative colitis (data not shown). Therefore, the increased effector function of Vδ2 T cells in inflamed human colon may not be restricted to CD alone. Since we used biopsy explant cultures to obtain sufficient Vδ2 T cells for the analyses presented in our report, we cannot exclude the possibility that some Vδ2 T cells were retained in the undigested mucosal tissue, or that small numbers of blood contaminants might have been present in our assays. However, since human blood contains multiple Vδ2 T cell population with distinct functional attributes (22), whereas intestinal Vδ2 T cells were exclusively CD45RA–CD27+ and displayed a cytokine profile that was highly consistent within patient groups, it is unlikely that our cultures contained substantial numbers of blood lymphocytes. Our in vitro data also demonstrated that RARα signaling is required to mediate TNF production by these cells, suggesting that the vitamin A metabolite RA may modulate inflammatory responses in the human gut. Taken together with our report that intestinal APC exhibit increased capacity to generate RA in patients with IBD (32), these findings suggest that RA synthesis by colonic APC may regulate Vδ2 T cell function and influence TNF production in the human intestine (31).
AZA-treated CD patients exhibit increased risk of lymphoproliferative disorders (42, 47), nonmelanoma skin cancers (48), and hepatosplenic T cell lymphoma (HSTCL) (49). Given that Vδ2 T cells are a key component of tumor surveillance in humans (41), selective depletion of this population in AZA-treated CD would be expected to confer increased risk of malignancy. Indeed, circulating Vδ2 T cells can suppress Epstein-Barr virus (50), have displayed therapeutic potential in a range of hematological and solid cancers (2, 51), and contain a recently identified population of skin-homing cells that may prove capable of destroying cutaneous tumors (11, 19). Cases of HSTCL in CD are known to occur primarily among young, male patients receiving long-term AZA therapy (49), and in an analysis of published reports and data collected via the MedWatch reporting system of the US Food and Drug Administration, 34 of 36 patients presenting with HSTCL were male, 90% were <35 years old, and most had been receiving thiopurine therapy for >2 years (49). It is notable therefore that the natural loss of Vδ2 T cells observed in adults over 30 years of age is more pronounced in males and that progressive depletion of the antitumor subset of IFNγ-producing Vδ2 T cells features only in men (24). AZA may therefore accelerate the natural decline of Vδ2 T cell tumor surveillance in CD patients more acutely in men than in women. Indeed, lymphoma risk in AZA-treated IBD has been reported to progressively increase with therapy duration (52), and older male patients are at the greatest risk of AZA-induced malignancy (53). Furthermore, HSTCL in AZA-treated CD is frequently of γδ T cell origin (49), and anti-TNF therapy reportedly increases the proliferation of γδ T cells in young, male CD patients (46). These data suggest that the Vδ2 T cell population itself may become dysregulated and eventually undergo malignant transformation in AZA-treated CD.
Our analyses revealed that the Vδ2 T cell population can be reconstituted in CD patients that cease AZA therapy, consistent with reports that lymphoma risk returns to normal levels after withdrawal of treatment (42). Since greater numbers of young patients are now receiving AZA for increasing lengths of time, either for CD or other types of chronic disease, new treatment strategies that spare the tumor-responsive Vδ2 T cell compartment may help to mitigate long-term risk of malignancy (42, 48). Preclinical studies have already identified methods of inducing Vδ2 T cell expansion for therapeutic benefit (40, 51), and similar approaches may also prove useful for preserving Vδ2 T cell function and reducing cancer risk in AZA-treated diseases. However, the potential benefits of these strategies will need to be weighed against the possibility of exacerbating mucosal inflammation by activating Vδ2 T cells in the intestine.
Methods
Study participants.
