Abstract
Ionizing radiation triggers a signal in human B-lymphocyte precursors that is intimately linked to an active protein-tyrosine kinase regulatory pathway. We show that in B-lymphocyte precursors, irradiation with gamma-rays leads to (i) stimulation of phosphatidylinositol turnover; (ii) downstream activation by covalent modification of multiple serine-specific protein kinases, including protein kinase C; and (iii) activation of nuclear factor kappa B. All of the radiation-induced signals were effectively prevented by the protein-tyrosine kinase inhibitors genistein and herbimycin A. Thus, tyrosine phosphorylation is an important and perhaps mandatory proximal step in the activation of the protein kinase C signaling cascade in human B-lymphocyte precursors. Our report expands current knowledge of the radiation-induced signaling cascade by clarifying the chronological sequence of biochemical events that follow irradiation.
Full text
PDF

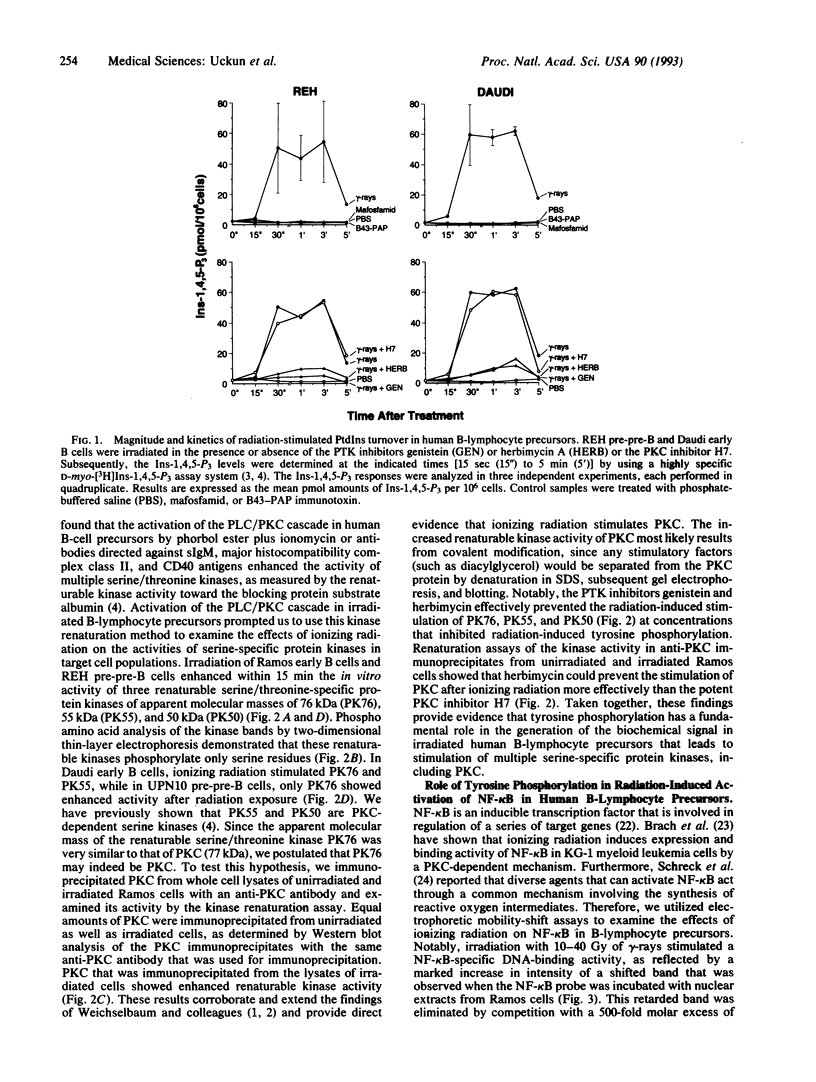


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Brach M. A., Hass R., Sherman M. L., Gunji H., Weichselbaum R., Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991 Aug;88(2):691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte D., Bellomo G., Thor H., Nicotera P., Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984 Dec;235(2):343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Thrombin stimulates the activities of multiple previously unidentified protein kinases in platelets. J Biol Chem. 1989 Dec 5;264(34):20723–20729. [PubMed] [Google Scholar]
- Hallahan D. E., Sukhatme V. P., Sherman M. L., Virudachalam S., Kufe D., Weichselbaum R. R. Protein kinase C mediates x-ray inducibility of nuclear signal transducers EGR1 and JUN. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2156–2160. doi: 10.1073/pnas.88.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Baldi C., Svensson S. A., Bellomo G., Orrenius S. Cystamine induces toxicity in hepatocytes through the elevation of cytosolic Ca2+ and the stimulation of a nonlysosomal proteolytic system. J Biol Chem. 1986 Nov 5;261(31):14628–14635. [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Rhee S. G., Carpenter G. Tyrosine phosphorylation of phospholipase C-II in vitro by the epidermal growth factor receptor. J Biol Chem. 1989 Jun 25;264(18):10335–10338. [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. L., Datta R., Hallahan D. E., Weichselbaum R. R., Kufe D. W. Ionizing radiation regulates expression of the c-jun protooncogene. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5663–5666. doi: 10.1073/pnas.87.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Dibirdik I., Smith R., Tuel-Ahlgren L., Chandan-Langlie M., Schieven G. L., Waddick K. G., Hanson M., Ledbetter J. A. Interleukin 7 receptor ligation stimulates tyrosine phosphorylation, inositol phospholipid turnover, and clonal proliferation of human B-cell precursors. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3589–3593. doi: 10.1073/pnas.88.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Gillis S., Souza L., Song C. W. Effects of recombinant growth factors on radiation survival of human bone marrow progenitor cells. Int J Radiat Oncol Biol Phys. 1989 Feb;16(2):415–435. doi: 10.1016/0360-3016(89)90338-6. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Mitchell J. B., Obuz V., Park C. H., Waddick K., Friedman N., Oubaha L., Min W. S., Song C. W. Radiation sensitivity of human B-lineage lymphoid precursor cells. Int J Radiat Oncol Biol Phys. 1991 Nov;21(6):1553–1560. doi: 10.1016/0360-3016(91)90332-x. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Ramakrishnan S., Houston L. L. Increased efficiency in selective elimination of leukemia cells by a combination of a stable derivative of cyclophosphamide and a human B-cell-specific immunotoxin containing pokeweed antiviral protein. Cancer Res. 1985 Jan;45(1):69–75. [PubMed] [Google Scholar]
- Uckun F. M., Schieven G. L., Dibirdik I., Chandan-Langlie M., Tuel-Ahlgren L., Ledbetter J. A. Stimulation of protein tyrosine phosphorylation, phosphoinositide turnover, and multiple previously unidentified serine/threonine-specific protein kinases by the Pan-B-cell receptor CD40/Bp50 at discrete developmental stages of human B-cell ontogeny. J Biol Chem. 1991 Sep 15;266(26):17478–17485. [PubMed] [Google Scholar]
- Uckun F. M., Tuel-Ahlgren L., Song C. W., Waddick K., Myers D. E., Kirihara J., Ledbetter J. A., Schieven G. L. Ionizing radiation stimulates unidentified tyrosine-specific protein kinases in human B-lymphocyte precursors, triggering apoptosis and clonogenic cell death. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9005–9009. doi: 10.1073/pnas.89.19.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Ward J. F. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]





