Introduction
Patients with homozygous familial hypercholesterolemia (hoFH) have severely elevated cholesterol levels with a high risk of premature cardiovascular disease, and they have limited therapeutic options, as many patients do not respond well to currently available drug therapies. 1, 2 In addition to routine pharmacologic therapy, lipoprotein apheresis is the current standard-of-care for these patients 3, because the procedure can temporarily reduce low-density lipoprotein (LDL) cholesterol (LDL-C) levels by 50–80%. However, lipoprotein apheresis is invasive, time consuming, and not readily available for many patients. 4, 5
The US Food and Drug Administration and the European Medicine Agency recently approved lomitapide (Aegerion Pharmaceuticals, Cambridge, MA, USA), an inhibitor of microsomal triglyceride transfer protein (MTP), as an add-on therapy for patients with hoFH. MTP is a key protein in the assembly and secretion of very-low-density lipoprotein (VLDL) in the liver and chylomicrons in the intestine, and its inhibition by lomitapide results in a significant decrease in the production of LDL apolipoprotein B 6 and significant reduction in serum LDL-C and triglycerides in patients with hoFH. 6, 7
However, MTP inhibition is associated with gastrointestinal adverse events and increases in liver fat content. Although gastrointestinal side effects can be controlled with a low-fat diet and gradual lomitapide dose titration, and liver fat content appears to stabilize after an initial increase, 6, 7 long-term safety of the drug is still being evaluated. Thus, reviewing available data on extended exposure is valuable.
We report here the case of a patient with hoFH who has been treated with lomitapide for over five years. She has had an excellent clinical response with LDL-C lowering, but has also had medication side effects and other challenges associated with her care.
Case Report
The patient is a 49-year-old Hispanic female with an initial diagnosis of hoFH at age 29 when she presented with premature coronary artery disease (CAD) and total cholesterol of over 600 mg/dL. Subsequent genetic analysis confirmed the presence of a homozygous mutation in the gene encoding for LDL reception (Ser156Leu). She was known to have hypercholesterolemia since age 11, along with the presence of xanthomas and bilateral corneal arcus, common signs of hoFH 8, but was not treated until age 29 when clinical manifestations of CAD first became apparent.
Cardiovascular History
At the age of 29, the patient presented with unstable angina. Coronary angiography revealed left main and severe three vessel coronary disease. She also had mild aortic and mitral valvular disease, and a significantly calcified ascending aorta. She required coronary artery bypass grafting (CABG) using a left internal mammary artery and vein grafts, which was challenging because the aortic calcification required that the three vein grafts to her coronary vessels arise from only a single proximal anastomosis. Six months following CABG she experienced recurrent angina and catheterization revealed patency of the individual grafts, but there was an occlusion of the vein grafts at their common proximal anastomosis. She underwent a second cardiac operation with successful bypass using the right internal mammary artery to the vein graft. Over the next eight years she experienced intermittent angina and developed slowly progressive mitral valve regurgitation and mixed aortic stenosis/aortic regurgitation. Her clinical course was further complicated when she presented with a thrombotic cerebrovascular accident attributed to atrial fibrillation. At the age of 37 she underwent further surgery which included aortic root replacement, placement of mechanical aortic and mitral valves, and reimplantation of the native left main coronary artery with successful preservation of her coronary bypass grafts. Her cardiac status has remained relatively stable since that time. She has been treated with statin therapy and apheresis, as described below.
Apheresis History
Given her diagnosis of hoFH with total cholesterol > 600 mg/dL, along with early coronary artery disease, substantial efforts were undertaken to achieve significant reductions in LDL-C. Statin and cholestyramine therapy had minimal effect. Prior to the availability of selective LDL apheresis, therapeutic plasma exchange (TPE) was performed from December of 1994 until March 1998. TPE was performed biweekly, with 1.5 plasma volumes (4.5 L) exchanged with 5% albumin using peripheral access via the anticubital veins. Completion of treatments were often challenging due to access-related issues.