Peripheral blood and colonic biopsies were obtained from patients undergoing colonoscopy as part of routine clinical care to assess CD activity, or for colorectal cancer screening or investigation of rectal bleeding but with no endoscopic abnormalities (controls). Additional samples of mucosal tissue were obtained from patients undergoing surgical resection for colorectal cancer, noninflammatory intestinal motility disorders, or severe CD. The CD cohort was heterogeneous; distinct subgroups of patients were recruited to address specific experimental questions (details in the corresponding results sections). The overall characteristics of the CD cohort were as follows: 41% male; mean age, 35 years (range 7–79); age at diagnosis, 21 years (range 4–51); disease duration, 14 years (range 1–60); C-reactive protein (CRP), 36 mg/l (range <5–318). Montreal classification: A1, 40.7%; A2, 51.9%; A3, 7.4%; L1, 25.9%; L2, 18.5%; L3, 55.6%; L4, 29.6%; B1, 42.1%; B2, 31.6%; B3, 26.3%; P, 7.4% (54). To assess the effects of thiopurine treatment, some CD patients were recruited immediately prior to commencing AZA therapy, and longitudinal blood sampling was conducted over a followup period of 6 months. The analyses of Vδ2 T cell subset distribution in peripheral blood were conducted in a subgroup of pediatric CD patients (mean age, 13 years [range 7–19]; 50% male; CRP, mean 12.5 mg/l [range 5–39 mg/l]) and sex/age/ethnicity-matched IBS controls (mean age, 13 years [range 6–20]; 50% male; CRP, <5 mg/l) in order to limit the effects of demography, historical pathogen exposure, and concomitant therapies.
Peripheral blood cells.
Human whole blood was directly labeled with monoclonal antibodies for 15 minutes at room temperature, and the red blood cells were lysed by addition of Optilyse C (Beckman Coulter). Labeled cell suspensions were washed twice in cold FACS buffer (PBS containing 2% fetal calf serum, 0.02% sodium azide, 1 mM EDTA) and fixed in 1% paraformaldehyde.
Lamina propria mononuclear cells.
Intestinal biopsies were washed in 1 mmol/l dithiothreitol (Sigma-Aldrich), and incubated in 1 mmol/l EDTA for 1 hour to thoroughly detach the epithelium and remove epithelial lymphocytes prior to biopsy culture in complete medium (Dutch-modified RPMI-1640 medium, 10% FCS, 2 mM l-glutamine, 100 μ/ml penicillin, 100 μg/ml streptomycin, 25 μg/ml gentamicin, 30 μ/ml recombinant human IL-2) in 24-well plates in the presence or absence of 2 nM all-trans RA (Sigma-Aldrich), or 1 μM RARα-selective antagonist Ro41-5253 (Enzo Life Sciences Inc.), for 3–5 days at 37°C, 5% CO2. Lamina propria mononuclear cells that migrated out of the biopsies were labeled with mAb for flow-cytometry or were reactivated with PMA (10 ng/ml) and ionomycin (2 μM) in the presence of monensin (3 μM) for 4 hours at 37°C, 5% CO2 prior to surface labelling. The reactivated cells were then permeabilized using Leucoperm reagents (AbD Serotec), labeled with anti-cytokine mAb, and fixed in 1% paraformaldehyde for analysis by flow cytometry.
Flow-cytometry.
Monoclonal antibodies were: PerCP-Cy5.5–conjugated CD3 (clone HIT3a); FITC-conjugated TCR Vδ2 (B6); Alexa Fluor 647–conjugated CD27 (O323) and CD103 (Ber-ACT8); PE/Cy7-conjugated CD45RA (HI100) and IFNγ (4S.B3); PE-conjugated integrin β7 (FIB504), CD69 (FN50), TNFα (MAb11), IL-17A (BL168), and IL-10 (JES3-9D7) from BioLegend UK; and FITC-conjugated TCR Vδ1 (REA173) from Miltenyi Biotec. Labeled cells were acquired on a FACSCanto II flow-cytometer using FACSDiva 6.1.2 software (BD Biosciences), and data were analyzed using WinList 6.0 (Verity Software House). Absolute cell numbers were determined using Flow-Count Fluorospheres (Beckman Coulter).
Statistics.
