Abstract
Objective
Pregnancies complicated by diabetes mellitus impair offspring memory functions during infancy and early childhood. The purpose of this study was to investigate the long term consequences of such pregnancies on memory and memory-related brain regions in 10-year-old children.
Method
Nineteen (19) children of diabetic mothers (CDMs) and thirty-five (35) children of non-diabetic mothers participated in this 10 year follow-up study. Memory performance was assessed using a continuous recognition memory task during which children made old/new judgments in response to pictures of concrete and abstract objects presented after different lags or delays. In addition, the volume of the hippocampal formation was measured using high resolution structural images.
Results
At 10 years of age, recognition memory performance of CDMs did not differ from children of non-diabetic mothers. Similarly, the volume of the hippocampal formation did not differ between groups. However, the size of the hippocampal formation in CDMs predicted the time those children needed to provide accurate responses in the continuous recognition memory task.
Conclusion
CDMs do not show memory impairments by 10 years of age, despite evidence for such impairments early in life. However, subtle differences in underlying neural processes may still be present. These results have important implications for long-term cognitive development of CDMs.
Keywords: declarative memory, development, hippocampal formation, developmental plasticity
INTRODUCTION
Diabetes mellitus during pregnancy constitutes an adverse fetal environment, and has been shown to negatively impact several cognitive domains later in life (1,2). These cognitive deficits are associated with poor maternal glycemic control during pregnancy (1,3), leading to fetal hypoxia, fetal hyper- and hypoglycemia, and fetal iron deficiency (3).
Given that as many as 10% of pregnancies are complicated by maternal diabetes (www.cdc.gov/Features/DiabetesPregnancy), studying the outcome of such pregnancies has substantial implications for public health. Moreover, it has been shown that such pregnancies increase the risk for neurodevelopmental disorders such as epilepsy (17) and schizophrenia (18). It is therefore particularly important to better understand this condition and its consequences on brain development.
One brain region that has been shown to be particularly sensitive to this adverse fetal environment is the hippocampal formation (HF), a fundamental brain structure for declarative memory functions. This brain region has a protracted developmental profile, as shown in humans and non-human primates (4–7), making it vulnerable to early insults. In addition, the high metabolic demands of the HF early in life place it at risk when vital substrates such as glucose, oxygen, and iron are deprived. For example, it has been shown that early iron deficiency will preferentially affect the developing HF, impacting its electrophysiology, CA1 dendritic arborization, and gene expression (8). Similarly, perinatal hypoxia impacts the dentate gyrus and CA1 regions of the HF in particular, altering the development of cholinergic and serotoninergic fibers (9).
Accordingly, memory impairments have been described in infants and toddlers of diabetic mothers. Studies have shown alterations of neonatal auditory recognition memory (10,11), visual recognition memory at 6 months of age (12), cross-modal recognition memory at 8 months of age (13), and delayed elicited imitation at both 12 months of age (14) and 3 to 4 years of age (15). As part of a longitudinal project (see 16 for a summary of previous findings with the sample), this study aimed to assess the long term outcomes of diabetic pregnancy on declarative memory function and hippocampal volume at 10 years of age. Specifically, we aimed to assess if memory and/or related brain processes’ impairments observed in infants of diabetic mothers (11–15), persist into childhood or are compensated for over time and thus no longer observable in childhood.
METHOD
Subjects
Pregnant women with and without pre-gestational or gestational diabetes mellitus were recruited at 28 weeks gestation as part of a longitudinal study assessing the consequences of diabetes mellitus during pregnancy on neural and cognitive development in infancy. Infants were included in this study if pregnancies were uncomplicated (lack of intrauterine growth restriction, significant maternal hypertension, chromosomal syndromes/non-chromosomal congenital anomalad sequences, and congenital infectious agents (TORCH)), if the labor and delivery were normal (delivery at 32 weeks gestation or greater, no significant heart rate decelerations and 5-min Apgar scores ≥ 6), and if the neonatal course was uncomplicated (no mechanical ventilation and no indication of acute perinatal or neonatal insult, such as asphyxia, sepsis, seizures, meningitis, prolonged or recurrent hypoglycemia, or intracranial hemorrhage)(see also 14).