In March 1998, after creation and maturation of an arteriovenous fistula, selective lipoprotein apheresis was begun using the Liposorber® LA-15 apheresis system, which selectively removed apo B containing lipoproteins as opposed to high-density lipoprotein (HDL). The procedure was performed biweekly, treating 4.5 L of plasma per treatment using access via the arteriovenous fistula. The main treatment challenge during this time was recurrent nausea, treated with oral ondansetron prior to each treatment. Using the LDL-C levels from 10 treatments between 2007 and 2008, the patient’s average acute drop in LDL-C was 223 mg/dL (49%) with a drop over time in pre-apheresis levels of approximately 60 mg/dL (13%). In general, the interval mean was 20–25% lower than baseline when performing lipoprotein apheresis biweekly, treating 1.5 plasma volumes.
Experience with lomitapide (Phase 2 and 3 Studies)
In 2003, the patient became eligible for and was enrolled in a Phase 2 study of lomitapide (BMS201038). As per the study protocol, all lipid-lowering therapies, including apheresis, were discontinued for at least 4 weeks before starting the study. At the baseline visit the lipid profile was TC 684 mg/dL, LDL-C 637 mg/dL, VLDL-C 12 mg/dL, TG 82 md/dL and HDL-C 35 mg/dL. Lomitapide was initiated at a dose of 0.03 mg/kg/day and titrated every four weeks up to 1 mg/kg/day. The patient achieved an LDL-C of 301 mg/dL after four weeks on the highest study dose, a 47% decrease from her baseline levels. 6 Liver enzymes remained within the normal range and hepatic fat remained below 10%. Her participation in the study lasted a total of 6 months, after which she was restarted on her pre-study treatment regimen.
In March, 2008 the patient became eligible for the Phase 3, single-arm, open-label study (NCT00730236) assessing the efficacy and safety of lomitapide, as add-on to other lipid-lowering treatment. Her daily lipid-lowering therapy at that time included rosuvastatin calcium 40mg, ezetimibe 10mg, and colesevelam hydrochloride 3750 mg. Additionally, she was receiving lipoprotein apheresis once a month. Other relevant concomitant medications included daily aspirin 81 mg, ferrous sulfate 325 mg, metoprolol tartrate 100 mg, and warfarin sodium 4 mg. As the patient had a tubal ligation in 1991, no additional contraception was required. At the first visit the patient met with a registered dietitian for dietary instructions, including how to consume ≤ 20% caloric intake from fat. Instructions included detailed information about how high fat intake could exacerbate the impact of lomitapide on gastro-intestinal symptoms. Additionally, the patient was instructed to limit alcohol to no more than 1 unit per day. International normalized ratio (INR) levels were monitored frequently throughout the study and the warfarin dose was adjusted accordingly to maintain a therapeutic target range of 2.5 – 3.5. As per the research protocol, background lipid-lowering therapy, including apheresis, was continued unaltered from six weeks prior to baseline through week 26 on drug therapy. Lomitapide was initiated at 5 mg/day and escalated according to protocol requirements to 10 mg after 2 weeks, and then to 20 mg, 40 mg and 60 mg once daily at 4 week intervals. She remained at a 60 mg dose for most of the remainder of the study. The primary endpoint was mean percent change in LDL-C from baseline to week 26 (intent to treat analysis), after which patients in the study remained on lomitapide for assessment of safety and long-term effects, and modifications in concomitant lipid lowering therapy were permitted. She was rolled into an extension study (NCT00943306) in October 2009 and transferred back to clinical care in July 2013, when the study was closed following FDA approval of the lomitapide. At that time she had been on drug therapy Juxtapid® (lomitapide) for more than five years (270 weeks). Her BMI was 28.3 kg/m2 at baseline and 22.3 kg/m2 at the time of her transition to clinical care.
