Abstract
Given conceptual frameworks of addiction as a disease of intercommunicating brain networks, examinations of network interactions may provide a holistic characterization of addiction-related dysfunction. One such methodological approach is the examination of resting-state functional connectivity, which quantifies correlations in low frequency fluctuations of the blood oxygen level–dependent magnetic resonance imaging signal between disparate brain regions in the absence of task performance. Here, evidence of differentiated effects of chronic nicotine exposure, which reduces the efficiency of network communication across the brain, and acute nicotine exposure, which increases connectivity within specific limbic circuits, is discussed. Several large-scale resting networks, including the salience, default, and executive control networks, have also been implicated in nicotine addiction. The dynamics of connectivity changes among and between these large-scale networks during nicotine withdrawal and satiety provide a heuristic framework with which to characterize the neurobiological mechanism of addiction. The ability to simultaneously quantify effects of both chronic (trait) and acute (state) nicotine exposure provides a platform to develop a neuroimaging-based addiction biomarker. While such development remains in its early stages, evidence of coherent modulations in resting-state functional connectivity at various stages of nicotine addiction suggests potential network interactions on which to focus future addiction biomarker development.
Keywords: addiction, nicotine, resting-state functional connectivity, biomarkers
Introduction
Despite both the prevalence of nicotine addiction and the well-known negative health consequences of long-term smoking, only approximately one in five smokers attempting to quit is successful using currently available pharmacotherapies. Among the explanations for this difficulty in maintaining long-term abstinence are a poor understanding of the neurobiological underpinnings of the disease, an inability to quantify the severity of addiction beyond behavioral reports of cigarettes per day and usage patterns, and an inability to proactively identify the most appropriate therapeutic plan for an individual (though see Refs. 1 and 2 for recent advances).
Nicotine addiction, much like other pharmacological addictions, is conceptualized as a heterogeneous collection of plastic effects that occur throughout various brain circuits and networks subserving disparate cognitive and affective processes (e.g., reward, learning, affect, executive control).3 As such, the neuroanatomical foci of dysfunction are not likely to be specific lesions or activation patterns in circumscribed brain regions, but instead interactions within and between several affected circuits and systems. Thus, network-based analysis paradigms employing resting-state functional connectivity may be able to more fully characterize these interactions in smokers and non-smokers, both within specific connections and across large-scale brain networks.
Resting-state functional connectivity
Resting-state functional connectivity (rsFC) is a task-independent metric of brain activity that is based on correlations between low-frequency fluctuations of the blood oxygen level–dependent (BOLD) signal between disparate brain areas in the absence of explicit task engagement.4 Increased temporal coherence in these signal fluctuations has been interpreted to reflect the strength of a functional connection between regions which, although generally in good agreement with the classical underlying neuroanatomy, often extend beyond simple monosynaptic connections and provide a systems-level understanding of brain function.5–7
The power of this method lies in its relative ease of acquisition and stability within and across scan sessions,8–11 making it ideal for longitudinal experimental designs, including pre–post treatment assessments. Data acquired at “rest” has been shown to correlate with both subsequent behavioral performance on a task and activation of brain regions that support task performance.12–14 Finally, task-free acquisition precludes equating levels of performance—or motivation—between individual participants or across comparison groups. This task independence also allows for better comparison across data sets, as the correlations in time course are not subject to the idiosyncrasies of a specific task design. Note, however, that this is mostly the case when resting scans are performed at the beginning of a scan session. Recent data suggest that cognitive efforts may “bleed” into the resting data when scans are performed immediately after especially effortful or emotionally powerful tasks.15–18
As the research community’s interest in rsFC grows, it becomes more challenging to fully review and interpret the extant literature. As such, we focus our discussion on nicotine addiction as an exemplar of common processes in drug addiction in general, and subsequently address theoretical aspects of rsFC in biomarker development. Here, we use the definition of a biomarker as a measurable biological signal that quantifies (1) normal or abnormal function and/or (2) response to treatment.19
While acquisition of rsFC data is relatively straightforward, data analysis is anything but, with head movement increasingly shown to present a large confound in data analysis and interpretation.20–22 In addition, the effects of global signal regression,23,24 confounds from cerebrospinal fluid and white matter signals,25 low-frequency cardiac signal fluctuations,26 and the effects of temporal filtering of rsFC data at various frequency bands27 must be taken into account during rsFC data processing. While numerous options for data analysis remain, recent work has sought to outline a set of preprocessing parameters that maximizes signal-to-noise ratio and data reliability.27 That said, in-depth description of rsFC analysis methods is beyond the scope of this review, and the reader is referred to a number of recent discussions of rsFC data–analysis methodology27–30 as well as cogent surveys of resting-state MRI more generally.31,32
Nonetheless, in order for the general reader to best appreciate the research described in this review, we briefly outline the three major types of rsFC analysis: seed-based, graph-theoretical, and independent component analysis. As is true with most neuroscience methods, there are advantages and trade-offs with each analysis tool.31 Seed-based analyses look at the correlation strength in the time courses between an a priori seed voxel or multivoxel region of interest and the rest of the brain.4 Seed-based analyses provide anatomically based, hypotheses-driven results, with the caveat of restricting brain exploration based on the seed selection. In contrast, graph-theoretical measures are based on hundreds or thousands of selected brain regions of interest (or indeed every voxel in the brain) and seek to characterize entire brain network topology.32,33 However, interpreting the sometimes abstract network parameters (e.g., modularity, small-worldness, partition coefficient), or relating them to known brain systems that underlie cognitive processes or that are affected in disease can be a challenge. Finally, independent component analysis (ICA) is a data-driven computational method to decompose an overall signal into independent, orthogonal components, segmenting the brain into large-scale components or networks that are generally well conserved across individuals.34,35 ICA provides a rich description of multiple spatially separated brain networks, but does not easily allow for the interrogation of a specific, predefined region.
Large-scale brain networks
Whether identified by ICA, graph methods or even a priori seeds, three consistently observed, large-scale brain networks are of particular relevance to the discussion of nicotine addiction. These networks include the default mode network (DMN),36–39 which is centered on nodes in the medial prefrontal cortex (mPFC), midline posterior cingulate cortex (PCC), and parahippocampal gyrus and has been implicated in ruminations, mind wandering, planning the future, and reflections on the past. The executive control network (ECN),40,41 centered on nodes in the dorsolateral prefrontal cortex (dlPFC) and the lateral posterior parietal cortex (PPC), has been associated with attending to and processing exogenous, attentionally driven executive functions, while the salience network (SN),42,43 centered on nodes in the dorsal anterior cingulate cortex (dACC) and the frontoinsular cortex (sometimes also including the ventral striatum), has been implicated in the facilitation of attentional orientation to internal or external stimuli. This tripartite network parcellation is seen not only in awake humans, but consistently and homologously in both awake and appropriately anesthetized rodents and non-human primates,44–46 suggesting a potentially important link for translational preclinical human research.
In general, the SN and ECN are identified as task-positive networks, in that these regions identified at rest are activated during performance of most cognitive tasks, while the DMN deactivates (i.e., reduces its activation level) during task performance. This antagonism is reflected in DMN and ECN time courses, which are anticorrelated at rest.36,40,43,47 The extent of this anticorrelation has been associated with subsequent task performance, with overactivation (i.e., reduced deactivation) of the DMN—at the cost of reduced ECN activation—leading to decrements in task performance both for healthy controls12,48,49 and those diagnosed with various neuropsychiatric diseases,50–52 including nicotine addiction.53 These networks are particularly relevant to nicotine addiction, as reflected by increases in expression of nicotinic receptors in the anterior cingulate and insular cortices—principal nodes of the SN.54
With this modest primer, we next synthesize recent findings on rsFC and nicotine addiction. Based on the patterns of dysfunction related to both chronic and acute nicotine exposure, we conclude by suggesting rsFC-based measures on which to focus future development of brain-based biomarkers of addiction. While the development of such rsFC biomarkers is in its early stages, the patterns of rsFC dysfunction described in the following sections provide potential avenues for future research.
