Abstract
Background
Recent descriptions of the clinical and laboratory features of subjects with acute porphyrias in the US are lacking. Our aim was to describe clinical, biochemical and genetic features of 108 subjects.
Methods
Between Sep 2010—Dec 2012, 108 subjects with acute porphyrias [90 acute intermittent porphyria, 9 hereditary coproporphyria, 9 variegate porphyria] were enrolled into an observational study. Genetic testing was performed at a central genetic testing laboratory and clinical information entered into a central data base. Selected features were compared to data for adults in the USA.
Results
Most subjects [88/108, 81%] were female with self-reported onset of symptoms in 2nd–4th decades of life. The most common symptom was abdominal pain. Appendectomies and cholecystectomies were common prior to a diagnosis of porphyria. The diagnosis was delayed by a mean of 15 years. Anxiety and depression were common, and 18% complained of chronic symptoms, especially neuropathic and other pains. The incidences of and systemic arterial hypertension, chronic kidney disease, seizure disorders, and psychiatric conditions were markedly increased. Mutations of the known causative genes were found in 102/105 of those tested, with novel mutations being found in 37 including in 7/8 subjects with hereditary coproporphyria. Therapy with intravenous hematin was the most effective therapy both for treatment of acute attacks and for prevention of recurrent attacks.
Conclusions
Acute porphyrias often remain undiagnosed for more than a decade after first symptoms develop. Intravenous hematin is the treatment of choice, both for treatment of acute attacks and for prevention of recurrent attacks.
Trial Registration
clinicaltrials.gov identifier: NCT01561157
Keywords: acute intermittent porphyria, clinical features, delta-aminolevulinic acid, hematin, heme, hereditary coproporphyria, hydroxymethylbilane synthase, porphobilinogen, porphobilinogen deaminase, porphyrins, variegate porphyria
The porphyrias are metabolic diseases that cause accumulation of intermediate compounds in the heme biosynthetic pathway. They occur chiefly due to mutations in the genes encoding the enzymes involved in heme production.1,2 The four hepatic acute porphyrias are δ-aminolevulinate dehydratase deficiency porphyria [a very rare autosomal recessive disorder], acute intermittent porphyria, hereditary coproporphyria, and variegate porphyria. When symptomatic, acute porphyrias present as acute attacks of pain, usually in the abdomen often accompanied by sympathetic nervous system over-activity [systemic arterial hypertension, tachycardia, sweating], sometimes with other neurologic manifestations such as weakness, delirium and seizures
The epidemiology, natural history and clinical, biochemical and genetic features of acute porphyrias in the US have not been described recently. Case series of patients with acute porphyria seen at single centers in the USA were published two to four decades ago4,5 as were series from other countries6–10. Since then, the clinical setting has changed, with less frequent use of barbiturates, which often precipitated or exacerbated acute attacks, and now greater availability of treatment with intravenous heme. Complicating the current picture of acute porphyria are many individuals who have been diagnosed erroneously, based on minor, non-specific increases in urinary porphyrins. We here describe the demographic, clinical, and biochemical features of 108 carefully confirmed cases of acute porphyria.
Methods
The 108 patients in this report had histories of consistent clinical features such as acute attacks of abdominal, back and/or limb pain or a relative with proven acute porphyria. Biochemical criteria for acute intermittent porphyria included a substantial increase in urinary PBG [>8 mg PBG /24 hours or > 8 mg PBG / g of creatinine, or >2 fold increase (relative to upper limit of normal (ULN) of 4 mg/24 h or mg/g creatinine)] or serum PBG [>0.2 ug/dL, or >2 fold increase (relative to ULN of 0.1 ug/dL)], with little or no increase in plasma or fecal porphyrins or with decreased activity of hydroxymethyl-bilane synthase [HMBS] in erythrocytes. Biochemical criteria for HEREDITARY COPROPORPHYRIA were a substantial increase in PBG an increase in urinary porphyrins [>450 nmol or >300 ug/24 h or /g of creatinine, or more than 1.5-fold increase (relative to ULN of 300 nmol or 200 ug/24 hours or /g of creatinine], normal or only slight increases in plasma porphyrins and a substantial increase in total fecal porphyrins, with a predominance of coproporphyrin III [>400 ug/g dry weight or >2-fold increase (relative to ULN of 200 ug/g dry weight, with a predominance of coproporphyrin III and a coproporphyin III/I ratio >1.5]. Biochemical criteria for VARIEGATE PORPHYRIA were a substantial increase in urinary or serum PBG and/or in urinary coproporphyrin III increases in plasma porphyrins [>2.7 ug/dL, or >3-fold increase (relative to ULN of 0.9 ug/dL)] and a fluorescence emission peak at 626–628 nm or fecal porphyrins [>400 ug/g dry weight or >2-fold increase (relative to ULN of 200 ug/d dry weight, with a predominance of coproporphyrin III and protoporphyrin]. Detailed mutational analyses were carried out on DNA from 105 subjects, and confirmatory disease-causing HMBS, CPOX or PPOX mutations were found in all but 3 cases [2 with ACUTE INTERMITTENT PORPHYRIA and 1 with HEREDITARY COPROPORPHYRIA].