LDL-C during lomitapide treatment
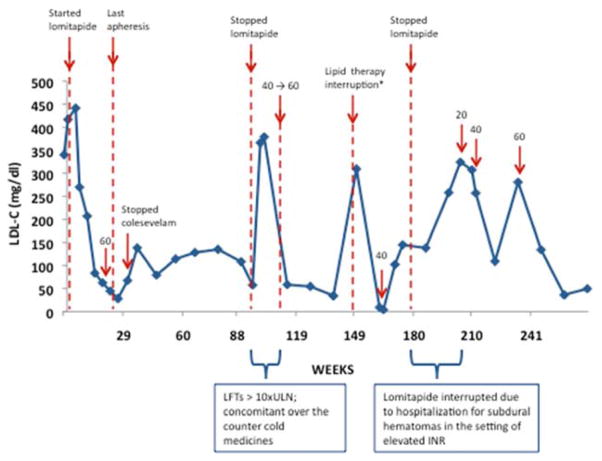
At the start of the phase 3 study the patient’s baseline LDL-C was 378.5 mg/dL, despite her intensive lipid-lowering regimen. The cumulative LDL-C response, along with comments on dose and liver function tests (LFT) changes are charted in Figure 1 and Figure 2. She started on 5 mg lomitapide per day and gradually increased to 60mg per day at week 14. During this period we observed a gradual decrease in LDL-C that reached 28 mg/dL (−92.6% change from baseline) at week 26. In light of the improvement in her LDL-C at the end of the efficacy phase her lipoprotein apheresis was discontinued. After 31 weeks, colesevelam hydrochloride was also discontinued. She generally maintained remarkably low LDL-C levels over time while taking lomitapide despite these changes in her lipid-lowering regimen.
Figure 1. LDL-C response to lomitapide over time.
LDL-C values from the time of patient enrollment in the phase 3 study are reported. Arrows indicate key events over the course of treatment. Numbers indicate lomitapide doses. *Prior to this lipid measurement, rosuvastatin and ezetimibe were not taken for 38 days, and lomitapide was not taken for 5 days for non-study-related reasons.
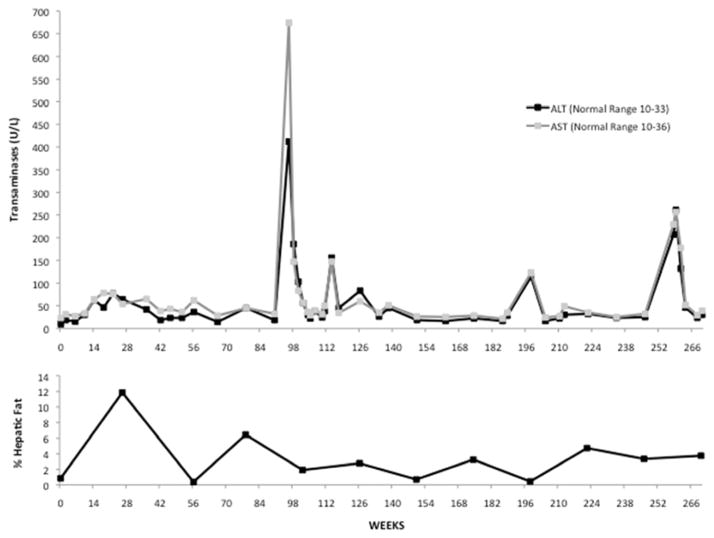
Figure 2. Transaminase levels and percent hepatic fat content over time.
Alanine aminotransferase and aspartate aminotransferase (ALT and AST) and percent hepatic fat content, measured by nuclear magnetic resonance spectroscopy, from the time of patient enrollment in the phase 3 study are reported.
During a routine study visit after 162 weeks on drug therapy, the patient was noted to have markedly decreased lipids (total cholesterol 53 mg/dL, triglycerides 8 mg/dL, HDL-C 39 mg/dL, and LDL-C 4 mg/dL) while on lomitapide 60 mg daily. LFTs were in the normal range. Her vitamin E and beta-carotene levels were also low (Vitamin E-alpha 0.29 mg/dL, range 0.50–1.75 and beta-carotene 0.023 mcg/mL, range 0.061–0.745.) She remained asymptomatic during this time and denied any neurological symptoms. Given a LDL-C value of 4 mg/dL, and because lomitapide may reduce absorption of fat soluble nutrients in the intestine, the study drug dose was reduced from 60 mg to 40 mg daily. Her LDL-C rebounded to 138 mg/dL. Similarly vitamin E-alpha and beta carotene levels returned to within normal range (Vitamin E-alpha 1.32 mg/dL, beta-carotene 0.312 mcg/dL). Following 6 months at 40 mg/day of lomitapide and stabilization of the LDL-C levels, the dose was increased back to 60 mg/day. After two weeks at the increased dose, LDL-C was 104 mg/dL. Vitamin results are not available from that measurement; however after another eight weeks Vitamin E-alpha and beta-carotene had returned to normal or supra-normal levels (1.84 mg/dL and 0.124 mcg/dL, respectively). By the time the patient was transitioned to commercial product, 270 weeks since the start of the Phase 3 studies, she was taking 60 mg daily and her LDL-C levels were 49 mg/dL – an 87% decrease from baseline.
