Abstract
A 27 year old female presented for fertility preservation prior to undergoing pelvic radiotherapy. She had previously undergone a radical laparoscopic hysterectomy for cervical carcinoma seven months earlier. A trans-vaginal oocyte aspiration was not advisable due to a vaginal recurrence of the disease. Due to a polycystic ovarian morphology (PCO), follicle stimulating hormone (FSH) priming with no human chorionic gonadotrophin (hCG) trigger was performed prior to oophorectomy followed by ex-vivo oocyte aspiration and in vitro maturation (IVM). All visualized follicles were punctured and follicular fluid aspirated. There were 22 immature oocytes identified and placed into maturation culture for 24 h. After this time, 15 oocytes were deemed to be mature and suitable for vitrification. Following an additional 24 h in maturation culture of the remaining 7 oocytes, three more were suitable for cryopreservation. The patient recovered well and progressed to radiotherapy three days later. This report demonstrates the use of IVM treatment to store oocytes for oncology patients in time-limited circumstances.
Keywords: Oophorectomy, IVM, Vitrification, Oocyte, Oncofertility
Highlights
-
•
A patient sought fertility preservation treatment prior to cancer therapy.
-
•
The patient had polycystic ovarian morphology.
-
•
We performed in vitro maturation on oocytes collected at the time of oophorectomy.
-
•
Twenty-two immature oocytes were collected.
-
•
A total of 18 oocytes were suitable for vitrification and were cryopreserved.
1. Introduction
Oncofertility is an emerging area of interdisciplinary practice whereby patients need to store their gametes and embryos prior to treatment for a range of cancers for which their future fertility is at risk (Woodruff, 2007). IVM plays an important role in fertility preservation and for some patients, such as those with oestrogen sensitive cancers, it maybe their only option (Shalom-Paz et al., 2010, González et al., 2011). Indeed, IVM is a viable treatment option when dealing with those patients diagnosed with polycystic ovaries (PCO), as it is the only treatment proven to eliminate the risk of ovarian hyperstimulation syndrome (OHSS) (Lindenberg, 2013). As cancer therapy may be initiated immediately after oocyte retrieval, OHSS may delay that lifesaving treatment. Oocyte collection and IVM following oophorectomy have been previously reported in only a handful of cases with only one live-birth reported worldwide (Prasath et al., 2014). There is very limited literature on this practice and this is the first reported case using this particular protocol of IVM.
2. Methods
2.1. Patient presentation, stimulation and oophorectomy
The patient presented with cervical cancer and was required to undergo laparoscopic radical hysterectomy, bilateral salpingectomies and pelvic lymphadenectomy with preservation of the ovaries, without ovarian transposition. Final pathology showed adequate clearance margins, and did not require adjuvant radiotherapy. Hence it was discussed with her that when the time arose for her to start a family she would be able to embark on a cycle of ovarian stimulation, oocyte retrieval and enter into a surrogacy arrangement for embryo replacement. However, during her follow-up an isolated vaginal vault recurrence was demonstrated seven months later. External beam radiotherapy treatment was required with curative intent. The ovaries would have been within the radiotherapy field, and it was not recommended to transpose the ovaries outside of the field, as the risk of metastatic disease in the ovaries was high. After discussion with her oncology surgeon (JT), the patient presented for a discussion of fertility preservation options. In view of the limited time prior to commencement of her radiotherapy treatment and the concern of a vaginal extension of her disease, it was felt that vaginal oocyte retrieval would risk further extending her disease, with the risk of significant haemorrhage. After counselling the patient, she elected to undergo her bilateral oophorectomy after several days of ovarian stimulation as a form of ovarian priming as part of a cycle of in-vitro maturation of oocytes after surgical oophorectomy.
