Highlights
-
•
Malignant transformation of deep infiltrating endometriosis involving the bladder is quite rare.
-
•
We review eight relevant cases which have been reported.
-
•
This is the second case fulfilling Sampson and Scott criteria.
Keywords: Endometrioid adenocarcinoma, Malignant transformation, Deep infiltrating endometriosis
1. Introduction
Endometriosis is a common disease and affects 10% of women of reproductive age. Lesions are commonly located in the ovaries, fallopian tubes, utero-sacral ligaments, pelvic wall, and cul-de-sac. However, endometriosis can sometimes infiltrate other organs, such as the bladder, ureter, sigmoid colon, and rectum. This is referred to as DIE and is defined as endometriosis infiltrating more than 5 mm beneath the peritoneum. DIE involving the bladder is extremely rare and occurs in only 1% of women with endometriosis (Donnez et al., 2000).
Malignant transformation of endometriosis is a well-established pathology, but only a few cases of malignant transformation of DIE involving the bladder have been reported. Here, we report a case of endometrioid carcinoma arising from DIE involving the bladder and review the relevant literature.
2. Case description
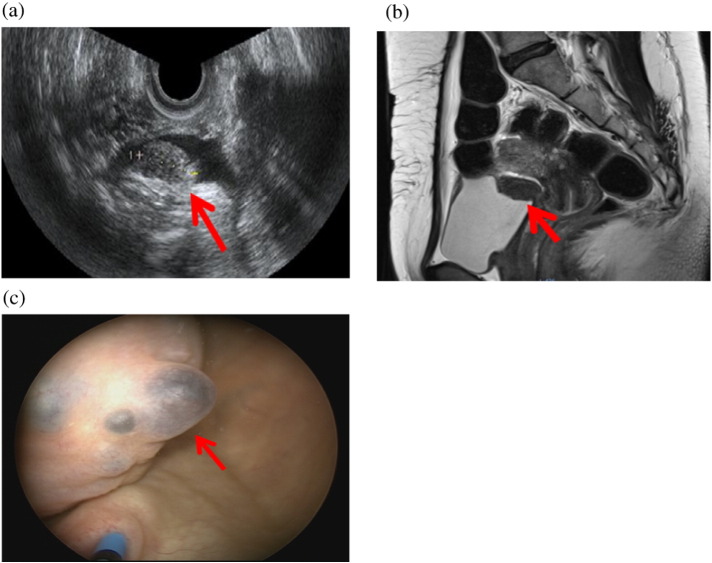
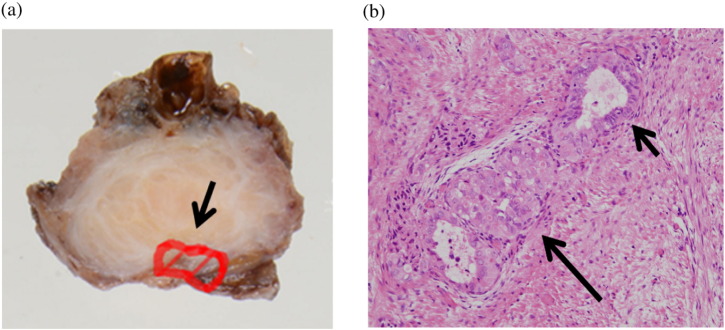
A 45 year-old, nulliparous woman had a history of left endometrioma and uterine leiomyoma, and had undergone laparoscopic cystectomy when 34 years old, and laparoscopic myomectomy 6 years later. She complained of frequent urination and miction pain during her menstrual period. Physical examination revealed a slight tenderness consistent with anterior fornix of the vagina. Transvaginal ultrasonography showed a 23-mm high echoic solid mass in the bladder (Fig. 1a). Pelvic magnetic resonance imaging (MRI) revealed a lesion in the posterior wall of the bladder, which showed slightly higher intensity on T1-weighted images, and low-intensity with high-intensity spots on T2-weighted images (Fig. 1b). The serum CA-125 level was slightly elevated (46.6 U/ml). Urine analysis revealed hematuria, but the urinary cytology was negative. Preoperative cystoscopy findings revealed focal lesions of endometriosis, and a blueberry spot on the mucous membrane of the bladder (Fig. 1c). The lesion was adjacent to the right ureteral orifice, but the urine outflow from the ureteral orifices was normal. Based on these findings, we preoperatively diagnosed DIE involving the bladder and performed total laparoscopic hysterectomy, partial bladder resection, and insertion of bilateral ureteral stents. Laparoscopic findings revealed a solid mass in the vesico-uterine pouch. The r-ASRM score was 36, moderate, and the ENZIAN score was FB (deep infiltration of the bladder). The blue-berry spot lesion in the bladder endometriosis was completely resected using cystoscopy with laparoscopy. Histopathological examination revealed the co-existence of endometriosis and endometrioid adenocarcinoma involving the bladder wall. Endometrioid carcinoma lesions were limited to the peritoneum side, and were very close to the serosa (Fig. 2a). Tumor-containing sections showed a continuum between a benign endometriotic focus and malignant glands (Fig. 2b). These histopathological findings strongly suggested that the endometrioid adenocarcinoma originated from DIE involving the bladder. Postoperative computed tomography (CT) findings showed no other malignant lesions. Clinically, we regarded this case as peritoneal carcinoma because the lesions were adjacent to the peritoneal serosa. We recommended staging laparotomy including bilateral oophorectomy and omentectomy for a more accurate diagnosis, but the patient refused this. She was administered 6 cycles of adjuvant chemotherapy (docetaxel/carboplatin, docetaxel; 70 mg/m2, carboplatin; AUC 6, q3 weeks, 6 cycles) as an alternative to paclitaxel/carboplatin therapy, which often induces peripheral neuropathy, as she was a piano teacher. There was no evidence of recurrence or metastasis after 10 months of follow-up.