Demographic, clinical, biochemical and genetic data were entered into a central database [maintained at Univ of South Florida], and descriptive statistics were generated. Bivariate analyses were performed using chi-square tests and non-parametric Wilcoxon rank sum tests. Indirect standardization methods were used to compare chronic medical conditions in subjects with acute intermittent porphyria with data from the NHANES 2009–2010 dataset or the United HealthCare database, 2009–2010. All analyses were performed using SAS software, v9.3.
Results
Demographic and Anthropomorphic Characteristics [Table 1]
Table 1.
Selected Demographic and BMI data of Subjects Studied
| AIP n=90 | HCP n=9 | VP n=9 | ||
|---|---|---|---|---|
| Median age at diagnosis in years [25th–75th Percentile] | 33 [22–49] | 42[29–46] | 39 [34–47] | |
| Gender | Female | 75 [83%] | 7/9 [77%] | 6/9 [66%] |
| Male | 15 [17%] | 2/9 [23%] | 3/9 [33%] | |
| Race | White 85 [94.4%] | White 7/9 | White 8/9 | |
| Asian 2 [2.2%] | Asian 1/9 | |||
| American Indian 3[3.3%] | African American 1/9 | African American 1/9 | ||
| Ethnicity | Non-Hispanic 79 [88%] | Non-Hispanic 9/9 | Non-Hispanic 9/9 | |
| Hispanic 7 [8%] | ||||
| Unknown 4 [4%] | ||||
| Family history | Mother + | 26/82 [32%] | 3/9 | 2/9 |
| Father + | 13/82 [16%] | 2/9 | 1/9 | |
| Median BMI kg/m2 [25th–75th Percentile] | Female | 24.6 [21.9–32.1] | 27.2 [20.9–34.4] | 22.3 [18.1–36.3] |
| Male | 30 [22.6–31.1] | 24.6 [23.8–25.3] | 23.8 [21.4–26.2] | |
Subjects were mainly female. A substantial majority were non-Hispanic Caucasians. Among those with acute intermittent porphyria, three were American Indian and two were of East Indian or Asian ancestry. One subject with hereditary coproporphyria was East Asian.
Symptoms and Family History of Acute Porphyria
Among subjects with acute intermittent porphyria, 81% reported onset of symptoms in the 2nd to 4th decades of life, with median age at diagnosis of 33 y. Similarly, in hereditary coproporphyria 7/9 and in variegate porphyria 9/9 reported onset of symptoms between 2nd and 4th decades [Table 1]. Among subjects with acute intermittent porphyria, 85% reported symptoms attributed to the disease. Symptoms were intermittent but frequent in 54%, occurred only during acute attacks in 28%, and nearly constant in 18%. In hereditary coproporphyria and variegate porphyria, 7/9 and 9/9, respectively, reported symptoms attributed to their disease.
Among subjects with cute intermittent porphyria, 48% reported parents with known disease. In coproporphyria and variegate porphyria, 5/9 and 3/9, respectively, reported having parents with porphyria [Table 1].
37% of subjects with acute intermittent porphyria reported ingestion of medications as triggers for acute attacks; 22% reported weight loss diets as triggers; 16% surgery; and 7% environmental toxins as other precipitating factors. Among the subjects who reported their histories of prior hospitalizations, 55% reported being hospitalized 1–5 times in their life times for acute attacks, whereas 15% reported no hospitalizations for porphyria..