Statistical analyses were performed using SigmaStat 3.5 (SYSTAT). Normal data were analyzed by 2-tailed t test or 1-way ANOVA as appropriate. Mann-Whitney and Wilcoxon signed-rank tests were used to evaluate non-normally distributed data. One-way repeated measures ANOVA was used to determine the effects of biopsy supplementation with RA or Ro41-5253. Two-way repeated measures ANOVA was used to test the influence of cell type and drug dose on proliferated cell number in the AZA in vitro assays. Kruskal-Wallis 1-way ANOVA on ranks was used to compare blood Vδ2 T cell frequencies between healthy controls and CD treatment groups. Correlations were assessed using Pearson product-moment and Spearman rank tests. Fisher exact test was used to assess Vδ2 T cell recovery after AZA withdrawal. Error bars in the figures indicate standard error of the mean. P < 0.05 was considered significant.
Study approval.
Ethical permissions for the study were granted by the appropriate local research ethics committees (approvals 05/Q0405/71 from Harrow Research Ethics Committee; 10/H0704/74 from East London Research Ethics Committee 2, London, UK; P/01/023 from East London and City Health Authority Research Ethics Committee, London, UK; and 7/H0805/46 from Bromley Local Research Ethics Committee). All volunteers gave written informed consent prior to inclusion in the study.
Supplementary Material
Acknowledgments
N.E. McCarthy was funded by Crohn’s and Colitis UK (M/13/5). C.R. Hedin was funded by Core Charity (COR002). T.J. Sanders was funded by The Broad Medical Research Program (IBD-0317R). The authors wish to thank Daniel Pennington for useful discussion, Samiul Hasan for providing the TCR Vδ1 monoclonal antibody, and Anna Vossenkämper, Francesca Ammoscato, Aneta Kucik, Luke Hanna, Carla Felice, Cian McGuire, John Broad, and Farah Barakat for their assistance in sample collection.
Footnotes
Conflict of interest: Kevin Whelan has received consultancy fees from Danone and lecturing fees from Yakult. James O. Lindsay has received lecturing fees and funding for research from MSD, and he is a member of the MSD Immunology advisory board. Andrew J. Stagg and James O. Lindsay have received funding for research from Takeda UK Limited.
Reference information:J Clin Invest. 2015;125(8):3215–3225. doi:10.1172/JCI80840.
References
- 1.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 2003;544(1–3):4–10. doi: 10.1016/s0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 2.Fournie JJ, et al. What lessons can be learned from γΔ T cell-based cancer immunotherapy trials? Cell Mol Immunol. 2013;10(1):35–41. doi: 10.1038/cmi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arpaia N, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali Z, et al. Prolonged (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-driven antimicrobial and cytotoxic responses of pulmonary and systemic Vγ2VΔ2 T cells in macaques. J Immunol. 2007;179(12):8287–8296. doi: 10.4049/jimmunol.179.12.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197(2):163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kistowska M, et al. Dysregulation of the host mevalonate pathway during early bacterial infection activates human TCR gamma delta cells. Eur J Immunol. 2008;38(8):2200–2209. doi: 10.1002/eji.200838366. [DOI] [PubMed] [Google Scholar]
- 7.Levy M, et al. Severe early-onset colitis revealing mevalonate kinase deficiency. Pediatrics. 2013;132(3):e779–e783. doi: 10.1542/peds.2012-3344. [DOI] [PubMed] [Google Scholar]
- 8.Bianco AM, Girardelli M, Vozzi D, Crovella S, Kleiner G, Marcuzzi A. Mevalonate kinase deficiency and IBD: shared genetic background. Gut. 2014;63(8):1367–1368. doi: 10.1136/gutjnl-2013-306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantarini L, et al. Weekly oral alendronate in mevalonate kinase deficiency. Orphanet J Rare Dis. 2013;8:196. doi: 10.1186/1750-1172-8-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human Vγ2VΔ2 T cells in vivo. J Clin Invest. 2001;108(9):1349–1357. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy NE, et al. Proinflammatory VΔ2+ T cells populate the human intestinal mucosa and enhance IFN-γ production by colonic αβ T cells. J Immunol. 2013;191(5):2752–2763. doi: 10.4049/jimmunol.1202959. [DOI] [PubMed] [Google Scholar]