Available biomarkers, such as the presence of drug in bodily fluid, cannot differentiate between chronic and incidental drug use, and are limited by the pharmacokinetics of drug metabolism in their detection time window. A number of measures, including subjective self-reports of nicotine craving55–57 and baseline nicotinic acetylcholine receptor (nAChR) upregulation as measured by positron emission tomography (PET),58 have been correlated with treatment outcomes. However, as stated above, cessation treatment outcomes remain poor, and a more mechanistic understanding of the dysfunctions across the brain networks associated with nicotine addiction is warranted. In contrast with other potential biomarkers, a single rsFC dataset can simultaneously characterize both state and trait differences among smokers, increasing the predictive efficacy of these measures. To this end, rsFC measures have the potential to provide more clinically useful diagnostic and, critically, predictive biomarkers of nicotine and other types of substance abuse.59,60
The relative ease and brief duration of data acquisition coupled with the stability of rsFC over extended periods of time suggests that such measures may also be useful to track disease progression over the course of treatment. Thus, rsFC measures have the potential to predict treatment outcomes as well as to fractionate individual smokers based on the specific network dysfunctions present, which would facilitate differential diagnosis of smokers based on specific network dysfunctions. Recent evidence has shown that personalized treatment for nicotine addiction is possible,1,2 and rsFC measures may thus serve to improve outcomes by identifying ideal treatment options for individuals. Thus, measures of chronic use and—perhaps more importantly—measures that track disease severity and trajectory are important missing pieces in the clinical arsenal of addiction medicine today.61,62
Chronic nicotine effects on rsFC
Long-term use of nicotine is associated with deficits in multiple cognitive domains.63 Such widespread effects suggest that chronic nicotine exposure and resulting addiction affect brain networks associated with executive function—a cognitive construct strongly linked with addiction.64,65 As has been shown for task activation,66–68 separable effects of chronic and acute administration of nicotine have been observed in a variety of resting-state measures. In the studies outlined in the section below, rsFC data was collected from sated smokers. Satiety is thought to characterize smokers in their baseline condition, in the absence of withdrawal, and provide evidence for the effects of chronic nicotine exposure that are separable from the effects of acute nicotine administration.
In seed-based rsFC analysis, reduced dACC–ventral striatum connectivity is correlated with increased addiction severity, independent of acute nicotine administration.69 Notably, Hong et al.69 identified a double dissociation such that acute nicotine (in the form of a transdermal nicotine patch) increased coherence strength in various cingulate–cortical circuits while having no effect on the dACC–ventral striatal circuit that correlated with FTND, perhaps helping to explain the poor efficacy of nicotine replacement therapy (NRT) in the vast majority of smokers. Predicated on these results, recent findings employing a range of rsFC methods show trait-level reductions in connectivity in smokers as compared to non-smokers.
Smokers, but not non-smokers, show an inverse relationship between the strength of rsFC between a right anterior insula seed and the vmPFC and higher personality trait alexithymia, which is in turn associated with a deficiency in subjective awareness and regulation of emotions.70 These effects, while correlated with nicotine craving during withdrawal, are observed independent of the presence or absence of nicotine. Thus, the reduction in rsFC is related to chronic nicotine exposure, and implicates reduced insula–vmPFC circuit strength with reduced affective processing and an increased incidence of negative personality traits in smokers. These findings are consistent with evidence implicating the anterior insula in self-regulation via modulations in functional connectivity.71
Studies examining smoking-related rsFC in patients with schizophrenia (SZ) have characterized separable and distinct effects of chronic nicotine exposure and disease comorbidities.72 These findings are in contrast to the related effects of SZ and smoking seen in previous studies.73,74 While the self-medication hypothesis has been a prevalent explanation of the coincidence of SZ and smoking,75,76 recent challenges77,78 and evidence of high rates of smoking in unaffected twins of SZ patients79 suggest that increased smoking comorbid with SZ may be related to a shared neurobiology.72 In fact, a dACC seed showed reduced rsFC in non-SZ smokers across a range of circuits, including a right limbic region subsuming ventral striatum, parahippocampal, amygdalar, and posterior insular areas.72 Importantly, these results were independent of SZ diagnosis, and replicate previous evidence showing this dACC–right limbic circuit to have reduced connectivity in smokers correlated with addiction severity.69,74
Across large-scale networks encompassing whole-brain analyses, similar disruptions in connectivity are observed. In a study of more than 600 individuals, smokers showed reduced connectivity between nodes of previously defined large-scale functional networks.80 For both the DMN and the left ECN, chronic smokers as compared to non-smokers showed reduced connectivity within each network. Furthermore, within key hub ROIs of both the ECN (dlPFC and parietal nodes) and DMN (mPFC and PCC nodes), network connectivity was degraded. The reduced connectivity strength in these key hubs was correlated with duration and frequency of chronic smoking.
Graph-theoretical measures also show disruptions in topological organization and network properties in chronic smokers. Heavy smokers have lower values for a number of parameters associated with global connectivity across disparate brain networks, as well as higher values than non-smokers for a number of network properties associated with local connectivity.81 Specifically, decreased global and increased local efficiencies in network connectivity were observed in heavy smokers in ROIs associated with the DMN, including the PCC and the medial superior frontal gyrus (SFG). In addition, increases in local efficiency were observed in visual areas, including the bilateral occipital cortex. This suggests that chronic exposure to nicotine reduces communication across the brain, while increasing the local connectivity within specific network nodes.81 As is seen with the decreases in DMN and ECN hub connectivity discussed above,80 each of these parameters was correlated with duration of lifetime cigarette use, suggesting that the deficits in network efficiency observed in chronic, heavy smokers were related to the duration of their nicotine exposure.
Beyond downregulation in rsFC across the brains of smokers, other evidence shows selective increases in rsFC associated with chronic exposure to nicotine. In many cases, these increases are related to cue reactivity and implicate nodes of the ECN attentional network. For example, coupling between a left frontoparietal network and an mPFC network were enhanced in female smokers when compared to non-smoking controls.82 The strength of this connectivity was correlated with smoking cue reactivity, suggesting that the enhanced coupling within networks associated with attentional control may contribute to cue reactivity in smokers. Subsequent work by the same group83 showed that increased cue reactivity was also correlated with increased connectivity between the insula and the dACC. The insula and dACC are key nodes of the SN,42 and their connectivity has been previously linked with smoking severity.69 Importantly, this insula–dACC coupling was insensitive to acute abstinence and thus may reflect a trait difference in network coherence for chronic smokers. Together, these results suggest that a component of the disordered resting connectivity seen in chronic smokers is an exaggerated coupling in attentional networks that may explain previously observed imbalances in cue reactivity.84
Regional homogeneity (ReHo)85 is an analysis method that characterizes intraregional resting-state BOLD fluctuations as opposed to coupling between spatially separate network nodes. Much like ICA and graph-theoretical methods, ReHo is a data-driven method and allows for interrogation of resting activity in the whole brain without any a priori neurobiological assumptions. To that end, smokers show decreased ReHo in brain regions associated with previously established networks relevant to addiction. Wu et al.86 found reduced ReHo within the DMN, frontoparietal attention, and inhibitory control networks. This rsFC data aligns with previous task-activation data in smokers showing decreased sensitivity in ROIs that are also nodes within the DMN,87 attention,88 and inhibitory control networks. 89 Using ReHo, reduced local synchronization in prefrontal regions, specifically the right inferior frontal cortex,90 right lateral prefrontal cortex, and left medial prefrontal cortex,91 have also been characterized. While these findings are in line with the decreases in rsFC reviewed above,80,81 ReHo has also revealed selectively increased intraregional connectivity strength in smokers in parietal,90 motor,86 posterior cingulate, and posterior insular regions.91
Gender differences in resting circuits have also been observed in smokers. Using arterial spin labeling (ASL), an alternative measure of cerebral blood flow,92 to quantify changes in resting connectivity, female smokers show stronger coupling between a variety of frontal and parietal networks.93 These findings are consistent with previous evidence in female smokers showing enhanced coupling between left frontoparietal and mfPFC networks,82 as well as evidence of reduced gray matter volume in the vmPFC and OFC in female smokers.94 These gender-specific differences may provide insight into the enhanced cue reactivity95,96 and lower cessation success rates for female smokers.56,97,98
In summary, chronic nicotine exposure leads to disordered functional connectivity across specific circuits and large-scale networks. These results are seen in seed-based,70,72 large-scale network,80 regional homogeneity,86,90,91 and graph-theoretical81 connectivity analyses. Evidence of both limited increases and more widespread decreases in rsFC in smokers are in line with evidence from other neuropsychiatric conditions like Alzheimer’s disease and schizophrenia, which generally show disordered network communication in patients.50,51 However, in nicotine addiction, the disruptions in functional connectivity appear to be focused within specific circuits centered on the ACC and insula and large-scale networks associated with attention and cognitive control.