In this paper, we report on a sample of fifty-six 9- to 10-year-old children from the original study: 36 children born to mothers who did not have diabetes during pregnancy (15 female) and 20 children born to mothers who did (7 female). All children were included in the final sample if they had good data from either the continuous recognition memory task or the structural MRI scan. Two children of diabetic mothers (CDMs) were excluded because they did not provide either good behavioral data or good structural data (see data analysis and results for exclusion criteria and attrition rate). All children were born at term (≥ 37 weeks of gestation) except one CDM (GA = 35 weeks) and one child of non-diabetic mother (GA = 32 weeks).
CDMs and children of non-diabetic mothers did not differ in age and IQ (see Table 1). No sex effects were found for any of the measured parameters, therefore, data from both sexes were combined for presentation.
Table 1.
Sample characteristics
| Characteristics | Children of non-diabetic mothers Mean (SD) | Children of diabetic mothers Mean (SD) | T-test results |
|---|---|---|---|
|
| |||
| Age at behavioral testing (months) | 117.05 (3.61) | 115.91 (3.90) | t(42) = .973, p = .336 |
| N | 28 | 16 | |
|
| |||
| IQ at 10 yrs | 117.48 (14.28) | 118.75 (10.37) | t(46) = −.334, p = .740 |
| N | 29 | 19 | |
|
| |||
| Head circumference at 10 yrs (cm) | 53.85 (1.29) | 54.24 (1.40) | t(52) = −1.027, p = .309 |
| N | 35 | 19 | |
|
| |||
| Weight at 10 yrs (lb) | 77.12 (13.87) | 78.27 (18.39) | t(44) = .−.239, p = .812 |
| N | 29 | 17 | |
|
| |||
| Birthweight (gr) | 3436.44 (596.42) | 3609.26 (628.98) | t(51) = −.992, p = .326 |
| N | 34 | 19 | |
|
| |||
| Head circumference at birth (cm) | 35.17 (2.49) | 36.52 (1.25) | t(47) = −2.04, p = .047 |
| N | 33 | 16 | |
|
| |||
| Gestational age (weeks) | 38.49 (2.65) | 38.21 (1.58) | t(52) = .413, p = .681 |
| N | 35 | 19 | |
The University of Minnesota’s Institutional Review Board approved all procedures and methods. All children and parents provided informed assent/consent.
Study design
Participants were tested across three sessions separated by approximately one month. During session one, a battery of behavioral measures (CANTAB tasks, neuropsychological tests; not reported here) and the Wechsler Abbreviated Scales of Intelligence (WASI) was administered. Children participated in a continuous memory task twice (once during EEG collection and once during fMRI scanning) at the second and third sessions (order of brain measures was randomly assigned). A structural scan was acquired during the second session. Only the behavioral data (the continuous memory tasks from the second and third sessions) and the structural data will be analyzed in this paper.
Continuous recognition memory task
Stimuli
Stimuli consisted of colorful images presented on a black background. Images were 3D objects that were classified as either concrete or abstract. Concrete images were photographs of easily named, real-world items (e.g., dog, car, banana, chair). Abstract images were photographs of three-dimensional objects with no identifiable name or label (e.g., abstract sculpture). A total of 120 unique images were used in this study, 60 abstract and 60 concrete. Separate images were used for practice rounds. During the EEG session, participants were seated 100 cm from a 19-inch computer monitor. During the fMRI session, the images were presented on a 6-inch monitor, 15 cm from the participant’s head. Images subtended approximately 11.5 degrees of visual angle in the center of the visual field.
Procedure
Stimulus presentation and behavioral data collection were completed using E-prime software (Psychology Software Tools, Pittsburgh, PA). Children took part in two brief practice rounds to ensure task comprehension. During the first practice round, each stimulus remained on the screen until the participant made the correct response. During the second practice round, the duration of both the stimulus and the inter-trial interval were identical to the actual experiment, and the child received no accuracy feedback. Behavioral data were collected in 3 runs of 72 trials each. Within each run, participants were presented with a total of 28 target stimuli (images that repeated after a lag of 2 or 5 intervening items), 4 foils stimuli (images that repeated after a lag of 1 or 3 intervening items) and 8 distractor stimuli (images that never repeated). Stimulus order within each run, as well as the order of runs was identical for all participants. Stimulus duration was 500 msec, followed by a 3500 msec inter-stimulus interval (ISI), consisting of a red fixation cross at the center of a black screen. These parameters were matched identically between the EEG and fMRI paradigms. Children were instructed to press one of two buttons on a response box for every image using their right hand. The first time a given image appeared (“new” trial), children were instructed to press with the index finger. For any image that appear a second time (“old” trial), the participant was instructed to press with the middle finger. Button press accuracy and reaction time were recorded.