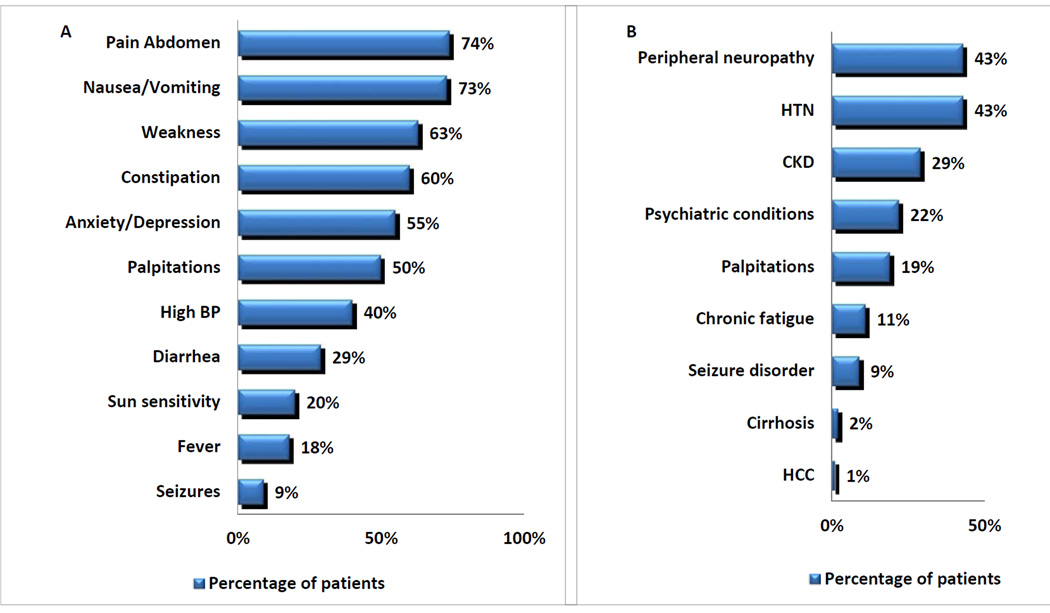
Abdominal pain (74%), nausea/vomiting (73%), weakness (63%), and constipation (60%) were the most commonly reported symptoms during acute attacks. [Figure 1A].
Figure 1.
(A) Symptoms and signs during acute attacks in ACUTE INTERMITTENT PORPHYRIA, (B) Medical conditions associated with ACUTE INTERMITTENT PORPHYRIA
Medical Conditions and Abdominal Surgeries associated with Acute Intermittent Porphyria [Table 2 and Figure 1B]
Table 2.
Patient reported prevalence of chronic medical conditions in AIP and comparison to age and sex matched controls
| AIP | Incidence in controls [NHANES & United Healthcare Databases, 2009–2010] |
Chi square test p-value | |
|---|---|---|---|
| Systemic arterial hypertension | 41% | 23% | <0.0001 |
| Chronic kidney disease | 29% | 1.0% | <0.0001 |
| Psychiatric conditions | 32% | 16% | 0.0041 |
| Chronic fatigue | 11% | 9.32% | 0.6942 |
| Seizure disorders | 9% | 0.63% | 0.0057 |
| Hepatocellular carcinoma | 1% | 0.09% | 0.382 |
| Cholecystectomy | 15% | 0.18% | <0.0001 |
| Hysterectomy | 18% | 14% | 0.459 |
Peripheral neuropathy (43%), systemic arterial hypertension (43%) and chronic kidney disease (29%) were the most common chronic medical conditions reported in subjects with acute intermittent porphyria. Two subjects, both without a history of viral hepatitis or excess alcohol use, aged 46 and 49 years, had cirrhosis. A 78 year old woman without cirrhosis had a history of hepatocellular carcinoma that had been resected ten years prior to her enrollment with no evidence of recurrence. She since has recurrent primary carcinoma of the lung. Of additional interest, her brother also had acute intermittent porphyria and died at 78 years due to hepatocellular carcinoma. The Hmbs mutation in this kindred is R167Q.