Acute nicotine effects on rsFC
In contrast to a number of other clinical disorders, nicotine (and other drug) addiction allows for the characterization of the effects of acute exposure in addition to chronic consequences. Recent meta-analysis68 and reviews67,99 have articulated a two-faceted effect of acute nicotine administration, enhancing cognition in both smokers and non-smokers while also assuaging withdrawal in abstinent smokers alone. The foci of the effects of acute nicotine administration largely overlap with nodes of large-scale brain networks relevant to cognition and addiction.68 Thus, acute nicotine intake may selectively enhance rsFC in the disordered networks of smokers and mitigate the deleterious effects of chronic exposure described above, at least in the short term.
Cognitive and circuit consequences of acute nicotine administration
Previous findings (see Refs. 30 and 100 for review) show that acute nicotine administration depresses DMN activity (i.e., more deactivation)87,101 and enhances cingulate–cortical connectivity across specific circuits in smokers.69 For non-smokers, in addition to enhanced deactivation of DMN activity, acute administration of nicotine enhances local network efficiency, as measured by graph-theoretical parameters.102 These effects are focused within the limbic and paralimbic areas and seem to be more pronounced in the right hemisphere, suggesting that, for non-smokers, local information transfer is facilitated in the presence of acute nicotine as the clustering of local connections is increased.
In smokers, the administration of acute nicotine enhances functional connectivity between the two brain hemispheres, and more specifically in rostral as opposed to caudal regions.103 This enhanced interhemispheric connectivity is observed only in smokers after ad lib smoking. Notably, when abstinent smokers and non-smokers were compared, no difference in rsFC was observed. Thus, the effect of recent smoking on interhemispheric rsFC is likely related to the enhancing effects of nicotine on cognition as opposed to a mitigation of the effects of withdrawal. Further, the localization of this enhanced interhemispheric coupling to the rostral third of the brain is broadly consistent with the foci of attentional control.104
In an examination of network topology, administration of nicotine to minimally abstinent smokers enhanced the efficiency of information transfer in right middle frontal gyrus. At the same time, clustering—that is, segregated interconnections between related nodes—was reduced within the left middle occipital gyrus, the right precentral sulcus, and the right posterior cingulate gyrus.105 Together, these effects in brain regions previously associated with attention and nicotinic stimulation88,106 suggest that the attention-enhancing effects of acute nicotine administration arise through a coordinated increase in global network efficiency along with a reduction in nodal clustering in specific attention-related ROIs. Importantly, the increases in global efficiency observed by Giessing et al.105 were correlated with greater behavioral benefits of nicotine, as well as increased frequency of cigarette smoking. For smokers, these increases in global and decreases in local connectivity 105 appear orthogonal to the effects of chronic nicotine exposure discussed above.81
In sum, across a variety of network nodes, acute nicotine administration is seen to bolster network communication in both smokers and non-smokers. Together, the enhancement of coordinated activity between hemispheres,103 large-scale networks 82 and globally across the brain105 provide a mechanistic foundation for previously observed modulations of activation associated with nicotine administration.67,68,99
Cognitive and circuit consequences of nicotine withdrawal
In contrast to the enhanced processing effects of acute administration, withdrawal from chronic nicotine precipitates negative affective states as well as deficits in cognitive abilities.107 As craving is hypothesized to be mediated by a network of brain regions,3 rsFC provides an appropriate analytic tool to characterize changes in brain connectivity and their relationship to behavioral measures of craving severity. In the abstinent state that precipitates withdrawal, distinct rsFC networks are enhanced. In addition, correlations between withdrawal-precipitated rsFC and subjective reports of craving severity further elucidate the neurobiological underpinnings of withdrawal.
For nicotine-dependent smokers, increases in craving occur over brief timescales. Changes in resting connectivity strength that correlate with self-reports of increased craving are observed during abstinence periods as short as 1 hour. Janes et al.108 found increased coupling between a large-scale resting network centered on the medial orbital prefrontal cortex and a variety of brain areas including the dorsal medial PFC, striatum, and visual cortex. This coupling increased as subjective reports of craving increased over the course of 1 h of acute abstinence, suggesting that abstinence-induced craving is related to the dynamic modulation in functional coupling between addiction-related resting-state networks.108
It should be noted that the pattern of decreases in global efficiency and long-range network connections and local increases in connectivity strength are not consistently observed across all comparisons between smokers and non-smokers. Evaluating network topology in ~ 2-h abstinent smokers as compared to non-smokers, no differences in global efficiency or clustering in whole-brain networks were observed.109
With longer abstinence durations, functional connectivity in additional brain areas is enhanced. Using psychophysiological interaction (PPI) analysis,110,111 which characterizes increases in correlations of the time course of BOLD activation between a seed region and other nodes in a functional network during task-dependent activation, 2-hour abstinent smokers show greater functional connectivity between a right insular seed and the precuneus (a node of the DMN) when exposed to smoking-related cues.112 Of note, the strength of the insula–precuneus circuit was positively correlated with the magnitude of craving, suggesting a role for functional connectivity in instantiating cue-induced craving in abstinent smokers.
Similar effects were observed in a cohort of 116 smokers exposed to smoking-related cues.113 Here, a PPI analysis showed correlations between left insula connectivity to the striatum, thalamus, cuneus and ACC. The strength of coupling in these insula-seeded circuits was correlated with nicotine dependence, as measured by the Fagerstrom test of nicotine dependence (FTND). While these PPI studies characterize functional connectivity during the viewing of smoking-related cues as opposed to a pure rest state, the similarity of the findings of enhanced coupling between insula and nodes associated with the DMN are consistent with other findings, including previously discussed evidence of craving-mediated connectivity strength between the insula and the vmPFC.70
Insula connectivity is also modulated by more prolonged abstinence. In a cohort of 12-h abstinent smokers, global brain connectivity (GBC)—a measure of connectivity between a given region of interest and the rest of the brain—was enhanced in both the insula and the superior frontal gyrus.114 Furthermore, when these abstinent smokers were administered nicotine and then rescanned, GBC in the bilateral insula and nodes of the DMN was decreased. This suggests that the connections heightened during nicotine withdrawal are downregulated when nicotine cravings are sated in chronic smokers. Additional evidence to this effect is discussed in the next section.115–117
Across the experimental results described above, a number of consistent patterns emerge concerning acute nicotine administration and absence. Acute nicotine increases rsFC in the visual attention network105 and regional efficiency in limbic and paralimbic nodes.81 In smokers, the absence of nicotine leads to increases in connectivity within specific limbic circuits. Increases in subjective ratings of withdrawal are associated with increased rsFC between orbital-medial PFC and a variety of cortical and striatal regions,108 as well as increases in coupling between the insula and nodes of the DMN.112 Following abstinence, acute nicotine administration downregulates these limbic connections while simultaneously upregulating connectivity in separable attention-related networks.114
Empirical evidence of a three-network model of nicotine addiction
Beyond the archipelago of individual nodal connections, large-scale network dynamics paint a more nuanced portrait of brain function as regulated by both chronic and acute nicotine. The observed individual connections modulated are in many cases components of large-scale networks, and the dynamics of changes in rsFC among these networks have been posited to be directly linked to effects of satiety and abstinence in smokers.100
On the basis of an influential three-network model of psychopathology,50 Sutherland et al. 100 theorized that nicotine addiction manifests as a dysfunction in the dynamic connections between large-scale brain networks. The three networks implicated are the previously defined SN, DMN, and ECN. Based on the anticorrelations between the DMN and the ECN,40 a role for the SN, and specifically the insula, in adjudicating between exogenous (ECN) and endogenous (DMN) attentional orientation during nicotine abstinence is predicted.100 As such, during acute abstinence, chronic smokers are hypothesized to show increased coupling between the SN and the DMN and decreased coupling between the SN and the ECN as withdrawal leads to an endogenous focus on internal craving state.118 Alternatively, following nicotine administration, smokers will show decreased coupling between the SN and the DMN and increased coupling between the SN and the ECN when nicotine administration facilitates exogenous attentional focus. This heuristic, network-level framework begins to provide a more complete characterization of the consequences of nicotine exposure and suggests that the dysfunctions of nicotine addiction can be classified as dynamic changes in the inter- and intranetwork connectivity between large-scale brain networks. Dynamic changes in coupling among the SN, DMN, and ECN have been empirically implicated in responses of the nicotine-addicted brain to abstinence (for a recent review, see Ref. 30).