Volumetric data
High-resolution structural images were obtained on a Siemens 3-Tesla TRIO whole-body scanner (Siemens Medical Systems, Iselin, NJ). Images were collected across the entire cortex in 176 sagittal slices with a voxel resolution of 1×1×1 mm3 (3D FLASH, TE=4.7, TR 20, flip angle=22, 1 mm slice thickness, 20% gap, FOV=256, matrix=256×256, phase encoding A to P) or in 240 sagittal slices (MPRAGE, TE=3.65, TR 2530, flip angle=7, 1 mm slice thickness, 50% gap, FOV=256, matrix=256×256, phase encoding A to P). No effect of image acquisition method was found, therefore, data from the two pulse sequence types were combined for analysis.
Data analysis
Continuous recognition memory task
For each child, we used the data from the first time they completed the recognition memory task (whether it was during the EEG or fMRI paradigm). Signal detection theory (SDT) was used for behavioral data analysis in order to control for response bias (the tendency to respond yes or no) and sensitivity (the degree of overlap between signal and noise distributions)(19). Correct responses were defined as a child correctly indicating via button press when an image was new (“hits”) or had previously been shown (“correct rejections”). Incorrect responses were defined as a child judging a previously seen image as new (“false alarms”) or a new image as previously seen (“misses”). The Hit rate (the probability of responding “new” on “new” trials; hits/hits+misses) and the false-alarm rate (the probability of responding “new” on “old” trials; false alarms/false alarms+correct rejections) were used to calculate the sensitivity index (d′, which is found by subtracting the z score that corresponds to the false-alarm rate from the z score that corresponds to the hit rate). A d’ value of 0 indicates an inability to distinguish signals from noise whereas a larger value represents the ability to differentiate signals from noise. Children unable to successfully recognize 75% of old and new targets (d′ < 1.35) were rejected from further behavioral analysis. In addition children who responded on fewer than 75% of the trials were not included in the analysis (see the results section for the attrition rate).
Volumetric data
All image files were converted from DICOM format to .mgz format and imported into Freeview a visualization tool of FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/fswiki/FreeviewGuide). Images were reoriented so that the anterior and posterior commissures were aligned, as well as the inferior and superior ends of the interhemispheric fissure. The right and left HFs (including the dentate gyrus, CA regions, subiculum, pre- and parasubiculum) were manually traced on every coronal slices following Schumann and colleagues’ (20) tracing criteria. All measurements were performed by the same experimenter (A.J.) who was blind to subject diagnosis (CDMs versus children of non-diabetic mothers). Twelve HF were randomly selected to be measured twice (14%). Intra-rater reliability was 96.65% (left HF: 96.77%; right HF: 96.52%).
Statistical analyses were conducted using SPSS software version 20.0 (IBM Corp., Armonk, NY). Behavioral data (d′ and reaction time) were analyzed using repeated-measures ANOVA with Greenhouse-Geisser corrected degrees of freedom. Post hoc analyses were performed with the Bonferroni test. Sample characteristics (table 1), hippocampal volume, and head circumference were compared between groups using independent t-tests with a significance level of p < 0.05. A Pearson correlation was performed to examine a possible relationship between the hippocampal volume and memory performance.
RESULTS
Table 1 reports characteristics of our sample at birth and at 10 years of age. Head circumference was marginally bigger for CDMs at birth (p = 0.047) but not at 10 years of age (p = 0.309; see table 1). No other group differences were found.
Continuous recognition memory task
Forty-four participants provided good behavioral data (16 CDMs). Two CDMs were excluded for providing insufficient trial number (< 75%), eight children (3 CDMs) were excluded for low behavioral performance (d′ < 1.35), and finally, two children (1 CDM) didn’t perform the task.