LFTs and liver fat content during lomitapide treatment
The patient’s participation in the study for the first 90 weeks was unremarkable. She experienced some expected non-serious adverse events of nausea, and some mild, transient LFT elevations, with aspartate aminotransferase (AST) and alanine aminotransferase (ALT) not reaching greater than 2.3 times the upper limit of normal (xULN.) (Figure 2).
After her study visit at week 90, elevated LFTs were detected (AST 16.8xULN, ALT 10.3xULN) during a routine laboratory study. Lomitapide administration was interrupted at that time. Laboratory studies were repeated biweekly until the LFTs normalized. The cause for the transient LFT elevation is not known, but the data safety and monitoring board concluded that the over-the-counter cold medications that the patient had taken for about four weeks prior to the elevated LFT measurement may have played a significant role. The medications included a combination of ibuprofen, guaifenesin, Robitussin® (dextromethorphan/guaifenesin/phenylephrine), and Nyquil® (acetaminophen/dextromethorphan/doxylamine/pseudoephedrine). Because lomitapide is metabolized primarily by CYP3A4, concomitant use of a CYP3A4 substrate, such as dextromethorphan, could cause increased levels of lomitapide, leading to greater liver toxicity. 9 The concomitant self-medication with acetaminophen up to twice daily for several days could also have contributed to the elevated LFTs. After the patient stopped these medications and lomitapide, the LFTs normalized and lomitapide was restarted at 40 mg/day, and up-titrated to 60 mg/day within three months.
Hepatic fat content was assessed using nuclear magnetic resonance spectroscopy technology at baseline, Week 26, 56 and 78, and then once every 24 weeks until the end of the extension study. The patient’s hepatic fat content was 0.86% at baseline and peaked at 11.85% at week 26 (Figure 2). During the rest of the study, hepatic fat content fluctuated, but never exceeded 6.45% (value at week 78) up to the last measurement available at Week 270. The observed fluctuation over time was not apparently related to notable changes in LFTs or patient-reported symptoms. Higher hepatic fat content was observed at lower LDL-C levels, suggesting that this finding may be dependent on the degree of MTP inhibition reached.
Hematology and INR related adverse events during lomitapide treatment
Following approximately 129 weeks on study treatment, the patient was hospitalized for the management of hypovolemic shock, secondary myocardial infarction and congestive heart failure caused by a period of prolonged menorrhagia and attendant anemia (hemoglobin 5.6 g/dL, reference range 12.0–16.0). At time of admission her INR was 2.9, which was within her target therapeutic range. Despite investigating the etiology of the menorrhagia no clear diagnosis was evident. Progesterone therapy was initiated, and the patient’s bleeding resolved. During this time the patient missed one dose of lomitapide.
After 197 weeks of treatment, the patient was referred to the emergency department for evaluation of stroke symptoms in the setting of suspected viral illness, weight loss and an elevated INR of 5.0 (therapeutic target range 2.5 – 3.5). MRI of the brain identified small bilateral posterior fossa subdural hematomas, an acute punctate stroke in the left frontal lobe, and several areas of microhemorrhages. Her anti-coagulation management was stabilized and over time she experienced the resolution of neurological symptoms. Lomitapide was stopped during hospital admission and restarted at 20 mg/day approximately one month after her hospitalization, with the recommendation of frequent INR monitoring before and after each dose change. Subdural hematomas were resolved on last follow-up MRI before the end of study. As stated in the product information, lomitapide increases plasma concentrations of warfarin. Increases in the dose of lomitapide may cause supra-therapeutic anticoagulation (22% increase in INR), and decreases in lomitapide dose may lead to sub-therapeutic anticoagulation. 9 This interaction may have contributed to this patient’s event, however other causes could have also lead to the event. After her re-titration period, the patient resumed 60 mg daily again by week 213 in the study. Since that time the patient has done well with very frequent INR checks and adjustment of warfarin dosing.