The dynamics of the rsFC changes influenced by nicotine administration are seen in heavy smokers scanned following 12 h of nicotine abstinence and again following nicotine replenishment via smoking.117 During abstinence, functional connectivity was enhanced within nodes of the SN and DMN. In contrast, following nicotine replenishment, connectivity was enhanced in the superior frontal gyrus within the SN and the precuneus within the DMN. A subsequent Granger causality analysis showed that nicotine administration reduced causal connections from the SN to posterior nodes of the DMN, whereas connections from both the ECN and anterior nodes of the DMN to the SN were enhanced.117 While the use of Granger causality in rsFC analysis remains controversial owing to potential variations in regional hemodynamic response unrelated to connectivity,119 these results corroborate the role of the SN and its connectivity with the DMN and the ECN in the effects of withdrawal across large-scale brain networks. The dynamics of the interactions observed—specifically downregulation of SN–DMN connectivity following nicotine administration—are consistent with the articulated heuristic framework.100
Further evidence of the sensitivity of rsFC among these three large-scale networks to the presence or absence of nicotine is seen in the direct effects of smoking-cessation pharmacotherapies.115 The administration of either varenicline—a nicotinic partial agonist/antagonist shown to be an effective smoking cessation aid120—or a nicotine replacement patch to 12-h abstinent smokers showed similar effects on connectivity between two specific circuits. Examining both an amygdala seed connected with the insula and an insular seed connected with nodes of the DMN, administration of a nicotinic agonist downregulated the connectivity between the seed region and the rest of the circuit. The reduction in connectivity between the insula—a prominent node of the SN—and nodes of the DMN provides mechanistic evidence of the role of acute nicotine in alleviating withdrawal. Importantly, the effects of varenicline or nicotine on connectivity strength were seen only in dependent smokers, not in non-smokers. Thus, the observed coupling patterns and their changes appear related to nicotine addiction, and not merely the presence of an acute nicotinic agonist.
While Sutherland et al.115 described a decrease in SN–DMN connectivity in the presence of nicotinic agonists following acute withdrawal, a subsequent study characterized concomitant modulations of both SN–DMN and SN–ECN coupling in a new cohort of smokers, scanned under both sated and 24-h abstinent conditions.116 This is an important distinction, as the examination of rsFC changes in both the DMN and ECN simultaneously can better characterize the anticorrelation between these networks40 as it relates to nicotine addiction. Resting SN–DMN and SN–ECN connectivity was quantified by a composite measure, resource allocation index (RAI), formulated to integrate SN–DMN and SN–ECN correlations into a single number. In the RAI, the sign of SN–DMN correlation is inverted, and thus SN–DMN and SN–ECN correlations do not cancel out when summed. As such, the RAI is able to quantify both changes in concurrent dynamic coupling between the networks better than measures of any two networks in isolation.
As compared to baseline smoking state, following 24 h of nicotine abstinence, RAI was reduced in smokers. This reduction in between-network correlation was driven by a significant reduction in temporal anticorrelations between the SN and the DMN. That is, the correlation between the two networks was increased during abstinence, with a trend-level decrease in the positive correlation between the SN and the ECN also observed during abstinence. Further, the reduction in RAI across the three large-scale networks was strongly correlated both with increases in subjective urge to smoke and deficits in working memory—an important construct of executive control. This suggests that the greater the dysfunction in dynamic network coupling between the SN and either the DMN or the ECN, the larger the subjective urge to smoke and the cognitive decline seen during abstinence.116
In sum, during abstinence, affective and subjective sensations orient smokers to endogenous craving signals, a process indexed by the coupling of the SN and the DMN. This endogenous orientation comes at the expense of an anticorrelated exogenous orientation, indexed by coupling between the SN and the ECN. In addition, the stronger coupling between nodes of the SN and the ECN following smoking may shed light on the mechanisms of cognitive enhancement seen with administration of acute nicotine in non-smokers, as discussed above.
Gaps in the current rsFC literature
Notably absent from the three-network model of nicotine addiction is any involvement of the mesocortical limbic dopamine (MCL DA) pathway. Reward dysfunction is implicated in addiction,3 yet the ICA methods used to identify the SN, DMN, and ECN across numerous cohorts of smokers and non-smokers do not consistently identify a large-scale MCL DA network. However, recent evidence in cocaine-dependent individuals suggests functional connections between striatal MCL DA seeds and the inhibitory control network121 as well as a variety of other cortical regions including the insula.122 In addition, enhanced rsFC in a cortical–striatial–amygdala circuit is observed123 (see Ref. 124 for a review). Further, in healthy controls, the insula exerts downstream influence on the nucleus accumbens and the ventral striatum.125 Thus, network connectivity among the previously discussed SN, DMN, and ECN, and the MCL DA pathway are implicated in addiction via novel analytical methods, although further empirical evidence is needed.
Other gaps in the rsFC literature include the absence of stronger links between the rsFC modulations described above and genetic differences between smokers and non-smokers. An established link exists between genetic variation and addiction,126–128 and evidence for a genetic basis of downregulated rsFC circuits in smokers has been observed.129 However, further interrogation of the influence of candidate single-nucleotide polymorphisms, including those within the CHRNA5 cluster and CYP2A6 gene, on rsFC in smokers is warranted. In addition, large-scale treatment studies should include the collection of resting-state data in an effort to characterize not only the initial differences between smokers and non-smokers, but also those changes that are plastic over the course of treatment. The ease of data collection and task independence allow for integration of resting-state measures into multisite clinical trials as well as future meta-analyses. A better understanding of network upregulation and downregulation associated with successful smoking cessation is an important step to improving treatment outcomes by influencing these dynamics.
Finally, beyond characterizing differences in rsFC between smokers and non-smokers, future research should focus on the ability to modulate specific rsFC circuits through interventional methods including neurofeedback,71,130,131 and mindfulness training.132 Recent evidence demonstrates the feasibility of these techniques, and experimental manipulation of rsFC circuits would provide a causal link between changes in rsFC and specific performance or treatment outcomes. In addition, transcranial magnetic stimulation (TMS) may prove useful in modulating addiction-related rsFC, as evidence to date holds promise for therapeutic applications.133
Value of resting-state functional connectivity in nicotine biomarker development
The development of quantifiable indices of nicotine addiction severity remains a major thrust of current neuroimaging research.59,61,134 It has been previously speculated that rsFC may be uniquely positioned to serve as a systems-level tool for neuropsychiatric disease diagnosis.50,135 Since both state and trait changes associated with nicotine exposure can be simultaneously characterized via functional connectivity, and since the stability of these large-scale network measures is maintained across multiple scanning sessions, rsFC measures may have the requisite consistency and sensitivity to characterize changes over the course of smoking cessation treatment.
Furthermore, the consistency of disruptions in functional circuits and networks associated with chronic nicotine exposure and the articulated theoretical framework of internetwork connectivity dynamics during acute nicotine withdrawal provide a foundation on which to develop rsFC-centered biomarkers. Chronic nicotine exposure is correlated with downregulation of a dACC–ventral striatal limbic connection,69,72,83 in addition to patterns of rsFC dysregulation—specifically reductions in global connectivity81—within highly connected regions of the ECN and the DMN.80 Conversely, acute nicotine administration in smokers also appears to counteract the decreased global connectivity and enhanced local clustering observed in chronic smokers.105 Finally, the tripartite network model of rsFC among the SN, DMN, and ECN100 provides a hypothesis-driven framework to combine with data-driven methods like graph-theoretical measures in the characterization of the effects of nicotine withdrawal. The ability to simultaneously quantify opposing effects of chronic and acute nicotine on network connectivity in smokers is a unique property of resting-state data.