Fig. 1.
a) Preoperative trans-vaginal ultrasonographic findings revealed a solid mass in the posterior wall of the bladder (arrow). b) Preoperative magnetic resonance imaging findings (T2 weighted image, sagittal section) revealed a solid lesion in the posterior wall of the bladder (arrow). c) Cystoscopy findings revealed blueberry spots on the mucous membrane of the bladder (arrow).
Fig. 2.
a) A specimen of deep infiltrating endometriosis arising from the bladder. Endometriosis was present in all layers of the section, whilst malignant cells were limited to the peritoneum side (delineated by a line in the image). b) The continuum between benign endometrial glands (short arrow) and endometrioid adenocarcinoma (long arrow) (hematoxylin and eosin, × 200).
3. Discussion
Malignant transformation of endometriosis is a recognized pathology, and is estimated to occur in about 0.7–1.6% of women suffering from endometriosis. However, most of these cases involve ovarian endometrioma, and malignant transformation from extraovarian sites accounts for about 20% of cases. The most common extraovarian sites are the rectovaginal septum, the colon, and the vagina, which together account for more than 50% of cases. Malignant transformation of endometriosis arising from the bladder is quite rare with only 8 cases to date in the English literature, as summarized in Table 1 (Al-Izzi et al., 1989, Vara et al., 1990, Chor et al., 1993, Balat et al., 1996, Garavan et al., 2004, Mann et al., 2012, Lah et al., 2013, Liu et al., 2014). Among these, 7 patients (77.8%) had undergone previous surgery. This suggests that the interfusion of endometrium into the bladder may contribute to carcinogenesis. The histological types of malignant transformation in endometriosis involving the bladder are primarily clear cell carcinoma (5/9; 55.6%), followed by endometrioid adenocarcinoma (2/9; 22.2%), mixed histology (clear cell carcinoma and endometrioid adenocarcinoma) (1/9; 11.1%), and endometrioid adenosarcoma (1/9; 11.1%), which is comparable with malignant transformation of endometriosis arising from other sites including ovarian endometrioma and the abdominal wall (Ijichi et al., 2014).
Table 1.
| No. | Reference | Age (year) | Previous surgery | Symptom | Histology | Coexisting endometriosis on histology | Surgical treatment | Chemotherapy | Follow up (month) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Al-Izzi et al. (1989) | 62 | TAH + BSO | Hematuria | EC and CCC | Unclear | Radical cystectomy | Unclear | 24 | NED |
| 2 | Vara et al. (1990) | 62 | TAH + BSO | Hematuria | endometrioid adenosarcoma | Unclear | Radical cystectomy | None | 12 | NED |
| 3 | Chor et al. (1993) | 35 | Laparotomy with lysis of adhesion of endometriosis | Hematuria | CCC | Unclear | Radical cystectomy | None | NA | – |
| 4 | Balat et al. (1996) | 40 | Diagnostic laparoscopy | Proctorrhagia | CCC | Unclear | Partial cystectomy RSO + LAR |
Carboplatin | NA | – |
| 5 | Garavan et al. (2004) | 53 | – | Hematuria | EC | Unclear | Partial cystectomy | Unclear | NA | – |
| 6 | Mann et al. (2012) | 54 | – | Hematuria | CCC | Yes | Partial cystectomy TAH + BSO + Pen | Unclear | NA | – |
| 7 | Lah et al. (2013) | 59 | CS | Hematuria | CCC | Unclear | Anterior pelvic exenteration | TC, 3 cycles | 12 | NED |
| 8 | Liu et al. (2014) | 39 | CS | Urgent urination | CCC | Unclear | Partial cystectomy TAH + BSO + Pen |
TC, 3 cycles | 12 | DOD |
| 9 | this case | 45 | Myomectomy | Miction pain | EC | Yes | Partial cystectomy TLH |
DC, 6 cycles | 10 | NED |
Note: CCC: clear cell adenocarcinoma, EC: endometrioid adenocarcinoma.
CS: cesarian section, TAH: total abdominal hysterectomy, BSO: bilateral salpingo-oophorectomy, RSO: right salpingo-oophorectomy.