Among subjects with acute intermittent porphyria, 13% reported having undergone appendectomies, 15% reported cholecystectomies, and 16% hysterectomies. 5/9 subjects with coproporphyria and 5/9 with variegate porphyria reported having undergone abdominal surgeries
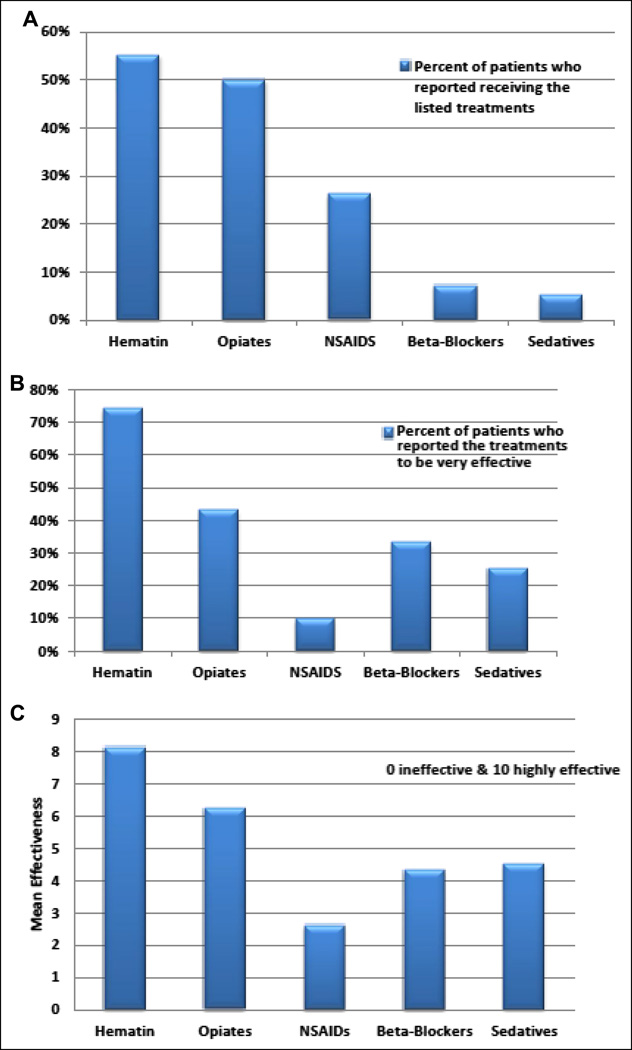
Summary of Data on Treatment and Prevention of Porphyric Attacks [Figure 2]
Figure 2.
Treatments during acute attacks in patients with ACUTE INTERMITTENT PORPHYRIA, (A) Percent of patients who reported receiving the listed treatments, (B) Percent of patients who reported the treatments to be effective, (C) Mean effectiveness of each treatment on a scale of 0–10 with 0 being ineffective and 10 being very effective
Among subjects with acute intermittent porphyria, 55% reported having received intravenous heme in the form of hematin [Panhematin], during acute attacks. 74% of them assessed hematin as very effective in improving abdominal pain and other manifestations. 50% of these patients reported treatment with opiates during acute attacks, and only 44% of them reported that they were effective. Sixty percent reported pursuing healthy life styles (physical exercise, adequate rest and sleep, efforts to minimize physical or psychological stress, avoidance of alcohol and certain medications). Twenty six percent reported receiving repeated hematin infusions, and 15% reported receiving glucose infusions for prevention. Thirty percent reported taking pain medications every day. By a substantial margin, hematin infusions were felt to be the most effective means of preventing acute attacks.
Among various methods for preventing acute attacks, hematin infusions were reported by patients to be most effective with a mean effectiveness of 7.9 [on a scale of 0 (least effective) – 10 (highly effective)]. Glucose infusions, high carbohydrate diets and pain medications scored 4.4, 4.7 and 4.2, respectively [p = 0.0781, 0.0021, and 0.0049, compared to hematin infusions]. Among 18 subjects with other forms of acute porphyria, 2 reported having received repeated hematin infusions for prevention of recurrent attacks, and both reported that they had been very effective.
Oral Contraceptive Use and Data on Pregnancy and Delivery
In women with acute intermittent porphyria, 30/63 reported severe pre-menstrual symptoms, 10/58 reported having experienced acute attacks during pregnancy (among whom 2 reported having received hematin for acute attacks during pregnancy), and 14/33 reported worsening symptoms after they had started oral contraceptive pills. Fifty-nine of 60 women with acute intermittent porphyria who had been pregnant carried pregnancies to term with delivery of living, normal newborns, including the two who had had received hematin while pregnant.
Summary of Selected Laboratory Data
Among subjects with acute intermittent porphyria, 63% had levels of urinary ALA that were increased with a mean value of 14.6 mg/ g creatinine [range 1.1–60.9]. 74% had levels of urinary PBG that were increased with a mean value of 48.9 mg/ g creatinine [range 0–473]. 5/9 had values of urinary porphyrins that were increased with a mean value of 1305 ug/g creatinine [range 77.4–6334]. The mean RBC HMBS activity was 17 nmol uroporphyrin /mL RBC/h [reference range 20–50]. Mean plasma and fecal porphyrins were within the reference ranges in all.