A baseline ultrasound scan was performed and she was noted to have a PCO appearance. After arranging her preliminary investigations a hormone profile confirmed her to have serum hormone profile consistent with the early follicular phase (oestradiol < 44 pmol/L, luteinising hormone < 1 U/L, follicle stimulating hormone (FSH) = 2 U/L, progesterone = 1.2 nmol/L). She commenced ovarian stimulation with Puregon 150 IU/day (Merck Sharp and Dohme, South Granville, NSW, Australia). She underwent a vaginal ultrasound examination on the seventh day and ninth day of ovarian stimulation. After nine days of ovarian stimulation due to a delayed response to FSH priming, she was scheduled to undergo bilateral oophorectomy. At the time the lead follicle was 11 mm, and there were noted to be 13 other antral follicles on the right ovary and 12 on the left ovary respectively. Surgery was performed by a standard laparoscopic approach in order to dissect the ovaries which were adherent to the external iliac vein, prior to ligation of the infundibular pelvic ligament with the intention to minimize time between ligation of blood supply and retrieval of ovaries. The ovaries were carefully extracted with ease through an extended laparoscopic port incision in a bag without compression trauma of the ovaries, and were passed to the embryology staff for oocyte extraction.
2.2. Oocyte recovery, IVM and vitrification
The ovaries were placed in Quinns Advantage media with Hepes (Sage, Cooper Scientific, USA) supplemented with 5 mg/ml human serum albumin (HSA) (SAGE, Cooper Surgical, USA) and transported to the laboratory in a portable incubator at 37 °C. All visualized follicles on the ovarian cortex were aspirated and flushed with Hartman's solution supplemented with heparin, using a 19 gauge needle. Follicular aspirates were examined under 10-40 × magnification, and oocytes were identified (Fig. 1) and placed into a wash solution containing G2plus culture medium (Vitrolife, Sweden) supplemented with 10% heat inactivated maternal serum. Oocytes were then moved into maturation culture media as previously described (Walls et al., 2015). Briefly, this involves oocytes being cultured in individual, 20 μL droplets of G-2Plus culture medium, supplemented with 10% heat inactivated maternal serum, 0.1 IU/mL rFSH (Puregon, Merck Sharp and Dohme, South Granville, NSW, Australia) and 0.5 IU/mL hCG (Pregnyl, Merck Sharp and Dohme, South Granville, NSW, Australia) under sterile mineral oil. The immature oocytes were cultured for 24 h at 37 °C in an atmosphere of 6% CO2, 5% O2 and 89% N2.
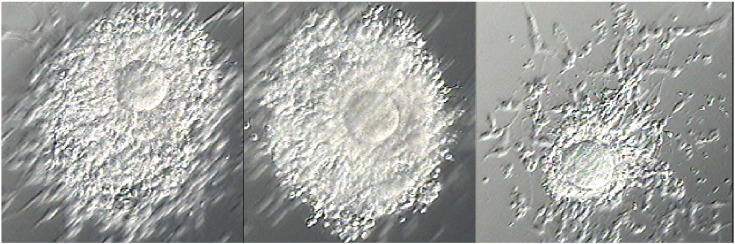
Fig. 1.
Immature oocytes following ex-vivo follicle aspiration.