Response accuracy (d′) was analyzed using a 2 (stimulus type: Concrete, Abstract) × 2 (lag: 2, 5) × 2 (group: CDMs versus children of non-diabetic mothers) repeated measures ANOVA. We found no effect of group (F(1,42) = .109, p = .743, η2p = .003) or interaction between group and lag (F(1,42) = .086, p = .771; η2p = .002) or group and stimulus type (F(1,42) = .215, p = .645; η2p = .005). There was no main effect of lag (Lag 2: M = 3.62, SD = .12; Lag 5: M = 3.53, SD = .11; F(1,42) = 1.409, p = .242; η2p = .032) but a main effect of stimulus type (F(1,42) = 9.203, p = .004; η = .180), with greater accuracy for concrete 2p pictures (M = 3.78; SD = .14) as compared to abstract pictures (M = 3.37; SD = .12; t(42) = 3.034; p = .004; d = 0.937). Finally, we found an interaction between lag and stimulus type (F(1, 42) = 6.504, p = .015; η2p =.134), with greater accuracy for lag 2 (M = 3.52; SD = .12) than lag 5 (M = 3.23; SD = .13) when judging abstract pictures (t(42) = 3.035; p = .004; d = 0.937) but not when judging concrete pictures (lag 2: M = 3.72; SD = .16; lag 5: M = 3.83; SD = .14; t(42) = 0.978; p = .334; d = 0.300). These data demonstrate that CDMs performed age-appropriately as compared to children of non-diabetic mothers, and that performance in both groups decreased as a function of task demand, with better memory for concrete, easy to name pictures and poorer memory for abstract objects, particularly after a longer delay.
Reaction times for correct responses (hits and correct rejections) were analyzed using a 2 (stimulus type: Concrete, Abstract) × 3 (new pictures, lag 2 and lag 5) × 2 groups (group: CDMs versus children of non-diabetic mothers) repeated measures ANOVA. We found no effect of group (F(1,42) = .001, p = .978, η2p < .001) or interaction between group and lag (F(1.656, 69.559) = .057, p = .917; η2p= .001) or group and stimulus type (F(1, 42) = .121, p = .730; η2p = .003). We found a main effect of lag (F(1.656, 69.559) = 9.764, p <.001; η2p = .189), with slower responses to new pictures (M = 1199.96; SD = 44.48) than to pictures repeated after a lag of 2 (M = 1141.01; SD = 40.30; p = .003) or a lag of 5 (M = 1153.24; SD = 42.17; p = .007). We found no difference between lag 2 and lag 5 (p = .789). Finally we found a main effect of stimulus type (F(1, 42) = 13.266, p = .001; η2p = .240), with children responding more slowly to abstract pictures (M = 1182.31; SD = 41.71) than to concrete pictures (M = 1147.16; SD = 41.97). In accordance with the response accuracy data, the reaction time data showed that performance by CDMs was age-appropriate as compared to children of non-diabetic mothers. In addition both groups were slower to correctly judge a picture “old” or “new” as a function of task demand. Specifically, reaction times were shorter for concrete, easy to name pictures than for abstract shapes.
Volumetric data
Forty-two children provided good structural MRI data (17 CDMs). Three children of non-diabetic mothers were rejected for unrelated brain anomaly (1 ventriculomegaly, 1 temporal cyst, 1 epilepsy) and thirteen children (5 CDMs) did not provide structural scans. We found no significant differences in total hippocampal volume between children of non-diabetic mothers (M = 5768.04, SD = 610.20) and CDMs (M = 5833.24, SD = 617.204; t(40) = −.338, p = .737). Finally, we found no significant group differences in left hippocampal volume (children of non-diabetic mothers: M = 2861.96, SD = 318.62; CDMs: M = 2845.29, SD = 345.56; t(40) = .161, p = .873) or right hippocampal volume (children of non-diabetic mothers: M = 2906.08, SD = 327.99; CDMs: M = 2987.94, SD = 343.57; t(40) = −.779, p = .441).
Our volumetric measures for both groups are in accordance with Schumann and colleagues’ data (20). By using the same tracing criteria our data are within 5% of their estimates of a 7.5–18.5-year-olds control population. They reported a right hippocampal volume of 2.75 cm3 ± 0.30 (current study: 2.91 cm3 ± 0.33) and a left hippocampal volume of 2.71 cm3 ± 0.32 (current study: 2.86 cm3 ± 0.32).