Discussion
Long-term medical management of hypercholesterolemia in patients with hoFH is challenging. Traditionally such patients have minimal improvement using statin therapy, and require combination drug therapy and often lipoprotein apheresis as well. MTP inhibitors represent a new therapeutic option for such patients, although drug and metabolic interactions represent important considerations for long-term use. In the patient presented here, long-term add-on treatment with lomitapide was successful in achieving a marked reduction in LDL-C levels, even reaching traditional target levels (LDL-C < 70 mg/dl for patients with CAD). Given the impressive reduction, other therapies including lipoprotein apheresis and use of colesevelam hydrochloride were successfully discontinued, with a consequent improvement of quality of life as self-reported by the patient. As demonstrated by this case, lomitapide is efficacious in the presence of apheresis, meaning it is a possible add-on treatment options for patients with or without apheresis and has been approved by the FDA for this indication.
Although average percent reduction in LDL-C levels at the end of the efficacy phase was 50%, a reduction of more than 75% was observed in 4 of the 23 patients that reached the end of the efficacy phase (17%). The approximately 90% reduction in LDL-C levels observed in this patient was the best response observed among the patients enrolled in the study. The reasons for her excellent response are not known, however several factors may have contributed. The patient’s specific LDL receptor mutation (Ser156Leu) is associated with some residual LDL-C activity, a less severe phenotype, and a better therapeutic response to statin and other conventional therapy as compared to carriers of a LDL receptor negative mutation10. It is possible that with this background, the concomitant treatment with other lipid modifying therapies, such as rosuvastatin and ezetimibe, may have caused an additive effect to treatment with lomitapide. Furthermore, the complete adherence to the low fat diet and the normalization of her BMI may have also contributed. Finally, we cannot exclude that other genetic and environmental factors may also have played a role.
Though lomitapide has known gastrointestinal side effects such as nausea, vomiting and diarrhea, this patient had relatively few instances of such events. Adherence to nutritional guidelines and the high level of compliance with the recommended low-fat diet, may have limited such side effects.
As noted in the FDA-approved lomitapide prescribing information, careful monitoring of LFTs and concomitant medication use are important considerations with the use of lomitapide. The patient’s transient and marked increases in LFTs associated with the use of a series of over-the-counter medications was instructive. Use of strong and moderate CYP3A4 inhibitors is contraindicated with lomitapide, and weak CYP3A4 inhibitors require a reduction in dose of lomitapide. Lomitapide may also increase exposure to statin, giving more cause for careful adverse event monitoring when initiating treatment11. In addition, a possible drug interaction with warfarin was seen resulting in transient INR elevation with critical clinical sequelae, indicating the need for close monitoring of patients with concomitant warfarin use. Because lomitapide is deemed to be teratogenic, it is contraindicated in women considering pregnancy and the use of an effective method of contraception is mandatory and should be appropriately discussed with patients.
The cause for the transient unintended reduction of LDL-C to a remarkably low level in this patient (LDL-C of 4mg/dL) is not known and further studies of potential mechanisms are warranted. As low levels of LDL-C may be associated with a reduction in lipid-soluble vitamin levels, reduction of lomitapide dose to restore higher LDL-C levels may be effective in these circumstances.
Conclusion
In summary, in this case, lomitapide was effective in maintaining low levels of LDL-C in a patient with hoFH over a 5-year period. Attention to diet, liver monitoring and concomitant medication usage were critical components of the patient’s management. For select patients with hoFH, lomitapide could be remarkably effective and may be tolerated with minimal side effects over a long treatment period. Continuing evaluation of long-term safety and drug interactions are warranted, but as this case report demonstrates, with careful attention to diet and safety monitoring, lomitapide is a viable long term treatment for such patients.
Case Report Highlights.