However, the question remains, what information would brain-based biomarkers of addiction severity provide that self-reports of craving severity (e.g., FTND) do not? For one, self-report measures provide little guidance about how treatment plans should differ among heterogeneous subgroups of smokers. As outlined above, recent evidence has shown that differences in potential treatment response can be identified and leveraged to provide treatment alternatives better suited to specific subgroups of smokers.1,2 In addition, specific changes in rsFC have been correlated with FTND,69,72,81,113 duration of chronic nicotine exposure,80,81,105 smoking cue reactivity,82,83,112 and subjective craving during nicotine withdrawal.108,116 These results suggest a link between the types of functional connectivity changes discussed above and clinically relevant behavioral and subjective reports. Thus, rsFC measures may ultimately provide additional, neurobiological mechanistic context and predictive validity beyond self-report measures of drug use and addiction severity.
Using smoking cessation and other variants of substance abuse as proofs of concept, a number of recent studies have linked differences in baseline functional connectivity with distal treatment outcomes. Increased relapse risk in treatment-seeking smokers was correlated with decreased rsFC between a bilateral, whole-insula seed and primary sensorimotor areas including the pre- and postcentral gyri.136 In addition, 30-day relapse risk and trait impulsivity were associated with reduced connectivity in a putamen–posterior insula–postcentral gyrus circuit in treatment-seeking cocaine users.137 Finally, decreases in rsFC in early alcohol abstinence were correlated with relapse, suggesting that low rsFC may decrease the ability to react appropriately to external cues and may precipitate relapse in addicted individuals.138
Specific targets for rsFC biomarker development
A growing body of evidence suggests that key hub regions exist across a range of resting state networks.139–141 A number of the brain regions discussed in this review have been implicated in other quantifications of network topology. For example, functional hubs within the frontal parietal network (an analog of the ECN) play a central role in adaptive cognitive control, independent of psychopathology.141 These hubs and their connections may further articulate the mechanisms of addiction and, perhaps more importantly, provide foci on which to develop biomarkers for diagnostic and treatment outcome predictions.
Specifically for rsFC of nicotine addiction, a number of potential hubs can be derived from the results reviewed above. Reductions in connections between the dlPFC, the parietal nodes, and the rest of ECN, as well as the mPFC, the PCC, and the rest of the DMN, are seen in smokers.80 In addition, rsFC within the middle superior frontal gyrus (mSFG) has been implicated in the effects of chronic nicotine exposure. Decreases in the efficiency of the mSFG as a network hub are negatively correlated with FTND score,81 and global brain connectivity in SFG is enhanced during abstinence.114 Furthermore, mSFG connectivity is a discriminatory feature in a machine-learning classifier to distinguish smokers and non-smokers.142 This suggests that. within the context of the larger ECN, the mSFG, dlPFC, and parietal nodes may be of particular importance when quantifying the effects of chronic nicotine exposure on rsFC.
The primacy of the insula node within the SN50 and the repeated occurrence of insular circuit modulations in nicotine addiction70,83,100,112–115 suggest that dynamic changes in connectivity between the insula and a variety of brain regions may be of special importance in characterizing the mechanistic dysfunctions of withdrawal. In addition, converging evidence from tractography and functional connectivity analysis illustrates an integrative hub in the rostral striatum that combines reward processing with inputs from the OFC, dlPFC and parietal cortex.143 These findings of a structurally instantiated network of reward and higher cognitive processes are in line with previous rsFC evidence144 and serve as a potential link in rsFC-based studies of addiction, which to date have not often included striatal nodes (see Ref. 124).
While the described correlations between rsFC and craving measures and treatment outcomes inform the neurobiological underpinnings of nicotine addiction, they are not sufficient to provide predictive biomarkers.145 Such correlational analyses rely on specific characteristics of an experimental cohort and can thus bias predictions made on future, independent samples.146 In order to furnish information useful in the clinic, biomarkers must provide data about the presence of a condition in an individual, as opposed to information about group differences between individuals with and without a condition.62 For a useful biomarker, the goal is to quantify how a test result predicts a condition. Correlational analyses instead quantify how a condition predicts a test result. As an alternative, machine-learning methods145,147 allow for progression beyond correlations between neurobiological signals and outcome and towards development of classifiers able to predict diagnostic group membership based on rsFC data. Supervised machine learning allows for the application of predictions at the level of individuals. While this work on biomarker development is in its infancy, the efficacy of predictive measures for group membership or disease severity goes beyond a mechanistic understanding of brain dysfunction and provides actionable information for interventions. The results are promising in that rsFC can be used either alone142 or in combination with other measures148 to predict—in independent cohorts of participants—group membership and eventually treatment outcomes.
Equipped with an organizing principle for large-scale network interaction in nicotine addiction, a more focused interrogation of the network interactions dependent on state and trait is advocated for future biomarker development. Leveraging the connectivity between hubs in the large-scale networks may ultimately prove to be a fruitful path in the development of addiction biomarkers. Indeed, a handful of recent studies142,148 provide proof of concept for the predictive validity of resting-state measures. Using the literature reviewed above to target specific nodes and edges on which to base predictions would seem an appropriate focus of future work.
Figure 1.
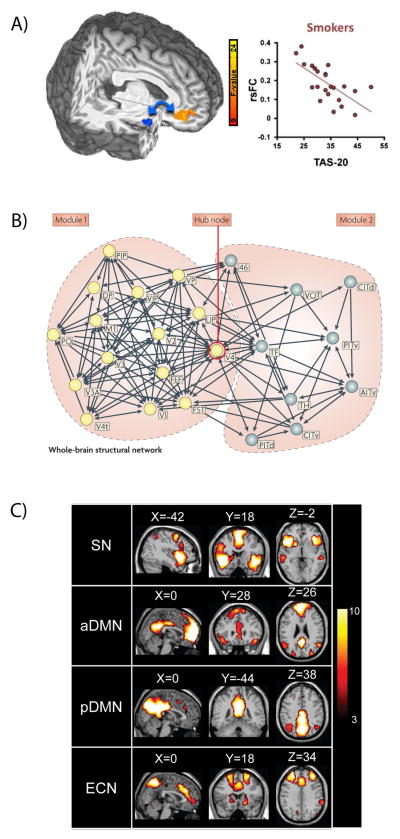
Examples of rsFC analysis methodologies. (A) Seed-based analysis showing decreased rsFC between an a priori right ventral–anterior insula seed (blue) and vmPFC associated with increased 20-item Toronto alexithymia scale (TAS-20) scores in smokers. Figure modified from Ref. 70. (B) Graph-theoretical analysis showing structural connections based on macaque cortex histological data. Two modules (yellow and gray nodes, respectively) show high clustering and limited connectivity between modules, and are linked by a hub at V4. Figure modified from Ref. 140. (C) Independent components analysis (ICA) showing distinct, correlated activation within the large-scale salience network (SN), the anterior and posterior default mode networks (aDMN, pDMN), and the executive control network (ECN). Figure modified from Ref. 117.
Figure 2.
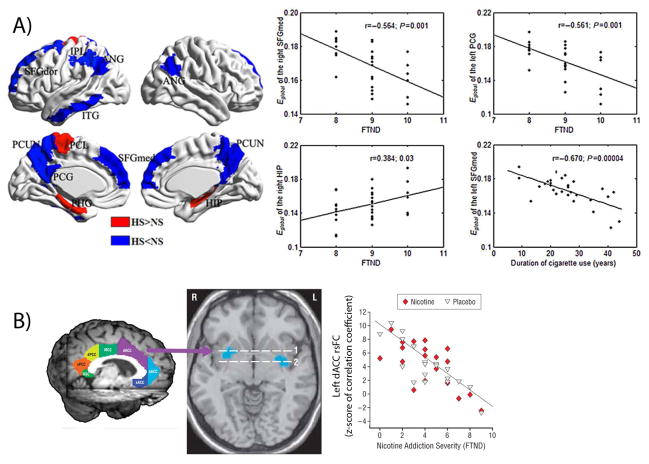
Chronic nicotine effects on rsFC. Decreases in rsFC across a range of brain networks are associated with increased duration and severity of nicotine addiction. (A) Blue regions show reduced global efficiency (E global) while red regions show increased E global in heavy smokers (HS) as compared to non-smokers (NS). Global efficiency is negatively correlated with nicotine dependence (as measured by FTND) and duration of cigarette use in HS group. Figure modified from Ref. 81. (B) Resting-state functional connectivity between a dACC seed (purple) and the bilateral ventral striatum (blue in axial brain slice) is negatively correlated with FTND in smokers. This negative correlation is unrelated to acute nicotine administration and is observed following administration of either nicotine or placebo. Figure modified from Ref. 69. ANG, angular gyrus; CUN, cuneus; FTND, Fagerström test for nicotine dependence; HIP, hippocampus; IFGtraing, triangular inferior frontal gyrus; IOG, inferior occipital gyrus; IPL, inferior parietal but supramarginal and angular gyri; ITG, inferior temporal gyrus; L, left hemisphere; LING, lingual gyrus; MOG, middle occipital gyrus; ORBsupmed, medial orbital superior frontal gyrus; PCG, posterior cingulate gyrus; PCL, paracentral lobule; PCUN, precuneus; PHG, parahippocampal gyrus; R, right hemisphere; SFGdor, dorsolateral superior frontal gyrus; SFGmed, medial superior frontal gyrus; SOG, superior occipital gyrus; dACC, dorsal anterior cingulate cortex (ACC); dPCC, dorsal posterior cingulate cortex (PCC); FTND, Fagerström test for nicotine dependence; MCC, middle cingulate cortex; oACC, rostral ACC; RSC, retrosplenial cortex; sACC, subcallosal ACC; vPCC, ventral PCC.