LAR: lower anterior resection of rectum, Pen: pelvic lymph nodes dissection, TLH: total laparoscopic hysterectomy.
TC: paclitaxel and carboplatin combination therapy, DC: docetaxel and carboplatin combination therapy.
DOD: died of disease, NED: no evidence of disease, REC: recurrence.
The criteria for the diagnosis of malignancy arising from endometriosis as defined by Sampson and Scott is as follows: 1) the presence of both benign and neoplastic endometrial tissues in the tumor, 2) histological findings compatible with an endometrial origin, 3) the discovery of no other primary tumor sites, and 4) a morphologic demonstration of a continuum between benign and malignant epithelium. The latter is challenging in practice because benign epithelial tissues in endometriosis have already been mostly replaced by malignant lesions. To date, the presence of benign and malignant tissue within the same histological section has only been demonstrated in a single case. The case we describe here is the second case of malignant transformation of endometriosis involving the bladder that genuinely meets the Sampson and Scott criteria.
The treatment for malignant transformation involving the bladder is poorly defined. Radical or partial cystectomy were conducted in all cases, and hysterectomy, oophorectomy or pelvic lymph node resection in addition to cystectomy were performed in 5 cases (55.6%). Only one patient from these 9 cases, who had multiple lymph metastasis and clear cell carcinoma from abdominal wall endometriosis, died from the disease despite undergoing radical resection and additional chemotherapy. Cisplatin based chemotherapy was administered in 3 of the 9 cases (33.3%), but there is no evidence that it actually improves the prognosis of this disease due to its rarity. However, local resection is now considered to be the primary treatment for this malignancy. In this case, we diagnosed peritoneal cancer arising from the serosa of the bladder wall and therefore suggested that the patient underwent an additional operation consisting of salpingo-oophorectomy with pelvic lymph node dissection in order to allow a more accurate diagnosis. However, the patient refused this.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Contributor Information
Yosuke Tarumi, Email: y-tarumi@koto.kpu-m.ac.jp.
Taisuke Mori, Email: moriman@koto.kpu-m.ac.jp.
Jo Kitawaki, Email: kitawaki@koto.kpu-m.ac.jp.
References
- Al-Izzi M.S., Horton L.W., Kelleher J., Fawcett D. Malignant transformation in endometriosis of the urinary bladder. Histopathology. 1989;14:191–198. doi: 10.1111/j.1365-2559.1989.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Balat O., Kudelka A.P., Edwards C.L., Silva E., Kavanagh J.J. Malignant transformation in endometriosis of the urinary bladder; case report of clear cell adenocarcinoma. Eur. J. Gynaecol. Oncol. 1996;17:13–16. [PubMed] [Google Scholar]
- Chor P.J., Gaum L.D., Young R.H. Clear cell adenocarcinoma of the urinary bladder: report of a case of probable mullerian origin. Mod. Pathol. 1993;6:225–228. [PubMed] [Google Scholar]
- Donnez J., Spada F., Squifflet J., Nisolle M. Bladder endometriosis must be considered as bladder adenomyosis. Fertil. Steril. 2000;74:1175–1181. doi: 10.1016/s0015-0282(00)01584-3. [DOI] [PubMed] [Google Scholar]
- Garavan F., Grainger R., Jeffers M. Endometrioid carcinoma of the urinary bladder complicating vesical mullerianosis: a case report and review of the literature. Virchows Arch. 2004;444:587–589. doi: 10.1007/s00428-004-1010-8. [DOI] [PubMed] [Google Scholar]
- Ijichi S., Mori T., Suganuma I., Yamamoto T., Matsushima H., Ito F., Akiyama M., Kusuki I., Kitawaki J. Clear cell carcinoma arising from cesarean section scar endometriosis: case report and review of the literature. Case Rep. Obstet. Gynecol. 2014;2014:642483. doi: 10.1155/2014/642483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lah K., Desai D., Hadway P., Perry-Keene J., Coughlin G. Primary vesical clear cell adenocarcinoma arising in endometriosis: a rare case of mullerian origin. Anticancer Res. 2013;33:615–617. [PubMed] [Google Scholar]
- Liu H., Leng J., Lang J., Cui Q. Clear cell carcinoma arising from abdominal wall endometriosis: a unique case with bladder and lymph node metastasis. World J. Surg. Oncol. 2014;12:51. doi: 10.1186/1477-7819-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S., Patel P., Matthews C.M., Pinto K., O'Connor J. Malignant transformation of endometriosis within the urinary bladder. Proc. (Baylor Univ. Med. Cent.) 2012;25:293–295. doi: 10.1080/08998280.2012.11928857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara A.R., Ruzics E.P., Moussabeck O., Martin D.C. Endometrioid adenosarcoma of the bladder arising from endometriosis. J. Uro. 1990;143:813–815. doi: 10.1016/s0022-5347(17)40105-4. [DOI] [PubMed] [Google Scholar]