Lomitapide was recently approved as an adjunct treatment for HoFH
The case of a hoFH patient treated with lomitapide for >5 years is presented
Lipid lowering response to lomitapide treatment has been significant
Careful monitoring of diet, liver function and concomitant medication was necessary
The possibility of drug-drug interactions should be carefully assessed
Acknowledgments
The phase 2 study was funded by the Doris Duke Charitable Foundation. The Phase 3 study was supported by a grant from the FDA Office of the Orphan Product Development (FR-R-003098) to Dr. Cuchel, by a grant for the NIH National Center for Research Resources (UL1-RR-024134) and by Aegerion Pharmaceuticals, Inc. The Extension Study was supported by Aegerion Pharmaceuticals, Inc. Dr. Cuchel has received lecture honoraria from Aegerion Pharmaceuticals. Dr. Sachais has received speaking honoraria from Kaneka Pharma America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 2863–2913. [Google Scholar]
- 2.Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, Kuivenhoven JA, Nordestgaard BG, Descamps OS, Steinhagen-Thiessen E, Tybjærg-Hansen A, Watts GF, Averna M, Boileau C, Borén J, Catapano AL, Defesche JC, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Ray KK, Stalenhoef AF, Stroes E, Taskinen MR, Wiegman A, Wiklund O, Chapman MJ. Homozygous familial hypercholesterolaemia: new insights for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014 Jul 22; doi: 10.1093/eurheartj/ehu274. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts GF, Gidding SS, Wierzbicki AS, Toth PP, Alonso R, Virgil Brown W, Bruckert E, Defesche J, Kah Lin K, Livingston M, Mata P, Parhofer KG, Raal F, Santos RD, Sijbrands EJG, Simpson WG, Sullivan DR, Susekov AV, Tomlinson B, Wiegman A, Yamashita S, Kastelein J. Integrated guidance on the care of famililal hypercholesterolaemia from the International FH Foundation. Int J Cardiol. 2014;171:309–325. doi: 10.1016/j.ijcard.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Hudgins LC, Gordon BR, Parker TS, Saal SD, Levine DM, Rubin AL. LDL Apheresis: an effective and safe treatment for refractory hypercholesterolemia. Cardiovasc Drug Rev. 2010;20:271–280. doi: 10.1111/j.1527-3466.2002.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 5.Thompson GR, Catapano A, Saheb S, Atassi-Dumont M, Barbir M, Eriksson M, Paulweber B, Sijbrands E, Anton F, Parhofer KG. Severe hypercholesterolaemia: therapeutic goals and eligibility criteria for LDL apheresis in Europe. Curr opin lipidol. 2010;21:492–498. doi: 10.1097/MOL.0b013e3283402f53. [DOI] [PubMed] [Google Scholar]
- 6.Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 7.Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Du Plessis AM, Propert KJ, Sasiela WJ, Bloedon LT, Rader DJ. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–46. doi: 10.1016/S0140-6736(12)61731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zech LA, Hoeg JM. Correlating corneal arcus with atherosclerosis in familial hypercholesterolemia. Lipids Health Dis. 2008;7 doi: 10.1186/1476-511X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aegerion Pharmaceuticals. Juxtapid (lomitapide) capsules: Highlights of prescribing information. 2013 Retrieved from: http://www.aegerion.com/Collateral/Documents/English-US/Prescribing_Information%20june%202013.pdf.
- 10.Kolansky DM, Cuchel M, Clark BJ, Paridon S, McCrindle BW, Wiegers SE, Araujo L, Vohra Y, Defesche JC, Wilson JM, Rader DJ. Longitudinal Evaluation and Assessment of Cardiovascular Disease in Patients with Homozygous Familial Hypercholesterolemia. Am J Cardiol. 2008;102:1438–1443. doi: 10.1016/j.amjcard.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Tuteja S, Duffy D, Dunbar RL, Movva R, Gadi R, Bloedon LT, Cuchel M. Pharmacokinetic Interactions of the Microsomal Triglyceride Transfer Protein Inhibitor, Lomitapide, with Drugs Commonly Used in the Management of Hypercholesterolemia. Pharmacotherapy. 2013;34:227–239. doi: 10.1002/phar.1351. [DOI] [PubMed] [Google Scholar]