Figure 3.
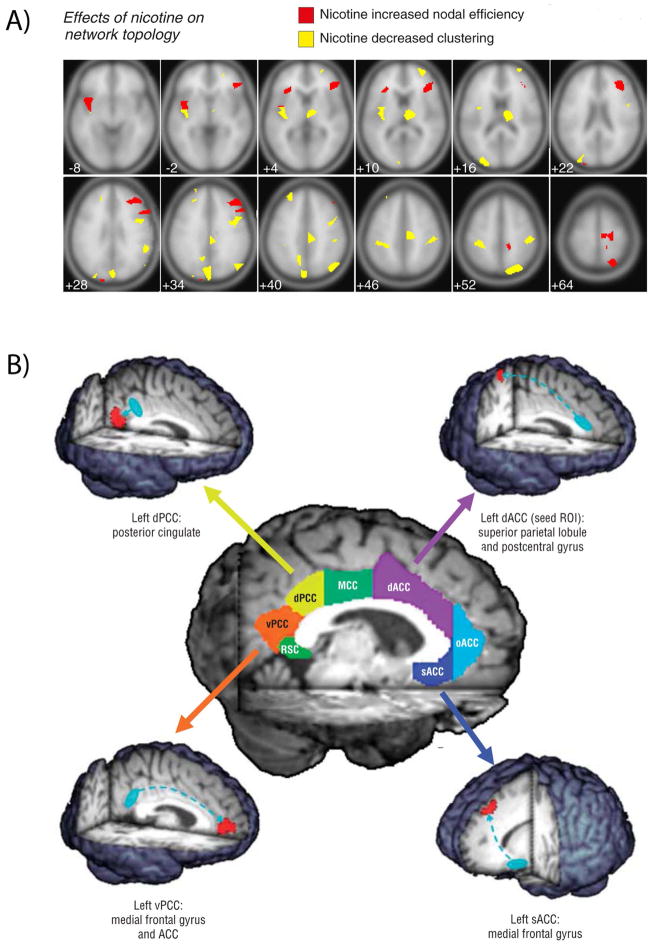
Acute nicotine effects on rsFC. In smokers, acute nicotine enhances rsFC throughout the brain. (A) Smokers administered nicotine gum display changes in nodal topology—increased nodal efficiency (red) and decreased clustering (yellow). Note the contrast with the reductions of network efficiency with chronic nicotine exposure in Figure 2A. Figure modified from Ref. 105. (B) Acute nicotine increases connectivity in circuits with a variety of cingulate seeds. Note the contrast to the effects of chronic nicotine in Figure 2B. Figure modified from Ref. 69. dACC, dorsal anterior cingulate cortex (ACC); dPCC, dorsal posterior cingulate cortex (PCC); MCC, middle cingulate cortex; oACC, rostral ACC; RSC, retrosplenial cortex; sACC, subcallosal ACC; vPCC, ventral PCC.
Table 1.
Summary of effects of nicotine on resting-state functional connectivity
| Chronic nicotine | ||||
|---|---|---|---|---|
| Ref # | Authors | Method | Circuit | Results |
| 69 | Hong et al., (2009) | Seed Based | Insula-ventral straitum | Decreased connectivity correlated with increased FTND |
|
| ||||
| 70 | Sutherland et al., (2013) | Seed Based | Insula-vmPFC | Decreased connectivity correlated with increased alexithymia |
|
| ||||
| 72 | Moran et al., (2013) | Seed Based | dACC-ventral striatum, parahippocampus, amygdala, posterior insula | Decreased connectivity in smokers |
|
| ||||
| 74 | Moran et al., (2012) | Seed Based | dACC-putamen, insula | Decreased connectivity correlated with increased addiction severity (FTND) |
|
| ||||
| 80 | Weiland et al., (2014) | ICA | DMN, left ECN | Decreased connectivity within each network correlated with duration and frequency of smoking |
|
| ||||
| 81 | Lin et al., (2014) | Graph Theory | PCC, mSFG Occipital cortex |
Decreased global efficiency correlated with lifetime cigarette use Increased local efficiency correlated with lifetime cigarette use |
|
| ||||
| 82 | Janes et al., (2012) | ICA | Fronto-parietal (ECN)-mfPFC (DMN) | Increased connectivity in female smokers correlated with smoking cue reactivity |
|
| ||||
| 83 | Janes et al., (2015) | Seed Based | Insula-dACC | Increased connectivity correlated with smoking cue reactivity |
|
| ||||
| 86 | Wu et al., (2015) | ReHo | DMN, fronto-parital, inhibitory control networks SMA, putamen, paracentral lobule |
Decreased in smokers Increased in smokers |
|
| ||||
| 90 | Tang et al., (2012) | ReHo | Right inferior frontal cortex Superior parietal gyrus |
Decreased in smokers Increased in smokers |
|
| ||||
| 91 | Yu et al., (2013) | ReHo | Right lPFC, left mPFC PCC, posterior insula |
Decreased in smokers Increased in smokers |
|
| ||||
| 93 | Wetherill et al., (2014) | ASL | Hippocampus/amygdala-rACC, anterior insula | Increased connectivity in female smokers |
|
| ||||
| Acute nicotine | ||||
|---|---|---|---|---|
| Ref # | Authors | Method | Circuit | Results |
| 69 | Hong et al., (2009) | Seed based | dACC–superior parietal lobule, sACC–medial frontal gyrus | Enhanced connectivity |
|
| ||||
| 101 | Tanabe et al., (2011) | ICA | DMN | Decreased activation within network |
|
| ||||
| 102 | Wylie et al., (2012) | Graph theory | Enhanced local network efficiency | |
|
| ||||
| 103 | Viswanth et al., (2015) | Seed based | Rostral left–right hemisphere | Enhanced connectivity |
|
| ||||
| 105 | Giessing et al., (2013) | Graph theory | MFG | Enhanced efficiency of information transfer correlated with frequency of cigarette smoking |
|
| ||||
| 115 | Sutherland et al., (2013) | Seed based | Insula–PCC | Reduced connectivity |
|
| ||||
| 117 | Ding et al., (2013) | ICA | Superior frontal gyrus (SN), precuneus (DMN) | Enhanced connectivity |
| Nicotine withdrawal | ||||
|---|---|---|---|---|
| Ref # | Authors | Method | Circuit | Results |
| 108 | Janes et al., (2014) | ICA | mOFC–dmPFC, striatum, visual cortex | Increased connectivity w/ 1 h of abstinence correlated with subjective nicotine craving |
|
| ||||
| 109 | Breckel et al., (2013) | Graph theory | No effects w/ 2 h of abstinence | |
|
| ||||
| 112 | Moran-Santa Maria et al., (2015) | PPI | Insula–precuneus | Increased connectivity during cue exposure w/ 2 h of abstinence correlated with subjective nicotine craving |
|
| ||||
| 113 | Claus et al., (2013) | PPI | Insula–striatum, thalamus, cuneus, ACC | Increased connectivity during cue exposure w/ 3 h of abstinence correlated with FTND |
|
| ||||
| 114 | Wang et al., (2014) | GBC | insula, superior frontal gyrus | Increased connectivity with rest of brain w/ 12 h of abstinence |
|
| ||||
| 115 | Sutherland et al., (2013) | Seed based | Insula–PCC | Enhanced connectivity w/ 12 h of abstinence |
|
| ||||
| 116 | Lerman et al., (2014) | ICA | SN–DMN,ECN | Reduced inhibition of DMN by SN w/ 24 h of abstinence correlated with subjective urge to smoke and working memory task performance (n-back) |
|
| ||||
| 117 | Ding et al., (2013) | ICA | SN, DMN | Enahnced connectivity within networks w/ 12 h of abstinence |
Acknowledgments
The authors thank X. Ding, J.L. Gowin, E. Lesage, and V. Pariyadath for helpful discussion. This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
References
- 1.Lerman C, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3:131–8. doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamoun M, Bergen AW, Shieh J, Wiggins A, Brody AL. Biomarkers of Response to Smoking Cessation Pharmacotherapies: Progress to Date. CNS Drugs. 2015 doi: 10.1007/s40263-015-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 5.Honey CJ, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–40. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–90. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–34. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, et al. Group independent component analysis reveals consistent resting-state networks across multiple sessions. Brain Res. 2008;1239:141–51. doi: 10.1016/j.brainres.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo XN, et al. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49:2163–77. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo CC, et al. One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage. 2012;61:1471–83. doi: 10.1016/j.neuroimage.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–37. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Baldassarre A, et al. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci U S A. 2012;109:3516–21. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou Q, et al. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Hum Brain Mapp. 2013;34:3204–15. doi: 10.1002/hbm.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grigg O, Grady CL. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS One. 2010;5:e13311. doi: 10.1371/journal.pone.0013311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison BJ, et al. Modulation of brain resting-state networks by sad mood induction. PLoS One. 2008;3:e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb Cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Gruttola VG, et al. Considerations in the Evaluation of Surrogate Endpoints in Clinical Trials. Control Clin Trials. 2001;22:485–502. doi: 10.1016/s0197-2456(01)00153-2. [DOI] [PubMed] [Google Scholar]
- 20.Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satterthwaite TD, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–32. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weissenbacher A, et al. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–16. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–65. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shmueli K, et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007;38:306–20. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirer WR, Jiang H, Price CM, Ng B, Greicius MD. Optimization of rs-fMRI Pre-processing for Enhanced Signal-Noise Separation, Test-Retest Reliability, and Group Discrimination. Neuroimage. 2015;117:67–79. doi: 10.1016/j.neuroimage.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Power JD, Schlaggar BL, Petersen SE. Studying Brain Organization via Spontaneous fMRI Signal. Neuron. 2014;84:681–696. doi: 10.1016/j.neuron.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland MT, Liang X, Yang Y, Stein EA. In: The Wiley Handbook on the Cognitive Neuroscience of Addiction. Wilson SJ, editor. Wiley-Blackwell; 2015. pp. 472–502. at < http://www.wiley.com/WileyCDA/WileyTitle/productCd-1118472241.html>. [Google Scholar]
- 31.Smith SM, et al. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;17:666–82. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sporns O. Networks of the Brain. 2010 at < http://dl.acm.org/citation.cfm?id=2024602>.