Table 3 provides additional detail on subjects who were documented to have experienced acute attacks of porphyria. Nearly all were relatively young women who often had markedly increased urinary excretions of PBG. They were not obese. All who reported having received Panhematin infusions for management reported prompt and excellent clinical responses, and some also reported good responses to ongoing prophylactic hematin.
Table 3.
Summary of AIP Subjects with Information about Prior or Current Acute Attacks
| Gender | Age | Age at Diagnosis [y] |
BMI | Urine PBG [mg/g creatinine] |
# prior Acute Attacks/ Hospitalizations |
Hmbs mutation |
Course; Comments |
|---|---|---|---|---|---|---|---|
| F | 26 | 22 | 19.2 | 143 | 4 | W283× | Urinary PBG was obtained during an acute attack, prior to Panhematin. She received Panhematin infusions with prompt clinical and biochemical response. The patient’s father also has the same genetically confirmed PBGD mutation. The father never had any porphyria symptoms/attack throughout his life. No urinary PBG available for the father. |
| F | 60 | 55 | N/A | 3.77 | 15 | A331V | nary PBG was obtained after a 4 day course of daily infusions of Panhematin. Patient continues to have frequent attacks and to receive Panhematin often. |
| F | 53 | 42 | 24.2 | 63 | 20 | G280E | Urinary PBG obtained when subject presented with acute attack. Hospital course complicated with shortness of breath and development of pneumonia, which was treated and subject improved. |
| F | 40 | 40 | 22.2 | 67 | 2 | R173W | Urine result was during acute attack, prior to Panhematin. The patient received Panhematin treatment for a single acute attack. She has not had not attacks since then. |
| F | 57 | 20 | 25.4 | 176.4 | 16 | R325× | Urinary PBG was a historical value when the patient had an acute attack. She received Panhematin during hospitalization and improved quickly. She now receives prophylactic Panhematin 1×/month |
| F | 28 | 29 | 21.4 | 100 | 0 | IVS6+1G>C | Patient enrolled just prior to her first acute attack. She now receives prophylactic Panhematin weekly. Her mother and sister also carry the same Hmbs mutation but have not experienced acute attacks. |
| F | 23 | 17 | 19.2 | 3.7 | 9 | L100P | Urinary PBG value was obtained at an office visit for abdominal pain. A decision was made to admit the patient given her overall condition, poor intake and symptoms of proximal muscle weakness. She was treated with Panhematin for 4 days. The patient also had a previous diagnosis of endometriosis and was started on Seasonique low OCP without a gap week by GYN during this hospital stay. She was discharged in 5 days at which time she had almost no abdominal pain. |
| M | 31 | 31 | 27.1 | 65 | 3 | R173W | Urine PBG was a value during an acute attack. He responded promptly to Panhematin. He has not had attacks since, except for one mild episode, which did not require hospitalization |
| F | 44 | 38 | N/A | 49.3 | 4 | IVS4-+1G>C | Urinary PBG was obtained Mar, 2011 during an acute attack, prior to Panhematin. The patient has been experiencing freuqent severe attack for 10+ years. |
Abbreviations used: BMI, body mass index; F, female; Hmbs, hydroxymethyl bilane synthase [also known as porphobilinogen deaminase]; M, male; N/A, not available; PBG, porphobilinogen
[Standard abbreviations and nomenclature are used for descriptions of the mutations.]
Among the five subjects with hereditary coproporphyria with available lab data, none had values of urinary ALA, PBG, or porphyrins, nor plasma porphyrin levels that were increased above upper limits of normal. Among the 9 subjects with variegate porphyria, 1/7 had urinary ALA that was slightly increased [7.33 mg/g creatinine]. 3/9 had increased urinary PBG. The mean urinary PBG was 8.6 mg/g creatinine [range 1.6–41.3]. We had urinary porphyrin levels for only one subject with variegate porphyria, and it was within the normal range [187 ug/g creatinine]. In this cohort, however, 2/5 had elevated levels of plasma total porphyrins; the mean plasma total porphyrin level was 1.3 ug/dL [reference range 0–0.9]. A fluorescence emission peak in diluted plasma at neutral pH at 626–628 nm, following excitation with light of ~400–410 nm [the Soret band], was observed in 1 of 3 of our subjects with variegate porphyria in whom these results were available.2,12–14
Genetic Findings and Clinical Correlations
In subjects with acute intermittent porphyria, mutations in the Hmbs gene were identified in 85/87; missense mutations were identified in 45, small insertions/deletions in 15, non-sense mutations in 8 and intronic mutations in 17. 27/85 were novel mutations. The most common missense mutation was R173W. [Despite repeated attempts, samples for genetic testing could not be obtained from two subjects; DNA analysis for one subject is pending.]