Correlational analysis
Correlations were performed to examine the relationship between volume of the hippocampal formation and performance on the continuous recognition memory task. The structural data included hippocampal volume (right, left and total). The behavioral data included the task performance index d′ and reaction time (RT). For both behavioral measures, two scores were included as a function of task demand (see above): low demand (d′ and RT measured for concrete pictures presented after a lag of 2 intervening items) or high demand (d′ and RT measured for abstract pictures presented after a lag of 5 intervening items). Participants were included in the correlation analysis if they provided both good structural and good behavioral data (children of non-diabetic mothers: N = 24; CDMs: N = 15). Bivariate correlations were run examining the relations of each behavioral measure with 3 volume measures, the total, left and right hippocampal volumes. Bonferroni correction was applied to account for multiple comparisons (i.e. a p-value of 0.05 was divided by 3, the number of comparisons for each behavioral measures; alpha <= 0.017).
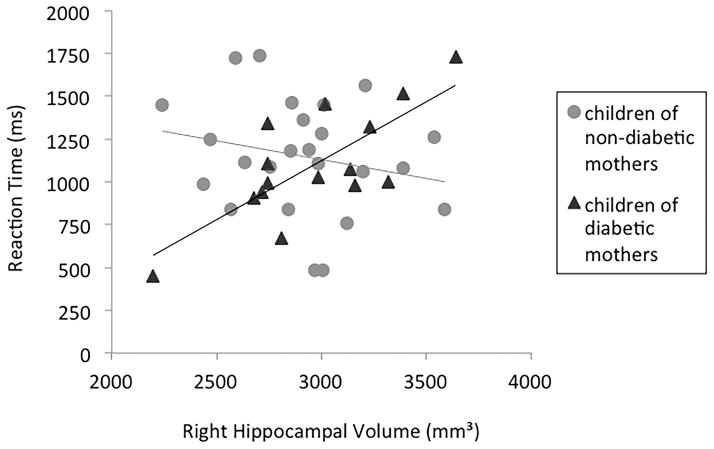
For children of non-diabetic mothers, no significant correlations were found between the volumetric measures and task performance (d′ or RT) for either low or high demand conditions. For CDMs, no correlations were found between brain measures and task accuracy (d′). However, we found a positive correlation between right hippocampal volume and RT in the high demand condition (r = .633; p = .015), such that the larger the right hippocampal volume, the more time this group needed to provide accurate responses on high demand trials. The correlation between right hippocampal volume and RT in the low demand condition was not significant (r = .477; p = .085).
DISCUSSION
The present follow up study examined continuous recognition memory performance and hippocampal integrity in 10-year-old CDMs versus children of non-diabetic mothers. Multiple studies have described the deleterious consequences of diabetic pregnancies on memory early in life. Behavioral impairments and brain electrophysiological differences have been described in infants and toddlers of diabetic mothers (10–16). Moreover, hippocampal abnormalities have been linked to conditions associated with diabetic pregnancies, such as iron deficiency or hypoxia (for reviews see 8–9). However, our study revealed that 10-year-old CDMs exhibit age-appropriate performance as compared to children of non-diabetic mothers on a measure of recognition memory, and do not present hippocampal volumetric abnormalities as compared to children of non-diabetic mothers. Similarly, a recent study on formerly iron-deficient children (one of the major consequences of diabetic pregnancies) showed no accuracy impairments on a similar continuous recognition memory task at 10 years of age (21) although delays in memory development have been suggested in iron-deficient infants (22).
Could memory deficits associated with diabetic pregnancies observed in infancy and early childhood (11–15) be overcome by 10 years of age? Interestingly the hippocampal formation is one of the latest brain regions to develop, exhibiting structural changes until adulthood (4–7). Several studies have highlighted its striking plasticity throughout development. For example in monkeys, whereas hippocampal lesions suffered in adulthood lead to severe spatial memory deficits (23), neonatal lesions to the hippocampal formation do not impair adult spatial memory function (24). Similarly, in humans, lesions of the hippocampus in adulthood generally impair both semantic and episodic memory processes (25; see also 26 for a perspective on the topic), whereas semantic memory is largely intact after early hippocampal insults (27). These findings suggest that early damage to the hippocampal formation is likely to be compensated by normative developmental conditions. The present participants were highly motivated and well educated. The mean IQ of these children was fairly high (children of non-diabetic mothers: 117.48 ± 14.28; CDMs: 118.75 ± 10.37). Early memory deficits observed in this cohort (see 16) might have been overcome due to a favorable developmental context.