- 33.Bullmore E, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–40. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- 34.Mckeown MJ, et al. Analysis of fMRI data by blind separation into independent spatial components. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. http://dx.doi.org/10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed]
- 35.Smith SM, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–33. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 40.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honey CJ, Kötter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci U S A. 2007;104:10240–5. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belcher AM, et al. Large-scale brain networks in the awake, truly resting marmoset monkey. J Neurosci. 2013;33:16796–804. doi: 10.1523/JNEUROSCI.3146-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu H, et al. Rat brains also have a default mode network. Proc Natl Acad Sci U S A. 2012;109:3979–84. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton JP, et al. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–33. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellanos FX, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–7. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prado J, Weissman DH. Heightened interactions between a key default-mode region and a key task-positive region are linked to suboptimal current performance but to enhanced future performance. Neuroimage. 2011;56:2276–82. doi: 10.1016/j.neuroimage.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 50.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–96. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 52.Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–86. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Cole DM, et al. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- 54.Picard F, et al. High density of nicotinic receptors in the cingulo-insular network. Neuroimage. 2013;79:42–51. doi: 10.1016/j.neuroimage.2013.04.074. [DOI] [PubMed] [Google Scholar]
- 55.Baker TB, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9 (Suppl 4):S555–70. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Japuntich SJ, et al. Smoker characteristics and smoking-cessation milestones. Am J Prev Med. 2011;40:286–94. doi: 10.1016/j.amepre.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fidler JA, Shahab L, West R. Strength of urges to smoke as a measure of severity of cigarette dependence: comparison with the Fagerström Test for Nicotine Dependence and its components. Addiction. 2011;106:631–8. doi: 10.1111/j.1360-0443.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- 58.Brody AL, et al. Brain nicotinic acetylcholine receptor availability and response to smoking cessation treatment: a randomized trial. JAMA psychiatry. 2014;71:797–805. doi: 10.1001/jamapsychiatry.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrison KA, Potenza MN. Neuroimaging and biomarkers in addiction treatment. Curr Psychiatry Rep. 2014;16:513. doi: 10.1007/s11920-014-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Volkow ND, Baler RD. Brain imaging biomarkers to predict relapse in alcohol addiction. JAMA psychiatry. 2013;70:661–3. doi: 10.1001/jamapsychiatry.2013.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volkow ND, Koob G, Baler R. Biomarkers in Substance Use Disorders. ACS Chem Neurosci. 2015;6:522–525. doi: 10.1021/acschemneuro.5b00067. [DOI] [PubMed] [Google Scholar]
- 62.Reske M, Paulus MP. In: Neuroimaging in Addiction. Adinoff B, Stein EA, editors. John Wiley & Sons; 2011. pp. 321–343. [Google Scholar]
- 63.Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev. 2007;17:259–73. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 64.Goldstein RZ, Volkow ND. Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rose EJ, et al. Acute nicotine differentially impacts anticipatory valence- and magnitude-related striatal activity. Biol Psychiatry. 2013;73:280–8. doi: 10.1016/j.biopsych.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jasinska AJ, Zorick T, Brody AL, Stein EA. Dual role of nicotine in addiction and cognition: A review of neuroimaging studies in humans. Neuropharmacology. 2014;84C:111–122. doi: 10.1016/j.neuropharm.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutherland MT, et al. Neurobiological impact of nicotinic acetylcholine receptor agonists: An ALE meta-analysis of pharmacological neuroimaging studies. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong LE, et al. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–41. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Stein EA. Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology (Berl) 2013;228:143–155. doi: 10.1007/s00213-013-3018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haller S, et al. Dynamic reconfiguration of human brain functional networks through neurofeedback. Neuroimage. 2013;81:243–52. doi: 10.1016/j.neuroimage.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 72.Moran LV, Sampath H, Kochunov P, Hong LE. Brain circuits that link schizophrenia to high risk of cigarette smoking. Schizophr Bull. 2013;39:1373–81. doi: 10.1093/schbul/sbs149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong LE, et al. A CHRNA5 allele related to nicotine addiction and schizophrenia. Genes, Brain Behav. 2011;10:530–535. doi: 10.1111/j.1601-183X.2011.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moran LV, Sampath H, Stein EA, Hong LE. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophr Res. 2012;142:223–9. doi: 10.1016/j.schres.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 76.Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021–34. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 77.Hahn B, et al. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biol Psychiatry. 2013;74:436–43. doi: 10.1016/j.biopsych.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hong LE, et al. Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophr Bull. 2011;37:416–25. doi: 10.1093/schbul/sbp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lyons MJ, et al. Nicotine and familial vulnerability to schizophrenia: A discordant twin study. 2002 doi: 10.1037//0021-843x.111.4.687. at < http://psycnet.apa.orgjournals/abn/111/4/687>. [DOI] [PubMed]
- 80.Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Hutchison KE. Reduced executive and default network functional connectivity in cigarette smokers. Hum Brain Mapp. 2014;36(3):872–82. doi: 10.1002/hbm.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin F, Wu G, Zhu L, Lei H. Altered brain functional networks in heavy smokers. Addict Biol. 2014;36:872–82. doi: 10.1111/adb.12155. [DOI] [PubMed] [Google Scholar]
- 82.Janes AC, Nickerson LD, Frederick BDB, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125:252–9. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Janes AC, Farmer S, Peechatka AL, Frederick B, de B, Lukas SE. Insula-Dorsal Anterior Cingulate Cortex Coupling is Associated with Enhanced Brain Reactivity to Smoking Cues. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 86.Wu G, Yang S, Zhu L, Lin F. Altered spontaneous brain activity in heavy smokers revealed by regional homogeneity. Psychopharmacology (Berl) 2015;232(14):2481–9. doi: 10.1007/s00213-015-3881-6. [DOI] [PubMed] [Google Scholar]
- 87.Hahn B, et al. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–89. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hahn B, et al. Performance effects of nicotine during selective attention, divided attention, and simple stimulus detection: an fMRI study. Cereb Cortex. 2009;19:1990–2000. doi: 10.1093/cercor/bhn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luijten M, et al. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci. 2014;39:149–69. doi: 10.1503/jpn.130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang J, et al. Altered spontaneous activity in young chronic cigarette smokers revealed by regional homogeneity. Behav Brain Funct. 2012;8:44. doi: 10.1186/1744-9081-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu R, et al. Regional homogeneity changes in heavy male smokers: a resting-state functional magnetic resonance imaging study. Addict Biol. 2013;18:729–31. doi: 10.1111/j.1369-1600.2011.00359.x. [DOI] [PubMed] [Google Scholar]