In subjects with hereditary coproporphyria, mutations in the coproporphyrinogen oxidase gene were identified in 8/9 subjects: missense mutations in 4, small insertions/deletions in 2, and non-sense mutations in 2. Seven of the eight were novel mutations [not found in the Human Gene Mutation Database [Univ of Cardiff, Wales]. Among subjects with variegate porphyria, mutations in the protoporphyrinogen oxidase gene were identified in 9/9 subjects: known missense mutations in 3 and nonsense mutations in 4. 2/9 were novel mutations. We found no statistically significant associations of mutations and symptoms, signs or biochemical abnormalities (Table 4). Additional details regarding the novel mutations and their functional significance will be published elsewhere [Desnick et al, in preparation].
Table 4.
Lack of Influence of Type of Mutation in the Causative Genes and Urinary Excretion of PBG
| Gender and Urinary PBG [mg/g creatinine] |
n | Type of Mutation [% of Total Cohort] |
||||
|---|---|---|---|---|---|---|
| Missense | Nonsense | Splice Site | Other IVS | Insertion/Deletion | ||
| Female | 88 | |||||
| PBG < 20 | 52 | 61.5 | 11.5 | 13.5 | 2.0 | 11.5 |
| PBG > 20 | 36 | 44.8 | 13.8 | 20.7 | 0.0 | 20.7 |
| Male | 20 | |||||
| PBG < 20 | 13 | 28.6 | 21.4 | 14.3 | 7.1 | 28.6 |
| PBG > 20 | 7 | 28.6 | 14.3 | 42.8 | 0.0 | 14.3 |
With but one exception [a woman with VP], all subjects with urinary PBG greater than 20 mg/g creatinine had AIP. Among the 75 women with AIP, 35 [50%] had urinary PBG > 20 mg/g creatinine. Among the 15 men with AIP, 7 [46.6%] had urinary PBG > 20 mg/g creatinine. The differences in distribution of types of mutations are not significant.
Discussion
We report the largest group of subjects with well-documented and -characterized acute porphyrias thus far assembled in North America. Our emphasis is on acute intermittent porphyria, which is the most common and severe form of acute porphyria in the USA. Among the important features are the following: 1. There is a substantial preponderance of females [83%] over males. 2. Fewer than half the subjects reported a parent with known acute porphyria, confirming the variable expression of the clinical phenotype, even within individual kindreds. 3. The onset of symptoms usually occurs during second through fourth decades of life [81%]. 4. Medications [37%] and weight loss diets [22%] are the most commonly reported triggers of acute attacks. 5. 18% feel they suffer from chronic, ongoing symptoms.
There are high prevalences of chronic medical conditions such as peripheral neuropathy [43%], systemic arterial hypertension [43%], chronic kidney disease [29%], and history of abdominal surgeries [appendectomy 13%, and cholecystectomy 15%. The prevalences of systemic arterial hypertension, psychiatric conditions, and seizures are significantly greater than that in the general population, matched for age and gender Cholecystectomies had been performed in a far higher percentage of subjects with acute intermittent porphyria than in a control cohort [15% vs 0.2%, p <0.001] (Table 2).
Recent results from a genetic analysis of French blood donors indicate that the frequency of genetic defects in the Hmbs gene in unselected subjects is as high as 1/1675 [59/100,000], which is much higher than previous estimates [~5/100,000].20 Fifty five percent of subjects reported having received hematin during acute attacks, and most (74%) felt that hematin was very effective in treatment of acute attacks 26% of subjects received repeated hematin infusions for prevention of acute attacks, and hematin was the most effective treatment for prevention of recurrent attacks. This is consistent with a previous study suggesting that approximately one third of patients treated with hematin in the U.S. receive prophylactic treatment17. In addition, 60% reported as beneficial life style modifications, such as avoidance of alcohol and certain medications and increasing physical exercise. Because of the perceived substantial therapeutic benefit from the use of intravenous hematin, such treatment should be widely and easily available to patients with well-established diagnoses of acute porphyria.