However, the present data suggest that differences in underlying neural processing may still exist in 10-year-old CDMs. Indeed, we found a positive correlation between the right hippocampal volume and the time CDMs needed to provide accurate responses under high memory demands. No such correlation was observed in children of non-diabetic mothers, suggesting subtle information processing differences between the two groups.
The right hippocampal hemisphere has been shown to subserve visuo-spatial memory function, whereas the left hippocampal hemisphere subserves verbal memory tasks (28). We found that the bigger the right hippocampal formation was, the slower CDMs were at correctly identifying abstract images as new or old after a longer delay. Interestingly, an increase in hippocampal volume has recently been shown to result from severe episodes of hypoglycemia in 7–17-year-olds children with type 1 diabetes (29). The authors suggested that a disruption of normal developmental pruning could be the cause of the enlargement of the hippocampal formation in this population. While a great number of connections between cells during infancy allows the system to be more plastic, pruning processes during development are necessary to refine brain networks by elimination of redundant synaptic connections. This mechanism allows for more efficient and therefore faster transmission of the information. Due to restricted episodes of hypoglycemia during fetal life (as compared to individuals experiencing severe hypoglycemia throughout development), potential structural differences in CDMs might not be revealed by the present measure. Indeed, a measure of the total hippocampal volume is likely to miss structural differences at the synaptic level suggested by animal studies (30). However, the fact that a bigger hippocampal formation is related to a delay in accurate responding suggests more subtle alterations in hippocampal circuitry that might be linked to deficits in information transfer.
Congdon and colleagues (21) found similar results in 10-year-old children who experienced anemia early in life (infants identified with anemia at 6, 12 or 18 months were enrolled in this study). Indeed, event-related potentials were recorded during a continuous recognition memory task similar to the one used here, and revealed reduced and slower neural response in formerly iron deficient children. In addition, a significant reaction time difference was observed between groups. Formerly iron-deficient children were slower to provide an old/new judgment than non-anemic children. The authors suggested that alterations in myelination and energy metabolism (two direct consequences of iron deficiency) might account for such differences.
Several other studies using neurophysiological measures, which constitute a more sensitive method for detecting subtle differences in neural processes, have linked diabetic pregnancies with information processing deficits. In CDMs, visual-evoked potentials and somatosensory-evoked potentials vary as a function of metabolic control during pregnancy with CDMs exhibiting delays in cortical evoked responses relative to children of non-diabetic mothers (31). Similar results have been previously obtained in our sample using electrophysiological measures. At birth, whereas infants of diabetic mothers could recognize their mothers’ voice compared to a strangers’ voice behaviorally, their ERP patterns differed, demonstrating subtle differences in the neural processing underlying memory (11). At 6 months of age, the positive slow wave (PSW) component, which has been associated with updating of memory for partially encoded items, was reduced or even absent in infants of diabetic mothers (12). At 8 months of age, the amplitude of the neural response was reduced as illustrated by a smaller PSW and smaller negative component (Nc) (i.e. a component reflecting attentional processes) (13). Moreover these data suggested that the Nc peaks later for infants of diabetic mothers, indicating slower information processing. Finally, preliminary results at 24 months of age also suggested Nc and PSW amplitude reduction in infants of diabetic mothers in the absence of memory deficits (16). Overall these studies demonstrate that even in the absence of behavioral differences, information processing mechanisms are impacted in CDMs throughout development, until at least 10 years of age (21). In the current study, the observed correlation between hippocampal volume and response speed during high demand trials suggests information processing differences, specifically revealed when the task is difficult. Future studies using more challenging memory tasks might show differences at the behavioral level as well.
It is worth mentioning that children participating in our study were born from mothers with generally well-controlled gestational and non-gestational diabetes. It is therefore possible that the impact of diabetic pregnancies on memory and brain related processes has been underestimated in our Study. Nevertheless, a range of control existed as would be expected, and it is clear that fetal neurologic risk in diabetic pregnancies is an inverse function of maternal glycemic control (10). Thus, in order to better characterize the information processing alteration our data suggested, it would be critical in future studies to apply additional and more precise brain imaging techniques (e.g. fMRI or EEG/ERP), as well as additional and more challenging memory tasks.