- 12.Brandes M, et al. Flexible migration program regulates γΔ T cell involvement in humoral immunity. Blood. 2003;102(10):3693–3701. doi: 10.1182/blood-2003-04-1016. [DOI] [PubMed] [Google Scholar]
- 13.McVay LD, et al. Changes in human mucosal γΔ T cell repertoire and function associated with the disease process in inflammatory bowel disease. Mol Med. 1997;3(3):183–203. [PMC free article] [PubMed] [Google Scholar]
- 14.Kanazawa H, Ishiguro Y, Munakata A, Morita T. Multiple accumulation of VΔ2+ γΔ T cell clonotypes in intestinal mucosa from patients with Crohn’s disease. Dig Dis Sci. 2001;46(2):410–416. doi: 10.1023/A:1005669319556. [DOI] [PubMed] [Google Scholar]
- 15.Hedin CR, Stagg AJ, Whelan K, Lindsay JO. Family studies in Crohn’s disease: new horizons in understanding disease pathogenesis, risk and prevention. Gut. 2012;61(2):311–318. doi: 10.1136/gut.2011.238568. [DOI] [PubMed] [Google Scholar]
- 16.Hedin CR, et al. Altered intestinal microbiota blood T cell phenotype are shared by patients with Crohn’s disease their unaffected siblings. Gut. 2014;63(10):1578–1586. doi: 10.1136/gutjnl-2013-306226. [DOI] [PubMed] [Google Scholar]
- 17.Issenman RM, Jenkins RT, Radoja C. Intestinal permeability compared in pediatric and adult patients with inflammatory bowel disease. Clin Invest Med. 1993;16(3):187–196. [PubMed] [Google Scholar]
- 18.Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53(1):1–4. doi: 10.1136/gut.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laggner U, et al. Identification of a novel proinflammatory human skin-homing Vγ9VΔ2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187(5):2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berlin C, et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74(1):185–195. doi: 10.1016/0092-8674(93)90305-A. [DOI] [PubMed] [Google Scholar]
- 21.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16(2):228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieli F, et al. Differentiation of effector/memory VΔ2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198(3):391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of γΔ T cell subsets in mouse and human. Immunology. 2012;136(3):283–290. doi: 10.1111/j.1365-2567.2012.03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caccamo N, Dieli F, Wesch D, Jomaa H, Eberl M. Sex-specific phenotypical and functional differences in peripheral human Vγ9/VΔ2 T cells. J Leukoc Biol. 2006;79(4):663–666. doi: 10.1189/jlb.1105640. [DOI] [PubMed] [Google Scholar]
- 25.Esin S, Shigematsu M, Nagai S, Eklund A, Wigzell H, Grunewald J. Different percentages of peripheral blood γΔ+ T cells in healthy individuals from different areas of the world. Scand J Immunol. 1996;43(5):593–596. doi: 10.1046/j.1365-3083.1996.d01-79.x. [DOI] [PubMed] [Google Scholar]
- 26.DeBarros A, Chaves-Ferreira M, d’Orey F, Ribot JC, Silva-Santos B. CD70-CD27 interactions provide survival and proliferative signals that regulate T cell receptor-driven activation of human gammadelta peripheral blood lymphocytes. Eur J Immunol. 2011;41(1):195–201. doi: 10.1002/eji.201040905. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura T, et al. CD70 is selectively expressed on Th1 but not on Th2 cells and is required for Th1-type immune responses. J Invest Dermatol. 2011;131(6):1252–1261. doi: 10.1038/jid.2011.36. [DOI] [PubMed] [Google Scholar]
- 28.Briskin M, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151(1):97–110. [PMC free article] [PubMed] [Google Scholar]
- 29.Pino-Lagos K, et al. A retinoic acid-dependent checkpoint in the development of CD4+ T cell-mediated immunity. J Exp Med. 2011;208(9):1767–1775. doi: 10.1084/jem.20102358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allie SR, Zhang W, Tsai CY, Noelle RJ, Usherwood EJ. Critical role for all-trans retinoic acid for optimal effector and effector memory CD8 T cell differentiation. J Immunol. 2013;190(5):2178–2187. doi: 10.4049/jimmunol.1201945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Sanders TJ, et al. Increased production of retinoic acid by intestinal macrophages contributes to their inflammatory phenotype in patients with Crohn’s disease. Gastroenterology. 2014;146(5):1278–1288. doi: 10.1053/j.gastro.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Horin S, et al. Early preservation of effector functions followed by eventual T cell memory depletion: a model for the delayed onset of the effect of thiopurines. Gut. 2009;58(3):396–403. doi: 10.1136/gut.2008.157339. [DOI] [PubMed] [Google Scholar]