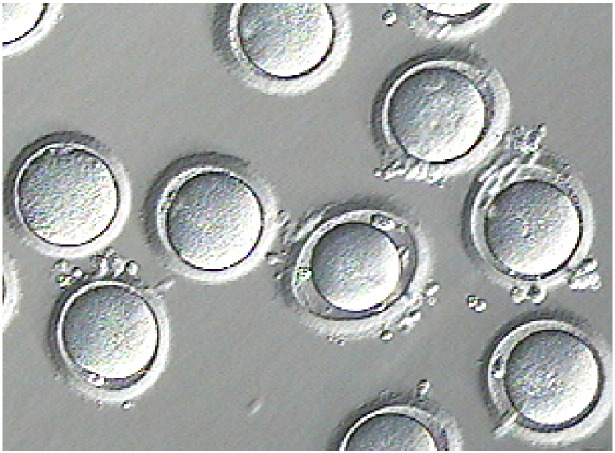
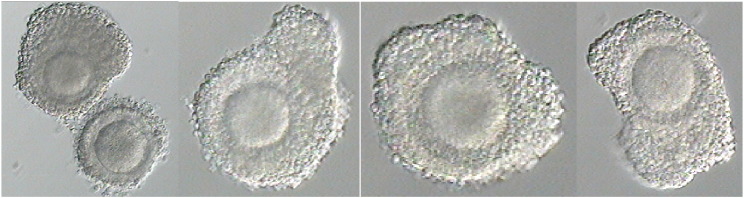
After 24 h (Fig. 2) the oocytes were briefly exposed to a hyaluronic acid solution and gently denuded to check maturation status (Fig. 3) at which point, seven oocytes were deemed to be immature (germinal vesicle and metaphase I stage oocytes) and placed back into maturation culture for an additional 24 h. The remaining 15 mature oocytes were vitrified using a Kitazato oocyte vitrification system (Kitazato, Japan) according to the manufacturer's instructions. The remaining seven oocytes were re-checked for maturation status following their additional 24 h culture and three more oocytes were deemed to be mature and also vitrified. A total of 18 oocytes were cryopreserved for the patient.
Fig. 2.
Oocytes following 24 h in maturation culture.
Fig. 3.
Denuded oocytes following 24 h in maturation culture.
2.3. Ethics approval and patient consent
Ethics approval for IVM treatment has been granted by the University of Western Australia's Human Research Ethics Committee. The patient consented to all procedures and to the use of her case details in any literature, media coverage or educational proceedings, providing no identifying information was used.
3. Discussion
In this case report we have successfully achieved a laparoscopic oophorectomy with oocyte collection ex-vivo, and matured and vitrified a large number of oocytes for the potential preservation of future fertility. In cases such as this where the patient is not currently in a relationship and under time limiting circumstances, oocyte cryopreservation, as opposed to embryo cryopreservation, is necessary. A live birth after in vitro maturation of oocytes collected after oophorectomy has been reported (Prasath et al., 2014), whereby matured oocytes were fertilized and embryos stored using slow freezing techniques. A systematic review has demonstrated that oocyte vitrification significantly improved oocyte survival, fertilisation and embryo quality as well as clinical and ongoing pregnancy rates compared with slow freezing techniques (Glujovsky et al., 2014).
In the literature there is a report of an unsuccessful embryo transfer following the vitrification/warming and fertilisation of oocytes collected by TVOA and matured in vitro prior to chemotherapy (Fadini et al., 2012), and oocyte vitrification has been reported without using IVM, after ovarian hyperstimulation and collection of matured oocytes following oophorectomy after laparoscopy (Bocca et al., 2011) and laparotomy (Fatemi et al., 2011). However, there has only been one other reported case of oocyte vitrification after IVM from a patient undergoing oophorectomy (Huang et al., 2007). In this case the patient was 43 years old and did not have ovarian stimulation. Four oocytes were collected, three of which matured and were suitable for vitrification. Additionally one case has been reported whereby IVM was performed on two immature oocytes collected in combination with ovarian tissue cryopreservation (González et al., 2011). We have shown that with minimal stimulation in a younger patient, significantly more oocytes can be collected, matured and cryopreserved. Successful oocyte fertilisation, embryo transfer to a suitable surrogate and live birth in the future will confirm the ultimate outcome for this patient. The benefit of this approach is the rapid time to oophorectomy as a shortened stimulation protocol is required, and as the ovary has undergone minimal stimulation the ovary can be retrieved through a small abdominal port incision enabling a quicker recovery and the ability to commence further oncology treatment rapidly.
Author's roles
All of the authors have contributed to the manuscript in the following manner:
-
1.
Substantial contributions to conception and design, and interpretation of results.