In conclusion, the present study revealed similar behavioral and neuroanatomical markers of memory in 10-year-old CDMs and children of non-diabetic mothers. Additionally, while we found no correlation between hippocampal size and memory performance in children of non-diabetic mothers, our data showed that for CDMs, the bigger the hippocampal formation was, the slower they were at performing the task during challenging trials. We suggest that this correlation reveals subtle information processing differences in CDMs, in accordance with several neurophysiological findings in this population. Moreover we suggest that this correlation might reflect memory deficits that could be revealed by more complex and challenging memory tasks than the one used here. Our findings could have important implications for CDMs performance on memory tasks with higher cognitive demand.
Figure 1.
Correlations between reaction time and right hippocampal volume in the high demand condition (recognition of abstract images after a lag of five intervening images) for children of non-diabetic mothers (light grey circles) and children of diabetic mothers (dark grey triangles).
Acknowledgments
Statement of financial support: This project received financial support from the National Institutes of Health and the McKnight Foundation. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank the many families who consented to participate in this study. We thank Ruskin Hunt, Lyric Jorgenson, Neely Miller, Jenie Cirilli-Raether, Jennifer Derham, Christopher Griggs, and Jennifer Wenner for assistance in data collection and processing. We thank Caterina Stamoulis for statistical support.
Abreviations
- CA
Cornu Ammonis
- CDMs
Children of Diabetic Mothers
- EEG
Electroencephalogram
- HF
Hippocampal Formation
- IQ
Intelligence Quotient
- ISI
Inter-Stimulus Interval
- MRI
Magnetic Resonance Imaging
- Nc
Negative Component
- PSW
Positive Slow Wave
- RT
Reaction Time
- SDT
Signal Detection Theory
- WASI
Weschler Abbreviated Scales of Intelligence
Footnotes
No conflicts of interest were declared.
Contributor Information
Adeline Jabès, Email: adeline.jabes@childrens.harvard.edu, Postdoctoral research fellow, Harvard Medical School/Boston Children’s Hospital, Division of Developmental Medicine, 1 Autumn Street, Boston, MA 02215, UNITED STATES, +1 617 355 0400, FAX: +1 617 730 0518
Kathleen M. Thomas, Institute of Child Development, University of Minnesota, Minneapolis, MN, USA
Sara Langworthy, Institute of Child Development, University of Minnesota, Minneapolis, MN, USA
Michael K. Georgieff, Institute of Child Development, University of Minnesota, Minneapolis, MN, USA.
Charles A. Nelson, Harvard Medical School, Boston Children’s Hospital, Division of Developmental Medicine, Boston, MA, USA
References
- 1.Rizzo T, Metzger BE, Burns WJ, et al. Correlations between antepartum maternal metabolism and intelligence of offspring. N Engl J Med. 1991;325:911–6. doi: 10.1056/NEJM199109263251303. [DOI] [PubMed] [Google Scholar]
- 2.Rizzo TA, Metzger BE, Dooley SL, et al. Early malnutrition and child neurobehavioral development: insights from the study of children of diabetic mothers. Child Dev. 1997;68:26–38. [PubMed] [Google Scholar]
- 3.Nold JL, Georgieff MK. Infants of diabetic mothers. Pediatr Clin North Am. 2004;51(3):619–637. doi: 10.1016/j.pcl.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Insausti R, Cebada-Sanchez S, Marcos P. Postnatal development of the human hippocampal formation. Adv Anat Embryol Cell Biol. 2010;206:1–86. [PubMed] [Google Scholar]
- 5.Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Jabès A, Lavenex PB, Amaral DG, et al. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur J Neurosci. 2010;31(2):273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabès A, Banta Lavenex P, Amaral DG, et al. Postnatal development of the hippocampal formation: a stereological study in macaque monkeys. J Comp Neurol. 2011;519(6):1051–1070. doi: 10.1002/cne.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fretham SJB, Carlson ES, Georgieff MK. The Role of Iron in Learning and Memory. Adv Nutr. 2011;2:112–121. doi: 10.3945/an.110.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyakas C, Buwalda B, Luiten PG. Hypoxia and brain development. Prog Neurobiol. 1996;49(1):1–51. doi: 10.1016/0301-0082(96)00007-x. [DOI] [PubMed] [Google Scholar]