One of our subjects with acute intermittent porphyria, a 36 year old woman with severe recurrent attacks, underwent orthotopic liver transplantation. The surgery was uneventful and resulted in rapid normalization of plasma and urinary ALA and PBG and to a rapid, complete, and ongoing resolution of symptoms related to acute intermittent porphyria. This case will be reported in detail elsewhere [Liu et al, personal communication]. The biochemical and clinical improvement seen in this subject is in agreement with recently published results of liver transplantation for acute intermittent porphyria18.
Genetic analyses of the genes known to be affected in acute intermittent porphyria, hereditary coproporphyria, and variegate porphyria detected mutations adequate to account for the occurrence of disease in nearly all subjects. Novel, not previously described mutations in the related genes were detected in 19% of the patients. There were no significant associations among clinical or laboratory abnormalities and the general types of mutations found, nor with specific mutations among those more frequently observed [Table 4].
Disease manifestations were less frequent and severe in hereditary coproporphyria and variegate porphyria than in acute intermittent porphyria. Most subjects [16/18] reported onset of symptoms between 2nd and 4th decades of life, but correct diagnosis was delayed by ~15 years. The clinical features were generally similar to those reported by subjects with acute intermittent porphyria. DNA studies were useful for diagnostic confirmation in 15/16 subjects with hereditary coproporphyria and variegate porphyria, including those with normal urinary porphyrins and porphyrin precursors.
Strengths of our study include use of a standardized protocol with rigorous biochemical and/or molecular inclusion criteria for diagnosis and the application of state-of-the art biochemical and genetic testing methods.
In conclusion, most patients with symptomatic acute porphyrias in the USA are women who first develop symptoms in the 2nd to 4th decades of life. The cardinal symptom is severe generalized abdominal pain, often with nausea and vomiting. There are significantly increased frequencies of systemic arterial hypertension, chronic renal and psychiatric disease and seizures. Genetic analyses reveal diverse mutations in the genes underlying these disorders.
Patients report that the most effective therapy of acute attacks is intravenous hematin. Thus,2,13 we believe that patients with acute porphyrias and with symptoms severe enough to come to the Emergency Department and/or to be hospitalized, should be treated as expeditiously as possible with intravenous hematin. In addition, prophylactic and repeated administration of intravenous hematin is of benefit to those prone to recurrent attacks,21 and hematin is safe for use in women who are pregnant. Hematin was the first drug approved under the Orphan Drug Act, and, it should be readily available to all symptomatic patients with well documented acute porphyrias.
Clinical Significance.
Recent descriptions of the clinical and laboratory features of subjects with acute porphyrias in the US are lacking.
Major findings are the acute porphyrias often remain undiagnosed for more than a decade after first symptoms develop; they are associated with increased systemic arterial hypertension, chronic kidney disease, seizures and psychiatric conditions;
Intravenous hematin is the treatment of choice, both for treatment of acute attacks and for prevention of recurrent attacks.
Acknowledgements
We thank the patients who enrolled and the research coordinators at the six clinical sites: Eric Gou, Csilla Hallberg, Akshata Moghe, Theora Cimino, Sam Zenhari, Jeanette Davis, Tiffanie Hales, Toni Seay, Gale Groseclose, and Hetanshi Naik. Supported by the American Porphyria Foundation and by a grant [HL117199] and contract [U54 DK083909] from NIH. The U54 funds the Porphyrias Consortium, part of the Rare Disease Clinical Research Network (RDCRN), a collaboration among the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science, and the National Institute of Diabetes and Digestive and Kidney Diseases. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balwani M, Desnick RJ. The porphyrias: advances in diagnosis and treatment. Blood. 2012 Nov 29;120(23):4496–4504. doi: 10.1182/blood-2012-05-423186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonkovsky HL, Guo JT, Hou W, Li T, Narang T, Thapar M. Porphyrin and heme metabolism and the porphyrias. Comprehensive Physiology. 2013 Jan;3(1):365–401. doi: 10.1002/cphy.c120006. [DOI] [PubMed] [Google Scholar]