- 34.Tiede I, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111(8):1133–1145. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322–328. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.French JD, Roark CL, Born WK, O’Brien RL. {gamma}{delta} T cell homeostasis is established in competition with {alpha}{beta} T cells and NK cells. Proc Natl Acad Sci U S A. 2005;102(41):14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302(18):981–987. doi: 10.1056/NEJM198005013021801. [DOI] [PubMed] [Google Scholar]
- 38.Coscia M, et al. Dysfunctional Vγ9VΔ2 T cells are negative prognosticators and markers of dysregulated mevalonate pathway activity in chronic lymphocytic leukemia cells. Blood. 2012;120(16):3271–3279. doi: 10.1182/blood-2012-03-417519. [DOI] [PubMed] [Google Scholar]
- 39.Siegers GM, et al. Anti-leukemia activity of in vitro-expanded human γΔ T cells in a xenogeneic Ph+ leukemia model. PLoS One. 2011;6(2):e16700. doi: 10.1371/journal.pone.0016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gertner-Dardenne J, et al. Human Vγ9VΔ2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immunol. 2012;188(9):4701–4708. doi: 10.4049/jimmunol.1103710. [DOI] [PubMed] [Google Scholar]
- 41.Meraviglia S, et al. In vivo manipulation of Vγ9VΔ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161(2):290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaugerie L, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374(9701):1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 43.Smith MA, Irving PM, Marinaki AM, Sanderson JD. Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32(2):119–130. doi: 10.1111/j.1365-2036.2010.04330.x. [DOI] [PubMed] [Google Scholar]
- 44.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134(2):577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 45.Soderstrom K, Bucht A, Halapi E, Gronberg A, Magnusson I, Kiessling R. Increased frequency of abnormal γΔ T cells in blood of patients with inflammatory bowel diseases. J Immunol. 1996;156(6):2331–2339. [PubMed] [Google Scholar]
- 46.Kelsen J, et al. Infliximab induces clonal expansion of γΔ-T cells in Crohn’s disease: a predictor of lymphoma risk? PLoS One. 2011;6(3):e17890. doi: 10.1371/journal.pone.0017890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54(8):1121–1125. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peyrin-Biroulet L, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141(5):1621–1628 e1621. doi: 10.1053/j.gastro.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 49.Kotlyar DS, et al. A systematic review of factors that contribute to hepatosplenic T cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9(1):36–41 e31. doi: 10.1016/j.cgh.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Landmeier S, et al. Activated human γΔ T cells as stimulators of specific CD8+ T cell responses to subdominant Epstein Barr virus epitopes: potential for immunotherapy of cancer. J Immunother. 2009;32(3):310–321. doi: 10.1097/CJI.0b013e31819b7c30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sicard H, et al. In vivo immunomanipulation of Vγ9VΔ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175(8):5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 52.Khan N, Abbas AM, Lichtenstein GR, Loftus EV, Jr, Bazzano LA. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology. 2013;145(5):1007–1015. doi: 10.1053/j.gastro.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 53.Beaugerie L. Lymphoma: the bete noire of the long-term use of thiopurines in adult and elderly patients with inflammatory bowel disease. Gastroenterology. 2013;145(5):927–930. doi: 10.1053/j.gastro.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 54.Silverberg MS, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.