-
2.
Drafting the article and revising it critically for important intellectual content.
-
3.
Final approval of the version submitted for review by The Journal of Human Reproduction.
Funding
No funding sources to declare.
Conflict of interest
Professor Hart is the Medical Director of Fertility Specialists of Western Australia and a shareholder of Western IVF. He has received educational sponsorship from MSD, Merck-Serono and Ferring Pharmaceuticals.
Acknowledgements
The authors would like to thank all of the staff at the Fertility Specialists of WA, Dr. Tan's Gynecologic Oncology practice and the theatre staff of the Bethesda Hospital, Claremont. Additionally the authors would like to thank the patient involved and wish her the best possible outcome following her cancer treatment.
References
- Bocca S., Dedmond D., Jones E., Stadtmauer L., Oehninger S. Successful extracorporeal mature oocyte harvesting after laparoscopic oophorectomy following controlled ovarian hyperstimulation for the purpose of fertility preservation in a patient with borderline ovarian tumor. J. Assist. Reprod. Genet. 2011;28(9):771–772. doi: 10.1007/s10815-011-9596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini R., Dal Canto M., Mignini Renzini M., Milani R., Fruscio R., Cantù M.G. Embryo transfer following in vitro maturation and cryopreservation of oocytes recovered from antral follicles during conservative surgery for ovarian cancer. J. Assist. Reprod. Genet. 2012;29(8):779–781. doi: 10.1007/s10815-012-9768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi H.M., Kyrou D., Al-Azemi M., Stoop D., De Sutter P., Bourgain C. Ex-vivo oocyte retrieval for fertility preservation. Fertil. Steril. 2011;95(5):1787.e15–1787.e17. doi: 10.1016/j.fertnstert.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Glujovsky D., Riestra B., Sueldo C., Fiszbajn G., Repping S., Nodar F. Vitrification versus slow freezing for women undergoing oocyte cryopreservation. Cochrane Database Syst. Rev. 2014;9 doi: 10.1002/14651858.CD010047.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González C., Devesa M., Boada M., Coroleu B., Veiga A., Barri P. Combined strategy for fertility preservation in an oncologic patient: vitrification of in vitro matured oocytes and ovarian tissue freezing. J. Assist. Reprod. Genet. 2011;28(12):1147–1149. doi: 10.1007/s10815-011-9628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.Y., Buckett W.M., Gilbert L., Tan S.L., Chian R.C. Retrieval of immature oocytes followed by in vitro maturation and vitrification: a case report on a new strategy of fertility preservation in women with borderline ovarian malignancy. Gynecol. Oncol. 2007;105(2):542–544. doi: 10.1016/j.ygyno.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Lindenberg S. New approach in patients with polycystic ovaries, lessons for everyone. Fertil. Steril. 2013;99(5):1170–1172. doi: 10.1016/j.fertnstert.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Prasath E.B., Chan M.L., Wong W.H., Lim C.J., Tharmalingam M.D., Hendricks M. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum. Reprod. 2014;29(2):276–278. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- Shalom-Paz E., Almog B., Shehata F., Huang J., Holzer H., Chian R.-C. Fertility preservation for breast-cancer patients using IVM followed by oocyte or embryo vitrification. Reprod. BioMed. Online. 2010;21(4):566–571. doi: 10.1016/j.rbmo.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Walls M.L., Hunter T., Ryan J.P., Keelan J.A., Nathan E., Hart R.J. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum. Reprod. 2015;30(1):88–96. doi: 10.1093/humrep/deu248. [DOI] [PubMed] [Google Scholar]
- Woodruff T. The Emergence of a New Interdiscipline: Oncofertility. In: Woodruff T., Snyder K., editors. Oncofertility Fertility Preservation for Cancer Survivors. Vol. 138. Springer US; 2007. pp. 3–11. (Cancer Treatment and Research). [Google Scholar]