- 3.Larion S, Caballes FR, Hwang SI, Bonkovsky HL. Circadian rhythms in acute intermittent porphyria - a pilot study. Eur J Clin Invest. 2013 Jul;43(7):727–739. doi: 10.1111/eci.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeans JB, Savik K, Gross CR, et al. Mortality in patients with acute intermittent porphyria requiring hospitalization: a United States case series. American Journal of Medical Genetics. 1996 Nov 11;65(4):269–273. doi: 10.1002/(SICI)1096-8628(19961111)65:4<269::AID-AJMG4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 5.Stein JA, Tschudy DP. Acute intermittent porphyria. A clinical and biochemical study of 46 patients. Medicine. 1970 Jan;49(1):1–16. [PubMed] [Google Scholar]
- 6.Meissner PN, Corrigall AV, Hift RJ. Fifty years of porphyria at the University of Cape Town. South African Medical Journal. 2012 Jun;102(6):422–426. doi: 10.7196/samj.5710. [DOI] [PubMed] [Google Scholar]
- 7.Hift RJ, Meissner PN. An analysis of 112 acute porphyric attacks in Cape Town, South Africa: Evidence that acute intermittent porphyria and variegate porphyria differ in susceptibility and severity. Medicine. 2005 Jan;84(1):48–60. doi: 10.1097/01.md.0000152454.56435.f3. [DOI] [PubMed] [Google Scholar]
- 8.Mustajoki P. Variegate porphyria. Twelve years' experience in Finland. The Quarterly Journal of Medicine. 1980 Spring;49(194):191–203. [PubMed] [Google Scholar]
- 9.Whatley SD, Puy H, Morgan RR, et al. Variegate porphyria in Western Europe: identification of PPOX gene mutations in 104 families, extent of allelic heterogeneity, and absence of correlation between phenotype and type of mutation. American Journal of Human Genetics. 1999 Oct;65(4):984–994. doi: 10.1086/302586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thunell S, Floderus Y, Henrichson A, Harper P. Porphyria in Sweden. Physiol Res. 2006;555(Suppl 2):S109–S118. doi: 10.33549/physiolres.930000.55.S2.109. [DOI] [PubMed] [Google Scholar]
- 11.Akagi RKN, Inoue R, Anderson KE, et al. delta-Aminolevulinate dehydratase (ALAD) porphyria: the first case in North America with two novel ALAD mutations. Mol Genet Metab. 2006 Apr;97(4):329–336. doi: 10.1016/j.ymgme.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Poh-Fitzpatrick MB. A plasma porphyrin fluorescence marker for variegate porphyria. Archives of Dermatology. 1980 May;116(5):543–547. [PubMed] [Google Scholar]
- 13.Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Int Med. 2005 Mar 15;142(6):439–450. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]
- 14.Whatley SD, Mason NG, Woolf JR, Newcombe RG, Elder GH, Badminton MN. Diagnostic strategies for autosomal dominant acute porphyrias: retrospective analysis of 467 unrelated patients referred for mutational analysis of the HMBS, CPOX, or PPOX gene. Clin Chem. 2009 Jul;55(7):1406–1414. doi: 10.1373/clinchem.2008.122564. [DOI] [PubMed] [Google Scholar]
- 15.Bonkovsky HL. Porphyrin and heme metabolism and the porphyrias. In: Zakim D, Boyer TD, editors. Hepatology-a Textbook of Liver Disease. 2nd Edn. Philadelphia: Elsevier; 1990. pp. 378–424. [Google Scholar]
- 16.Wahlin S, Harper P, Sardh E, Andersson C, Andersson DE, Ericzon BG. Combined liver and kidney transplantation in acute intermittent porphyria. Transplant International. 2010 Jun;23(6):e18–e21. doi: 10.1111/j.1432-2277.2009.01035.x. [DOI] [PubMed] [Google Scholar]
- 17.Anderson KE, Collins S. Open-label study of hemin for acute porphyria: clinical practice implications. Amer Journal of Medicine. 2006 Sep;119(9):801, e819–e824. doi: 10.1016/j.amjmed.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Dowman JK, Gunson BK, Mirza DF, et al. Liver transplantation for acute intermittent porphyria is complicated by a high rate of hepatic artery thrombosis. Liver Transplantation. 2012 Feb;18(2):195–200. doi: 10.1002/lt.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson KE, SS S, DF B, Desnick RJ. The Metabolic Basis of Inherited Disease. 8th edn. New York: McGraw-Hill; 2001. pp. 2991–3062. [Google Scholar]
- 20.Elder G, Harper P, Badminton M, Sandberg S, Deybach JC. The incidence of inherited porphyrias in Europe. Journal of Inherited Metabolic Disease. 2012 Nov 1;26(4):476–483. doi: 10.1007/s10545-012-9544-4. [DOI] [PubMed] [Google Scholar]
- 21.Badminton MN, Deybach JC. Treatment of an acute attack of porphyria during pregnancy. Europ J Neurol. 2006 Jun;13(6):668–669. doi: 10.1111/j.1468-1331.2006.01238.x. [DOI] [PubMed] [Google Scholar]