- 10.Siddappa AM, Georgieff MK, Wewerka S, et al. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55(6):1034–41. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- 11.de Regnier R-A, Nelson CA, Thomas KM, et al. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr. 2000;137(6):777–784. doi: 10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- 12.Nelson CA, Wewerka S, Thomas KM, et al. Neurocognitive sequelae of infants of diabetic mothers. Behav Neurosci. 2000;114(5):950–956. [PubMed] [Google Scholar]
- 13.Nelson CA, Wewerka SS, Borscheid AJ, et al. Electrophysiologic evidence of impaired cross-modal recognition memory in 8-month-old infants of diabetic mothers. J Pediatr. 2003;142(5):575–82. doi: 10.1067/mpd.2003.210. [DOI] [PubMed] [Google Scholar]
- 14.DeBoer T, Wewerka S, Bauer PJ, et al. Explicit memory performance in infants of diabetic mothers at 1 year of age. Dev Med Child Neurol. 2005;47(8):525–31. doi: 10.1017/s0012162205001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riggins T, Miller NC, Bauer PJ, et al. Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood. Dev Neuropsychol. 2009;34(6):762–779. doi: 10.1080/87565640903265145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson CA. A developmental cognitive neuroscience approach to the study of atypical development: a model system involving infants of diabetic mothers. In: Dawson G, Fischer K, Coch D, editors. Human behavior and the developing brain. 2. New York: Guilford Press; 2007. pp. 1–27. [Google Scholar]
- 17.Glass HC, Pharm TN, Danielsen B, et al. Antenatal and Intrapartum Risk Factors for Seizures in Term Newborns: A Population-Based Study, California 1998–2002. J Pediatr. 2009;154(1):24–28. doi: 10.1016/j.jpeds.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Lieshout RJ, Voruganti LP. Diabetes mellitus during pregnancy and increased risk of schizophrenia in offspring_: a review of the evidence and putative mechanisms. J Psychiatry Neurosci. 2008;33(5):395–404. [PMC free article] [PubMed] [Google Scholar]
- 19.Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers. 1999;31(1):137–49. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- 20.Schumann CM, Hamstra J, Goodlin-Jones BL, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Congdon EL, Westerlund A, Algarin CR, et al. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J Pediatr. 2012;160(6):1027–33. doi: 10.1016/j.jpeds.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burden MJ, Westerlund AJ, Armony-Sivan R, et al. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120(2):e336–45. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banta Lavenex P, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci. 2006;26(17):4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavenex P, Banta Lavenex P, Amaral DG. Spatial relational learning persists following neonatal hippocampal lesions in macaque monkeys. Nat Neurosci. 2007;10(2):234–239. doi: 10.1038/nn1820. [DOI] [PubMed] [Google Scholar]
- 25.Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci USA. 1996;93(24):13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishkin M, Vargha-Khadem F, Gadian DG. Amnesia and the organization of the hippocampal system. Hippocampus. 1998;8(3):212–216. doi: 10.1002/(SICI)1098-1063(1998)8:3<212::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Vargha-Khadem F, Gadian DG, Watkins KE, et al. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(5324):376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 28.Bohbot VD, Kalina M, Stepankova K, et al. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36(11):1217–38. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- 29.Hershey T, Perantie DC, Wu J, et al. Hippocampal Volumes in Youth With Type 1 Diabetes. Diabetes. 2010;59:236–241. doi: 10.2337/db09-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran PV, Dakoji S, Reise KH, et al. Fetal iron deficiency alters the proteome of adult rat hippocampal synaptosomes. Am J Physiol - Regul Integr Comp Physiol. 2013;305(11):R1297–R1306. doi: 10.1152/ajpregu.00292.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinciotti M, Napoli A, Mittica A, et al. Cortical evoked potentials in children of diabetic mothers. Exp Diabetes Res. 2011;2011:640535. doi: 10.1155/2011/640535. [DOI] [PMC free article] [PubMed] [Google Scholar]