- 92.Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging. 2003;18:649–55. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- 93.Wetherill RR, Jagannathan K, Shin J, Franklin TR. Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addict Behav. 2014;39:789–92. doi: 10.1016/j.addbeh.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Franklin TR, et al. The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One. 2014;9:e104102. doi: 10.1371/journal.pone.0104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–57. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- 96.Field M, Duka T. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78:647–52. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 97.Wetter DW, et al. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67:555–62. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 98.Perkins KA. Smoking Cessation in Women. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- 99.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210:453–69. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:1–15. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tanabe J, et al. Nicotine effects on default mode network during resting state. Psychopharmacology (Berl) 2011;216:287–95. doi: 10.1007/s00213-011-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wylie KP, Rojas DC, Tanabe J, Martin LF, Tregellas JR. Nicotine increases brain functional network efficiency. Neuroimage. 2012;63:73–80. doi: 10.1016/j.neuroimage.2012.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Viswanath H, et al. Alterations in interhemispheric functional and anatomical connectivity are associated with tobacco smoking in humans. Front Hum Neurosci. 2015;9:116. doi: 10.3389/fnhum.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 105.Giessing C, Thiel CM, Alexander-Bloch AF, Patel AX, Bullmore ET. Human brain functional network changes associated with enhanced and impaired attentional task performance. J Neurosci. 2013;33:5903–14. doi: 10.1523/JNEUROSCI.4854-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim J, et al. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage. 2010;49:3426–35. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res. 2007;9:329–39. doi: 10.1080/14622200701188927. [DOI] [PubMed] [Google Scholar]
- 108.Janes AC, Farmer S, Frederick B, de B, Nickerson LD, Lukas SE. An increase in tobacco craving is associated with enhanced medial prefrontal cortex network coupling. PLoS One. 2014;9:e88228. doi: 10.1371/journal.pone.0088228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Breckel TPK, Thiel CM, Giessing C. The efficiency of functional brain networks does not differ between smokers and non-smokers. Psychiatry Res. 2013;214:349–56. doi: 10.1016/j.pscychresns.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 110.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 111.O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7:604–9. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moran-Santa Maria MM, et al. Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addict Biol. 2015;20:407–14. doi: 10.1111/adb.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38:2363–72. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang K, et al. The neural mechanisms underlying the acute effect of cigarette smoking on chronic smokers. PLoS One. 2014;9:e102828. doi: 10.1371/journal.pone.0102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sutherland MT, et al. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013;74:538–546. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lerman C, et al. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA psychiatry. 2014;71:523–30. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ding X, Lee SW. Changes of functional and effective connectivity in smoking replenishment on deprived heavy smokers: a resting-state FMRI study. PLoS One. 2013;8:e59331. doi: 10.1371/journal.pone.0059331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Craig ADB. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 119.Smith SM, et al. Network modelling methods for FMRI. Neuroimage. 2011;54:875–91. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 120.Oncken C, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–7. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- 121.Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired Functional Connectivity Within and Between Frontostriatal Circuits and Its Association With Compulsive Drug Use and Trait Impulsivity in Cocaine Addiction. JAMA psychiatry. 2015;72(6):584–92. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- 122.Gu H, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Contreras-Rodríguez O, et al. Increased corticolimbic connectivity in cocaine dependence versus pathological gambling is associated with drug severity and emotion-related impulsivity. Addict Biol. 2015:n/a–n/a. doi: 10.1111/adb.12242. [DOI] [PubMed] [Google Scholar]
- 124.Pariyadath V, Gowin JL, Stein EA. doi: 10.1016/bs.pbr.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 125.Cho YT, et al. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage. 2013;66:508–21. doi: 10.1016/j.neuroimage.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr Clin North Am. 2012;35:495–519. doi: 10.1016/j.psc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wassenaar CA, et al. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103:1342–6. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen LS, et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am J Psychiatry. 2012;169:735–42. doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hong LE, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107:13509–14. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hanlon CA, et al. Reduction of cue-induced craving through realtime neurofeedback in nicotine users: the role of region of interest selection and multiple visits. Psychiatry Res. 2013;213:79–81. doi: 10.1016/j.pscychresns.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim D-Y, Yoo S-S, Tegethoff M, Meinlschmidt G, Lee J-H. The Inclusion of Functional Connectivity Information into fMRI-based Neurofeedback Improves Its Efficacy in the Reduction of Cigarette Cravings. J Cogn Neurosci. 2015:1–21. doi: 10.1162/jocn_a_00802. [DOI] [PubMed] [Google Scholar]
- 132.Paulus MP, Stewart JL, Haase L. Treatment approaches for interoceptive dysfunctions in drug addiction. Front psychiatry. 2013;4:137. doi: 10.3389/fpsyt.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci. 2014;1327:79–93. doi: 10.1111/nyas.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bough KJ, et al. Biomarkers for smoking cessation. Clin Pharmacol Ther. 2013;93:526–38. doi: 10.1038/clpt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;24:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 136.Addicott MA, Sweitzer MM, Froeliger B, Rose JE, McClernon FJ. Increased Functional Connectivity in an Insula-Based Network is Associated with Improved Smoking Cessation Outcomes. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McHugh MJ, et al. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am J Drug Alcohol Abuse. 2013;39:424–32. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- 138.Camchong J, Stenger A, Fein G. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb Cortex. 2013;23:2086–99. doi: 10.1093/cercor/bhs190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 141.Cole MW, et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–55. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pariyadath V, Stein EA, Ross TJ. Machine learning classification of resting state functional connectivity predicts smoking status. Front Hum Neurosci. 2014;8:425. doi: 10.3389/fnhum.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jarbo K, Verstynen TD. Converging Structural and Functional Connectivity of Orbitofrontal, Dorsolateral Prefrontal, and Posterior Parietal Cortex in the Human Striatum. J Neurosci. 2015;35:3865–3878. doi: 10.1523/JNEUROSCI.2636-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Choi EY, Yeo BTT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–63. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gabrieli JDE, Ghosh SS, Whitfield-Gabrieli S. Prediction as a Humanitarian and Pragmatic Contribution from Human Cognitive Neuroscience. Neuron. 2015;85:11–26. doi: 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Whelan R, Garavan H. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biol Psychiatry. 2014;75:746–8. doi: 10.1016/j.biopsych.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 147.Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. Neuroimage. 2009;45:S199–209. doi: 10.1016/j.neuroimage.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deshpande G, et al. Recursive Cluster Elimination Based Support Vector Machine for Disease State Prediction Using Resting State Functional and Effective Brain Connectivity. PLoS One. 2010;5:e14277. doi: 10.1371/journal.pone.0014277. [DOI] [PMC free article] [PubMed] [Google Scholar]